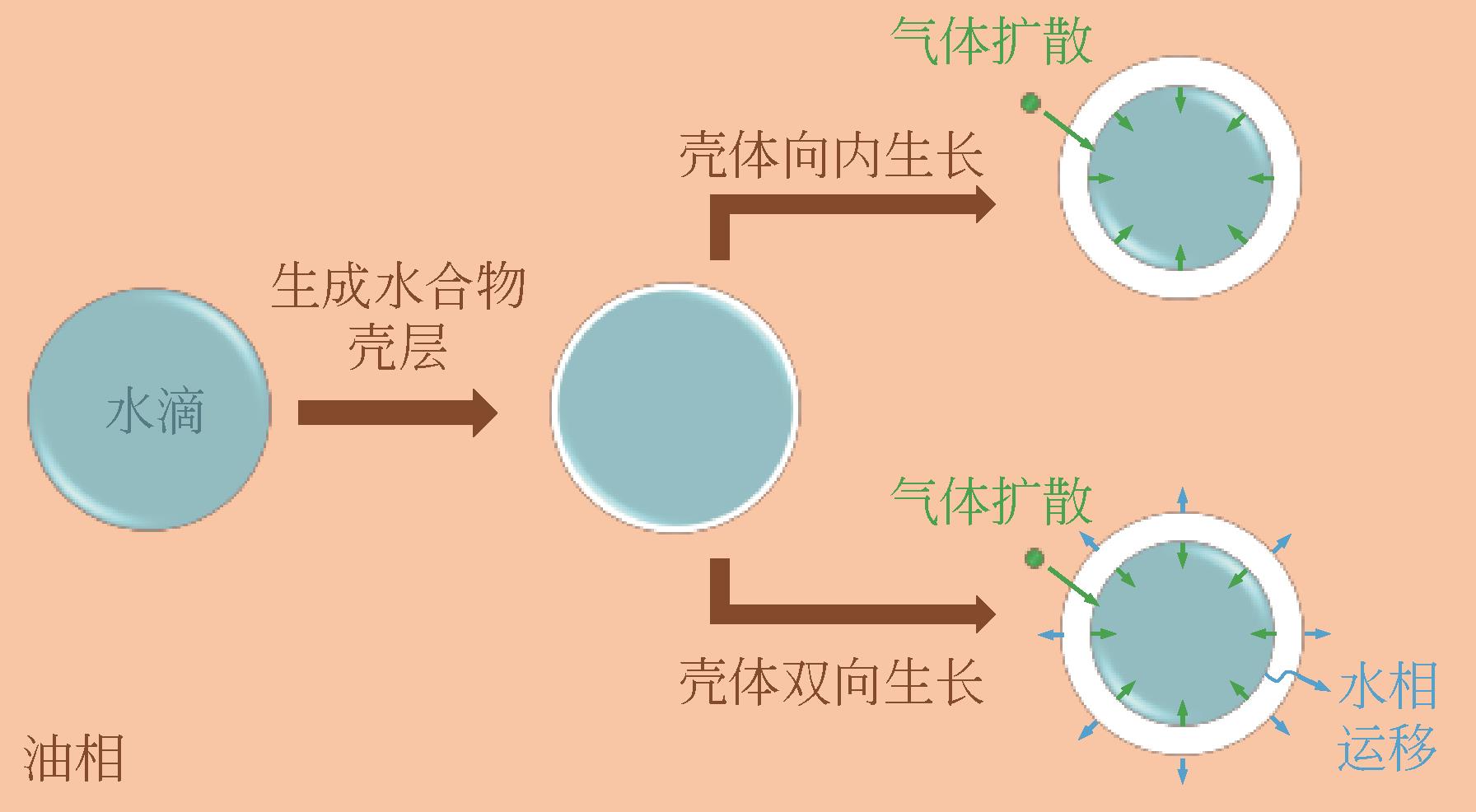
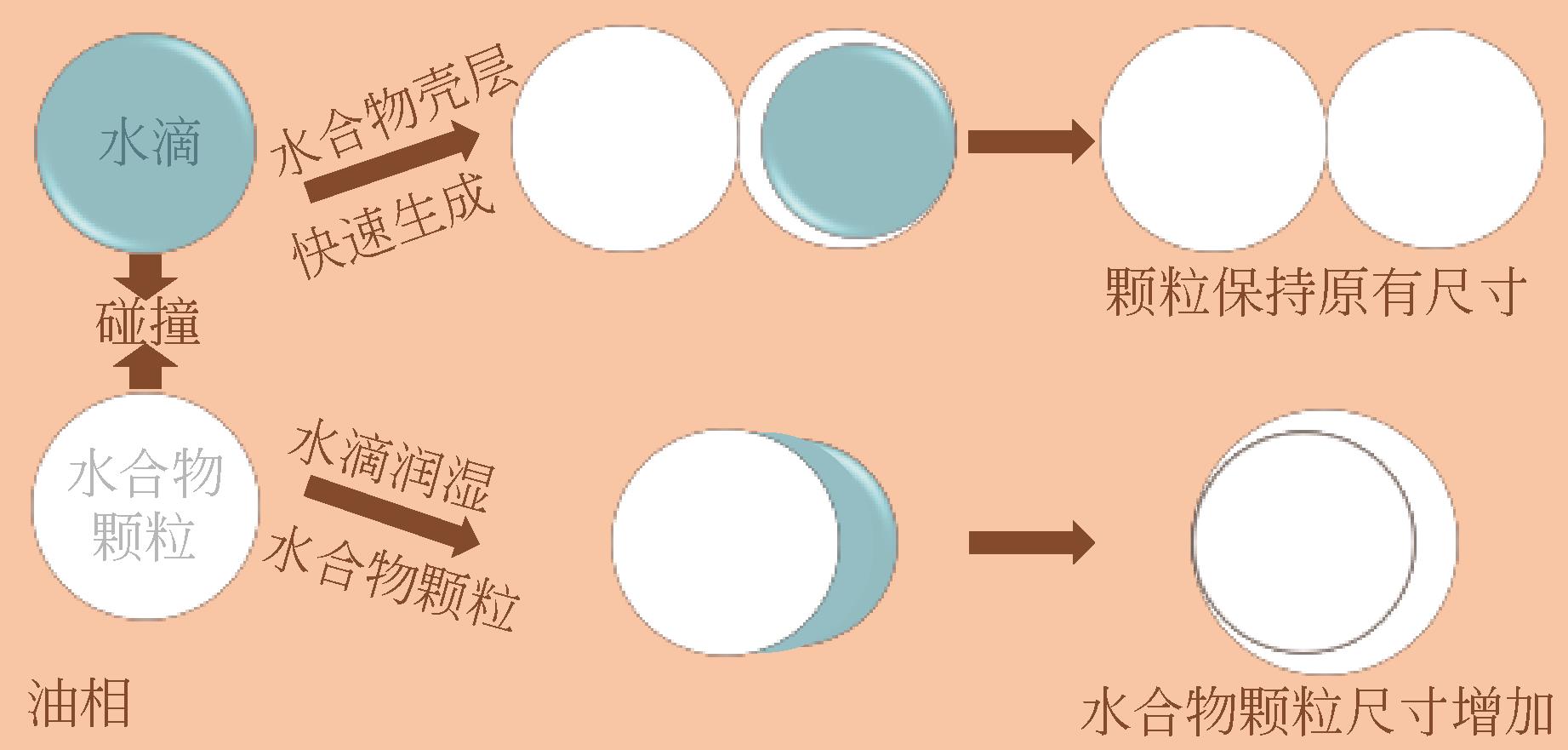
Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (3): 1155-1166.DOI: 10.16085/j.issn.1000-6613.2022-1736
• Chemical processes and equipment • Previous Articles Next Articles
Research progress on the growth behavior of hydrates in water-in-oil emulsion systems
WANG Wei1( ), ZHANG Dongxu1, LI Zunzhao1, WANG Xiaolin1, HUANG Qiyu2
), ZHANG Dongxu1, LI Zunzhao1, WANG Xiaolin1, HUANG Qiyu2
- 1.SINOPEC Dalian Research Institute of Petroleum and Petrochemicals Company Limited, Dalian 116045, Liaoning, China
2.National Engineering Laboratory for Pipeline Safety, Beijing Key Laboratory of Urban Oil and Gas Distribution Technology, College of Mechanical and Transportation Engineering, China University of Petroleum (Beijing), Beijing 102249, China
-
Received:2022-09-19Revised:2022-11-13Online:2023-04-10Published:2023-03-15 -
Contact:WANG Wei
油包水乳状液体系中水合物生长行为研究进展
- 1.中国石化(大连)石油化工研究院有限公司,大连 辽宁 116045
2.中国石油大学(北京)机械与储运工程学院,油气管道输送安全国家工程实验室,城市油气输配技术北京市重点实验室,北京 102249
-
通讯作者:王唯 -
作者简介:王唯(1992—),男,博士,工程师,研究方向为水合物流动保障技术及集输系统工艺优化。E-mail:wangwei9248@163.com。 -
基金资助:国家自然科学基金重点基金(21736005)
CLC Number:
Cite this article
WANG Wei, ZHANG Dongxu, LI Zunzhao, WANG Xiaolin, HUANG Qiyu. Research progress on the growth behavior of hydrates in water-in-oil emulsion systems[J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1155-1166.
