Chemical Industry and Engineering Progress ›› 2022, Vol. 41 ›› Issue (3): 1309-1317.DOI: 10.16085/j.issn.1000-6613.2022-0244
• Renewable energy development and usage • Previous Articles Next Articles
Liquid sunshine methanol
WANG Jijie1( ), HAN Zhe1, CHEN Siyu1,2, TANG Chizhou1,2, SHA Feng1,3, TANG Shan1,2, YAO Tingting1, LI Can1(
), HAN Zhe1, CHEN Siyu1,2, TANG Chizhou1,2, SHA Feng1,3, TANG Shan1,2, YAO Tingting1, LI Can1( )
)
- 1.State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, Liaoning, China
2.University of Chinese Academy of Sciences, Beijing 100049, China
3.School of Materials Science and Engineering and National Institute for Advanced Materials, Nankai University, Tianjin, 300350, China
-
Received:2022-02-16Revised:2022-03-04Online:2022-03-28Published:2022-03-23 -
Contact:LI Can
太阳燃料甲醇合成
王集杰1( ), 韩哲1, 陈思宇1,2, 汤驰洲1,2, 沙峰1,3, 唐珊1,2, 姚婷婷1, 李灿1(
), 韩哲1, 陈思宇1,2, 汤驰洲1,2, 沙峰1,3, 唐珊1,2, 姚婷婷1, 李灿1( )
)
- 1.中国科学院大连化学物理研究所,催化基础国家重点实验室,辽宁 大连 116023
2.中国科学院大学,北京 100049
3.南开大学材料科学与工程学院,天津 300350
-
通讯作者:李灿 -
作者简介:王集杰(1985—),男,研究员,研究方向为液态阳光及二氧化碳资源化利用。E-mail:jjwang@dicp.ac.cn 。 -
基金资助:人工光合成基础科学中心(22088102);国家自然科学基金(22172160);中国科学院A类先导专项(XDA21090200);中国科学院青年创新促进会项目(2019183)
CLC Number:
Cite this article
WANG Jijie, HAN Zhe, CHEN Siyu, TANG Chizhou, SHA Feng, TANG Shan, YAO Tingting, LI Can. Liquid sunshine methanol[J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1309-1317.
王集杰, 韩哲, 陈思宇, 汤驰洲, 沙峰, 唐珊, 姚婷婷, 李灿. 太阳燃料甲醇合成[J]. 化工进展, 2022, 41(3): 1309-1317.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-0244
| 成本 | 工艺名称 | 煤制甲醇 /×108CNY | 液态阳光甲醇 /×108CNY |
|---|---|---|---|
| 固定资产 | 造气 | 6.60 | |
| 空分 | 4.20 | ||
| 变换 | 0.84 | ||
| 净化 | 1.88 | ||
| 电解水装置 | 5.70① | ||
| 其他/公用工程等 | 21.08 | 14.05② | |
| 小计 | 34.60 | 19.75 | |
| 运行成本 | 煤消耗 | 9.00③ | |
| 空分 | 1.80 | ||
| 制氢电耗 | 9.11① | ||
| 二氧化碳的捕获 | 1.34④ | ||
| 其他 | 1.50 | 1.50 | |
| 小计 | 12.30 | 11.95 | |
| 甲醇成本 | 2627CNY/t | 2321CNY/t | |
| 考虑碳税成本 | 2777CNY/t⑤ | 2261CNY/t⑥ |
