Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (5): 2516-2535.DOI: 10.16085/j.issn.1000-6613.2022-1299
• Materials science and technology • Previous Articles Next Articles
Research progress on enzyme immobilization on porous framework materials
MAO Menglei( ), MENG Lingding, GAO Rui, MENG Zihui, LIU Wenfang(
), MENG Lingding, GAO Rui, MENG Zihui, LIU Wenfang( )
)
- School of Chemistry and Chemical Engineering, Beijing Institute of Technology, Beijing 102488, China
-
Received:2022-07-11Revised:2022-08-31Online:2023-06-02Published:2023-05-10 -
Contact:LIU Wenfang
多孔框架材料固定化酶研究进展
- 北京理工大学化学与化工学院,北京 102488
-
通讯作者:刘文芳 -
作者简介:毛梦雷(1997—),女,硕士研究生,研究方向为功能材料与催化。E-mail:3464744667@qq.com。
CLC Number:
Cite this article
MAO Menglei, MENG Lingding, GAO Rui, MENG Zihui, LIU Wenfang. Research progress on enzyme immobilization on porous framework materials[J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2516-2535.
毛梦雷, 孟令玎, 高蕊, 孟子晖, 刘文芳. 多孔框架材料固定化酶研究进展[J]. 化工进展, 2023, 42(5): 2516-2535.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-1299
| 方法 | 酶 | 载体 | 应用 | 固定化效率或 负载量 | 重复利用性 | 稳定性 | 文献 |
|---|---|---|---|---|---|---|---|
| 共沉积法 | 水解酶 | ||||||
| 脂肪酶 | Fe-BTC | 水解p-NPA | 87% | — | — | [ | |
| NH2-MIL-53(Al) | 水解p-NPA | 99%(NaOH),86%(TEA), 95%(NH4OH) | — | — | [ | ||
| ZIF-8 | 水解外消旋萘 普生甲酯 | 240mg/g | 重复利用6次后,固定化酶可保持初始活性的83% | 在4℃下储存5周后,固定化酶可保留初始活性的73%。在60℃时,固定化酶为35℃时活性的84%,而游离酶几乎完全失活 | [ | ||
| AmpC | 降解头孢菌素 | 97% | 重复利用6次后,固定化酶可保持初始活性的约90% | 在80℃时,固定化酶为25℃时活性的87%,而游离酶仅保留16.9%的活性。在二甲基甲酰胺、甲醇、乙腈溶剂中处理1h后,固定化酶的活性均约为初始活性的90%,而游离酶仅为初始活性的50%。在pH为5和10的体系中,固定化酶的活性均可保留在水溶液中活性的94%以上,而游离酶分别降至49.15%和66.2% | [ | ||
| 胰蛋白酶 | 分解蛋白质 | 176mg/g | 重复利用5次后,固定化酶可以保持初始活性的80%以上 | — | [ | ||
| GOx/HRP | 氧化荧光红 染料 | 40/25.8mg/g | — | 在室温下储存3周后,固定化酶的催化活性基本不变 | [ | ||
| GOx/HRP/GLB1 | 氧化荧光红 染料 | 25.6/30.5/18.8mg/g | — | 在室温下储存3周后,固定化酶的催化活性基本不变 | [ | ||
| ADH/LDH | 还原丙酮酸 | 23.8/17.9mg/g | — | 在室温下储存3周后,固定化酶体系的催化活性仅保留初始活性的30%~40% | [ | ||
| 细胞色素c | 检测H2O2、 过氧化丁酮、 叔丁基过氧化氢 | 82.3% | — | — | [ | ||
| 漆酶 | Fe-BTC | 氧化ABTS | 99% | — | — | [ | |
| 裂合酶 | |||||||
| PAL | ZIF-8 | 苯丙氨酸脱氨 | 94.07% | 重复使用10次后,固定化酶能保持初始酶活的20%左右 | 在室温下储存19天后,固定化酶可保持70%的初始活性;pH=5条件下,固定化酶能保持50%~60%的酶活,游离酶基本丧失活性;pH=11条件下,固定化酶的保留活性为83.96%,游离酶62.47% | [ | |
| 仿生矿化法 | 水解酶 | ||||||
| CRL | Fe@ZIF-8 | 水解对硝基苯基棕榈酸酯 | 7.2mg/g | 重复使用5次后,固定化酶保留了初始活性的80% | 在4℃下储存5周后,固定化酶的保留活性为68%。在酸性(pH=4)条件下,固定化酶仍能保持pH=8时活性的40%以上 | [ | |
| QLM | Bio@MOF | 葵花籽油与甲醇合成生物柴油 | 15.9% | — | 在室温下储存4周后,固定化酶保留了初始活性的32%;90℃时,固定化酶的活性为65℃时酶活性的66.3%,而游离酶的活性仅为60℃时酶活性的11.7% | [ | |
| Ur | ZIF-90 | 尿素水解 | — | 重复利用5次后,固定化酶的催化活性为初始值的97.7% | 在35℃、pH=7.4条件下,储存35天后其催化活性为初始值的96.1% | [ | |
| 仿生矿化法 | 合成酶 | ||||||
| NHase1229 | ZIF-67 | 3-氰吡啶水合 | — | 重复利用6次后,固定化酶活性无明显 下降 | 在70℃时,固定化酶的活性为50℃时酶活性的40%,而游离酶已完全失活 | [ | |
| 孔道扩散法 | 氧化还原酶 | ||||||
| HRP/GOx | ZIF-8 | 氧化葡萄糖 | 122/141mg/g | — | 在95℃时,固定化双酶活性可保持初始值的27.5%,而游离酶基本无活性。在pH=2和pH=9的条件下孵育1h后,固定化双酶体系分别保持了初始活性的50%和65.6%,游离酶活性低于20% | [ | |
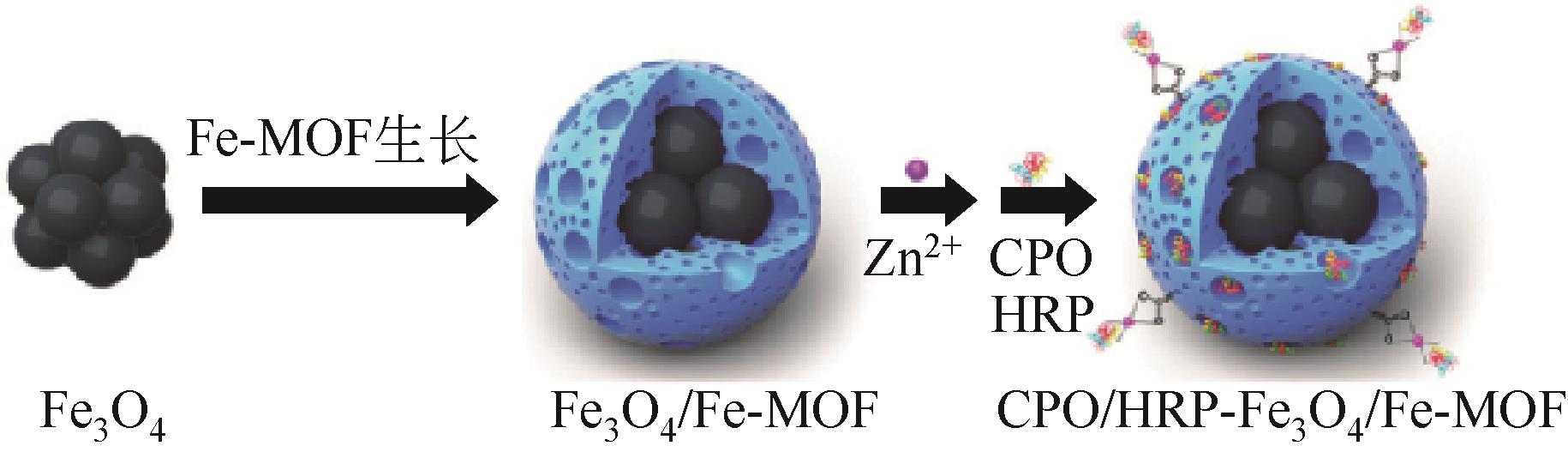
| HRPCPO | Fe-MOF | 降解废水中的有机毒素、异丙脲或2,4-二氯苯酚 | — | 重复利用10次后,固定化酶可保留初始活性的94.1% | 在70℃下,固定化CPO和HRP的保留活性分别为84.6%和94% | [ | |
| 细胞色素c | NU-1000 | 氧化邻苯三酚 | 13% | 重复利用3次后,固定化酶活性基本保持不变 | 在丙酮中,固定化酶活性基本不变;在己烷、四氢呋喃和甲醇中,活性略微降低;在二氧六烷中,保留活性为79% | [ | |
| MP8 | MIL-101(Cr) | 氧化ABTS | — | 在5次催化循环后,固定化酶可保持初始活性的66%。 | 在4℃下储存4周后,固定化酶和游离酶的保留活性分别为87%和85% | [ | |
| 水解酶 | |||||||
| CalA | ZIF-67 | 硝基醛醇反应 | 26.5% | 重复使用5次后,催化产率仍高于80% | — | [ | |
| 转移酶 | |||||||
| ANL | ZIF-8 | 大豆油制备生物柴油 | — | 重复利用5次后,固定化酶保持了初始活性的68% | 在100℃下,固定化酶保持了40℃时活性的67%,而游离酶的残余活性仅为20% | [ | |
| 表面吸附法 | 氧化还原酶 | ||||||
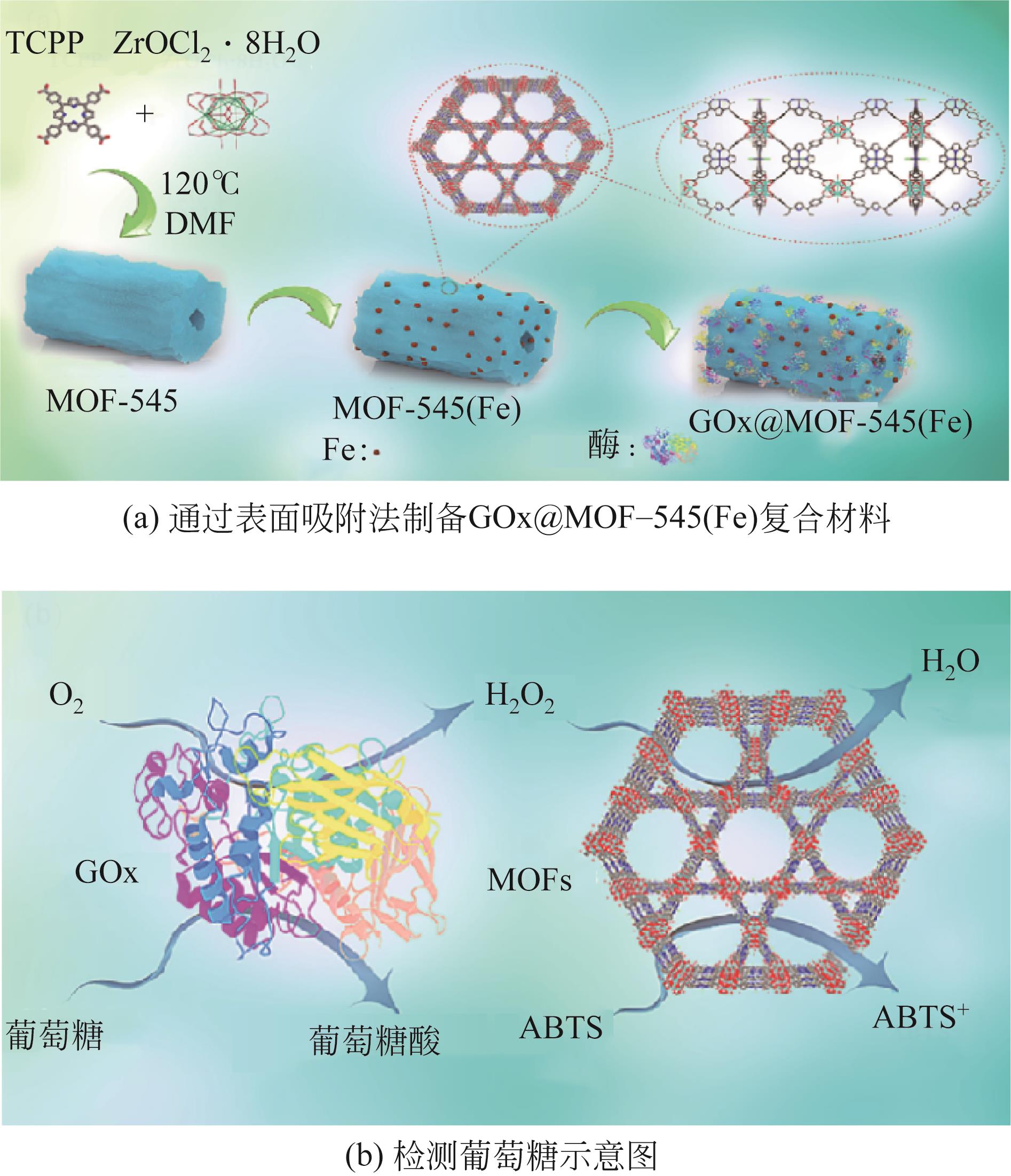
| GOx | MOF-545(Fe) | 检测葡萄糖 | 296mg/g | 在5次循环后,固定化酶仍保持初始活性的71% | 在室温下保存7天后,固定化酶的保留活性为92%,而游离酶的保留活性为40%。在65℃时,固定化酶活性为初始值的62.3%,而游离酶仅保持了11.9%。在四氢呋喃、乙腈和二甲基亚砜中处理1h后,固定化酶的保留活性分布为87.5%、75.6%和70%,而游离酶为43.8%、54.3%和38.3% | [ | |
| PCN-222(Fe) | 氧化葡萄糖和ABTS | 10.45%,0.1mg/g | 在6次循环后,固定化酶体系的催化效率为初始值的95.5% | 在65℃时,固定化酶体系催化ABTS的转化率约为初始值的80%;pH<2条件下,固定化酶体系仍保持初始催化转化率的40% | [ | ||
| CA | ZIF-8 | CO2水合 | >95% | 9次循环利用后,固定化酶活性约为初始值的85%。 | 在60℃时,固定化酶保留活性为40%,而游离酶几乎无活性。浸入2%的SDS溶液30min后,固定化酶的保留活性约为93%,而游离酶仅为2% | [ | |
