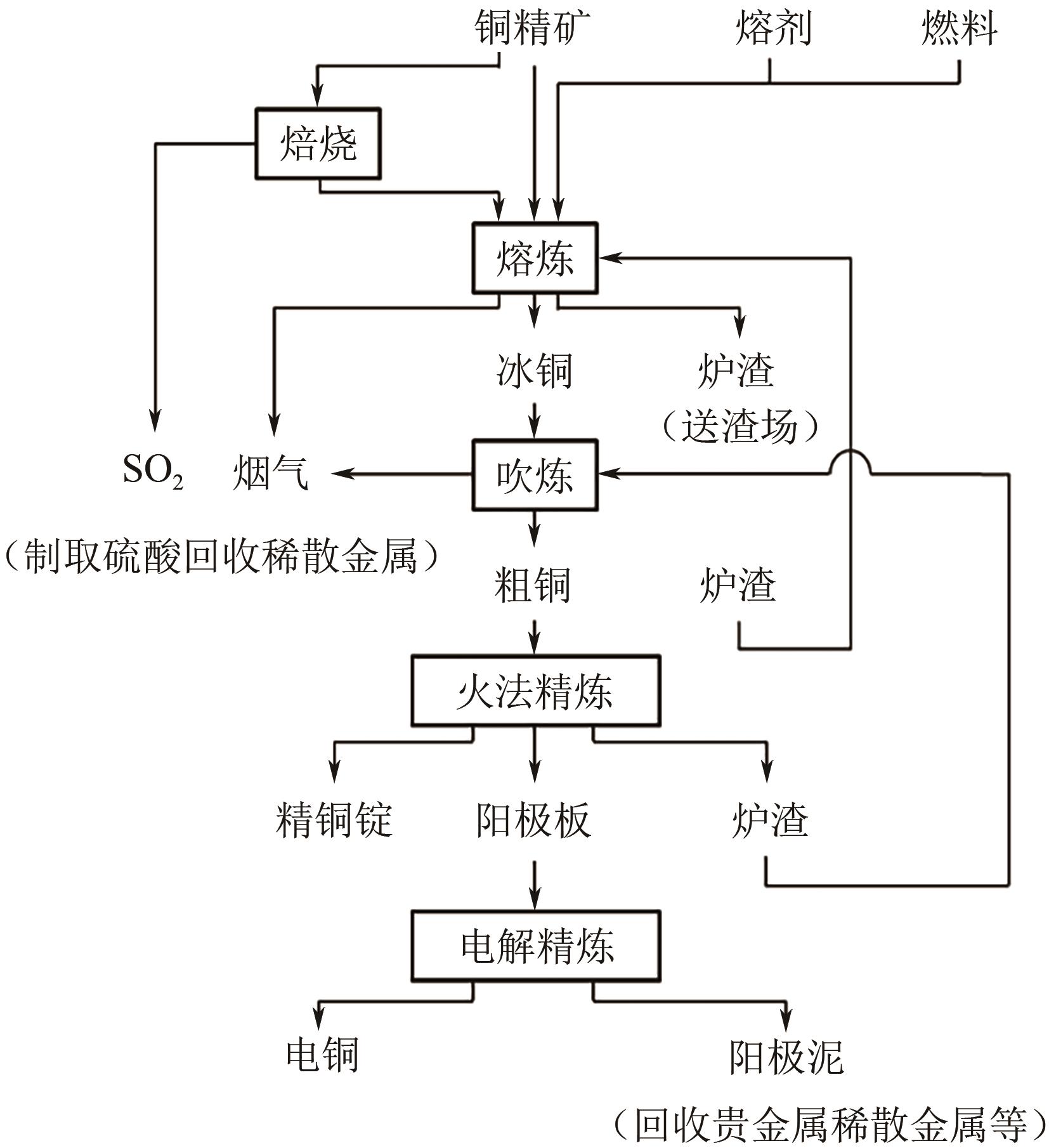
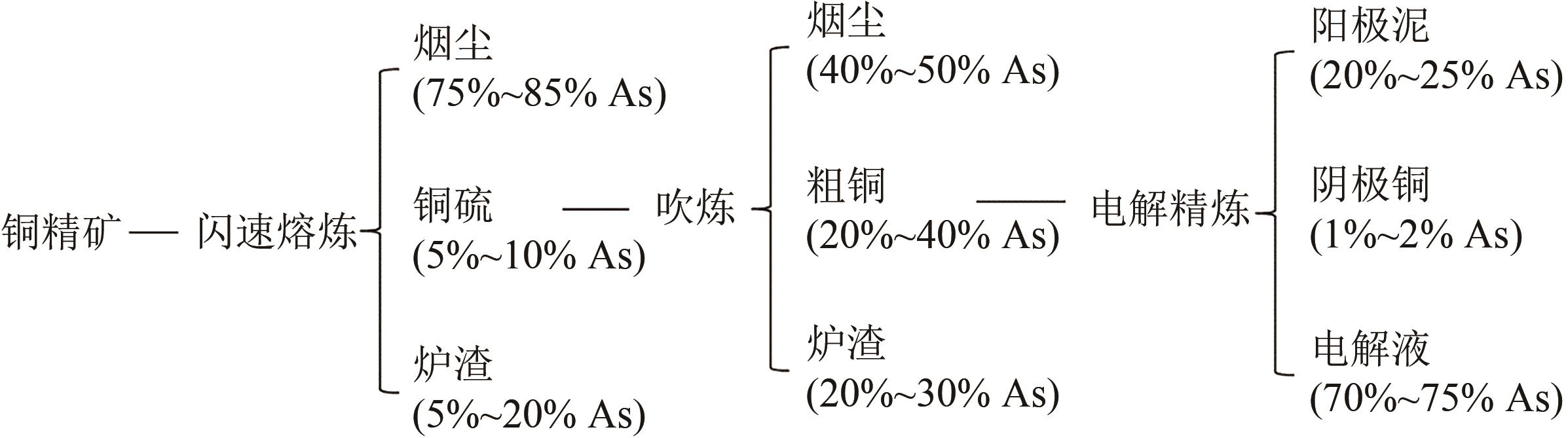
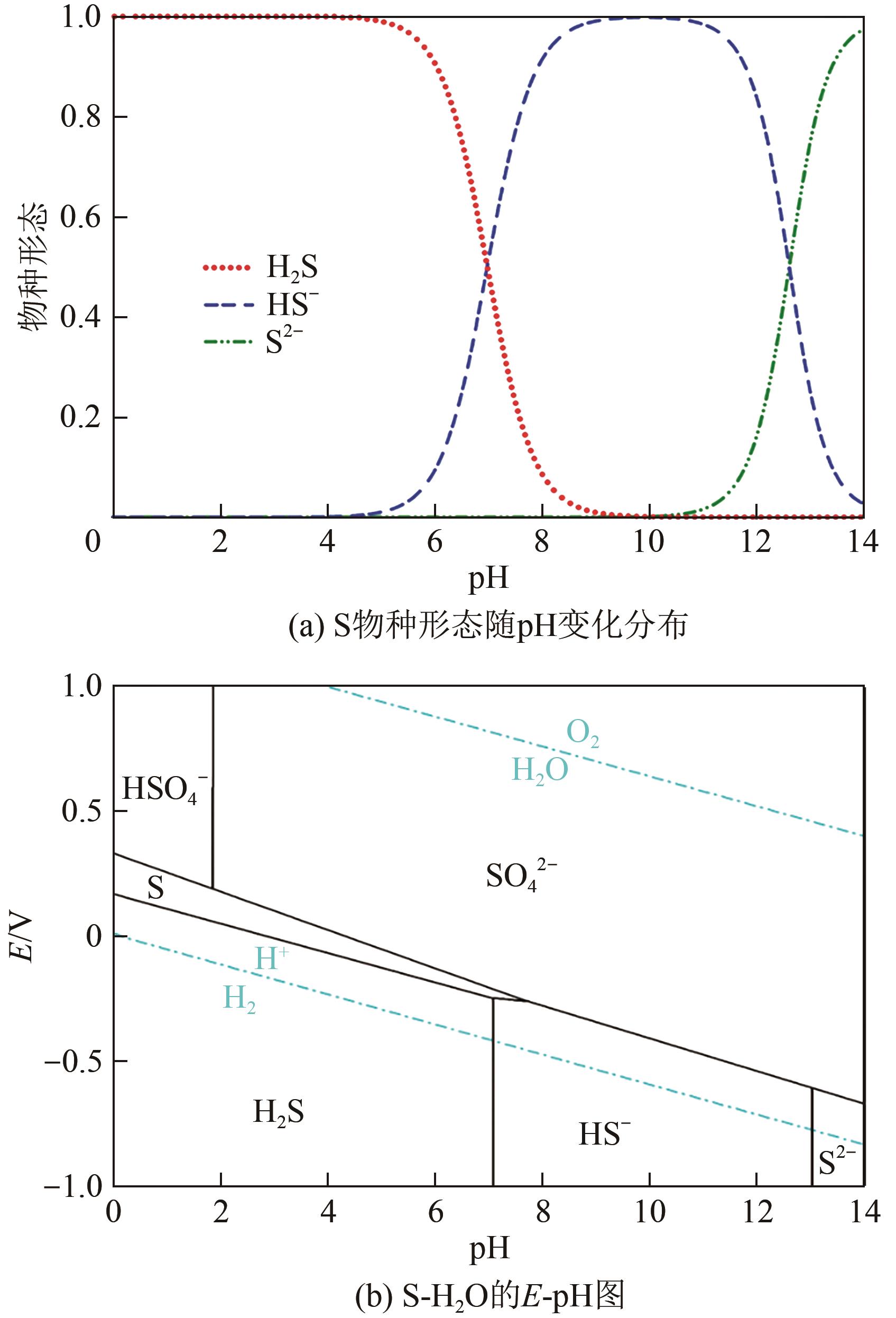
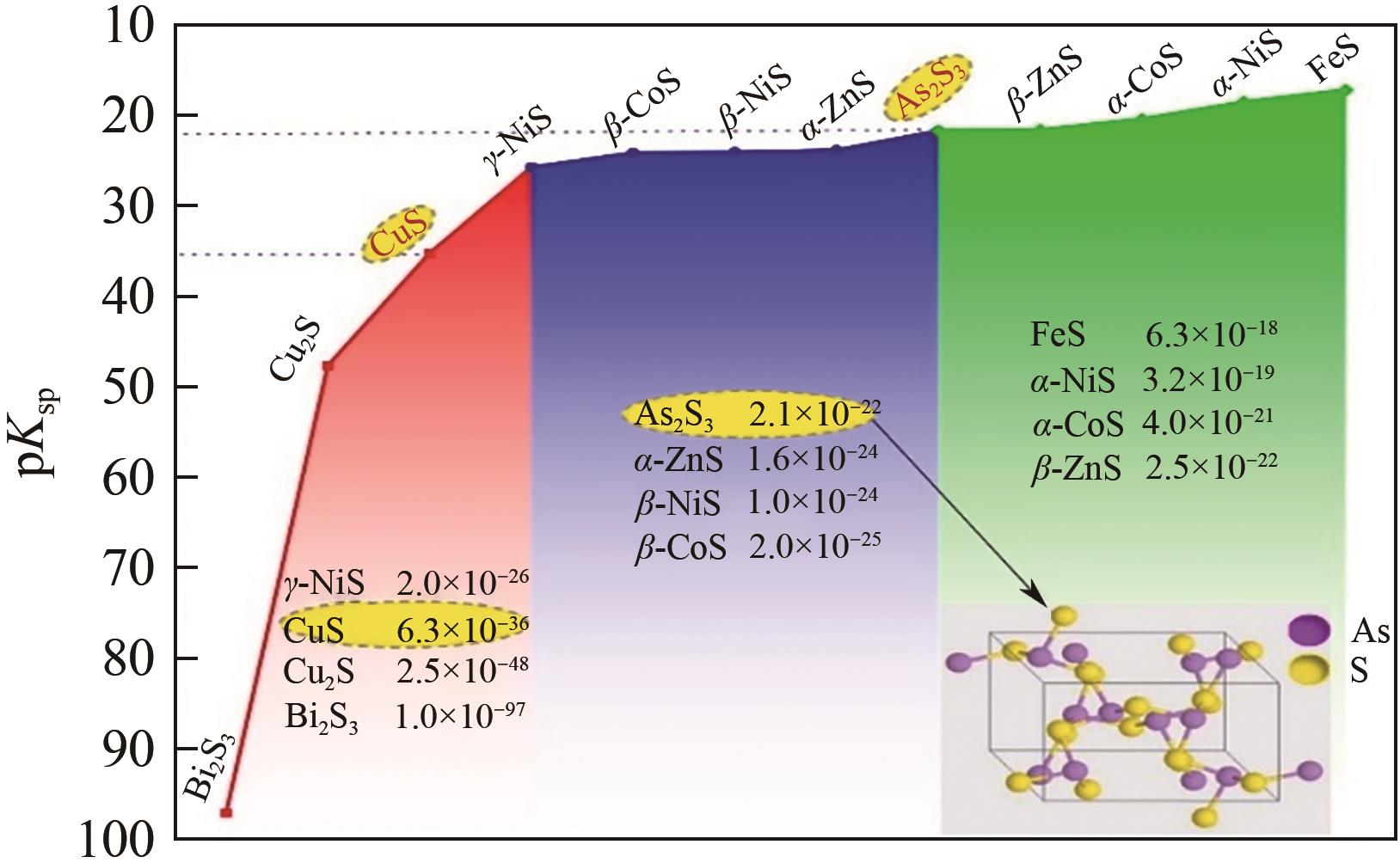
Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (9): 5301-5314.DOI: 10.16085/j.issn.1000-6613.2024-1061
• Resources and environmental engineering • Previous Articles
Treatment of copper-arsenic polymetallic acidic wastewater by sulfide precipitation: A review