| 成本 | 工艺名称 | 煤制甲醇 /×108CNY | 液态阳光甲醇 /×108CNY |
|---|---|---|---|
| 固定资产 | 造气 | 6.60 | |
| 空分 | 4.20 | ||
| 变换 | 0.84 | ||
| 净化 | 1.88 | ||
| 电解水装置 | 5.70① | ||
| 其他/公用工程等 | 21.08 | 14.05② | |
| 小计 | 34.60 | 19.75 | |
| 运行成本 | 煤消耗 | 9.00③ | |
| 空分 | 1.80 | ||
| 制氢电耗 | 9.11① | ||
| 二氧化碳的捕获 | 1.34④ | ||
| 其他 | 1.50 | 1.50 | |
| 小计 | 12.30 | 11.95 | |
| 甲醇成本 | 2627CNY/t | 2321CNY/t | |
| 考虑碳税成本 | 2777CNY/t⑤ | 2261CNY/t⑥ |
| 煤制甲醇 | 液态阳光甲醇 | ||
|---|---|---|---|
煤炭价格 /CNY·t-1 | 甲醇成本 /CNY·t-1 | 可再生能源发电 成本/CNY·(kW·h-1) | 甲醇成本 /CNY·t-1 |
| 500 | 1800 | 0.1 | 1600 |
| 1000 | 2600 | 0.2 | 2600 |
| 1500 | 3300 | 0.3 | 3600 |
| 2000 | 4100 | 0.4 | 4600 |
| 煤制甲醇 | 液态阳光甲醇 | ||
|---|---|---|---|
煤炭价格 /CNY·t-1 | 甲醇成本 /CNY·t-1 | 可再生能源发电 成本/CNY·(kW·h-1) | 甲醇成本 /CNY·t-1 |
| 500 | 1800 | 0.1 | 1600 |
| 1000 | 2600 | 0.2 | 2600 |
| 1500 | 3300 | 0.3 | 3600 |
| 2000 | 4100 | 0.4 | 4600 |
| 1 | STOCKER T F, QIN D, G-K PLATTNER, et al. Climate change 2013: the physical science basis[R]. New York: Intergovernmental Panel on Climate Change, 2014. |
| 2 | OLAH George A. Beyond oil and gas: the methanol economy[J]. Angewandte Chemie International Edition, 2005, 44(18): 2636-2639. |
| 3 | SHIH Choon Fong, ZHANG Tao, LI Jinghai, et al. Powering the future with liquid sunshine[J]. Joule, 2018, 2(10): 1925-1949. |
| 4 | ZHANG Jian, WANG Tao, LIU Pan, et al. Efficient hydrogen production on MoNi4 electrocatalysts with fast water dissociation kinetics[J]. Nature Communications, 2017, 8: 15437. |
| 5 | ZHANG Jian, WANG Tao, LIU Pan, et al. Engineering water dissociation sites in MoS2 nanosheets for accelerated electrocatalytic hydrogen production[J]. Energy & Environmental Science, 2016, 9(9): 2789-2793. |
| 6 | ZHANG Zhicheng, LIU Guigao, CUI Xiaoya, et al. Crystal phase and architecture engineering of lotus-thalamus-shaped Pt-Ni anisotropic superstructures for highly efficient electrochemical hydrogen evolution[J]. Advanced Materials, 2018, 30(30): e1801741. |