| 共价连接法 | 氧化还原酶 | ||||||
| GOx | MIL-88B-(Fe) | 葡萄糖传感器 | — | 循环利用5次后,固定化酶活性基本不变 | 60天后,生物传感器性能约为初始性能的90% | [ | |
| MIL-101 | 葡萄糖传感器 | — | 循环利用5次后,固定化酶活性可保留初始活性的85% | 30天后,固定化酶的保留活性为90% | [ | ||
| HRP | MIL-88B(Fe) | 降解BPA | — | 循环利用4次后,固定化酶的残余活性仍高于80% | 在4℃下储存30天后,固定化酶的保留活性为70%以上,而游离酶只有26.2%;经过60℃热处理后,固定化酶仍能保持70.2%的活性,而游离酶只有55.9% | [ | |
| 水解酶 | |||||||
| Rha | Fe3O4@PDA@MOF | 水解芦丁 | — | 循环利用30次后,固定化酶体系的转化率仍为最初的55%。 | — | [ | |
| 脂肪酶 | MPAME对映体水解 | — | 循环利用4 次后,固定化酶的催化活性基本不变 | 在60℃时,固定化酶体系的产率仍高于70%,而游离酶体系由59.04%降至46.99% | [ | ||
| 转移酶 | |||||||
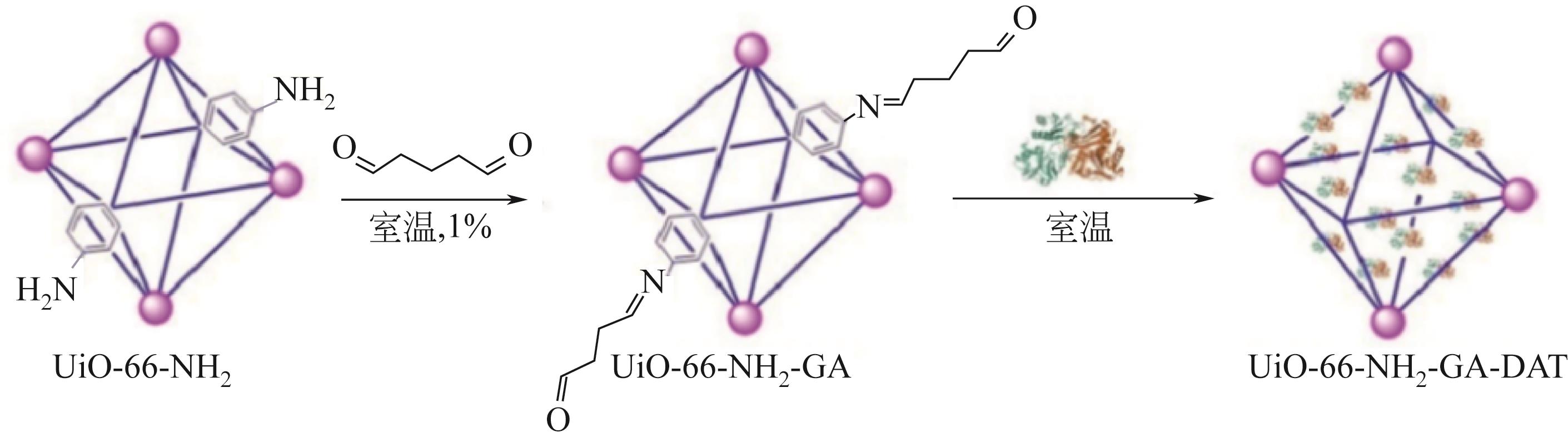
| DAT | 合成D-氨基酸 | 95% | — | 在80℃时,固定化酶可保持40℃时活性的65%,而游离酶仅保持了10%的活性 | [ |
| 方法 | 酶 | 载体 | 应用 | 固定化效率或 负载量 | 重复利用性 | 稳定性 | 文献 |
|---|---|---|---|---|---|---|---|
| 共沉积法 | 水解酶 | ||||||
| 脂肪酶 | Fe-BTC | 水解p-NPA | 87% | — | — | [ | |
| NH2-MIL-53(Al) | 水解p-NPA | 99%(NaOH),86%(TEA), 95%(NH4OH) | — | — | [ | ||
| ZIF-8 | 水解外消旋萘 普生甲酯 | 240mg/g | 重复利用6次后,固定化酶可保持初始活性的83% | 在4℃下储存5周后,固定化酶可保留初始活性的73%。在60℃时,固定化酶为35℃时活性的84%,而游离酶几乎完全失活 | [ | ||
| AmpC | 降解头孢菌素 | 97% | 重复利用6次后,固定化酶可保持初始活性的约90% | 在80℃时,固定化酶为25℃时活性的87%,而游离酶仅保留16.9%的活性。在二甲基甲酰胺、甲醇、乙腈溶剂中处理1h后,固定化酶的活性均约为初始活性的90%,而游离酶仅为初始活性的50%。在pH为5和10的体系中,固定化酶的活性均可保留在水溶液中活性的94%以上,而游离酶分别降至49.15%和66.2% | [ | ||
| 胰蛋白酶 | 分解蛋白质 | 176mg/g | 重复利用5次后,固定化酶可以保持初始活性的80%以上 | — | [ | ||
| GOx/HRP | 氧化荧光红 染料 | 40/25.8mg/g | — | 在室温下储存3周后,固定化酶的催化活性基本不变 | [ | ||
| GOx/HRP/GLB1 | 氧化荧光红 染料 | 25.6/30.5/18.8mg/g | — | 在室温下储存3周后,固定化酶的催化活性基本不变 | [ | ||
| ADH/LDH | 还原丙酮酸 | 23.8/17.9mg/g | — | 在室温下储存3周后,固定化酶体系的催化活性仅保留初始活性的30%~40% | [ | ||
| 细胞色素c | 检测H2O2、 过氧化丁酮、 叔丁基过氧化氢 | 82.3% | — | — | [ | ||
| 漆酶 | Fe-BTC | 氧化ABTS | 99% | — | — | [ | |
| 裂合酶 | |||||||
| PAL | ZIF-8 | 苯丙氨酸脱氨 | 94.07% | 重复使用10次后,固定化酶能保持初始酶活的20%左右 | 在室温下储存19天后,固定化酶可保持70%的初始活性;pH=5条件下,固定化酶能保持50%~60%的酶活,游离酶基本丧失活性;pH=11条件下,固定化酶的保留活性为83.96%,游离酶62.47% | [ | |
| 仿生矿化法 | 水解酶 | ||||||
| CRL | Fe@ZIF-8 | 水解对硝基苯基棕榈酸酯 | 7.2mg/g | 重复使用5次后,固定化酶保留了初始活性的80% | 在4℃下储存5周后,固定化酶的保留活性为68%。在酸性(pH=4)条件下,固定化酶仍能保持pH=8时活性的40%以上 | [ | |
| QLM | Bio@MOF | 葵花籽油与甲醇合成生物柴油 | 15.9% | — | 在室温下储存4周后,固定化酶保留了初始活性的32%;90℃时,固定化酶的活性为65℃时酶活性的66.3%,而游离酶的活性仅为60℃时酶活性的11.7% | [ | |
| Ur | ZIF-90 | 尿素水解 | — | 重复利用5次后,固定化酶的催化活性为初始值的97.7% | 在35℃、pH=7.4条件下,储存35天后其催化活性为初始值的96.1% | [ | |
| 仿生矿化法 | 合成酶 | ||||||
| NHase1229 | ZIF-67 | 3-氰吡啶水合 | — | 重复利用6次后,固定化酶活性无明显 下降 | 在70℃时,固定化酶的活性为50℃时酶活性的40%,而游离酶已完全失活 | [ | |
| 孔道扩散法 | 氧化还原酶 | ||||||
| HRP/GOx | ZIF-8 | 氧化葡萄糖 | 122/141mg/g | — | 在95℃时,固定化双酶活性可保持初始值的27.5%,而游离酶基本无活性。在pH=2和pH=9的条件下孵育1h后,固定化双酶体系分别保持了初始活性的50%和65.6%,游离酶活性低于20% | [ | |
| HRPCPO | Fe-MOF | 降解废水中的有机毒素、异丙脲或2,4-二氯苯酚 | — | 重复利用10次后,固定化酶可保留初始活性的94.1% | 在70℃下,固定化CPO和HRP的保留活性分别为84.6%和94% | [ | |
| 细胞色素c | NU-1000 | 氧化邻苯三酚 | 13% | 重复利用3次后,固定化酶活性基本保持不变 | 在丙酮中,固定化酶活性基本不变;在己烷、四氢呋喃和甲醇中,活性略微降低;在二氧六烷中,保留活性为79% | [ | |
| MP8 | MIL-101(Cr) | 氧化ABTS | — | 在5次催化循环后,固定化酶可保持初始活性的66%。 | 在4℃下储存4周后,固定化酶和游离酶的保留活性分别为87%和85% | [ | |
| 水解酶 | |||||||
| CalA | ZIF-67 | 硝基醛醇反应 | 26.5% | 重复使用5次后,催化产率仍高于80% | — | [ | |
| 转移酶 | |||||||
| ANL | ZIF-8 | 大豆油制备生物柴油 | — | 重复利用5次后,固定化酶保持了初始活性的68% | 在100℃下,固定化酶保持了40℃时活性的67%,而游离酶的残余活性仅为20% | [ | |
| 表面吸附法 | 氧化还原酶 | ||||||
| GOx | MOF-545(Fe) | 检测葡萄糖 | 296mg/g | 在5次循环后,固定化酶仍保持初始活性的71% | 在室温下保存7天后,固定化酶的保留活性为92%,而游离酶的保留活性为40%。在65℃时,固定化酶活性为初始值的62.3%,而游离酶仅保持了11.9%。在四氢呋喃、乙腈和二甲基亚砜中处理1h后,固定化酶的保留活性分布为87.5%、75.6%和70%,而游离酶为43.8%、54.3%和38.3% | [ | |
| PCN-222(Fe) | 氧化葡萄糖和ABTS | 10.45%,0.1mg/g | 在6次循环后,固定化酶体系的催化效率为初始值的95.5% | 在65℃时,固定化酶体系催化ABTS的转化率约为初始值的80%;pH<2条件下,固定化酶体系仍保持初始催化转化率的40% | [ | ||
| CA | ZIF-8 | CO2水合 | >95% | 9次循环利用后,固定化酶活性约为初始值的85%。 | 在60℃时,固定化酶保留活性为40%,而游离酶几乎无活性。浸入2%的SDS溶液30min后,固定化酶的保留活性约为93%,而游离酶仅为2% | [ | |
| 共价连接法 | 氧化还原酶 | ||||||
| GOx | MIL-88B-(Fe) | 葡萄糖传感器 | — | 循环利用5次后,固定化酶活性基本不变 | 60天后,生物传感器性能约为初始性能的90% | [ | |
| MIL-101 | 葡萄糖传感器 | — | 循环利用5次后,固定化酶活性可保留初始活性的85% | 30天后,固定化酶的保留活性为90% | [ | ||
| HRP | MIL-88B(Fe) | 降解BPA | — | 循环利用4次后,固定化酶的残余活性仍高于80% | 在4℃下储存30天后,固定化酶的保留活性为70%以上,而游离酶只有26.2%;经过60℃热处理后,固定化酶仍能保持70.2%的活性,而游离酶只有55.9% | [ | |
| 水解酶 | |||||||
| Rha | Fe3O4@PDA@MOF | 水解芦丁 | — | 循环利用30次后,固定化酶体系的转化率仍为最初的55%。 | — | [ | |