HONG Kai1( ), FAN Huan2, TIAN Jia3,4, ZHANG Xingfei5(
), FAN Huan2, TIAN Jia3,4, ZHANG Xingfei5( )
)
- 1.Hubei Light Industry Technology Institute, Wuhan 430070, Hubei, China
2.Water Resources and Environmental Engineering Division, Hunan Provincial Communications Planning, Survey & Design Institute Co. , Ltd. , Changsha 410219, Hunan, China
3.School of Environmental and Resource Engineering, Southwest University of Science and Technology, Mianyang 621000, Sichuan, China
4.School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, Hunan, China
5.School of Resources Engineering, Xi’an University of Architecture and Technology, Xi’an 710055, Shaanxi, China
-
Received:2024-07-01Revised:2024-08-01Online:2025-09-30Published:2025-09-25 -
Contact:ZHANG Xingfei
硫化沉淀法处理铜砷多金属酸性废水研究进展
- 1.湖北轻工职业技术学院,湖北 武汉 430070
2.湖南省交通规划勘察设计院有限公司水利与环保事业部,湖南 长沙 410219
3.西南科技大学环境与资源工程学院,四川 绵阳 621000
4.中南大学资源加工与生物 工程学院,湖南 长沙 410083
5.西安建筑科技大学资源工程学院,陕西 西安 710055
-
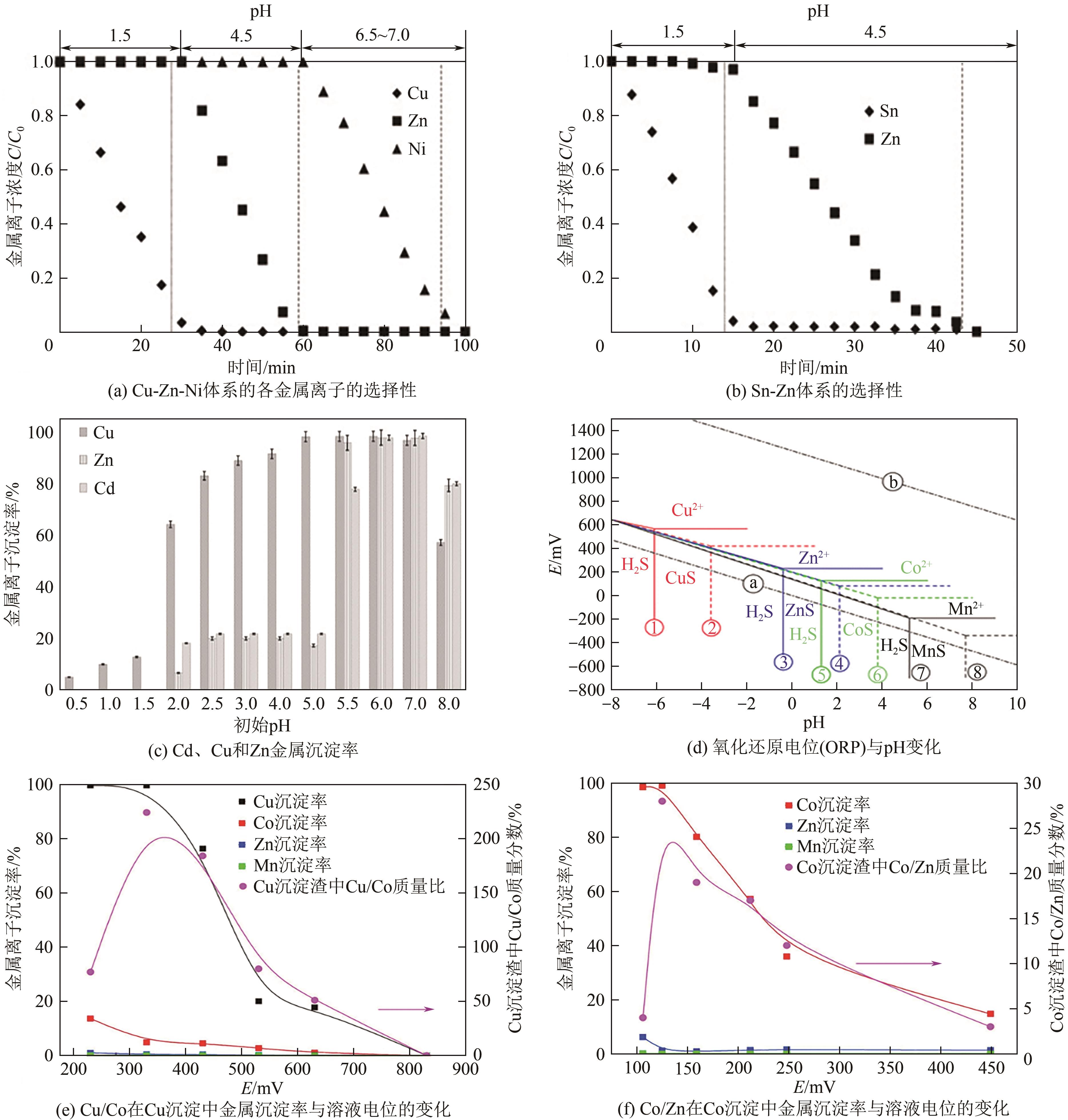
通讯作者:张荥斐 -
作者简介:洪凯(1966—),男,硕士,副教授。E-mail:408769310@qq.com。 -
基金资助:国家自然科学基金青年项目(52304315);湖南省重大科技攻关项目(2023ZJ1110)
CLC Number:
Cite this article
HONG Kai, FAN Huan, TIAN Jia, ZHANG Xingfei. Treatment of copper-arsenic polymetallic acidic wastewater by sulfide precipitation: A review[J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5301-5314.