| 7 | YE Sheng, DING Chunmei, LIU Mingyao, et al. Water oxidation catalysts for artificial photosynthesis[J]. Advanced Materials, 2019, 31(50): e1902069. |
| 8 | ZOU Xiaoxin, ZHANG Yu. Noble metal-free hydrogen evolution catalysts for water splitting[J]. Chemical Society Reviews, 2015, 44(15): 5148-5180. |
| 9 | KING Laurie A, HUBERT McKenzie A, CAPUANO Christopher, et al. A non-precious metal hydrogen catalyst in a commercial polymer electrolyte membrane electrolyser[J]. Nature Nanotechnology, 2019, 14(11): 1071-1074. |
| 10 | LI Ailong, KONG Shuang, GUO Chenxi, et al. Enhancing the stability of cobalt spinel oxide towards sustainable oxygen evolution in acid[J]. Nature Catalysis, 2022, 5(2): 109-118. |
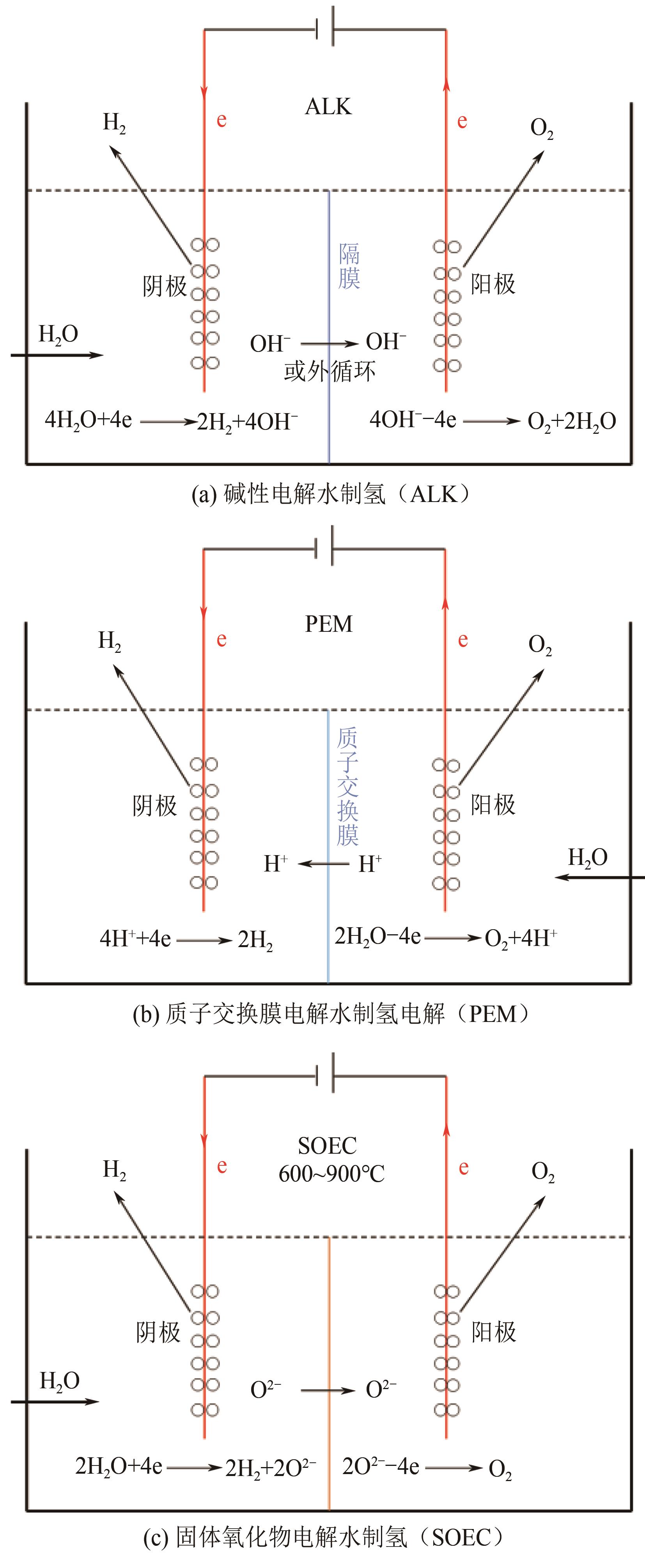
| 11 | 何泽兴, 史成香, 陈志超, 等. 质子交换膜电解水制氢技术的发展现状及展望[J]. 化工进展, 2021, 40(9): 4762-4773. |
| HE Zexing, SHI Chengxiang, CHEN Zhichao, et al. Development status and prospects of proton exchange membrane water electrolysis[J]. Chemical Industry and Engineering Progress, 2021, 40(9): 4762-4773. | |
| 12 | LI Ailong, SUN Yimeng, YAO Tingting, et al. Earth-abundant transition-metal-based electrocatalysts for water electrolysis to produce renewable hydrogen[J]. Chemistry, 2018, 24(69): 18334-18355. |
| 13 | NAKAMURA J, CHOI Y, FUJITANI T. On the issue of the active site and the role of ZnO in Cu/ZnO methanol synthesis catalysts[J]. Topics in Catalysis, 2003, 22(3/4): 277-285. |
| 14 | KUNKES Edward L, STUDT Felix, Frank ABILD-PEDERSEN, et al. Hydrogenation of CO2 to methanol and CO on Cu/ZnO/Al2O3: is there a common intermediate or not? [J]. Journal of Catalysis, 2015, 328: 43-48. |
| 15 | FICHTL Matthias B, SCHLERETH David, JACOBSEN Nikolas, et al. Kinetics of deactivation on Cu/ZnO/Al2O3 methanol synthesis catalysts[J]. Applied Catalysis A: General, 2015, 502: 262-270. |
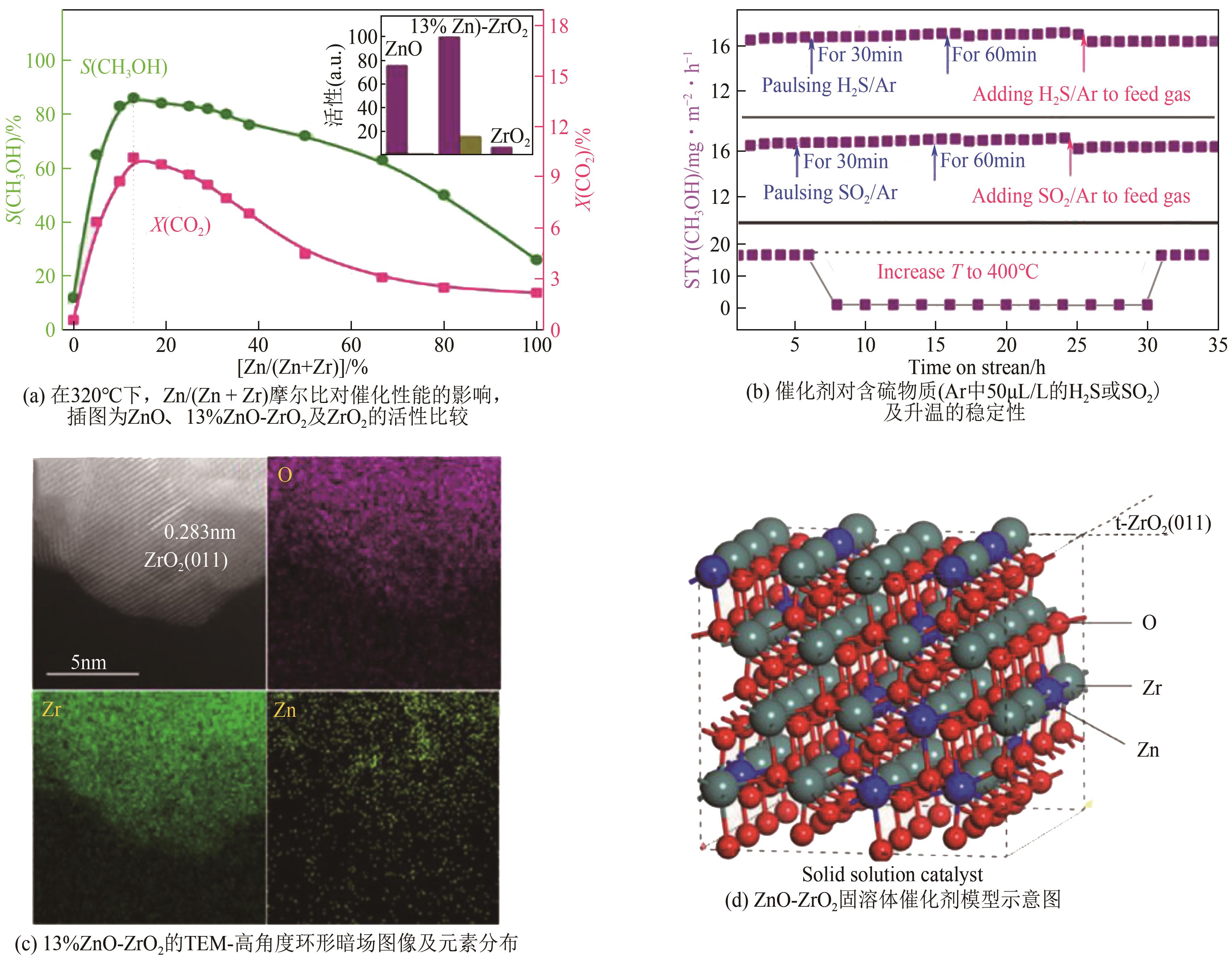
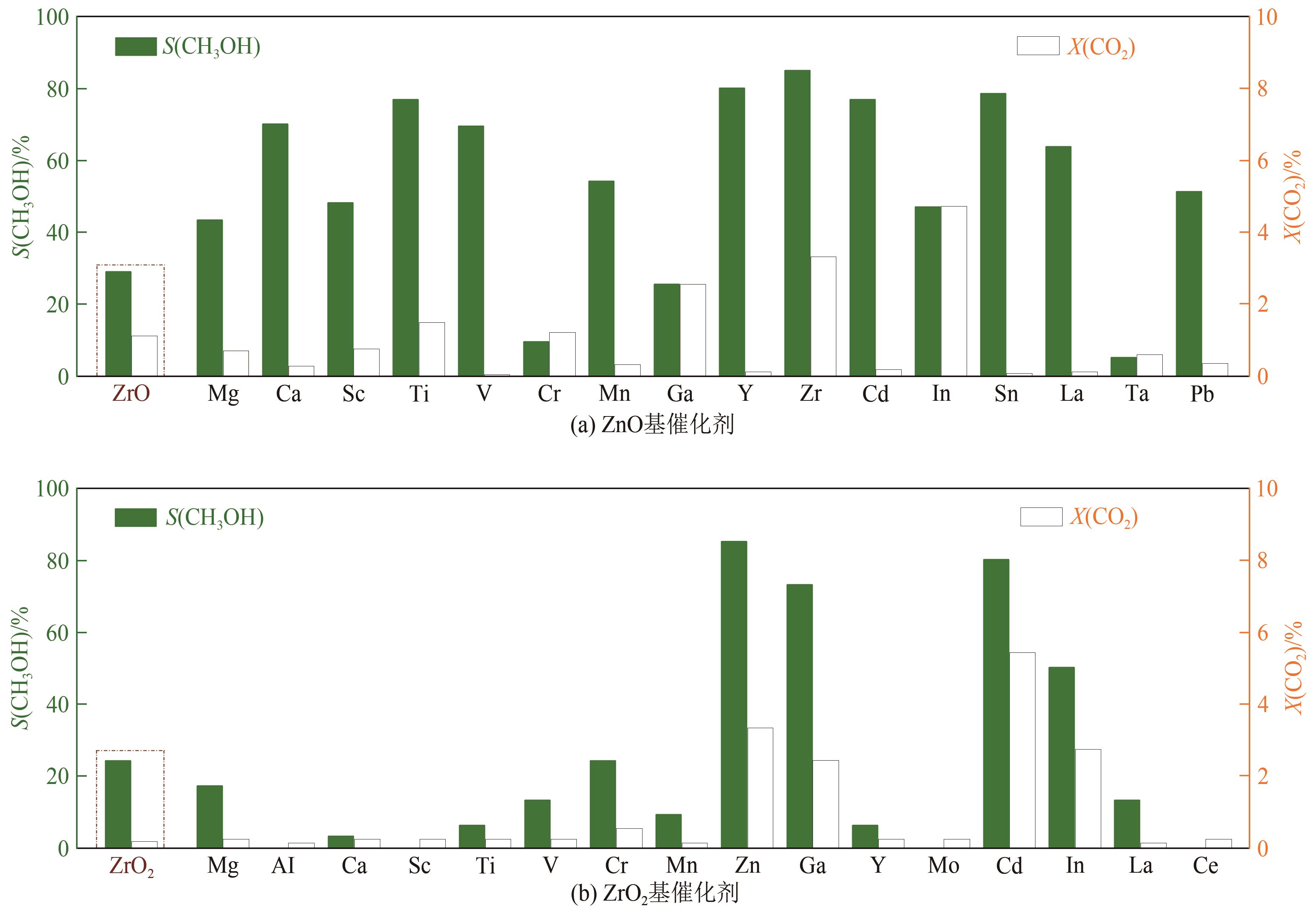