| 脂肪酶 | MPAME对映体水解 | — | 循环利用4 次后,固定化酶的催化活性基本不变 | 在60℃时,固定化酶体系的产率仍高于70%,而游离酶体系由59.04%降至46.99% | [ | ||
| 转移酶 | |||||||
| DAT | 合成D-氨基酸 | 95% | — | 在80℃时,固定化酶可保持40℃时活性的65%,而游离酶仅保持了10%的活性 | [ |
| 方法 | 酶 | 载体 | 应用 | 负载量 | 重复利用性 | 稳定性 | 文献 |
|---|---|---|---|---|---|---|---|
| 孔道扩散法 | 水解酶 | ||||||
| 脂肪酶 | COF-ETTA-EDDA | 酯交换反应,制备外消旋1-苯乙醇 | 780mg/g | 循环利用5次后,固定化酶的催化活性略微下降 | — | [ | |
| COF-OMe | 制备外消旋1-苯乙醇 | 890mg/g | — | 在120℃下暴露24h后,固定化酶和游离酶的保留活性分别约为75%和2%;在苯甲腈中处理1h后,固定化酶的保留活性约为37%,游离酶几乎完全失活 | [ | ||
| COF-V,COF-OH,COF-ONa,POP-OMe,POP-V | 780mg/g,750mg/g,590mg/g,580mg/g,500mg/g | ||||||
| 溶菌酶 | TPB-DMTP-COF | 溶菌病微球菌细胞 分解 | 710mg/g | — | 在80℃和100℃时以及在甲醇中处理3h后,固定化酶活性基本不变,游离酶的保留活性为15%、14%和50% | [ | |
| 氧化还原酶 | |||||||
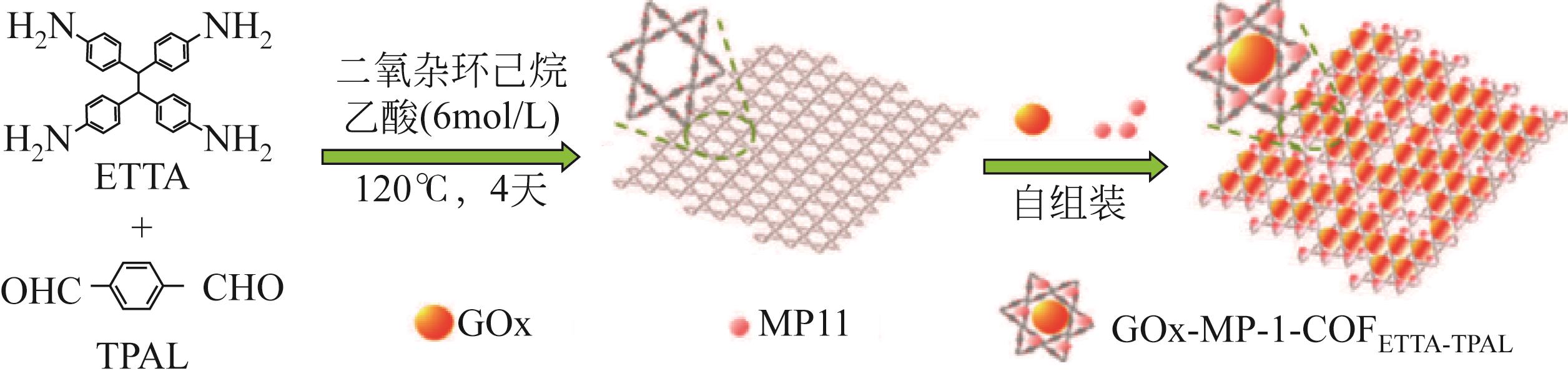
| MP-11/GOx | COF-ETTA-TPAL | 葡萄糖传感器 | 0.78mg/g | — | 在4℃下储存15天后,传感器性能为初始值的96.2% | [ | |
| 表面吸附法 | 氧化还原酶 | ||||||
| HRP/GOx | TpBD | 检测葡萄糖 | 42mg/g | 循环利用6次后,固定化酶保持了的初始活性的84% | 在4℃下储存8天后,固定化酶活性为初始值的78.4% | [ | |
| 水解酶 | |||||||
| 胰蛋白酶 | DhaTab | 水解N-苯甲酰-L-精氨酸4-硝基苯胺 | 0.0155mmol/g | — | — | [ | |
| RML | Fe3O4@COF-OMe | 麻风树油制备生物 柴油 | — | 循环利用10次后,固定化酶保持了初始活性的90%以上 | 在60℃时,固定化酶体系的产率约为60%,而游离酶体系约为20% | [ | |
| 共价连接法 | 水解酶 | ||||||
| 溶菌酶 | COF1 | 水解壳聚糖 | 22mmol/g | 循环利用5次后,固定化酶保持了初始活性的90%以上 | 经加热、超声和多种溶剂处理后,固定化酶保持了初始活性的85%以上,游离酶几乎全部失活 | [ | |
| 氧化还原酶 | |||||||
| GOx | COFHD | 葡萄糖传感器 | — | — | 在4℃下储存100天后,固定化酶的活性为初始值的85% | [ | |
| 包埋法 | 氧化还原酶 | ||||||
| CAT | COF-42-B | 分解H2O2 | 1660mg/g | 循环利用10次后,固定化酶活性基本不变 | 在pH=4、丙酮、蛋白酶和60℃条件下,固定化酶活性分别为初始值的85%、95%、约100%和88%,而游离酶仅为35%、25%、25%和20% | [ |
| 方法 | 酶 | 载体 | 应用 | 负载量 | 重复利用性 | 稳定性 | 文献 |
|---|---|---|---|---|---|---|---|
| 孔道扩散法 | 水解酶 | ||||||
| 脂肪酶 | COF-ETTA-EDDA | 酯交换反应,制备外消旋1-苯乙醇 | 780mg/g | 循环利用5次后,固定化酶的催化活性略微下降 | — | [ | |
| COF-OMe | 制备外消旋1-苯乙醇 | 890mg/g | — | 在120℃下暴露24h后,固定化酶和游离酶的保留活性分别约为75%和2%;在苯甲腈中处理1h后,固定化酶的保留活性约为37%,游离酶几乎完全失活 | [ | ||
| COF-V,COF-OH,COF-ONa,POP-OMe,POP-V | 780mg/g,750mg/g,590mg/g,580mg/g,500mg/g | ||||||
| 溶菌酶 | TPB-DMTP-COF | 溶菌病微球菌细胞 分解 | 710mg/g | — | 在80℃和100℃时以及在甲醇中处理3h后,固定化酶活性基本不变,游离酶的保留活性为15%、14%和50% | [ | |
| 氧化还原酶 | |||||||
| MP-11/GOx | COF-ETTA-TPAL | 葡萄糖传感器 | 0.78mg/g | — | 在4℃下储存15天后,传感器性能为初始值的96.2% | [ | |
| 表面吸附法 | 氧化还原酶 | ||||||
| HRP/GOx | TpBD | 检测葡萄糖 | 42mg/g | 循环利用6次后,固定化酶保持了的初始活性的84% | 在4℃下储存8天后,固定化酶活性为初始值的78.4% | [ | |
| 水解酶 | |||||||
| 胰蛋白酶 | DhaTab | 水解N-苯甲酰-L-精氨酸4-硝基苯胺 | 0.0155mmol/g | — | — | [ | |
| RML | Fe3O4@COF-OMe | 麻风树油制备生物 柴油 | — | 循环利用10次后,固定化酶保持了初始活性的90%以上 | 在60℃时,固定化酶体系的产率约为60%,而游离酶体系约为20% | [ | |
| 共价连接法 | 水解酶 | ||||||
| 溶菌酶 | COF1 | 水解壳聚糖 | 22mmol/g | 循环利用5次后,固定化酶保持了初始活性的90%以上 | 经加热、超声和多种溶剂处理后,固定化酶保持了初始活性的85%以上,游离酶几乎全部失活 | [ | |
| 氧化还原酶 | |||||||
| GOx | COFHD | 葡萄糖传感器 | — | — | 在4℃下储存100天后,固定化酶的活性为初始值的85% | [ | |
| 包埋法 | 氧化还原酶 | ||||||
| CAT | COF-42-B | 分解H2O2 | 1660mg/g | 循环利用10次后,固定化酶活性基本不变 | 在pH=4、丙酮、蛋白酶和60℃条件下,固定化酶活性分别为初始值的85%、95%、约100%和88%,而游离酶仅为35%、25%、25%和20% | [ |
| 61 | ZHONG Xue, XIA Huan, HUANG Wenquan, et al. Biomimetic metal-organic frameworks mediated hybrid multi-enzyme mimic for tandem catalysis[J]. Chemical Engineering Journal, 2020, 381: 122758. |
| 62 | TAN Wenlong, WEI Ting, HUO Jia, et al. Electrostatic interaction-induced formation of enzyme-on-MOF as chemo-biocatalyst for cascade reaction with unexpectedly acid-stable catalytic performance[J]. ACS Applied Materials & Interfaces, 2019, 11(40): 36782-36788. |
| 63 | WANG Deqing, ZHENG Pu, CHEN Pengcheng, et al. Immobilization of alpha-L-rhamnosidase on a magnetic metal-organic framework to effectively improve its reusability in the hydrolysis of rutin[J]. Bioresource Technology, 2021, 323: 124611. |
| 64 | 蔡文婷, 许嘉鑫, 杜克斯, 等. MIL-88B(Fe)固定辣根过氧化物酶去除双酚A[J]. 环境工程学报, 2021, 15(7): 2295-2304. |
| CAI Wenting, XU Jiaxin, DU Kesi, et al. Degradation of bisphenol A using horseradish peroxidase immobilized on MIL-88B(Fe)[J]. Chinese Journal of Environmental Engineering, 2021, 15(7): 2295-2304. | |
| 65 | XU Weiqing, JIAO Lei, YAN Hongye, et al. Glucose oxidase-integrated metal-organic framework hybrids as biomimetic cascade nanozymes for ultrasensitive glucose biosensing[J]. ACS Applied Materials & Interfaces, 2019, 11(25): 22096-22101. |