洪凯, 樊欢, 田佳, 张荥斐. 硫化沉淀法处理铜砷多金属酸性废水研究进展[J]. 化工进展, 2025, 44(9): 5301-5314.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-1061
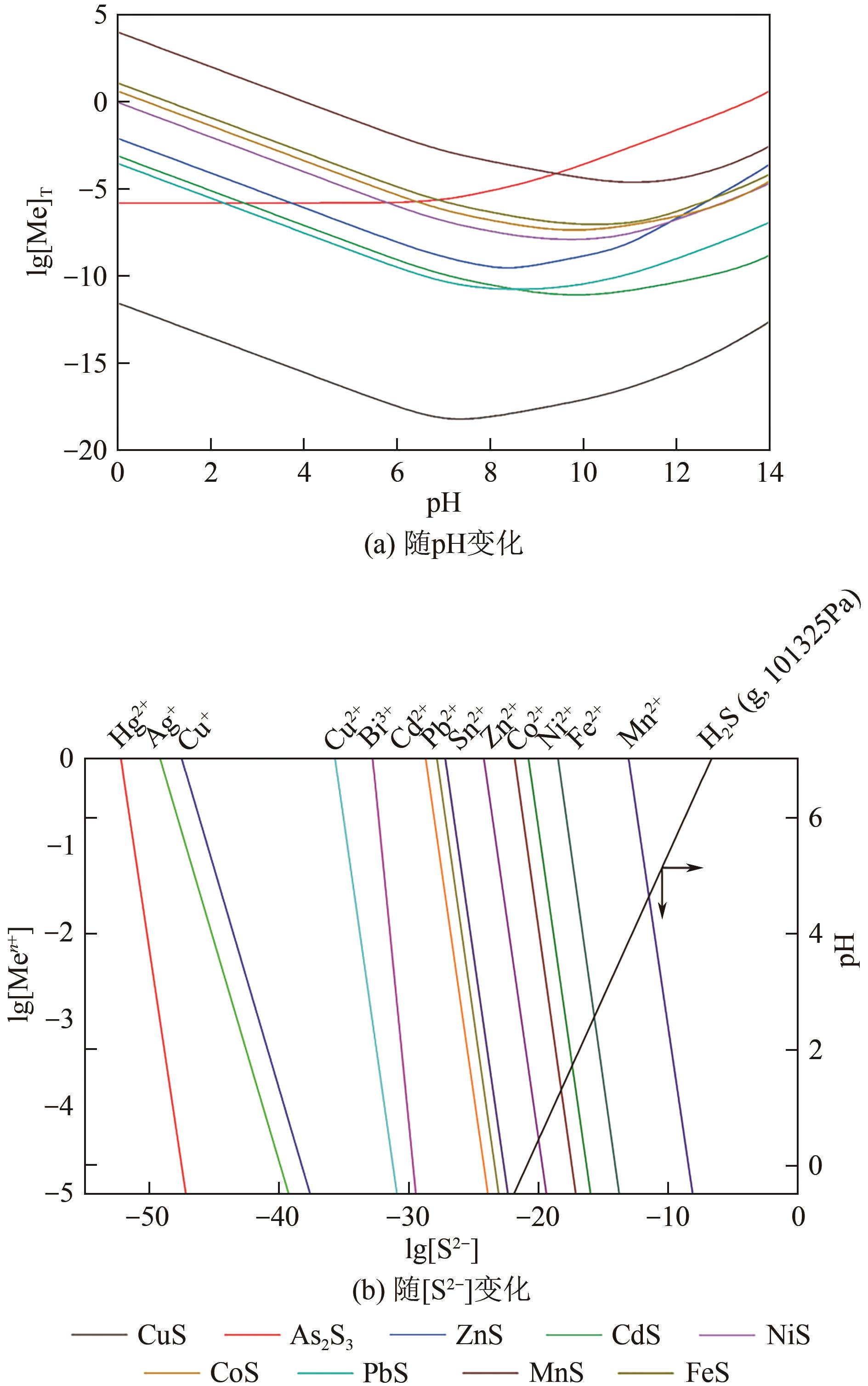
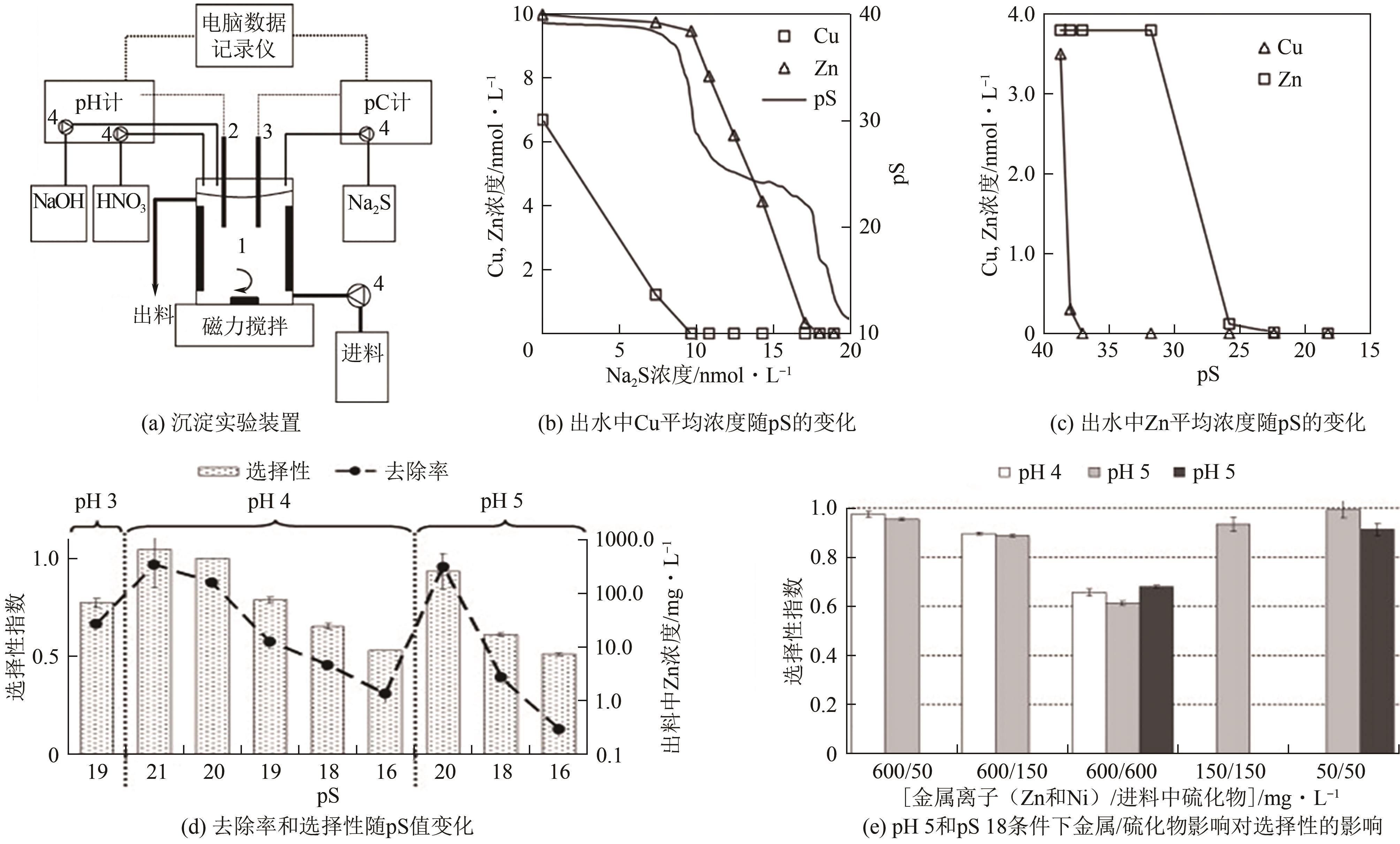
| 反应式 | 活度积 | ||
|---|---|---|---|
| 298K | 323K | 373K | |
| Ag2S ⇌ 2Ag++S2- | 49.14 | 45.37 | 39.70 |