| 16 | WANG Jijie, LI Guanna, LI Zelong, et al. A highly selective and stable ZnO-ZrO2 solid solution catalyst for CO2 hydrogenation to methanol[J]. Science Advances, 2017, 3(10): e1701290. |
| 17 | WANG Jijie, TANG Chizhou, LI Guanna, et al. High-performance MaZrO x (Ma = Cd, Ga) solid-solution catalysts for CO2 hydrogenation to methanol[J]. ACS Catalysis, 2019, 9(11): 10253-10259. |
| 18 | YE Jingyun, LIU Changjun, GE Qingfeng. DFT study of CO2 adsorption and hydrogenation on the In2O3 surface[J]. The Journal of Physical Chemistry C, 2012, 116(14): 7817-7825. |
| 19 | YE Jingyun, LIU Changjun, MEI Donghai, et al. Active oxygen vacancy site for methanol synthesis from CO2 hydrogenation on In2O3(110): a DFT study[J]. ACS Catalysis, 2013, 3(6): 1296-1306. |
| 20 | SUN Kaihang, FAN Zhigang, YE Jingyun, et al. Hydrogenation of CO2 to methanol over In2O3 catalyst[J]. Journal of CO2 Utilization, 2015, 12: 1-6. |
| 21 | MARTIN Oliver, MARTÍN Antonio J, MONDELLI Cecilia, et al. Indium oxide as a superior catalyst for methanol synthesis by CO2 hydrogenation[J]. Angewandte Chemie International Edition, 2016, 55(21): 6261-6265. |
| 22 | JIANG Xiao, NIE Xiaowa, GUO Xinwen, et al. Recent advances in carbon dioxide hydrogenation to methanol via heterogeneous catalysis[J]. Chemical Reviews, 2020, 120(15): 7984-8034. |
| 23 | KATTEL Shyam, RAMÍREZ Pedro J, CHEN Jingguang, et al. Active sites for CO2 hydrogenation to methanol on Cu/ZnO catalysts[J]. Science, 2017, 355(6331): 1296-1299. |
| 24 | KATTEL Shyam, RAMÍREZ Pedro J, CHEN Jingguang, et al. Response to comment on “active sites for CO2 hydrogenation to methanol on Cu/ZnO catalysts”[J]. Science, 2017, 357(6354): eaan8210. |
| 25 | BEHRENS Malte, STUDT Felix, KASATKIN Igor, et al. The active site of methanol synthesis over Cu/ZnO/Al2O3 industrial catalysts[J]. Science, 2012, 336(6083): 893-897. |