| 66 | JING Wenjie, KONG Fanbo, TIAN Sijia, et al. Glucose oxidase decorated fluorescent metal-organic frameworks as biomimetic cascade nanozymes for glucose detection through the inner filter effect[J]. The Analyst, 2021, 146(13): 4188-4194. |
| 67 | WANG Bin, ZHOU Jin, ZHANG Xiangyang, et al. Covalently immobilize crude d-amino acid transaminase onto UiO-66-NH2 surface for d-Ala biosynthesis[J]. International Journal of Biological Macromolecules, 2021, 175: 451-458. |
| 68 | CHEN Jing, SUN Bizhu, SUN Chenrui, et al. Immobilization of lipase AYS on UiO-66-NH2 metal-organic framework nanoparticles as a recyclable biocatalyst for ester hydrolysis and kinetic resolution[J]. Separation and Purification Technology, 2020, 251: 117398. |
| 69 | DÍAZ DE GREÑU Borja, TORRES Juan, Javier GARCÍA-GONZÁLEZ, et al. Microwave-assisted synthesis of covalent organic frameworks: a review[J]. ChemSusChem, 2021, 14(1): 208-233. |
| 70 | GUAN Xinyu, CHEN Fengqian, FANG Qianrong, et al. Design and applications of three dimensional covalent organic frameworks[J]. Chemical Society Reviews, 2020, 49(5): 1357-1384. |
| 71 | SONG Yanpei, SUN Qi, AGUILA Briana, et al. Opportunities of covalent organic frameworks for advanced applications[J]. Advanced Science, 2018, 6(2): 1801410. |
| 72 | SUN Qi, AGUILA Briana, LAN Pui Ching, et al. Tuning pore heterogeneity in covalent organic frameworks for enhanced enzyme accessibility and resistance against denaturants[J]. Advanced Materials, 2019, 31(19): e1900008. |
| 73 | GAN Jiansong, BAGHERI Ahmad Reza, ARAMESH Nahal, et al. Covalent organic frameworks as emerging host platforms for enzyme immobilization and robust biocatalysis—A review[J]. International Journal of Biological Macromolecules, 2021, 167: 502-515. |
| 74 | GUAN Qun, WANG Guangbo, ZHOU Lele, et al. Nanoscale covalent organic frameworks as theranostic platforms for oncotherapy: Synthesis, functionalization, and applications[J]. Nanoscale Advances, 2020, 2(9): 3656-3733. |
| 75 | VVARDHAN Harsh, NAFADY Ayman, AL-ENIZI Abdullah M, et al. Pore surface engineering of covalent organic frameworks: Structural diversity and applications[J]. Nanoscale, 2019, 11(45): 21679-21708. |
| 76 | WANG Li, LIANG Huihui, XU Mengli, et al. Ratiometric electrochemical biosensing based on double-enzymes loaded on two-dimensional dual-pore COFETTA-TPAL[J]. Sensors and Actuators B: Chemical, 2019, 298: 126859. |
| 77 | CHEN Haixin, JIN Chaonan, CHEN Xuepeng, et al. Covalent organic frameworks as crystalline sponges for enzyme extraction and production from natural biosystems[J]. Chemical Engineering Journal, 2022, 444: 136624. |
| 78 | YANG Xiaolian, TAN Zheng, SUN Hanjun, et al. Fabrication of a hierarchical nanoreactor based on COFs for cascade enzyme catalysis[J]. Chemical Communications, 2022, 58(24): 3933-3936. |
| 79 | KANDAMBETH Sharath, VENKATESH V, SHINDE Digambar B, et al. Self-templated chemically stable hollow spherical covalent organic framework[J]. Nature Communications, 2015, 6(1): 6786. |
| 80 | WANG Minghui, PAN Yanhong, WU Shuai, et al. Detection of colorectal cancer-derived exosomes based on covalent organic frameworks[J]. Biosensors & Bioelectronics, 2020, 169: 112638. |
| 81 | ZHOU Ziwen, CAI Chunxian, XING Xiu, et al. Magnetic COFs as satisfied support for lipase immobilization and recovery to effectively achieve the production of biodiesel by maintenance of enzyme activity[J]. Biotechnology for Biofuels, 2021, 14(1): 1-12. |
| 82 | ZHANG Sainan, ZHENG Yunlong, AN Hongde, et al. Covalent organic frameworks with chirality enriched by biomolecules for efficient chiral separation[J]. Angewandte Chemie International Edition, 2018, 57(51): 16754-16759. |
| 83 | YUE Jieyu, DING Xiuli, WANG Ling, et al. Correction: Novel enzyme-functionalized covalent organic frameworks for the colorimetric sensing of glucose in body fluids and drinks[J]. Materials Chemistry Frontiers, 2021, 5(24): 8398. |
