Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (9): 4731-4745.DOI: 10.16085/j.issn.1000-6613.2022-1905
• Materials science and technology • Previous Articles Next Articles
Properties and research progress of magnesium based hydrogen storage materials
SHI Keke( ), LIU Muzi, ZHAO Qiang, LI Jinping, LIU Guang(
), LIU Muzi, ZHAO Qiang, LI Jinping, LIU Guang( )
)
- Shanxi Key Laboratory of Gas Energy Efficient and Clean Utilization, College of Chemical Engineering and Technology, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
-
Received:2022-10-13Revised:2023-01-01Online:2023-09-28Published:2023-09-15 -
Contact:LIU Guang
镁基储氢材料的性能及研究进展
- 太原理工大学化学工程与技术学院,气体能源高效清洁利用山西省重点实验室,山西 太原 030024
-
通讯作者:刘光 -
作者简介:史柯柯(1998—),女,硕士研究生,研究方向为镁基固态储氢技术。E-mail:1349177646@qq.com。 -
基金资助:国家自然科学基金(21878204);山西省重点研发计划国际合作项目(201903D421073)
CLC Number:
Cite this article
SHI Keke, LIU Muzi, ZHAO Qiang, LI Jinping, LIU Guang. Properties and research progress of magnesium based hydrogen storage materials[J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4731-4745.
史柯柯, 刘木子, 赵强, 李晋平, 刘光. 镁基储氢材料的性能及研究进展[J]. 化工进展, 2023, 42(9): 4731-4745.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-1905
| 合金 | Tonset/℃ | 焓变ΔH /kJ·mol-1 H2 | 氢质量分数 /% | 参考 文献 |
|---|---|---|---|---|
| Mg2Ni | 254 | 64.5 | 3.6 | [ |
| Mg2FeH6 | — | 77.4 | 5.5 | [ |
| Mg2CoH5 | 317 | 83.2 | 4.4 | [ |
| Mg2Si | — | 36.4 | 5.0 | [ |
| Mg2Cu | 273 | 72.6 | 2.53 | [ |
| Mg17Al12 | 250 | 73.8 | 5.7 | [ |
| Mg3Cd | — | 65.5 | 2.8 | [ |
| Mg0.95In0.05 | — | 68.1 | 5.3 | [ |
| 合金 | Tonset/℃ | 焓变ΔH /kJ·mol-1 H2 | 氢质量分数 /% | 参考 文献 |
|---|---|---|---|---|
| Mg2Ni | 254 | 64.5 | 3.6 | [ |
| Mg2FeH6 | — | 77.4 | 5.5 | [ |
| Mg2CoH5 | 317 | 83.2 | 4.4 | [ |
| Mg2Si | — | 36.4 | 5.0 | [ |
| Mg2Cu | 273 | 72.6 | 2.53 | [ |
| Mg17Al12 | 250 | 73.8 | 5.7 | [ |
| Mg3Cd | — | 65.5 | 2.8 | [ |
| Mg0.95In0.05 | — | 68.1 | 5.3 | [ |
| 纳米材料 | Tonset/℃ | 焓变ΔH/kJ·mol-1 H2 | 活化能Ea/kJ·mol-1 H2 | 氢质量分数/% | 参考文献 | ||
|---|---|---|---|---|---|---|---|
| Abs | Des | Abs | Des | ||||
| MgH2-0.1TiH2 | 180 | — | 68 | — | 54.8 | 6.2 | [ |
| MgH2/c-NbH x | 237.2 | — | — | — | 50.4 | 6.1 | [ |
| Mg NCs/PMMA | — | 25 | 79 | — | — | 6 | [ |
| MgH2胶体 | 100 | — | — | — | — | 7.6 | [ |
| Mg-HDA | 115 | — | — | — | — | — | [ |
| 超细MgH2 | 30 | — | 59.5 | 28 | 80 | 6.7 | [ |
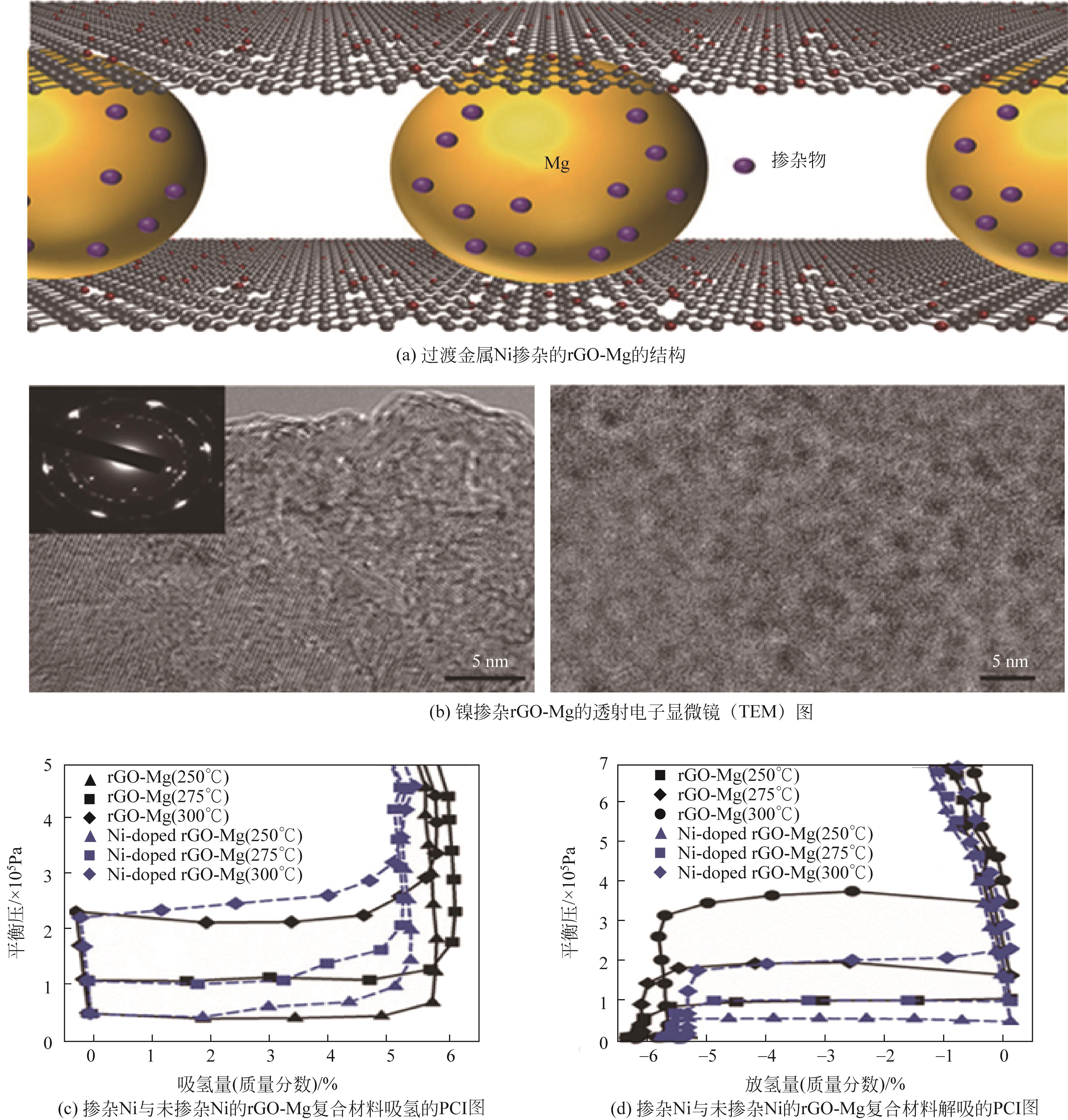
| rGO-Mg | — | 65.6 | 69.4 | 60.8 | 92.9 | 6.5 | [ |
| Ni-doped rGO-Mg | — | 63.9 | 66.9 | — | — | 6.5 | [ |
| MgH2/ACF | — | 63.8 | — | — | 52 | — | [ |
| 20% MgH2/CMK3 | 253 | — | 52.38 | — | — | 1.8 | [ |
| Ni-MHGH-75 | — | 62.1 | — | 22.7 | 64.7 | 5.4 | [ |
| MHCH-5 | — | 46.9 | 49.2 | 31 | 43 | 6.63 | [ |
| MgH2/Ni@pCNF | 200 | — | — | 25.4 | 96.58 | 4.1 | [ |
| MgH2@CoS-NBs | — | 65.6 | 68.1 | 57.4 | 120.8 | 3.23 | [ |
| 纳米线 | — | 63.3 | — | 33.5 | 38.8 | — | [ |
| Mg92V8@C | — | — | — | 41 | 67 | 5.2 | [ |
| 纳米材料 | Tonset/℃ | 焓变ΔH/kJ·mol-1 H2 | 活化能Ea/kJ·mol-1 H2 | 氢质量分数/% | 参考文献 | ||
|---|---|---|---|---|---|---|---|
| Abs | Des | Abs | Des | ||||
| MgH2-0.1TiH2 | 180 | — | 68 | — | 54.8 | 6.2 | [ |
| MgH2/c-NbH x | 237.2 | — | — | — | 50.4 | 6.1 | [ |
| Mg NCs/PMMA | — | 25 | 79 | — | — | 6 | [ |