| 26 | LUNKENBEIN Thomas, SCHUMANN Julia, BEHRENS Malte, et al. Formation of a ZnO overlayer in industrial Cu/ZnO/Al2O3 catalysts induced by strong metal-support interactions[J]. Angewandte Chemie International Edition, 2015, 54(15): 4544-4548. |
| 27 | NAKAMURA Junji, FUJITANI Tadahiro, KULD Sebastian, et al. Comment on “active sites for CO2 hydrogenation to methanol on Cu/ZnO catalysts”[J]. Science, 2017, 357(6354): eaan8074. |
| 28 | BANSODE Atul, TIDONA Bruno, ROHR Philipp Rudolf VON, et al. Impact of K and Ba promoters on CO2 hydrogenation over Cu/Al2O3 catalysts at high pressure[J]. Catalysis Science & Technology, 2013, 3(3): 767-778. |
| 29 | BAN Hongyan, LI Congming, ASAMI Kenji, et al. Influence of rare-earth elements (La, Ce, Nd and Pr) on the performance of Cu/Zn/Zr catalyst for CH3OH synthesis from CO2 [J]. Catalysis Communications, 2014, 54: 50-54. |
| 30 | NOMURA Naofumi, TAGAWA Tomohiko, GOTO Shigeo. Effect of acid-base properties on copper catalysts for hydrogenation of carbon dioxide[J]. Reaction Kinetics and Catalysis Letters, 1998, 63(1): 21-25. |
| 31 | PHONGAMWONG Thanaree, CHANTAPRASERTPORN Usanee, WITOON Thongthai, et al. CO2 hydrogenation to methanol over CuO-ZnO-ZrO2-SiO2 catalysts: effects of SiO2 contents[J]. Chemical Engineering Journal, 2017, 316: 692-703. |
| 32 | ARENA F, MEZZATESTA G, ZAFARANA G, et al. How oxide carriers control the catalytic functionality of the Cu-ZnO system in the hydrogenation of CO2 to methanol[J]. Catalysis Today, 2013, 210: 39-46. |
| 33 | Mei Kee KOH, KHAVARIAN Mehrnoush, CHAI Siang Piao, et al. The morphological impact of siliceous porous carriers on copper-catalysts for selective direct CO2 hydrogenation to methanol[J]. International Journal of Hydrogen Energy, 2018, 43(19): 9334-9342. |
| 34 | WANG Guannan, CHEN Limin, SUN Yuhai, et al. Carbon dioxide hydrogenation to methanol over Cu/ZrO2/CNTs: effect of carbon surface chemistry[J]. RSC Advances, 2015, 5(56): 45320-45330. |