王唯, 张东旭, 李遵照, 王晓霖, 黄启玉. 油包水乳状液体系中水合物生长行为研究进展[J]. 化工进展, 2023, 42(3): 1155-1166.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-1736
| 生成方法 | 操作过程 | 方法特性 |
|---|---|---|
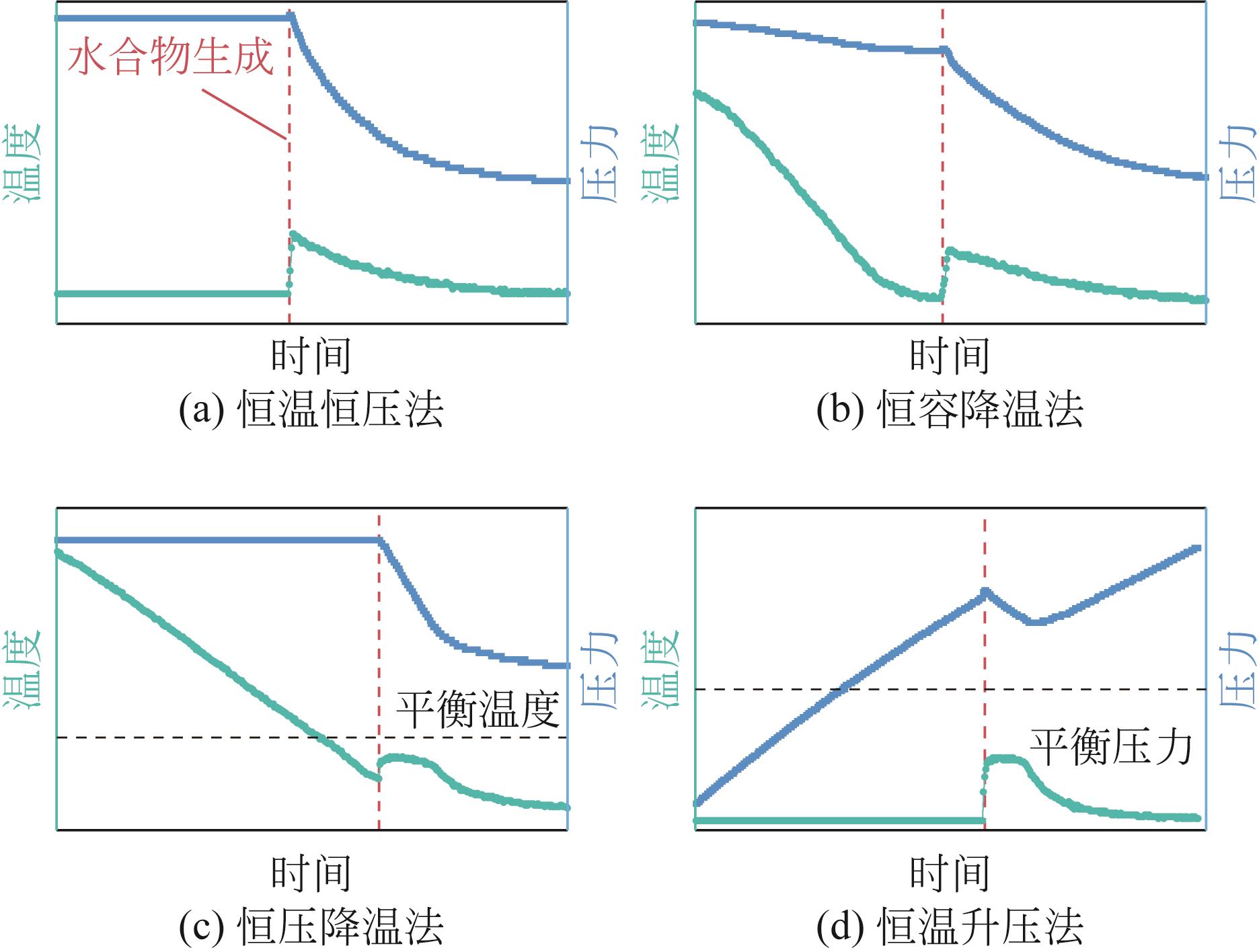
| 恒温恒压法 | 控制多相体系在短时间内降温和升压,直至达到三相平衡条件下的某一状态,而后维持体系稳定直至水合物生成[ | 可使不同组成的体系中水合物开始生长时的温度和压力相同,便于比较水合物生长速率和生成量 |
| 恒容降温法 | 将多相体系升压至高压状态,而后关闭进气阀进行降温,使体系在恒容条件下逐渐降温至水合物生成[ | 应用范围广,操作相对简单,但由于不同组成的体系中水合物的诱导期存在差异,所以水合物开始生长时的初始压力和温度很难保持一致,因而不利于水合物生长速率的比较 |
| 恒压降温法 | 将多相体系升压至高压状态后降温直至水合物生成,降温过程中调节装置进气量以维持水合物生成前体系压力恒定[ | 减弱了不同组成的体系中水合物生长开始时温度、压力不同对水合物生长速率的干扰,有利于水合物生长速率的比较 |
| 恒温升压法 | 将多相体系降温达到恒定过冷度,而后以恒定速率升压直至水合物生成[ | 操作相对简单,但与海底管道内温度、压力变化趋势不同 |
| 诱导转化法 | 将冰粒放置于水合物生成介质中促使水合物颗粒快速形成[ | 水合物生成速度快,多用于研究水合物颗粒间黏附力变化规律,但操作相对复杂 |
| 生成方法 | 操作过程 | 方法特性 |
|---|---|---|
| 恒温恒压法 | 控制多相体系在短时间内降温和升压,直至达到三相平衡条件下的某一状态,而后维持体系稳定直至水合物生成[ | 可使不同组成的体系中水合物开始生长时的温度和压力相同,便于比较水合物生长速率和生成量 |
| 恒容降温法 | 将多相体系升压至高压状态,而后关闭进气阀进行降温,使体系在恒容条件下逐渐降温至水合物生成[ | 应用范围广,操作相对简单,但由于不同组成的体系中水合物的诱导期存在差异,所以水合物开始生长时的初始压力和温度很难保持一致,因而不利于水合物生长速率的比较 |
| 恒压降温法 | 将多相体系升压至高压状态后降温直至水合物生成,降温过程中调节装置进气量以维持水合物生成前体系压力恒定[ | 减弱了不同组成的体系中水合物生长开始时温度、压力不同对水合物生长速率的干扰,有利于水合物生长速率的比较 |
| 恒温升压法 | 将多相体系降温达到恒定过冷度,而后以恒定速率升压直至水合物生成[ | 操作相对简单,但与海底管道内温度、压力变化趋势不同 |
| 诱导转化法 | 将冰粒放置于水合物生成介质中促使水合物颗粒快速形成[ | 水合物生成速度快,多用于研究水合物颗粒间黏附力变化规律,但操作相对复杂 |
| 量化描述方法 | 所需参数/计算公式 | 研究者(研究装置) |
|---|---|---|
| 参数变化速率 | 温度或压力 | Zhang等[ |
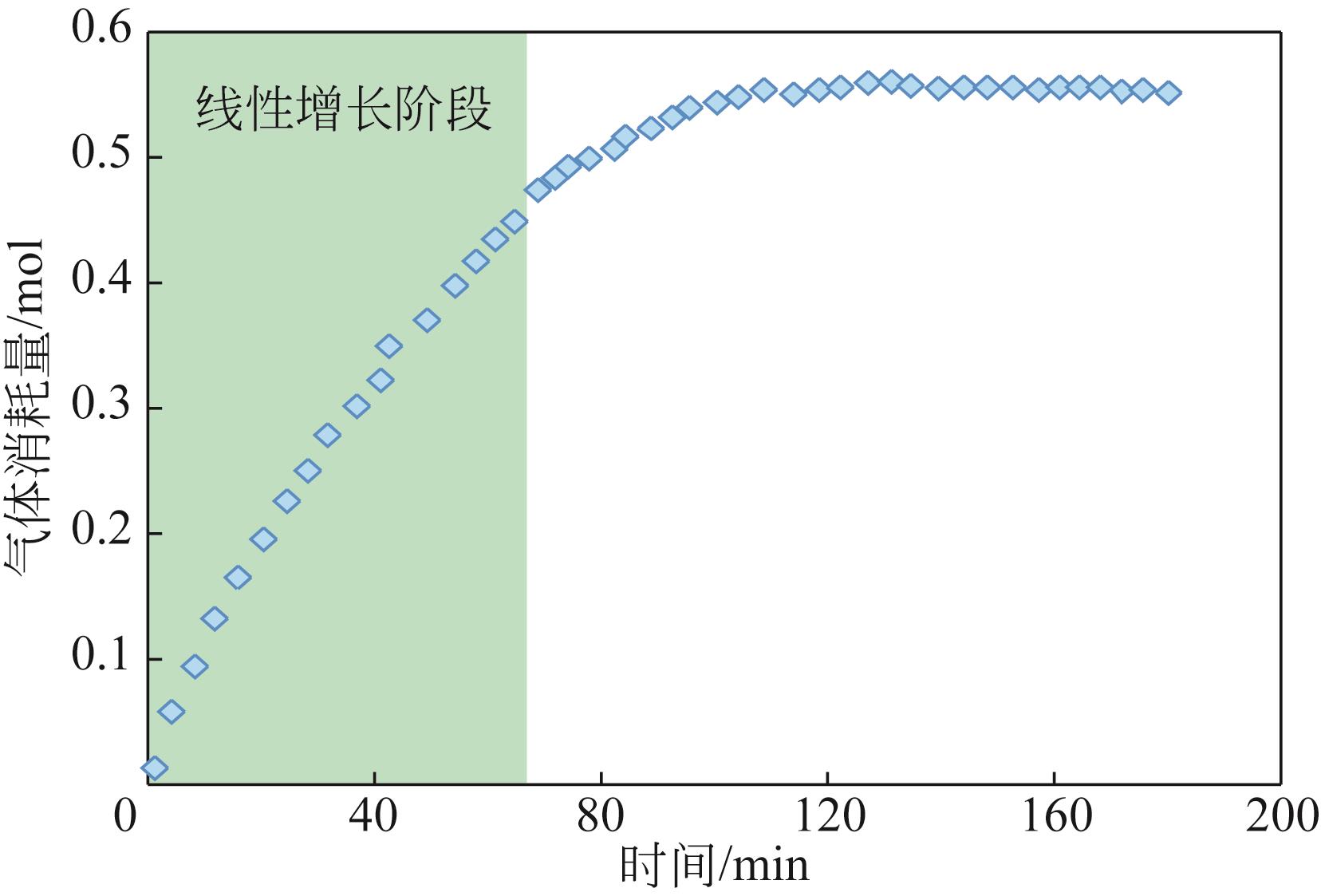
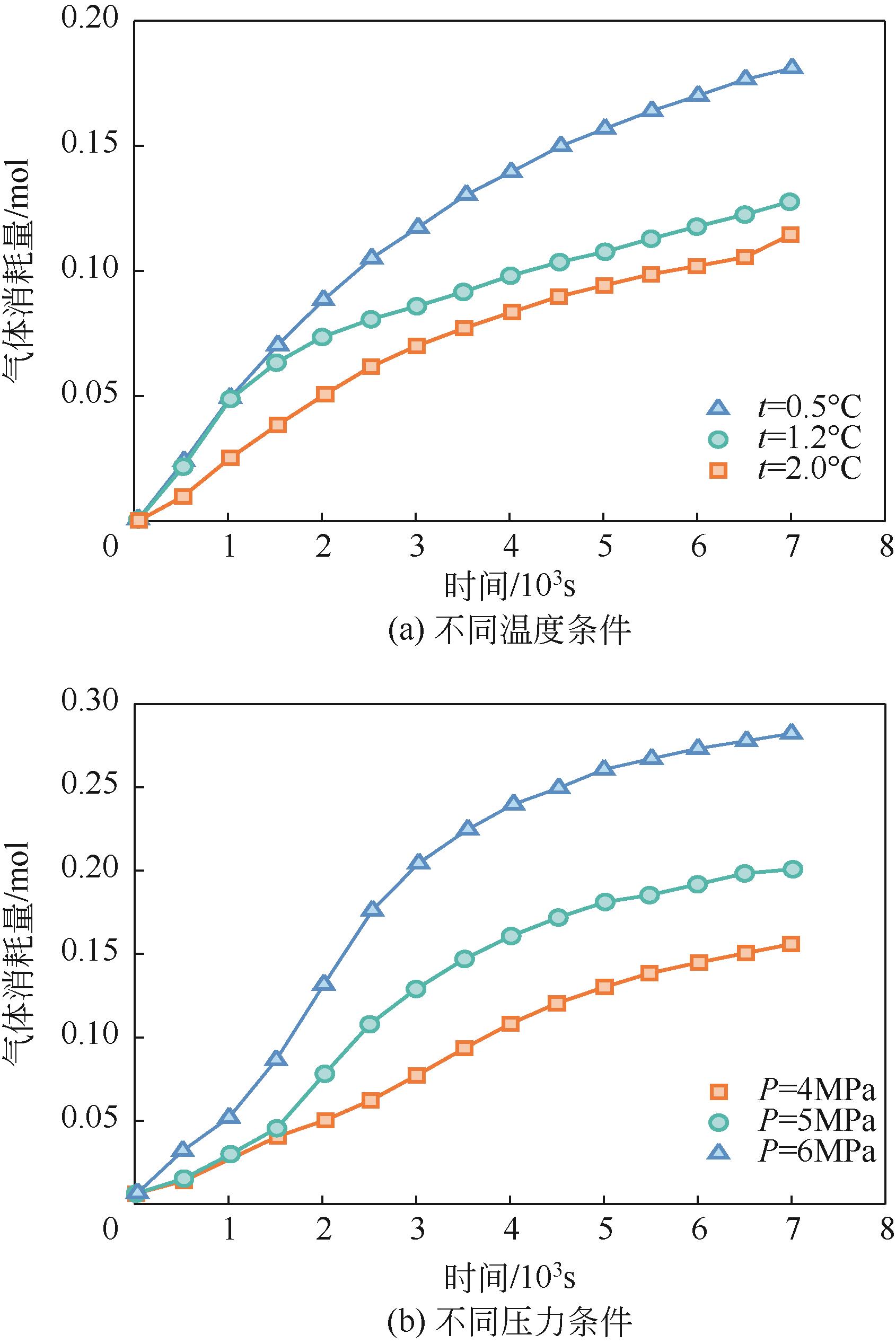
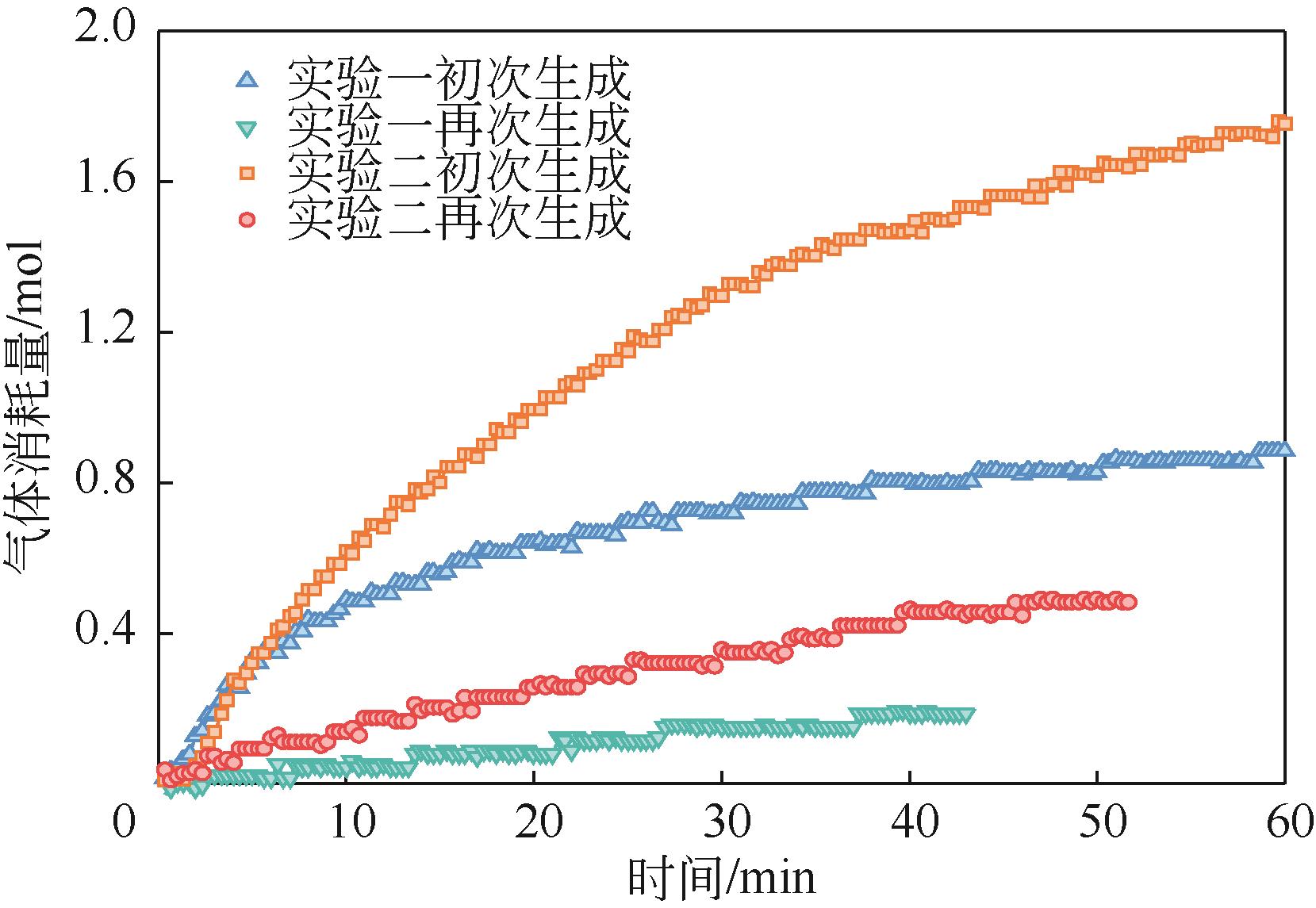
| 气体消耗量 | Wang等[ Shi等[ Mu等[ | |
| 初始气体消耗速率 | Turner等[ | |
| 90%生成量平均速率 | Liu等[ | |
| 水相转化率 | Zi等[ Davies等[ | |
| 水合物体积分数 | 柳扬[ Qin等[ Akhfash等[ | |
| 壳体生长速率 | 水合物壳体比表面积和时间 | Song等[ |
| 量化描述方法 | 所需参数/计算公式 | 研究者(研究装置) |
|---|---|---|
| 参数变化速率 | 温度或压力 | Zhang等[ |
| 气体消耗量 | Wang等[ Shi等[ Mu等[ | |
| 初始气体消耗速率 | Turner等[ | |
| 90%生成量平均速率 | Liu等[ | |
| 水相转化率 | Zi等[ Davies等[ | |
| 水合物体积分数 | 柳扬[ Qin等[ Akhfash等[ | |
| 壳体生长速率 | 水合物壳体比表面积和时间 | Song等[ |
| 原油重质组分 | 研究者 | 生成方法 | 速率表征方法 | 主要研究结论 |
|---|---|---|---|---|
| 蜡 | Chen等[ | 恒容降温法 | 水合物体积分数 | 蜡降低了水合物平均生长速率 |
| 周诗岽等[ | 恒温恒压法 | 气体消耗量 | 水合物生成量随含蜡量的升高而增加 | |
| Wang等[ | 恒压降温法和恒温升压法 | 气体消耗量 | 蜡可增加水合物生成量 | |
| Liu等[ | 恒容降温法 | 气体消耗量 | 蜡促进了水合物初始生长过程 | |
| Song等[ | 诱导转化法 | 壳体平均生长速率 | 蜡降低了水合物壳体生长速率 | |
| Liu等[ | 恒容降温法 | 气体消耗量 | 蜡晶析出减少了水合物生成量 | |
| 沥青质 | Zi等[ | 恒容降温法 | 水相转化率 | 模拟沥青质的加入降低了水合物生成量 |
| Zhang等[ | 恒压降温法和恒温升压法 | 气体消耗量 | 沥青质提高了水合物初始生长速率但降低了最终生成量 | |
| Ning等[ | 恒容降温法 | 初始耗气速率 | 沥青质降低了水合物初始生长速率 | |
| Prasad等[ | 恒温恒压法 | 气体消耗量 | 低含量沥青质增加了水合物生长过程中的气体消耗量 | |
| Song等[ | 诱导转化法 | 壳体平均生长速率 | 水合物壳体生长速率随沥青质含量增加而逐渐降低 | |
| 胶质 | Zhang等[ | 恒压降温法 | 参数变化速率 | 胶质降低了水合物生长速率 |
| 蜡+沥青质 | Zhang等[ | 恒压降温法 | 气体消耗量 | 组分间协同作用增强了沥青质对水合物生长的抑制效果 |
| 原油重质组分 | 研究者 | 生成方法 | 速率表征方法 | 主要研究结论 |
|---|---|---|---|---|
| 蜡 | Chen等[ | 恒容降温法 | 水合物体积分数 | 蜡降低了水合物平均生长速率 |
| 周诗岽等[ | 恒温恒压法 | 气体消耗量 | 水合物生成量随含蜡量的升高而增加 | |
| Wang等[ | 恒压降温法和恒温升压法 | 气体消耗量 | 蜡可增加水合物生成量 | |
| Liu等[ | 恒容降温法 | 气体消耗量 | 蜡促进了水合物初始生长过程 | |
| Song等[ | 诱导转化法 | 壳体平均生长速率 | 蜡降低了水合物壳体生长速率 | |
| Liu等[ | 恒容降温法 | 气体消耗量 | 蜡晶析出减少了水合物生成量 | |
| 沥青质 | Zi等[ | 恒容降温法 | 水相转化率 | 模拟沥青质的加入降低了水合物生成量 |
| Zhang等[ | 恒压降温法和恒温升压法 | 气体消耗量 | 沥青质提高了水合物初始生长速率但降低了最终生成量 | |
| Ning等[ | 恒容降温法 | 初始耗气速率 | 沥青质降低了水合物初始生长速率 | |
| Prasad等[ | 恒温恒压法 | 气体消耗量 | 低含量沥青质增加了水合物生长过程中的气体消耗量 | |
| Song等[ | 诱导转化法 | 壳体平均生长速率 | 水合物壳体生长速率随沥青质含量增加而逐渐降低 | |
| 胶质 | Zhang等[ | 恒压降温法 | 参数变化速率 | 胶质降低了水合物生长速率 |
| 蜡+沥青质 | Zhang等[ | 恒压降温法 | 气体消耗量 | 组分间协同作用增强了沥青质对水合物生长的抑制效果 |
| 1 | WANG Wei, HUANG Qiyu, HU Sijia, et al. Influence of wax on cyclopentane clathrate hydrate cohesive forces and interfacial properties[J]. Energy & Fuels, 2020, 34(2): 1482-1491. |