| MgH2胶体 | 100 | — | — | — | — | 7.6 | [ |
| Mg-HDA | 115 | — | — | — | — | — | [ |
| 超细MgH2 | 30 | — | 59.5 | 28 | 80 | 6.7 | [ |
| rGO-Mg | — | 65.6 | 69.4 | 60.8 | 92.9 | 6.5 | [ |
| Ni-doped rGO-Mg | — | 63.9 | 66.9 | — | — | 6.5 | [ |
| MgH2/ACF | — | 63.8 | — | — | 52 | — | [ |
| 20% MgH2/CMK3 | 253 | — | 52.38 | — | — | 1.8 | [ |
| Ni-MHGH-75 | — | 62.1 | — | 22.7 | 64.7 | 5.4 | [ |
| MHCH-5 | — | 46.9 | 49.2 | 31 | 43 | 6.63 | [ |
| MgH2/Ni@pCNF | 200 | — | — | 25.4 | 96.58 | 4.1 | [ |
| MgH2@CoS-NBs | — | 65.6 | 68.1 | 57.4 | 120.8 | 3.23 | [ |
| 纳米线 | — | 63.3 | — | 33.5 | 38.8 | — | [ |
| Mg92V8@C | — | — | — | 41 | 67 | 5.2 | [ |
| 复合材料 | Tonset/℃ | 焓变ΔH/kJ·mol-1 H2 | 活化能Ea/kJ·mol-1 H2 | 氢质量分数/% | 参考文献 | ||
|---|---|---|---|---|---|---|---|
| Abs | Des | Abs | Des | ||||
| MgH-4%Ni NFs | 143 | — | — | — | 81.5 | 7.02 | [ |
| o-Nb2O5 | 195 | — | 74.7 | — | 101 | 6.4 | [ |
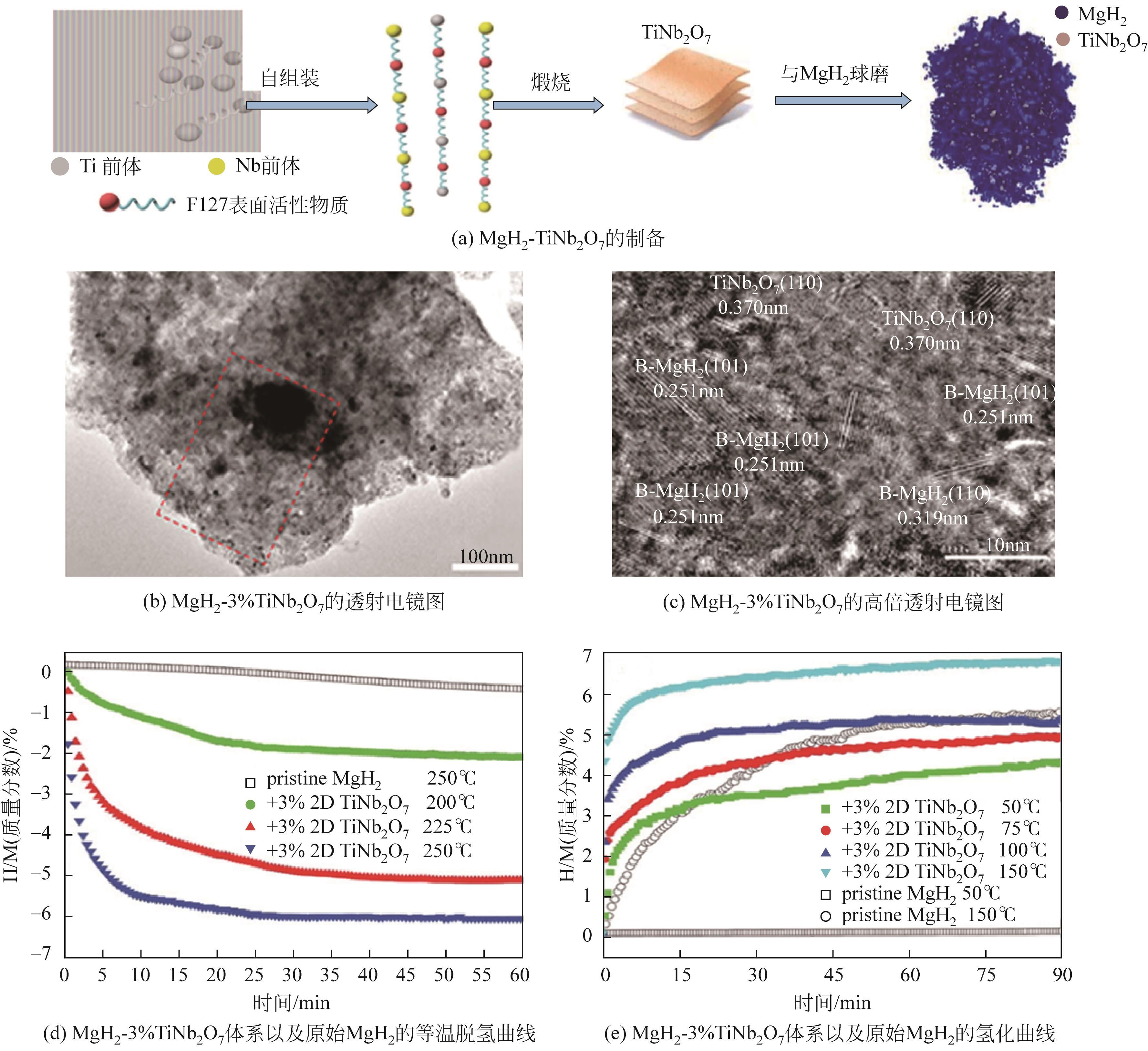
| 2D-TiNb2O7 nanoflakes | 178 | — | 75.2 | — | 100.4 | 7.0 | [ |
| MgH2-10%TiC | — | — | — | — | 144.62 | 6.01 | [ |
| MgH2:Fe3O4@GS | 262 | 60.62 | 66.34 | — | 90.53 | 6.2 | [ |
| 10%-TiFe+5%-CNTs | 210 | — | 80.6 | 60.7 | — | 6.2 | [ |
| MgH2-Co/Pd@B-CNTs | 198.9 | — | — | — | 76.66 | 6.67 | [ |
| MgH2-10% TiO2@C | 205 | — | 73.6 | 38 | 106 | 6.5 | [ |
| MgH2-TiO2 SCNPs/AC | 163.5 | — | — | — | 69.2 | 6.5 | [ |
| 复合材料 | Tonset/℃ | 焓变ΔH/kJ·mol-1 H2 | 活化能Ea/kJ·mol-1 H2 | 氢质量分数/% | 参考文献 | ||
|---|---|---|---|---|---|---|---|
| Abs | Des | Abs | Des | ||||
| MgH-4%Ni NFs | 143 | — | — | — | 81.5 | 7.02 | [ |
| o-Nb2O5 | 195 | — | 74.7 | — | 101 | 6.4 | [ |
| 2D-TiNb2O7 nanoflakes | 178 | — | 75.2 | — | 100.4 | 7.0 | [ |
| MgH2-10%TiC | — | — | — | — | 144.62 | 6.01 | [ |
| MgH2:Fe3O4@GS | 262 | 60.62 | 66.34 | — | 90.53 | 6.2 | [ |
| 10%-TiFe+5%-CNTs | 210 | — | 80.6 | 60.7 | — | 6.2 | [ |
| MgH2-Co/Pd@B-CNTs | 198.9 | — | — | — | 76.66 | 6.67 | [ |
| MgH2-10% TiO2@C | 205 | — | 73.6 | 38 | 106 | 6.5 | [ |
| MgH2-TiO2 SCNPs/AC | 163.5 | — | — | — | 69.2 | 6.5 | [ |
| 1 | SCHLAPBACH Louis, Andreas ZÜTTEL. Hydrogen-storage materials for mobile applications[J]. Nature, 2001, 414(6861): 353-358. |
| 2 | SADHASIVAM T, KIM Hee-Tak, JUNG Seunghun, et al. Dimensional effects of nanostructured Mg/MgH2 for hydrogen storage applications: A review[J]. Renewable and Sustainable Energy Reviews, 2017, 72: 523-534. |
| 3 | MURADOV Nazim Z, Nejat VEZIROĞLU T. “Green” path from fossil-based to hydrogen economy: An overview of carbon-neutral technologies[J]. International Journal of Hydrogen Energy, 2008, 33(23): 6804-6839. |
| 4 | 张秋雨, 邹建新, 任莉, 等. 核壳结构纳米镁基复合储氢材料研究进展[J]. 材料科学与工艺, 2020, 28(3): 58-67. |
| ZHANG Qiuyu, ZOU Jianxin, REN Li, et al. Research development of core-shell nanostructured Mg-based hydrogen storage composite materials[J]. Materials Science and Technology, 2020, 28(3): 58-67. | |
| 5 | ABE J O, POPOOLA A P I, AJENIFUJA E, et al. Hydrogen energy, economy and storage: Review and recommendation[J]. International Journal of Hydrogen Energy, 2019, 44(29): 15072-15086. |
| 6 | AHMED Alauddin, SETH Saona, PUREWAL Justin, et al. Exceptional hydrogen storage achieved by screening nearly half a million metal-organic frameworks[J]. Nature Communications, 2019, 10(1): 1568. |