| 35 | TISSERAUD Céline, COMMINGES Clément, HABRIOUX Aurélien, et al. Cu-ZnO catalysts for CO2 hydrogenation to methanol: morphology change induced by ZnO lixiviation and its impact on the active phase formation[J]. Molecular Catalysis, 2018, 446: 98-105. |
| 36 | LI Molly M J, CHEN Chunping, Ayvalı TUĞÇE, et al. CO2 hydrogenation to methanol over catalysts derived from single cationic layer CuZnGa LDH precursors[J]. ACS Catalysis, 2018, 8(5): 4390-4401. |
| 37 | ZHANG Chen, YANG Haiyan, GAO Peng, et al. Preparation and CO2 hydrogenation catalytic properties of alumina microsphere supported Cu-based catalyst by deposition-precipitation method[J]. Journal of CO2 Utilization, 2017, 17: 263-272. |
| 38 | BEHRENS Malte. Coprecipitation: an excellent tool for the synthesis of supported metal catalysts—From the understanding of the well known recipes to new materials[J]. Catalysis Today, 2015, 246: 46-54. |
| 39 | FREI M S, CAPDEVILA-CORTADA M, GARCÍA-MUELAS R, et al. Mechanism and microkinetics of methanol synthesis via CO2 hydrogenation on indium oxide[J]. Journal of Catalysis, 2018, 361: 313-321. |
| 40 | DANG Shanshan, QIN Bin, YANG Yong, et al. Rationally designed indium oxide catalysts for CO2 hydrogenation to methanol with high activity and selectivity[J]. Science Advances, 2020, 6(25): eaaz2060. |
| 41 | TSOUKALOU Athanasia, ABDALA Paula M, STOIAN Dragos, et al. Structural evolution and dynamics of an In2O3 catalyst for CO2 hydrogenation to methanol: an operando XAS-XRD and in situ TEM study[J]. Journal of the American Chemical Society, 2019, 141(34): 13497-13505. |
| 42 | RUI Ning, WANG Zongyuan, SUN Kaihang, et al. CO2 hydrogenation to methanol over Pd/In2O3: effects of Pd and oxygen vacancy[J]. Applied Catalysis B: Environmental, 2017, 218: 488-497. |
| 43 | YE Jingyun, LIU Changjun, MEI Donghai, et al. Methanol synthesis from CO2 hydrogenation over a Pd4/In2O3 model catalyst: a combined DFT and kinetic study[J]. Journal of Catalysis, 2014, 317: 44-53. |