| Bi2S3 ⇌ 2Bi3++3S2- | 104.05 | 98.37 | 90.58 |
| CdS ⇌ Cd2++S2- | 27.19 | 25.70 | 23.73 |
| CoS ⇌ Co2++S2- | 19.74 | 18.94 | 18.1 |
| CuS ⇌ Cu2++S2- | 35.85 | 33.76 | 30.85 |
| Cu2S ⇌ 2Cu++S2- | 47.64 | 44.13 | 38.89 |
| FeS ⇌ Fe2++S2- | 16.47 | 15.75 | 15.17 |
| HgS ⇌ Hg2++S2- | 52.7 | 49.1 | 43.9 |
| NiS ⇌ Ni2++S2- | 21.03 | 20.19 | 19.26 |
| MnS ⇌ Mn2++S2- | 12.95 | 12.59 | 12.4 |
| PbS ⇌ Pb2++S2- | 28.06 | 26.28 | 23.89 |
| SnS ⇌ Sn2++S2- | 27.53 | 26.04 | 24.08 |
| ZnS ⇌ Zn2++S2- | 23.10 | 22.07 | 20.83 |
| 反应式 | 活度积 | ||
|---|---|---|---|
| 298K | 323K | 373K | |
| Ag2S ⇌ 2Ag++S2- | 49.14 | 45.37 | 39.70 |
| Bi2S3 ⇌ 2Bi3++3S2- | 104.05 | 98.37 | 90.58 |