| 1 | LIANG Weibin, WIED Peter, CARRARO Francesco, et al. Metal-organic framework-based enzyme biocomposites[J]. Chemical Reviews, 2021, 121(3): 1077-1129. |
| 2 | DU Yingjie, JIA Xiaotong, ZHONG Le, et al. Metal-organic frameworks with different dimensionalities: An ideal host platform for enzyme@MOF composites[J]. Coordination Chemistry Reviews, 2021, 454: 214327. |
| 3 | LIANG Shan, WU Xiaoling, XIONG Jun, et al. Metal-organic frameworks as novel matrices for efficient enzyme immobilization: An update review[J]. Coordination Chemistry Reviews, 2020, 406: 213149. |
| 4 | HAO Yun, DENG Suimin, WANG Ruoxin, et al. Development of dual-enhancer biocatalyst with photothermal property for the degradation of cephalosporin[J]. Journal of Hazardous Materials, 2022, 429: 128294. |
| 5 | YANG Xiaoyu, CHEN Lihua, LI Yu, et al. Hierarchically porous materials: Synthesis strategies and structure design[J]. Chemical Society Reviews, 2017, 46(2): 481-558. |
| 6 | WU Liang, LI Yu, FU Zhengyi, et al. Hierarchically structured porous materials: Synthesis strategies and applications in energy storage[J]. National Science Review, 2020, 7(11): 1667-1701. |
| 7 | SUN Minghui, HUANG Shaozhuan, CHEN Lihua, et al. Applications of hierarchically structured porous materials from energy storage and conversion, catalysis, photocatalysis, adsorption, separation, and sensing to biomedicine[J]. Chemical Society Reviews, 2016, 45(12): 3479-3563. |
| 8 | 王斓懿, 于学华, 赵震. 无机多孔材料的合成及其在环境催化领域的应用[J]. 物理化学学报, 2017, 33(12): 2359-2376. |
| WANG Lanyi, YU Xuehua, ZHAO Zhen. Synthesis of inorganic porous materials and their applications in the field of environmental catalysis[J]. Acta Physico-Chimica Sinica, 2017, 33(12): 2359-2376. | |
| 9 | 杨文艳. 基于乙烯基POSS的有机-无机杂化多孔聚合物的设计合成及其应用[D]. 济南: 山东大学, 2015. |
| YANG Wenyan. Design, synthesis and application of the organic-inorganic hybrid porous polymer based on POSS[D]. Jinan: Shandong University, 2015. | |
| 10 | 周瑕. 湿化学法制备Sr2TiO4和锰基有机-无机杂化材料及其介电性能研究[D]. 杭州: 浙江大学, 2020. |
| ZHOU Xia. Preparation of Sr2TiO4 and Mn-based organic-inorganic hybrid materials by wet chemical method and investigation of the dielectric properties[D]. Hangzhou: Zhejiang University, 2020. | |
| 11 | 杜昕. 功能化有机微孔聚合物的合成、表征及其催化性能研究[D]. 兰州: 兰州大学, 2010. |
| DU Xin. Synthesis, characterization and catalytic performance of functionalized organic microporous polymer[D]. Lanzhou: Lanzhou University, 2010. | |
| 12 | ZHANG Shuaihua, YANG Qian, WANG Chun, et al. Porous organic frameworks: Advanced materials in analytical chemistry[J]. Advanced Science, 2018, 5(12): 1801116. |
| 13 | FENG Xiao, DING Xuesong, JIANG Donglin. Covalent organic frameworks[J]. Chemical Society Reviews, 2012, 41(18): 6010-6022. |
| 14 | 侯晨, 陈文强, 付琳慧,等. 共价有机框架材料在固定化酶及模拟酶领域的应用[J]. 化学进展, 2020, 32(7): 895-905. |
| HOU Chen, CHEN Wenqiang, FU Linhui, et al. Covalent organic frameworks(COFs) materials in enzyme immobilization and mimic enzymes[J]. Progress in Chemistry, 2020, 32(7): 895-905. | |
| 15 | Asunción MOLINA M, Victoria GASCÓN-PÉREZ, Manuel SÁNCHEZ-SÁNCHEZ, et al. Sustainable one-pot immobilization of enzymes in/on metal-organic framework materials[J]. Catalysts, 2021, 11(8): 1002. |
| 16 | NADAR Shamraja S, VAIDYA Leena, RATHOD Virendra K. Enzyme embedded metal organic framework (enzyme-MOF): De novo approaches for immobilization[J]. International Journal of Biological Macromolecules, 2020, 149: 861-876. |
| 17 | HUANG Siming, KOU Xiaoxue, SHEN Jun, et al. “Armor-plating” enzymes with metal-organic frameworks (MOFs)[J]. Angewandte Chemie International Edition, 2020, 59(23): 8786-8798. |
| 18 | SUN Qi, FU Chung-Wei, AGUILA Briana, et al. Pore environment control and enhanced performance of enzymes infiltrated in covalent organic frameworks[J]. Journal of the American Chemical Society, 2018, 140(3): 984-992. |
| 19 | WANG Cui’e, LIAO Kaiming. Recent advances in emerging metal-and covalent-organic frameworks for enzyme encapsulation[J]. ACS Applied Materials & Interfaces, 2021, 13(48): 56752-56776. |