| 44 | HAN Zhe, TANG Chizhou, WANG Jijie, et al. Atomically dispersed Pt n + species as highly active sites in Pt/In2O3 catalysts for methanol synthesis from CO2 hydrogenation[J]. Journal of Catalysis, 2021, 394: 236-244. |
| 45 | SUN Kaihang, RUI Ning, ZHANG Zhitao, et al. A highly active Pt/In2O3 catalyst for CO2 hydrogenation to methanol with enhanced stability[J]. Green Chemistry, 2020, 22(15): 5059-5066. |
| 46 | WANG Jing, SUN Kaihang, JIA Xinyu, et al. CO2 hydrogenation to methanol over Rh/In2O3 catalyst[J]. Catalysis Today, 2021, 365: 341-347. |
| 47 | RUI Ning, ZHANG Feng, SUN Kaihang, et al. Hydrogenation of CO2 to methanol on a Au δ +-In2O3- x catalyst[J]. ACS Catalysis, 2020, 10(19): 11307-11317. |
| 48 | JIA Xinyu, SUN Kaihang, WANG Jing, et al. Selective hydrogenation of CO2 to methanol over Ni/In2O3 catalyst[J]. Journal of Energy Chemistry, 2020, 50: 409-415. |
| 49 | BAVYKINA Anastasiya, YARULINA Irina, ABDULGHANI Abdullah J AL, et al. Turning a methanation Co catalyst into an In-Co methanol producer[J]. ACS Catalysis, 2019, 9(8): 6910-6918. |
| 50 | HAN Zhe, TANG Chizhou, SHA Feng, et al. CO2 hydrogenation to methanol on ZnO-ZrO2 solid solution catalysts with ordered mesoporous structure[J]. Journal of Catalysis, 2021, 396: 242-250. |
| 51 | HALPER Mark. Forget storing carbon; re-use it: a company in Iceland is turning CO2 into methanol to power cars[J]. Renewable Energy Focus, 2011, 12(1): 56-58. |
| 52 | SINGH Surinder P, HAO Pingjiao, LIU Xiao, et al. Large-scale affordable CO2 capture is possible by 2030[J]. Joule, 2019, 3(9): 2154-2164. |
| 53 | 姬加良. 煤与不同原料重整气化制甲醇对CO2排放的影响[J]. 能源科技, 2020, 18(2): 62-66. |
| JI Jialiang. Effect of reforming gasification to methanol by coal and different raw materials on carbon dioxide emission[J]. Energy Science and Technology, 2020, 18(2): 62-66. |
| [1] | SHI Yongxing, LIN Gang, SUN Xiaohang, JIANG Weigeng, QIAO Dawei, YAN Binhang. Research progress on active sites in Cu-based catalysts for CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 287-298. |