| 7 | LEE Seul-Yi, LEE Jong-Hoon, KIM Yeong-Hun, et al. Recent progress using solid-state materials for hydrogen storage: A short review[J]. Processes, 2022, 10(2): 304. |
| 8 | BARDHAN Rizia, RUMINSKI Anne M, BRAND Alyssa, et al. Magnesium nanocrystal-polymer composites: A new platform for designer hydrogen storage materials[J]. Energy & Environmental Science, 2011, 4(12): 4882-4895. |
| 9 | Kondo-Francois AGUEY-ZINSOU, José-Ramón ARES-FERNÃNDEZ. Hydrogen in magnesium: new perspectives toward functional stores[J]. Energy & Environmental Science, 2010, 3(5): 526-543. |
| 10 | STAMPFER J F, HOLLEY C E, SUTTLE J F. The magnesium-hydrogen System1-3 [J]. Journal of the American Chemical Society, 1960, 82(14): 3504-3508. |
| 11 | BOHMHAMMEL K, WOLF U, WOLF G, et al. Thermodynamic optimization of the system magnesium-hydrogen[J]. Thermochimica Acta, 1999, 337(1/2): 195-199. |
| 12 | 雍辉, 李玉钏, 胡季帆, 等. 镁基储氢合金的研究现状[J]. 金属功能材料, 2021, 28(5): 50-56. |
| YONG Hui, LI Yuchuan, HU Jifan, et al. Recent research of Mg-based hydrogen storage material[J]. Metallic Functional Materials, 2021, 28(5): 50-56. | |
| 13 | Andreas ZÜTTEL. Materials for hydrogen storage[J]. Materials Today, 2003, 6(9): 24-33. |
| 14 | BANERJEE S, PILLAI C G S, MAJUMDER C. Dissociation and diffusion of hydrogen on the Mg(0001) surface: Catalytic effect of V and Ni double substitution[J]. The Journal of Physical Chemistry C, 2009, 113(24): 10574-10579. |
| 15 | MARTINO Paola, CHIESA Mario, CRISTINA PAGANINI Maria, et al. Coadsorption of NO and H2 at the surface of MgO monitored by EPR spectroscopy. Towards a site specific discrimination of polycrystalline oxide surfaces[J]. Surface Science, 2003, 527(1/2/3): 80-88. |
| 16 | LUO Qun, LI Jianding, LI Bo, et al. Kinetics in Mg-based hydrogen storage materials: Enhancement and mechanism[J]. Journal of Magnesium and Alloys, 2019, 7(1): 58-71. |
| 17 | REILLY James J, WISWALL Richard H. Reaction of hydrogen with alloys of magnesium and nickel and the formation of Mg2NiH4 [J]. Inorganic Chemistry, 1968, 7(11): 2254-2256. |
| 18 | POZZO M, ALFÈ D. Structural properties and enthalpy of formation of magnesium hydride from quantum Monte Carlo calculations[J]. Physical Review B, 2008, 77(10): 104103. |
| 19 | OUYANG Liuzhang, LIU Fen, WANG Hui, et al. Magnesium-based hydrogen storage compounds: A review[J]. Journal of Alloys and Compounds, 2020, 832: 154865. |
| 20 | MORINAGA M, YUKAWA H. Nature of chemical bond and phase stability of hydrogen storage compounds[J]. Materials Science and Engineering: A, 2002, 329/330/331: 268-275. |
| 21 | Borislav BOGDANOVIĆ, REISER Alexander, SCHLICHTE Klaus, et al. Thermodynamics and dynamics of the Mg-Fe-H system and its potential for thermochemical thermal energy storage[J]. Journal of Alloys and Compounds, 2002, 345(1/2): 77-89. |
| 22 | GENNARI F C, CASTRO F J, J J Andrade GAMBOA. Synthesis of Mg2FeH6 by reactive mechanical alloying: Formation and decomposition properties[J]. ChemInform, 2003, 34(12): 261-267. |
| 23 | CHEN J, TAKESHITA H T, CHARTOUNI D, et al. Synthesis and characterization of nanocrystalline Mg2CoH5 obtained by mechanical alloying[J]. Journal of Materials Science, 2001, 36(24): 5829-5834. |