| 84 | XU Shujuan, WANG Yuying, LI Wang, et al. Covalent organic framework incorporated chiral polymer monoliths for capillary electrochromatography[J]. Journal of Chromatography A, 2019, 1602: 481-488. |
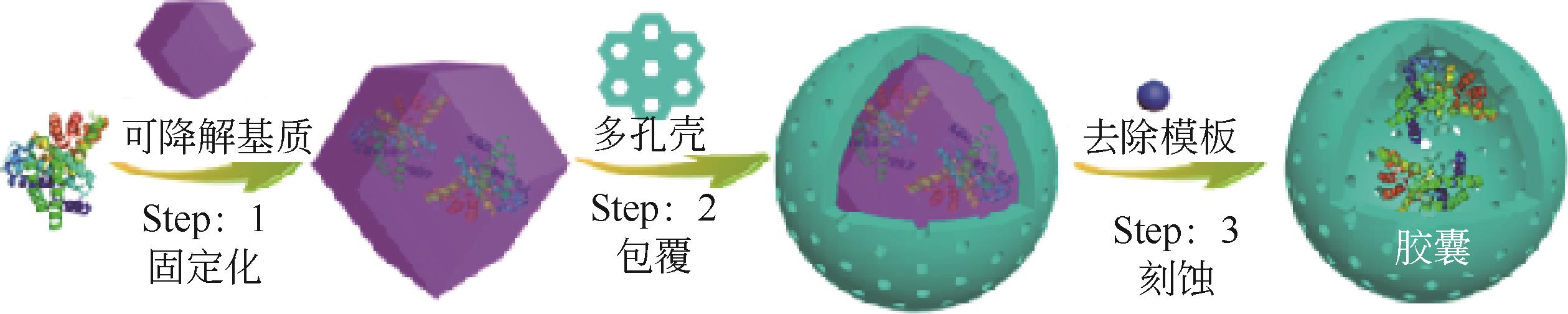
| 85 | LI Mingmin, QIAO Shan, ZHENG Yunlong, et al. Fabricating covalent organic framework capsules with commodious microenvironment for enzymes[J]. Journal of the American Chemical Society, 2020, 142(14): 6675-6681. |
| 20 | HOWARTH Ashlee J, LIU Yangyang, LI Peng, et al. Chemical, thermal and mechanical stabilities of metal-organic frameworks[J]. Nature Reviews Materials, 2016, 1(3): 1-15. |
| 21 | FURUKAWA Hiroyasu, CORDOVA Kyle E, Michael O'KEEFFE, et al. The chemistry and applications of metal-organic frameworks[J]. Science, 2013, 341(6149): 1230444. |
| 22 | 陆顺. 金属有机框架化合物(MOF)的制备、表征与电化学性质研究[D]. 重庆: 西南大学, 2017. |
| LU Shun. Preparation, characterization and electrochemical properties of metal organic framework (MOF)[D]. Chongqing: Southwest University, 2017. | |
| 23 | YUAN Shuai, FENG Liang, WANG Kecheng, et al. Stable metal-organic frameworks: Design, synthesis, and applications[J]. Advanced Materials, 2018, 30(37): e1704303. |
| 24 | FALCARO Paolo, RICCO Raffaele, DOHERTY Cara M, et al. MOF positioning technology and device fabrication[J]. Chemical Society Reviews, 2014, 43(16): 5513-5560. |
| 25 | ZHOU Kui, ZHANG Chen, XIONG Ziyu, et al. Template-directed growth of hierarchical MOF hybrid arrays for tactile sensor[J]. Advanced Functional Materials, 2020, 30(38): 2001296. |
| 26 | FARHA Omar K, ERYAZICI Ibrahim, JEONG Nak Cheon, et al. Metal-organic framework materials with ultrahigh surface areas: Is the sky the limit?[J]. Journal of the American Chemical Society, 2012, 134(36): 15016-15021. |
| 27 | EDDAOUDI M, KIM Jaheon, ROSI N, et al. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage[J]. Science, 2002, 295(5554): 469-472. |
| 28 | PRESTIPINO C, REGLI L, VITILLO J G, et al. Local structure of framework Cu( Ⅱ ) in HKUST-1 metallorganic framework: Spectroscopic characterization upon activation and interaction with adsorbates[J]. Chemistry of Materials, 2006, 18(5): 1337-1346. |
| 29 | CHUI Stephen S, Samuel M LO, CHARMANT Jonathan P, et al. A chemically functionalizable nanoporous material[J]. Science, 1999, 283(5405): 1148-1150. |
| 30 | PARK Kyo Sung, NI Zheng, CÔTÉ Adrien P, et al. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks[J]. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(27): 10186-10191. |
| 31 | LOISEAU Thierry, SERRE Christian, HUGUENARD Clarisse, et al. A rationale for the large breathing of the porous aluminum terephthalate (MIL-53) upon hydration[J]. Chemistry: A European Journal, 2004, 10(6): 1373-1382. |
| 32 | LLEWELLYN Philip L, BOURRELLY Sandrine, SERRE Christian, et al. High uptakes of CO2 and CH4 in mesoporous metal-organic frameworks MIL-100 and MIL-101[J]. Langmuir, 2008, 24(14): 7245-7250. |
| 33 | MA Shengqian, ZHOU Hongcai. A metal-organic framework with entatic metal centers exhibiting high gas adsorption affinity[J]. Journal of the American Chemical Society, 2006, 128(36): 11734-11735. |
| 34 | WANG Xisen, MA Shengqian, FORSTER Paul M, et al. Enhancing H2 uptake by “close-packing” alignment of open copper sites in metal-organic frameworks[J]. Angewandte Chemie International Edition, 2008, 47(38): 7263-7266. |
| 35 | MA Shengqian, WANG Xisen, COLLIER Christopher D, et al. Ultramicroporous metal-organic framework based on 9,10-anthracenedicarboxylate for selective gas adsorption[J]. Inorganic Chemistry, 2007, 46(21): 8499-8501. |
| 36 | BOSCH Mathieu, YUAN Shuai, RUTLEDGE William, et al. Stepwise synthesis of metal-organic frameworks[J]. Accounts of Chemical Research, 2017, 50(4): 857-865. |
| 37 | CAVKA Jasmina Hafizovic, Søren JAKOBSEN, OLSBYE Unni, et al. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability[J]. Journal of the American Chemical Society, 2008, 130(42): 13850-13851. |
| 38 | COHEN Seth M. The postsynthetic renaissance in porous solids[J]. Journal of the American Chemical Society, 2017, 139(8): 2855-2863. |