| [2] | LIU Chang, LIU Zhongwen. Perspective on the one-step CO2 hydrogenation to dimethyl ether [J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1115-1120. |
| [3] | XIANG Hongwei, YANG Yong, LI Yongwang. Transformation and development of coal chemical industry under the goal of carbon neutralization [J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1399-1408. |
| [4] | ZHENG Xuewen, ZHAO Rui, WU Jiazhe, WANG Menglong, CHEN Yubin. Design and modification of electrocatalysts for seawater splitting: a review [J]. Chemical Industry and Engineering Progress, 2022, 41(11): 5800-5810. |
| [5] | ZHOU Ying, LI Yeqing, ZHOU Hongjun, XU Chunming. Exploration of bio-energy in promoting rural revitalization in China [J]. Chemical Industry and Engineering Progress, 2022, 41(11): 6195-6199. |
| [6] | ZHAO Yu, ZHOU Fei, ZHANG Weiwei, LI Ning, LI Shiyou, LI Guixian. Research progress of cobalt phosphide materials in the field of electrochemical energy [J]. Chemical Industry and Engineering Progress, 2021, 40(4): 2188-2205. |
| [7] | CHU Tingting,WANG Hui,FENG Caixia,MAO Liqun. Progress of photocatalysts for water splitting by solar energy [J]. Chemical Industry and Engineering Progree, 2012, 31(10): 2228-2233. |
| [8] | GUO Xiaoming,MAO Dongsen,LU Guanzhong,WANG Song. Progress in catalysts for methanol synthesis from CO2 hydrogenation [J]. Chemical Industry and Engineering Progree, 2012, 31(03): 477-488. |
| [9] |
GUO Xinbin,QIAO Qingdong.
Research progress of hydrogen production on photocatalysts for water splitting [J]. Chemical Industry and Engineering Progree, 2006, 25(7): 729-. |
| [10] | JIANG Qin,ZHANG Shouchen,WANG Liqiu,LIU Changhou. Preparation of perovskite photocatalysts and its applications progress [J]. Chemical Industry and Engineering Progree, 2006, 25(2): 136-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||