| 24 | VAJO John J, MERTENS Florian, Channing C AHN, et al. Altering hydrogen storage properties by hydride destabilization through alloy formation: LiH and MgH2 destabilized with Si[J]. The Journal of Physical Chemistry B, 2004, 108(37): 13977-13983. |
| 25 | REILLY James J, WISWALL Richard H. Reaction of hydrogen with alloys of magnesium and copper[J]. Inorganic Chemistry, 1967, 6(12): 2220-2223. |
| 26 | LIU Tong, QIN Chenggong, ZHANG Tongwen, et al. Synthesis of Mg@Mg17Al12 ultrafine particles with superior hydrogen storage properties by hydrogen plasma-metal reaction[J]. Journal of Materials Chemistry, 2012, 22(37): 19831-19838. |
| 27 | SKRIPNYUK V M, RABKIN E. Mg3Cd: A model alloy for studying the destabilization of magnesium hydride[J]. International Journal of Hydrogen Energy, 2012, 37(14): 10724-10732. |
| 28 | ZHONG H C, WANG H, LIU J W, et al. Altered desorption enthalpy of MgH2 by the reversible formation of Mg(In) solid solution[J]. Scripta Materialia, 2011, 65(4): 285-287. |
| 29 | KIM Ki Chul, DAI Bing, KARL JOHNSON J, et al. Assessing nanoparticle size effects on metal hydride thermodynamics using the Wulff construction[J]. Nanotechnology, 2009, 20(20): 204001. |
| 30 | VAJEESTON P, RAVINDRAN P, FICHTNER M, et al. Influence of crystal structure of bulk phase on the stability of nanoscale phases: Investigation on MgH2 derived nanostructures[J]. The Journal of Physical Chemistry C, 2012, 116(35): 18965-18972. |
| 31 | VAJEESTON P, SARTORI S, RAVINDRAN P, et al. MgH2 in carbon scaffolds: A combined experimental and theoretical investigation[J]. The Journal of Physical Chemistry C, 2012, 116(40): 21139-21147. |
| 32 | LU Jun, CHOI Young Joon, FANG Zhigang Zak, et al. Hydrogen storage properties of nanosized MgH2-0.1TiH2 prepared by ultrahigh-energy-high-pressure milling[J]. Journal of the American Chemical Society, 2009, 131(43): 15843-15852. |
| 33 | ZHANG Liuting, XIAO Xuezhang, XU Chenchen, et al. Remarkably improved hydrogen storage performance of MgH2 catalyzed by multivalence NbH x nanoparticles[J]. The Journal of Physical Chemistry C, 2015, 119(16): 8554-8562. |
| 34 | SUN Yahui, Kondo-Francois AGUEY-ZINSOU. Synthesis of magnesium nanofibers by electroless reduction and their hydrogen interaction properties[J]. Particle & Particle Systems Characterization, 2017, 34(4): 1600276. |
| 35 | LIU Wei, Kondo-Francois AGUEY-ZINSOU. Hydrogen storage properties of in situ stabilised magnesium nanoparticles generated by electroless reduction with alkali metals[J]. International Journal of Hydrogen Energy, 2015, 40(47): 16948-16960. |
| 36 | LIU Wei, Kondo-Francois AGUEY-ZINSOU. Size effects and hydrogen storage properties of Mg nanoparticles synthesised by an electroless reduction method[J]. Journal of Materials Chemistry A, 2014, 2(25): 9718-9726. |
| 37 | JEON Ki-Joon, MOON Hoi Ri, RUMINSKI Anne M, et al. Air-stable magnesium nanocomposites provide rapid and high-capacity hydrogen storage without using heavy-metal catalysts[J]. Nature Materials, 2011, 10(4): 286-290. |