| 39 | DENNY Michael S, COHEN Seth M. In situ modification of metal-organic frameworks in mixed-matrix membranes[J]. Angewandte Chemie International Edition, 2015, 54(31): 9029-9032. |
| 40 | WANG Zhenqiang, COHEN Seth M. Postsynthetic modification of metal-organic frameworks[J]. Chemical Society Reviews, 2009, 38(5): 1315-1329. |
| 41 | COHEN Seth M. Postsynthetic methods for the functionalization of metal-organic frameworks[J]. Chemical Reviews, 2012, 112(2): 970-1000. |
| 42 | YIN Zheng, WAN Shuang, YANG Jian, et al. Recent advances in post-synthetic modification of metal-organic frameworks: New types and tandem reactions[J]. Coordination Chemistry Reviews, 2019, 378: 500-512. |
| 43 | Victoria GASCÓN, JIMÉNEZ Mayra B, BLANCO Rosa M, et al. Semi-crystalline Fe-BTC MOF material as an efficient support for enzyme immobilization[J]. Catalysis Today, 2018, 304: 119-126. |
| 44 | Victoria GASCÓN-PÉREZ, JIMÉNEZ Mayra Belen, MOLINA Asunción, et al. Efficient one-step immobilization of CaLB lipase over MOF support NH2-MIL-53(Al)[J]. Catalysts, 2020, 10(8): 918. |
| 45 | OZYILMAZ Elif, ASCIOGLU Sebahat, YILMAZ Mustafa. Calix[4]arene tetracarboxylic acid-treated lipase immobilized onto metal-organic framework: Biocatalyst for ester hydrolysis and kinetic resolution[J]. International Journal of Biological Macromolecules, 2021, 175: 79-86. |
| 46 | ZHONG Chao, LEI Zhixian, HUANG Huan, et al. One-pot synthesis of trypsin-based magnetic metal-organic frameworks for highly efficient proteolysis[J]. Journal of Materials Chemistry B, 2020, 8(21): 4642-4647. |
| 47 | CHEN Weihai, Margarita VÁZQUEZ-GONZÁLEZ, ZOABI Amani, et al. Biocatalytic cascades driven by enzymes encapsulated in metal-organic framework nanoparticles[J]. Nature Catalysis, 2018, 1(9): 689-695. |
| 48 | Fengjiao LYU, ZHANG Yifei, ZARE Richard N, et al. One-pot synthesis of protein-embedded metal-organic frameworks with enhanced biological activities[J]. Nano Letters, 2014, 14(10): 5761-5765. |
| 49 | 孙宝婷, 邱萌霞, 王子辰, 等. 半胱氨酸辅助的酶@ZIF-8固定化酶制备及其特性研究[J]. 生物技术通报, 2021, 37(8): 221-232. |
| SUN Baoting, QIU Mengxia, WANG Zichen, et al. Preparation of @ZIF-8 immobilized enzyme by using cysteine as auxiliary reagent and its characterization[J]. Biotechnology Bulletin, 2021, 37(8): 221-232. | |
| 50 | PEI Xiaolin, WU Yifeng, WANG Jiapao, et al. Biomimetic mineralization of nitrile hydratase into a mesoporous cobalt-based metal-organic framework for efficient biocatalysis[J]. Nanoscale, 2020, 12(2): 967-972. |
| 51 | OZYILMAZ Elif, Sami BILTEKIN M, CAGLAR Ozge, et al. Design of MOF-based nanobiocatalyst with super-catalytic properties with iron mineralization approach[J]. Materials Letters, 2021, 305: 130768. |
| 52 | LI Qing, CHEN Yingxuan, BAI Shaowei, et al. Immobilized lipase in bio-based metal-organic frameworks constructed by biomimetic mineralization: A sustainable biocatalyst for biodiesel synthesis[J]. Colloids and Surfaces B: Biointerfaces, 2020, 188: 110812. |
| 53 | CHENG Yujun, CHEN Tao, FU Donglei, et al. A molecularly imprinted nanoreactor based on biomimetic mineralization of bi-enzymes for specific detection of urea and its analogues[J]. Sensors and Actuators B: Chemical, 2022, 350: 130909. |
| 54 | SHA Fanrui, CHEN Yijing, DROUT Riki J, et al. Stabilization of an enzyme cytochrome c in a metal-organic framework against denaturing organic solvents[J]. iScience, 2021, 24(6): 102641. |
| 55 | GKANIATSOU Effrosyni, SICARD Clémence, RICOUX Rémy, et al. Enzyme encapsulation in mesoporous metal-organic frameworks for selective biodegradation of harmful dye molecules[J]. Angewandte Chemie International Edition, 2018, 57(49): 16141-16146. |
| 56 | GAO Xia, ZHAI Quanguo, HU Mancheng, et al. Hierarchically porous magnetic Fe3O4/Fe-MOF used as an effective platform for enzyme immobilization: A kinetic and thermodynamic study of structure-activity[J]. Catalysis Science & Technology, 2021, 11(7): 2446-2455. |