| 2 | HU Sijia, Carolyn A KOH. CH4/C2H6 gas hydrate interparticle interactions in the presence of anti-agglomerants and salinity[J]. Fuel, 2020, 269: 117208. |
| 3 | DIEKER Laura E, AMAN Zachary M, GEORGE Nathan C, et al. Micromechanical adhesion force measurements between hydrate particles in hydrocarbon oils and their modifications[J]. Energy & Fuels, 2009, 23(12): 5966-5971. |
| 4 | 宫敬, 史博会, 陈玉川, 等. 含天然气水合物的海底多相管输及其堵塞风险管控[J]. 天然气工业, 2020, 40(12): 133-142. |
| GONG Jing, SHI Bohui, CHEN Yuchuan, et al. Submarine multiphase pipeline transport containing natural gas hydrate and its plugging risk prevention and control[J]. Natural Gas Industry, 2020, 40(12): 133-142. | |
| 5 | 宋光春, 李玉星, 王武昌, 等. 柴油+水+天然气体系中水合物堵管实验研究[J]. 化工进展, 2017, 36(8): 2838-2846. |
| SONG Guangchun, LI Yuxing, WANG Wuchang, et al. Experimental investigation on hydrate plugging in diesel oil+water+natural gas systems[J]. Chemical Industry and Engineering Progress, 2017, 36(8): 2838-2846. | |
| 6 | SLOAN E Dendy. Fundamental principles and applications of natural gas hydrates[J]. Nature, 2003, 426: 353-359. |
| 7 | TURNER Douglas J, MILLER Kelly T, DENDY Sloan E. Methane hydrate formation and an inward growing shell model in water-in-oil dispersions[J]. Chemical Engineering Science, 2009, 64(18): 3996-4004. |
| 8 | ZHENG Haimin, HUANG Qiyu, WANG Wei, et al. Induction time of hydrate formation in water-in-oil emulsions[J]. Industrial & Engineering Chemistry Research, 2017, 56(29): 8330-8339. |
| 9 | SONG Guangchun, LI Yuxing, WANG Wuchang, et al. Hydrate formation in oil-water systems: Investigations of the influences of water cut and anti-agglomerant[J]. Chinese Journal of Chemical Engineering, 2020, 28(2): 369-377. |
| 10 | CHEN Yuchuan, SHI Bohui, FU Shunkang, et al. Kinetic and rheological investigation of cyclopentane hydrate formation in waxy water-in-oil emulsions[J]. Fuel, 2021, 287: 119568. |
| 11 | WALSH M R, KOH C A, SLOAN E D, et al. Microsecond simulations of spontaneous methane hydrate nucleation and growth[J]. Science, 2009, 326(5956): 1095-1098. |
| 12 | 周守为, 李清平, 吕鑫, 等. 天然气水合物开发研究方向的思考与建议[J]. 中国海上油气, 2019, 31(4): 1-8. |
| ZHOU Shouwei, LI Qingping, Xin LYU, et al. Thinking and suggestions on research direction of natural gas hydrate development[J]. China Offshore Oil and Gas, 2019, 31(4): 1-8. | |
| 13 | KIM Shol, LEE Seong Hyuk, KANG Yong Tae. Characteristics of CO2 hydrate formation/dissociation in H2O + THF aqueous solution and estimation of CO2 emission reduction by district cooling application[J]. Energy, 2017, 120: 362-373. |
| 14 | CAO Xuewen, YANG Kairan, BIAN Jiang. Investigation of CO2 hydrate slurry flow characteristics with particle dissociation for carbon storage and transportation[J]. Process Safety and Environmental Protection, 2021, 152: 427-440. |
| 15 | 黄婷, 李长俊, 李清平, 等. 全透明高压反应釜甲烷水合物动力学实验[J]. 化工进展, 2020, 39(7): 2624-2631. |
| HUANG Ting, LI Changjun, LI Qingping, et al. Experiment on methane hydrate kinetics in a high-pressure transparent autoclave[J]. Chemical Industry and Engineering Progress, 2020, 39(7): 2624-2631. | |
| 16 | CHEN Yuchuan, SHI Bohui, LIU Yang, et al. Experimental and theoretical investigation of the interaction between hydrate formation and wax precipitation in water-in-oil emulsions[J]. Energy & Fuels, 2018, 32(9): 9081-9092. |
| 17 | WANG Wei, HUANG Qiyu, ZHENG Haimin, et al. Effect of wax on hydrate formation in water-in-oil emulsions[J]. Journal of Dispersion Science and Technology, 2020, 41(12): 1821-1830. |