| 38 | Kondo-Francois AGUEY-ZINSOU, José-Ramón ARES-FERNÁNDEZ. Synthesis of colloidal magnesium: A near room temperature store for hydrogen[J]. Chemistry of Materials, 2008, 20(2): 376-378. |
| 39 | KALIDINDI Suresh Babu, JAGIRDAR Balaji R. Highly monodisperse colloidal magnesium nanoparticles by room temperature digestive ripening[J]. Inorganic Chemistry, 2009, 48(10): 4524-4529. |
| 40 | NORBERG Nick S, ARTHUR Timothy S, FREDRICK Sarah J, et al. Size-dependent hydrogen storage properties of Mg nanocrystals prepared from solution[J]. Journal of the American Chemical Society, 2011, 133(28): 10679-10681. |
| 41 | ZHANG Xin, LIU Yongfeng, REN Zhuanghe, et al. Realizing 6.7 wt% reversible storage of hydrogen at ambient temperature with non-confined ultrafine magnesium hydrides[J]. Energy & Environmental Science, 2021, 14(4): 2302-2313. |
| 42 | WAN Liwen F, LIU Yisheng, CHO Eun Seon, et al. Atomically thin interfacial suboxide key to hydrogen storage performance enhancements of magnesium nanoparticles encapsulated in reduced graphene oxide[J]. Nano Letters, 2017, 17(9): 5540-5545. |
| 43 | CHO Eun Seon, RUMINSKI Anne M, ALONI Shaul, et al. Graphene oxide/metal nanocrystal multilaminates as the atomic limit for safe and selective hydrogen storage[J]. Nature Communications, 2016, 7: 10804. |
| 44 | CHO Eun Seon, RUMINSKI Anne M, LIU Yisheng, et al. Hierarchically controlled inside-out doping of Mg nanocomposites for moderate temperature hydrogen storage[J]. Advanced Functional Materials, 2017, 27(47): 1704316. |
| 45 | CHO YongJun, KANG ShinYoung, WOOD Brandon C, et al. Heteroatom-doped graphenes as actively interacting 2D encapsulation media for Mg-based hydrogen storage[J]. ACS Applied Materials & Interfaces, 2022, 14(18): 20823-20834. |
| 46 | NIELSEN Thomas K, MANICKAM Kandavel, HIRSCHER Michael, et al. Confinement of MgH2 nanoclusters within nanoporous aerogel scaffold materials[J]. ACS Nano, 2009, 3(11): 3521-3528. |
| 47 | Zhirong ZHAO-KARGER, HU Jianjiang, ROTH Arne, et al. Altered thermodynamic and kinetic properties of MgH(2) infiltrated in microporous scaffold[J]. Chemical Communications, 2010, 46(44): 8353-8355. |
| 48 | JIA Yi, SUN Chenghua, CHENG Lina, et al. Destabilization of Mg-H bonding through nano-interfacial confinement by unsaturated carbon for hydrogen desorption from MgH2 [J]. Physical Chemistry Chemical Physics, 2013, 15(16): 5814-5820. |
| 49 | KONAROVA Muxina, TANKSALE Akshat, NORBERTO BELTRAMINI Jorge, et al. Effects of nano-confinement on the hydrogen desorption properties of MgH2 [J]. Nano Energy, 2013, 2(1): 98-104. |
| 50 | XIA Guanglin, TAN Yingbin, CHEN Xiaowei, et al. Monodisperse magnesium hydride nanoparticles uniformly self-assembled on graphene[J]. Advanced Materials, 2015, 27(39): 5981-5988. |
| 51 | SHINDE S S, KIM Dong Hyung, YU Jin Young, et al. Self-assembled air-stable magnesium hydride embedded in 3-D activated carbon for reversible hydrogen storage[J]. Nanoscale, 2017, 9(21): 7094-7103. |
| 52 | REN Li, ZHU Wen, ZHANG Qiuyu, et al. MgH2 confinement in MOF-derived N-doped porous carbon nanofibers for enhanced hydrogen storage[J]. Chemical Engineering Journal, 2022, 434: 134701. |