| 57 | CHENG Kaipeng, SVEC Frantisek, Yongqin LYU, et al. Hierarchical micro- and mesoporous Zn-based metal-organic frameworks templated by hydrogels: Their use for enzyme immobilization and catalysis of Knoevenagel reaction[J]. Small, 2019, 15(49): e1906245. |
| 58 | HU Yingli, ZHOU Hao, DAI Lingmei, et al. Lipase immobilization on macroporous ZIF-8 for enhanced enzymatic biodiesel production[J]. ACS Omega, 2021, 6(3): 2143-2148. |
| 59 | DUTTA Soumen, KUMARI Nitee, DUBBU Sateesh, et al. Highly mesoporous metal-organic frameworks as synergistic multimodal catalytic platforms for divergent cascade reactions[J]. Angewandte Chemie International Edition, 2020, 59(9): 3416-3422. |
| 60 | REN Sizhu, FENG Yuxiao, WEN Huan, et al. Immobilized carbonic anhydrase on mesoporous cruciate flower-like metal organic framework for promoting CO2 sequestration[J]. International Journal of Biological Macromolecules, 2018, 117: 189-198. |
| [1] | ZHANG Lihong, JIN Yaoru, CHENG Fangqin. Resource utilization of coal gasification slag [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4447-4457. |
| [2] | CHEN Sen, YIN Pengyuan, YANG Zhenglu, MO Yiming, CUI Xili, SUO Xian, XING Huabin. Advances in the intelligent synthesis of functional solid materials [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3340-3348. |
| [3] | ZHANG Yaodan, SUN Ruoxi, CHEN Pengcheng. Advances of multi-enzyme co-immobilization carrier based on cascade reactions [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3167-3176. |
| [4] | KONG Xiangru, ZHANG Xiaoyang, SUN Pengxiang, CUI Lin, DONG Yong. Research progress of solid porous materials for direct CO2 capture from air [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1471-1483. |
| [5] | JIN Xin, LI Yushan, XIE Qingqing, WANG Mengyu, XIA Xingfan, YANG Chaohe. Progress on solketal synthesis catalyzed by porous materials [J]. Chemical Industry and Engineering Progress, 2023, 42(2): 731-743. |
| [6] | ZHANG Yan, WANG Wei, XIE Rui, JU Xiaojie, LIU Zhuang, CHU Liangyin. Controllable fabrication of polymeric microparticles loaded with enzyme@ZIF-8 [J]. Chemical Industry and Engineering Progress, 2022, 41(4): 2022-2028. |
| [7] | MAO Menglei, SUN Danyang, MENG Zihui, LIU Wenfang. Enzyme immobilization on graphene oxide and transition metal carbon/nitrogen compounds [J]. Chemical Industry and Engineering Progress, 2022, 41(4): 1941-1955. |
| [8] | MENG Zihao, LI Qingyun, LIU Youyan, LIN Dongliang, TANG Aixing. MOF-immobilized lipase-catalyzed epoxidation of limonene in a single-phase system [J]. Chemical Industry and Engineering Progress, 2022, 41(12): 6540-6548. |
| [9] | WANG Wenxia, LIU Xiaofeng, CHEN Xi, XU Yanhong, MENG Zhenbang, ZHENG Junxia, AN Taicheng. Research advances of synthesis and applications of porous g-C3N4-based photocatalyst [J]. Chemical Industry and Engineering Progress, 2022, 41(1): 300-309. |
| [10] | Yonghou XIAO, Kerun ZHU, Xiaoying DONG, Gaohong HE. Research progress on porous materials for desulfurization of fuel by selective adsorption [J]. Chemical Industry and Engineering Progress, 2020, 39(6): 2241-2250. |
| [11] | Jie ZHU, Wenhui LI, Bangjian LIU, Minchen MU, Xinwen GUO. Preparation of high-surface-area ZrO2 and its application in catalysis [J]. Chemical Industry and Engineering Progress, 2019, 38(01): 315-323. |
| [12] | HE Meizhi, YANG Luwei, ZHANG Zhentao. Research progress of organic-inorganic composite phase change materials [J]. Chemical Industry and Engineering Progress, 2018, 37(12): 4709-4718. |
| [13] | LIN Yuanqing, LI Xialan, ZHANG Guangya. Recent research progress of enzyme self-immobilization methods [J]. Chemical Industry and Engineering Progress, 2018, 37(12): 4523-4532. |
| [14] | WU Bingfeng, YANG Lina, LI Jian, BAI Jin. Application of biomass templates in the preparation of mesoporous materials [J]. Chemical Industry and Engineering Progress, 2018, 37(07): 2686-2693. |
| [15] | MA Zhi, LI Yingqian, DING Tong, DONG Junjie, QIN Yongning. Research progress of halloysite nanotubes in biomedical science application [J]. Chemical Industry and Engineering Progress, 2017, 36(08): 3032-3039. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||