| 18 | ZHANG Dongxu, HUANG Qiyu, LI Rongbin, et al. Effects of waxes on hydrate behaviors in water-in-oil emulsions containing asphaltenes[J]. Chemical Engineering Science, 2021, 244: 116831. |
| 19 | ZHANG Dongxu, HUANG Qiyu, WANG Wei, et al. Effects of waxes and asphaltenes on CO2 hydrate nucleation and decomposition in oil-dominated systems[J]. Journal of Natural Gas Science and Engineering, 2021, 88: 103799. |
| 20 | AMAN Zachary M, DIEKER Laura E, ASPENES Guro, et al. Influence of model oil with surfactants and amphiphilic polymers on cyclopentane hydrate adhesion forces[J]. Energy & Fuels, 2010, 24(10): 5441-5445. |
| 21 | AMAN Zachary M, SLOAN E Dendy, Amadeu K SUM, et al. Adhesion force interactions between cyclopentane hydrate and physically and chemically modified surfaces[J]. Physical Chemistry Chemical Physics, 2014, 16(45): 25121-25128. |
| 22 | ZHANG Dongxu, HUANG Qiyu, LI Rongbin, et al. Hydrate formation in water-in-oil emulsions in the presence of resins[C]//13th International Pipeline Conference, 2020. |
| 23 | XIANG Changsheng, PENG Baozi, LIU Huang, et al. Hydrate formation/dissociation in (natural gas + water + diesel oil) emulsion systems[J]. Energies, 2013, 6(2): 1009-1022. |
| 24 | SHI Bohui, GONG Jing, SUN Changyu, et al. An inward and outward natural gas hydrates growth shell model considering intrinsic kinetics, mass and heat transfer[J]. Chemical Engineering Journal, 2011, 171(3): 1308-1316. |
| 25 | SONG Guangchun, LI Yuxing, WANG Wuchang, et al. Experimental study of hydrate formation in oil-water systems using a high-pressure visual autoclave[J]. AIChE Journal, 2019, 65(9): e16667. |
| 26 | INKONG Katipot, RANGSUNVIGIT Pramoch, KULPRATHIPANJA Santi, et al. Effects of temperature and pressure on the methane hydrate formation with the presence of tetrahydrofuran (THF) as a promoter in an unstirred tank reactor[J]. Fuel, 2019, 255: 115705. |
| 27 | LIU Zaixing, SONG Yongchen, LIU Weiguo, et al. Formation of methane hydrate in oil-water emulsion governed by the hydrophilic and hydrophobic properties of non-ionic surfactants[J]. Energy & Fuels, 2019, 33(6): 5777-5784. |
| 28 | ZI Mucong, WU Guozhong, WANG Jiang, et al. Investigation of gas hydrate formation and inhibition in oil-water system containing model asphaltene[J]. Chemical Engineering Journal, 2021, 412: 128452. |
| 29 | QIN Yahua, AMAN Zachary M, PICKERING Paul F, et al. High pressure rheological measurements of gas hydrate-in-oil slurries[J]. Journal of Non-Newtonian Fluid Mechanics, 2017, 248: 40-49. |
| 30 | SONG Guangchun, NING Yuanxing, LI Yuxing, et al. Investigation on hydrate growth at the oil-water interface: In the presence of wax and kinetic hydrate inhibitor[J]. Langmuir, 2020, 36(48): 14881-14891. |
| 31 | NING Y, LI Y, SONG G, et al. Investigation on hydrate formation and growth characteristics in dissolved asphaltene-containing water-in-oil emulsion[J]. Langmuir, 2021, 37(37): 11072-11083. |
| 32 | AZAM Muhammad Zeshan, XIN Feng, SONG Yuexiao, et al. Rate enhancement of methane hydration in slurry of ice by phase change of water-in-oil emulsions[J]. Fuel, 2019, 244: 296-303. |
| 33 | Yining LYU, SUN Changyu, LIU Bei, et al. A water droplet size distribution dependent modeling of hydrate formation in water/oil emulsion[J]. AIChE Journal, 2017, 63(3): 1010-1023. |