| 53 | MA Zhewen, PANDA Subrata, ZHANG Qiuyu, et al. Improving hydrogen sorption performances of MgH2 through nanoconfinement in a mesoporous CoS nano-boxes scaffold[J]. Chemical Engineering Journal, 2021, 406: 126790. |
| 54 | 张秋雨, 杜四川, 马哲文, 等. 镁基储氢材料的研究进展[J]. 科学通报, 2022, 67(19): 2158-2171. |
| ZHANG Qiuyu, DU Sichuan, MA Zhewen, et al. Recent advances in Mg-based hydrogen storage materials[J]. Chinese Science Bulletin, 2022, 67(19): 2158-2171. | |
| 55 | MATSUMOTO I, AKIYAMA T, NAKAMURA Y, et al. Controlled shape of magnesium hydride synthesized by chemical vapor deposition[J]. Journal of Alloys and Compounds, 2010, 507(2): 502-507. |
| 56 | LI Weiyang, LI Chunsheng, MA Hua, et al. Magnesium nanowires: Enhanced kinetics for hydrogen absorption and desorption[J]. Journal of the American Chemical Society, 2007, 129(21): 6710-6711. |
| 57 | WU Xinxing, ZHANG Ruiqi, YANG Jinlong. A first-principles study of the thermodynamic and electronic properties of Mg and MgH2 nanowires[J]. Physical Chemistry Chemical Physics, 2016, 18(28): 19412-19419. |
| 58 | CHEN Ming, HU Miaomiao, XIE Xiubo, et al. High loading nanoconfinement of V-decorated Mg with 1 nm carbon shells: Hydrogen storage properties and catalytic mechanism[J]. Nanoscale, 2019, 11(20): 10045-10055. |
| 59 | POZZO M, ALFÈ D. Hydrogen dissociation and diffusion on transition metal (=Ti, Zr, V, Fe, Ru, Co, Rh, Ni, Pd, Cu, Ag)-doped Mg(0001) surfaces[J]. International Journal of Hydrogen Energy, 2009, 34(4): 1922-1930. |
| 60 | CHEN Haipeng, YU Hao, ZHANG Qianqian, et al. Enhancement in dehydriding performance of magnesium hydride by iron incorporation: A combined experimental and theoretical investigation[J]. Journal of Power Sources, 2016, 322: 179-186. |
| 61 | LIANG G, HUOT J, BOILY S, et al. Catalytic effect of transition metals on hydrogen sorption in nanocrystalline ball milled MgH2-Tm (Tm=Ti, V, Mn, Fe and Ni) systems[J]. Journal of Alloys and Compounds, 1999, 292(1/2): 247-252. |
| 62 | CUI Jie, LIU Jiangwen, WANG Hui, et al. Mg-TM (TM: Ti, Nb, V, Co, Mo or Ni) core-shell like nanostructures: Synthesis, hydrogen storage performance and catalytic mechanism[J]. Journal of Materials Chemistry A, 2014, 2(25): 9645-9655. |
| 63 | CHEN Jie, XIA Guanglin, GUO Zaiping, et al. Porous Ni nanofibers with enhanced catalytic effect on the hydrogen storage performance of MgH2 [J]. Journal of Materials Chemistry A, 2015, 3(31): 15843-15848. |
| 64 | ZHANG J, YAN S, QU H. Recent progress in magnesium hydride modified through catalysis and nanoconfinement[J]. International Journal of Hydrogen Energy, 2018, 43(3): 1545-1565. |
| 65 | TERZIEVA M, KHRUSSANOVA M, PESHEV P. Dehydriding kinetics of mechanically alloyed mixtures of magnesium with some 3d transition metal oxides[J]. International Journal of Hydrogen Energy, 1991, 16(4): 265-270. |
| 66 | OELERICH W, KLASSEN T, BORMANN R. Metal oxides as catalysts for improved hydrogen sorption in nanocrystalline Mg-based materials[J]. Journal of Alloys and Compounds, 2001, 315(1/2): 237-242. |
| 67 | FRIEDRICHS O, AGUEY-ZINSOU F, J R Ares FERNÁNDEZ, et al. MgH2 with Nb2O5 as additive, for hydrogen storage: Chemical, structural and kinetic behavior with heating[J]. Acta Materialia, 2006, 54(1): 105-110. |