| 34 | Xiaofang LYU, SHI Bohui, ZHOU Shidong, et al. Study on the growth rate of natural gas hydrate in water-in-oil emulsion system using a high-pressure flow loop[J]. RSC Advances, 2018, 8(64): 36484-36492. |
| 35 | ZHOU Shidong, YAN Hongyu, SU Di, et al. Investigation on the kinetics of carbon dioxide hydrate formation using flow loop testing[J]. Journal of Natural Gas Science and Engineering, 2018, 49: 385-392. |
| 36 | MU Liang, VON SOLMS Nicolas. Inhibition of natural gas hydrate in the system containing salts and crude oil[J]. Journal of Petroleum Science and Engineering, 2020, 188: 106940. |
| 37 | AKHFASH Masoumeh, AMAN Zachary M, Sang Yoon AHN, et al. Gas hydrate plug formation in partially-dispersed water-oil systems[J]. Chemical Engineering Science, 2016, 140: 337-347. |
| 38 | DAVIES Simon R, BOXALL John A, DIEKER Laura E, et al. Predicting hydrate plug formation in oil-dominated flowlines[J]. Journal of Petroleum Science and Engineering, 2010, 72(3/4): 302-309. |
| 39 | 柳扬. 蜡与水合物共存W/O体系流动及沉积规律研究[D]. 北京: 中国石油大学(北京), 2019. |
| LIU Yang. Study on the flow and deposition mechanisms of W/O systems containing wax and hydrates[D]. Beijing: China University of Petroleum (Beijing), 2019. | |
| 40 | SHI Bohui, CHAI Shuai, WANG Linyan, et al. Viscosity investigation of natural gas hydrate slurries with anti-agglomerants additives[J]. Fuel, 2016, 185: 323-338. |
| 41 | GUO Penghao, SONG Guangchun, NING Yuanxing, et al. Investigation on hydrate growth at oil-water interface: In the presence of wax[J]. Energy & Fuels, 2021, 35(15): 11884-11895. |
| 42 | 宋光春, 施政灼, 李玉星, 等. 油水体系内水合物的生成: 温度、压力和搅拌速率影响[J]. 化工进展, 2019, 38(3): 1338-1345. |
| SONG Guangchun, SHI Zhengzhuo, LI Yuxing, et al. Hydrate formation in oil-water systems: Investigations of the influences of temperature, pressure and rotation rate[J]. Chemical Industry and Engineering Progress, 2019, 38(3): 1338-1345. | |
| 43 | LI You, LI Xingang, ZHOU Wentao, et al. Kinetics of ethylene hydrate formation in water-in-oil emulsion[J]. Journal of the Taiwan Institute of Chemical Engineers, 2017, 70: 79-87. |
| 44 | 刘朝阳, 栾石柱, 韩文超, 等. 含蜡原油蜡沉积影响因素对比试验[J]. 油气储运, 2021, 40(1): 78-83. |
| LIU Zhaoyang, LUAN Shizhu, HAN Wenchao, et al. Comparison test of factors affecting wax deposition of waxy crude oil[J]. Oil & Gas Storage and Transportation, 2021, 40(1): 78-83. | |
| 45 | 周诗岽, 于雪薇, 江坤, 等. 蜡晶析出对天然气水合物生成动力学特性的影响[J]. 天然气工业, 2018, 38(3): 103-109. |
| ZHOU Shidong, YU Xuewei, JIANG Kun, et al. Effect of wax crystal precipitation on the kinetic characteristics of hydrate formation[J]. Natural Gas Industry, 2018, 38(3): 103-109. | |
| 46 | LIU Yang, SHI Bohui, DING Lin, et al. Study of hydrate formation in water-in-waxy oil emulsions considering heat transfer and mass transfer[J]. Fuel, 2019, 244: 282-295. |
| 47 | LIU Jia, WANG Jing, DONG Ti, et al. Effects of wax on CH4 hydrate formation and agglomeration in oil-water emulsions[J]. Fuel, 2022, 322: 124128. |
| 48 | ZHANG Dongxu, HUANG Qiyu, LI Rongbin, et al. Nucleation and growth of gas hydrates in emulsions of water in asphaltene-containing oils[J]. Energy & Fuels, 2021, 35(7): 5853-5866. |
| 49 | PRASAD Siddhant Kumar, NAIR Vishnu Chandrasekharan, SANGWAI Jitendra S. Effect of asphaltenes on the kinetics of methane hydrate formation and dissociation in oil-in-water dispersion systems containing light saturated and aromatic hydrocarbons[J]. Energy & Fuels, 2021, 35(21): 17410-17423. |
| 50 | SONG Guangchun, NING Yuanxing, LI Yuxing, et al. Investigation on hydrate growth at the oil-water interface: In the presence of asphaltene[J]. Chinese Journal of Chemical Engineering, 2022, 45: 211-218. |
| 51 | LI Weidong, LI Huiyuan, Hongju DA, et al. Influence of pour point depressants (PPDs) on wax deposition: A study on wax deposit characteristics and pipeline pigging[J]. Fuel Processing Technology, 2021, 217: 106817. |
| 52 | 范开峰, 黄启玉, 李思, 等. 油水乳状液蜡沉积规律与扩散系数[J]. 油气储运, 2015, 34(10): 1067-1072. |
| FAN Kaifeng, HUANG Qiyu, LI Si, et al. Wax deposition laws and diffusion coefficient of oil-water emulsion[J]. Oil & Gas Storage and Transportation, 2015, 34(10): 1067-1072. | |
| 53 | 黄启玉, 毕权, 李男. 油水两相流蜡沉积研究进展[J]. 化工进展, 2016, 35(S1): 69-74. |
| HUANG Qiyu, BI Quan, LI Nan. Research progress of wax deposition in oil-water two-phase flow[J]. Chemical Industry and Engineering Progress, 2016, 35(S1): 69-74. | |
| 54 | 史博会, 柴帅, 柳扬, 等. 含蜡晶天然气水合物浆液黏度的影响因素[J]. 天然气工业, 2017, 37(5): 97-105. |
| SHI Bohui, CHAI Shuai, LIU Yang, et al. Factors influencing the viscosity of natural gas hydrate slurry with wax crystal[J]. Natural Gas Industry, 2017, 37(5): 97-105. | |
| 55 | ZHANG Dongxu, HUANG Qiyu, ZHENG Haimin, et al. Effect of wax crystals on nucleation during gas hydrate formation[J]. Energy & Fuels, 2019, 33(6): 5081-5090. |
| 56 | HUO Z, FREER E, LAMAR M, et al. Hydrate plug prevention by anti-agglomeration[J]. Chemical Engineering Science, 2001, 56(17): 4979-4991. |
| 57 | AZIZI Azlinda, JOHNS Michael L, AMAN Zachary M, et al. Effect of hydrate anti-agglomerants on water-in-crude oil emulsion stability[J]. Journal of Petroleum Exploration and Production Technology, 2020, 10(1): 139-148. |
| 58 | NORRIS Bruce W E, ZERPA Luis E, Carolyn A KOH, et al. Rapid assessments of hydrate blockage risk in oil-continuous flowlines[J]. Journal of Natural Gas Science and Engineering, 2016, 30: 284-294. |
| 59 | SHARIFI Hassan, RIPMEESTER John, WALKER Virginia K, et al. Kinetic inhibition of natural gas hydrates in saline solutions and heptane[J]. Fuel, 2014, 117: 109-117. |
| 60 | KANG Seong Pil, SHIN Ju Young, Jong Se LIM, et al. Experimental measurement of the induction time of natural gas hydrate and its prediction with polymeric kinetic inhibitor[J]. Chemical Engineering Science, 2014, 116: 817-823. |
| 61 | 陈光进, 孙长宇, 马庆兰. 气体水合物科学与技术[M]. 北京: 化学工业出版社, 2008. |
| CHEN Guangjin, SUN Changyu, MA Qinglan. Gas hydrate science and technology[M]. Beijing: Chemical Industry Press, 2008. | |
| 62 | 张剑波, 王志远, 刘书杰, 等. 深水气井测试过程中水合物流动障碍防治方法[J]. 石油勘探与开发, 2020, 47(6): 1256-1264. |
| ZHANG Jianbo, WANG Zhiyuan, LIU Shujie, et al. A method for preventing hydrates from blocking flow during deep-water gas well testing[J]. Petroleum Exploration and Development, 2020, 47(6): 1256-1264. | |
| 63 | KUMAR Rajnish, LEE Ju dong, SONG Myungho, et al. Kinetic inhibitor effects on methane/propane clathrate hydrate-crystal growth at the gas/water and water/n-heptane interfaces[J]. Journal of Crystal Growth, 2008, 310(6): 1154-1166. |
| 64 | ANDERSON Brian J, TESTER Jefferson W, BORGHI Gian Paolo, et al. Properties of inhibitors of methane hydrate formation via molecular dynamics simulations[J]. Journal of the American Chemical Society, 2005, 127(50): 17852-17862. |
| 65 | YI Lizhi, ZHAO Lili, TAO Shunhui. Methane hydrate formation in an oil-water system in the presence of lauroylamide propylbetaine[J]. RSC Advances, 2020, 10(21): 12255-12261. |
| 66 | 闫柯乐. 水合物阻聚剂在油水体系中阻聚性能实验研究[J]. 应用化工, 2021, 50(11): 3006-3010. |