| 68 | PUKAZHSELVAN D, SANDHYA K S, RAMASAMY Devaraj, et al. Active catalytic species generated in situ in zirconia incorporated hydrogen storage material magnesium hydride[J]. Journal of Magnesium and Alloys, 2022, 10(3): 786-796. |
| 69 | ZHANG Xuelian, WANG Ke, ZHANG Xin, et al. Synthesis process and catalytic activity of Nb2O5 hollow spheres for reversible hydrogen storage of MgH2 [J]. International Journal of Energy Research, 2021, 45(2): 3129-3141. |
| 70 | SUN Gaili, LI Yuanyuan, ZHAO Xinxin, et al. First-principles investigation of energetics and electronic structures of Ni and Sc co-doped MgH2 [J]. American Journal of Analytical Chemistry, 2016, 7(1): 34-42. |
| 71 | XIAN Kaicheng, WU Meihong, GAO Mingxia, et al. A unique nanoflake-shape bimetallic Ti-Nb oxide of superior catalytic effect for hydrogen storage of MgH2 [J]. Small, 2022, 18(43): 2107013. |
| 72 | JIN Seon-Ah, SHIM Jae-Hyeok, CHO Young Whan, et al. Dehydrogenation and hydrogenation characteristics of MgH2 with transition metal fluorides[J]. Journal of Power Sources, 2007, 172(2): 859-862. |
| 73 | MALKA I E, PISAREK M, CZUJKO T, et al. A study of the ZrF4, NbF5, TaF5, and TiCl3 influences on the MgH2 sorption properties[J]. International Journal of Hydrogen Energy, 2011, 36(20): 12909-12917. |
| 74 | FAN Mei-Qiang, LIU Shusheng, ZHANG Yao, et al. Superior hydrogen storage properties of MgH2-10 wt.% TiC composite[J]. Energy, 2010, 35(8): 3417-3421. |
| 75 | BHATNAGAR Ashish, PANDEY Sunita K, VISHWAKARMA Alok K, et al. Fe3O4@graphene as a superior catalyst for hydrogen de/absorption from/in MgH2/Mg[J]. Journal of Materials Chemistry A, 2016, 4(38): 14761-14772. |
| 76 | LOTOSKYY Mykhaylo, DENYS Roman, YARTYS Volodymyr A, et al. An outstanding effect of graphite in nano-MgH2-TiH2 on hydrogen storage performance[J]. Journal of Materials Chemistry A, 2018, 6(23): 10740-10754. |
| 77 | LU Xiong, ZHANG Liuting, YU Haijie, et al. Achieving superior hydrogen storage properties of MgH2 by the effect of TiFe and carbon nanotubes[J]. Chemical Engineering Journal, 2021, 422: 130101. |
| 78 | LIU Meijia, XIAO Xuezhang, ZHAO Shuchun, et al. Facile synthesis of Co/Pd supported by few-walled carbon nanotubes as an efficient bidirectional catalyst for improving the low temperature hydrogen storage properties of magnesium hydride[J]. Journal of Materials Chemistry A, 2019, 7(10): 5277-5287. |
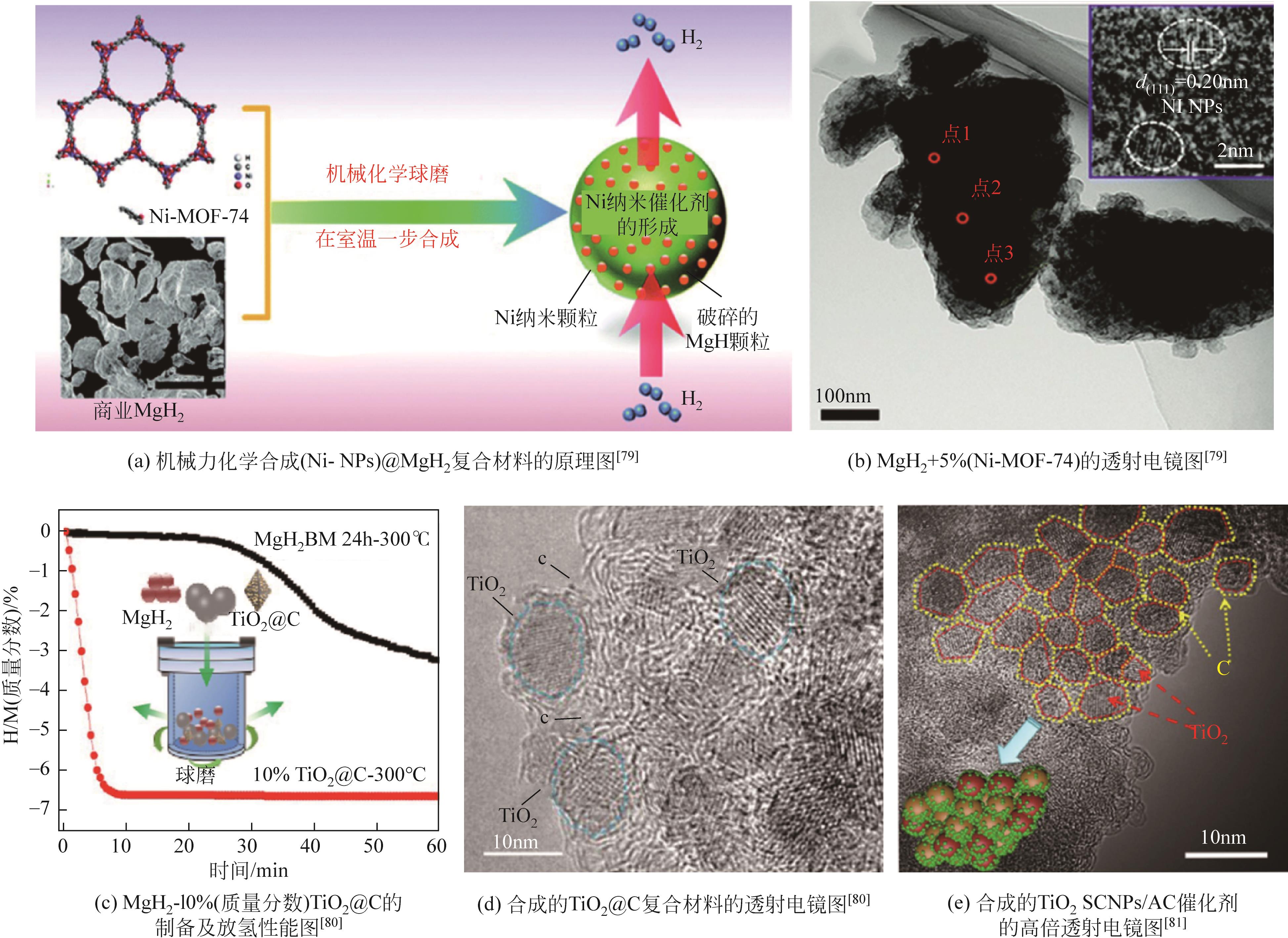
| 79 | JIA Yi, SUN Chenghua, PENG Ye, et al. Metallic Ni nanocatalyst in situ formed from a metal-organic-framework by mechanochemical reaction for hydrogen storage in magnesium[J]. Journal of Materials Chemistry A, 2015, 3(16): 8294-8299. |
| 80 | ZHANG Xin, LENG Zihan, GAO Mingxia, et al. Enhanced hydrogen storage properties of MgH2 catalyzed with carbon-supported nanocrystalline TiO2 [J]. Journal of Power Sources, 2018, 398: 183-192. |
| 81 | ZHANG Meng, XIAO Xuezhang, MAO Jianfeng, et al. Synergistic catalysis in monodispersed transition metal oxide nanoparticles anchored on amorphous carbon for excellent low-temperature dehydrogenation of magnesium hydride[J]. Materials Today Energy, 2019, 12: 146-154. |