| YAN Kele. Experimental study on a new class of hydrate anti-agglomerant in oil-water system[J]. Applied Chemical Industry, 2021, 50(11): 3006-3010. | |
| 67 | NING Fulong, GUO Dongdong, DIN Shahab Ud, et al. The kinetic effects of hydrate anti-agglomerants/surfactants[J]. Fuel, 2022, 318: 123566. |
| 68 | MU Liang, LI Shi, MA Qinglan, et al. Experimental and modeling investigation of kinetics of methane gas hydrate formation in water-in-oil emulsion[J]. Fluid Phase Equilibria, 2014, 362: 28-34. |
| 69 | LI Shifeng, FAN Shuanshi, WANG Jinqu, et al. Clathrate hydrate capture of CO2 from simulated flue gas with cyclopentane/water emulsion[J]. Chinese Journal of Chemical Engineering, 2010, 18(2): 202-206. |
| 70 | LIU Huang, WANG Jin, CHEN Guangjin, et al. High-efficiency separation of a CO2/H2 mixture via hydrate formation in W/O emulsions in the presence of cyclopentane and TBAB[J]. International Journal of Hydrogen Energy, 2014, 39(15): 7910-7918. |
| 71 | DING Kun, ZHONG Dongliang, LU Yiyu, et al. Enhanced precombustion capture of carbon dioxide by gas hydrate formation in water-in-oil emulsions[J]. Energy & Fuels, 2015, 29(5): 2971-2978. |
| 72 | TANG Cuiping, ZHAO Xiangyong, LI Dongliang, et al. Investigation of the flow characteristics of methane hydrate slurries with low flow rates[J]. Energies, 2017, 10(1): 145. |
| 73 | LACHANCE Jason W, SLOAN E Dendy, Carolyn A KOH. Effect of hydrate formation/dissociation on emulsion stability using DSC and visual techniques[J]. Chemical Engineering Science, 2008, 63(15): 3942-3947. |
| 74 | GUO Dongdong, Wenjia OU, NING Fulong, et al. The effects of hydrate formation and dissociation on the water-oil interface: Insight into the stability of an emulsion[J]. Fuel, 2020, 266: 116980. |
| 75 | 阮超宇, 史博会, 丁麟, 等. 天然气水合物生长及堵管规律研究进展[J]. 油气储运, 2016, 35(10): 1027-1037. |
| RUAN Chaoyu, SHI Bohui, DING Lin, et al. Research progress on the growth and plugging laws of gas hydrates[J]. Oil & Gas Storage and Transportation, 2016, 35(10): 1027-1037. | |
| 76 | YIN Zhenyuan, KHURANA Maninder, TAN Hoon Kiang, et al. A review of gas hydrate growth kinetic models[J]. Chemical Engineering Journal, 2018, 342: 9-29. |
| 77 | TAYLOR Craig J, MILLER Kelly T, Carolyn A KOH, et al. Macroscopic investigation of hydrate film growth at the hydrocarbon/water interface[J]. Chemical Engineering Science, 2007, 62(23): 6524-6533. |
| 78 | LEE Ju dong, SUSILO Robin, ENGLEZOS Peter. Methane-ethane and methane-propane hydrate formation and decomposition on water droplets[J]. Chemical Engineering Science, 2005, 60(15): 4203-4212. |
| 79 | TURNER D J, MILLER K T, SLOAN E D. Direct conversion of water droplets to methane hydrate in crude oil[J]. Chemical Engineering Science, 2009, 64(23): 5066-5072. |
| 80 | ZERPA Luis E, SLOAN E Dendy, Amadeu K SUM, et al. Overview of CSMHyK: A transient hydrate formation model[J]. Journal of Petroleum Science and Engineering, 2012, 98/99: 122-129. |
| 81 | DALMAZZONE Didier, HAMED Néjib, DALMAZZONE Christine. DSC measurements and modelling of the kinetics of methane hydrate formation in water-in-oil emulsion[J]. Chemical Engineering Science, 2009, 64(9): 2020-2026. |
| 82 | 吕一宁. 油水体系水合物生长过程与盐析效应研究[D]. 北京: 中国石油大学(北京), 2017. |
| Yining LYU. Study on hydrate growth and desalination effects in water/oil dispersion[D]. Beijing: China University of Petroleum (Beijing), 2017. | |
| 83 | ENGLEZOS P, KALOGERAKIS N, DHOLABHAI P D, et al. Kinetics of formation of methane and ethane gas hydrates[J]. Chemical Engineering Science, 1987, 42(11): 2647-2658. |
| [1] | SHENG Weiwu, CHENG Yongpan, CHEN Qiang, LI Xiaoting, WEI Jia, LI Linge, CHEN Xianfeng. Operating condition analysis of the microbubble and microdroplet dual-enhanced desulfurization reactor [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 142-147. |
| [2] | HUANG Yiping, LI Ting, ZHENG Longyun, QI Ao, CHEN Zhenglin, SHI Tianhao, ZHANG Xinyu, GUO Kai, HU Meng, NI Zeyu, LIU Hui, XIA Miao, ZHU Kai, LIU Chunjiang. Hydrodynamics and mass transfer characteristics of a three-stage internal loop airlift reactor [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 175-188. |
| [3] | YANG Hanyue, KONG Lingzhen, CHEN Jiaqing, SUN Huan, SONG Jiakai, WANG Sicheng, KONG Biao. Decarbonization performance of downflow tubular gas-liquid contactor of microbubble-type [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 197-204. |
| [4] | CHEN Kuangyin, LI Ruilan, TONG Yang, SHEN Jianhua. Structure design of gas diffusion layer in proton exchange membrane fuel cell [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 246-259. |
| [5] | GU Yongzheng, ZHANG Yongsheng. Dynamic behavior and kinetic model of Hg0 adsorption by HBr-modified fly ash [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 498-509. |
| [6] | WANG Peng, ZHANG Yang, FAN Bingqiang, HE Dengbo, SHEN Changshuai, ZHANG Hedong, ZHENG Shili, ZOU Xing. Process and kinetics of hydrochloric acid leaching of high-carbon ferrochromium [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 510-517. |
| [7] | SHAO Boshi, TAN Hongbo. Simulation on the enhancement of cryogenic removal of volatile organic compounds by sawtooth plate [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 84-93. |
| [8] | WANG Jinhang, HE Yong, SHI Lingli, LONG Zhen, LIANG Deqing. Progress of gas hydrate anti-agglomerants [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4587-4602. |
| [9] | LI You, WU Yue, ZHONG Yu, LIN Qixuan, REN Junli. Pretreatment of wheat straw with acidic molten salt hydrate for xylose production and its effect on enzymatic hydrolysis efficiency [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4974-4983. |
| [10] | YIN Xinyu, PI Pihui, WEN Xiufang, QIAN Yu. Application of special wettability materials for anti-hydrate-nucleation and anti-hydrate-adhesion in oil and gas pipelines [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4076-4092. |
| [11] | LI Dong, WANG Qianqian, ZHANG Liang, LI Jun, FU Qian, ZHU Xun, LIAO Qiang. Performance of series stack of non-aqueous nano slurry thermally regenerative flow batteries [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4238-4246. |
| [12] | ZHANG Kai, LYU Qiunan, LI Gang, LI Xiaosen, MO Jiamei. Morphology and occurrence characteristics of methane hydrates in the mud of the South China Sea [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3865-3874. |
| [13] | WANG Junjie, PAN Yanqiu, NIU Yabin, YU Lu. Molecular level catalytic reforming model construction and application [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3404-3412. |
| [14] | LI Ruidong, HUANG Hui, TONG Guohu, WANG Yueshe. Hygroscopic properties and corrosion behavior of ammonium salt in a crude oil distillation column [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2809-2818. |
| [15] | ZHANG Kai, JIN Hanyu, LIU Siyu, WANG Shuai. Simulation of mass transfer process under the bubble interaction in bubbling fluidization [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2828-2835. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||