| [1] | LIU Muzi, SHI Keke, ZHAO Qiang, LI Jinping, LIU Guang. Research progress of solid hydrogen storage materials [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4746-4769. |
| [2] | SUN Chongzheng, FAN Xin, LI Yuxing, XU Jie, HAN Hui, LIU Liang. Coupling characteristics of hydrogen heat transfer and normal-parahydrogen conversion in offshore porous media channels [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1281-1290. |
| [3] | YUE Zihan, LONG Zhen, ZHOU Xuebing, ZANG Xiaoya, LIANG Deqing. State of the art on hydrogen storage of sⅡ clathrate hydrate [J]. Chemical Industry and Engineering Progress, 2023, 42(10): 5121-5134. |
| [4] | HAN Li, LI Qi, LENG Guoyun, WEI Wenzhen, LI Yuying, WU Yuting. Latest research progress of hydrogen energy storage technology [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 108-117. |
| [5] | LI Haoyang, ZHANG Wei, LI Xiaosen, XU Chungang. Research process of hydrate-based hydrogen storage [J]. Chemical Industry and Engineering Progress, 2022, 41(12): 6285-6294. |
| [6] | GAO Jiajia, MI Yuanyuan, ZHOU Yang, ZHOU Hongjun, XU Quan. Recent developments in new hydrogen storage materials [J]. Chemical Industry and Engineering Progress, 2021, 40(6): 2962-2971. |
| [7] | Yanghao ZHENG, Heping LI, Jianzhong LIU, Daolun LIANG, Junhu ZHOU. Research progress of aluminum hydride used in fuel cells [J]. Chemical Industry and Engineering Progress, 2021, 40(1): 130-138. |
| [8] | Yan LI,Yuzhen DENG,Jingling YU,Sifang LI. Research progress in hydrogen production from decomposition of ammonia borane and its regeneration [J]. Chemical Industry and Engineering Progress, 2019, 38(12): 5330-5338. |
| [9] | QU Wenmin, HUA Zhengli, LI Xiongying, GU Chaohua, ZHENG Jingyang, ZHAO Yongzhi. Application of TDS technology in the study of hydrogen traps in the materials of hydrogen storage vessels [J]. Chemical Industry and Engineering Progress, 2017, 36(11): 4160-4169. |
| [10] | WANG Feng, YANG Yunquan, HU Yongjun, ZENG Yonglin. Advances in the development of methylcyclohexane as a hydrogen storage medium [J]. Chemical Industry and Engineering Progree, 2017, 36(02): 538-547. |
| [11] | ZHANG Yuanyuan, ZHAO Jing, LU Xilan, ZHANG Dexiang. Progress in liquid organic hydrogen storage materials [J]. Chemical Industry and Engineering Progree, 2016, 35(09): 2869-2874. |
| [12] | SONG Xiaofei, HAO Jianmin, HAN Limin, FENG Xuemin. Research progress of quasicrystal application in catalysis, reinforced composites and hydrogen storage [J]. Chemical Industry and Engineering Progree, 2015, 34(04): 1037-1042. |
| [13] | YUAN Huatang,WANG Yijing,YAN Chao,SONG Dawei . Progress in rare earth-based high performance hydrogen storage alloys [J]. Chemical Industry and Engineering Progree, 2012, 31(02 ): 253-258. |
| [14] | XIE Yingming,GONG Jinming,LIU Daoping,LI Gang,LIU Ni,QI Yingxia. Hydrogen stored in hydrates——A novel hydrogen storage method [J]. Chemical Industry and Engineering Progree, 2010, 29(5): 796-. |
| [15] |
CHEN Zhuo,YANG Yunquan,BAO Jianguo,WANG Weiyan,JIANG Xinmin.
Catalytic performance of Ni/γ-Al2O3 for hydrogen carrier methylcyclohexane dehydrogenation [J]. Chemical Industry and Engineering Progree, 2010, 29(3): 484-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||