| CdS ⇌ Cd2++S2- | 27.19 | 25.70 | 23.73 |
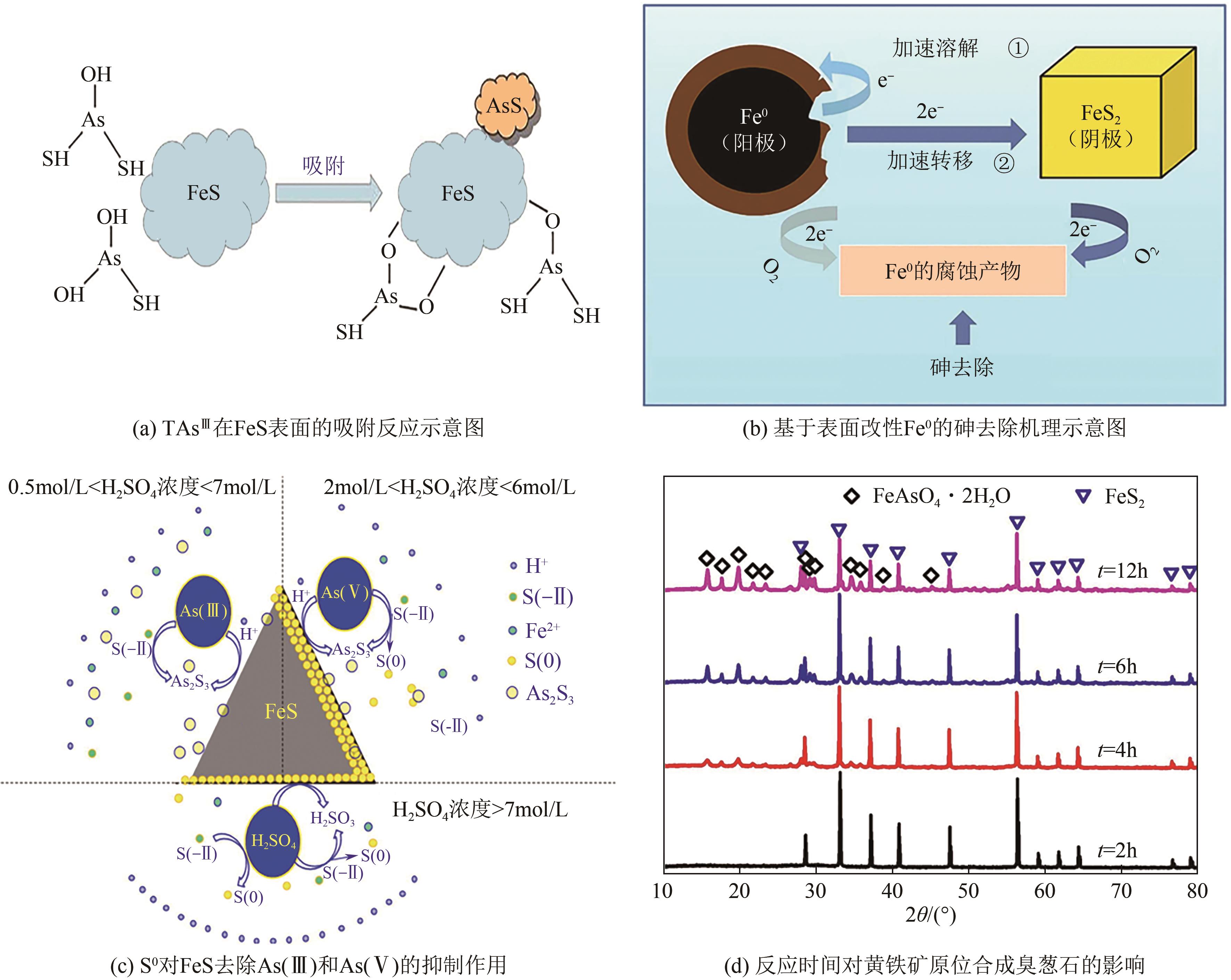
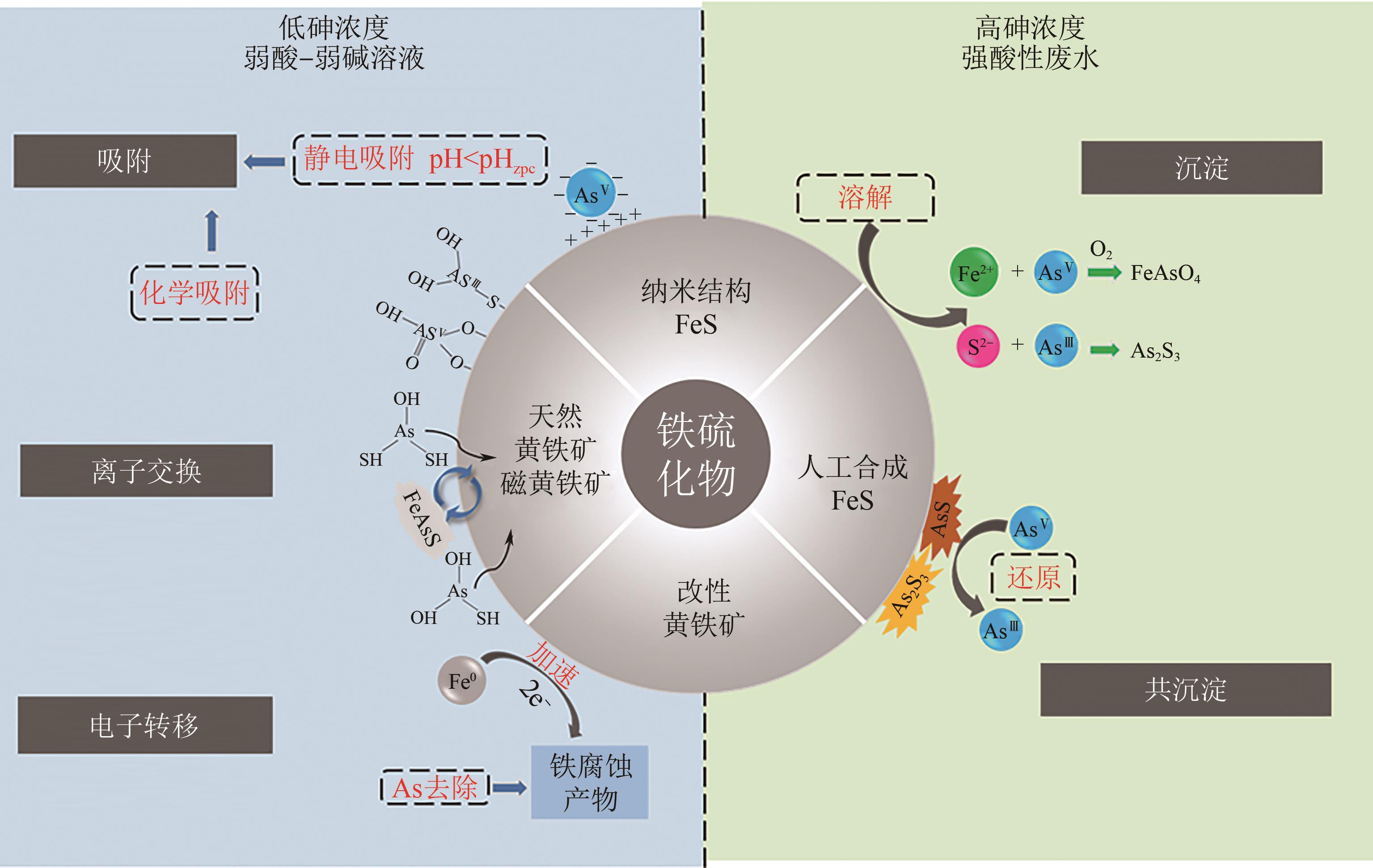
| CoS ⇌ Co2++S2- | 19.74 | 18.94 | 18.1 |
| CuS ⇌ Cu2++S2- | 35.85 | 33.76 | 30.85 |
| Cu2S ⇌ 2Cu++S2- | 47.64 | 44.13 | 38.89 |
| FeS ⇌ Fe2++S2- | 16.47 | 15.75 | 15.17 |
| HgS ⇌ Hg2++S2- | 52.7 | 49.1 | 43.9 |
| NiS ⇌ Ni2++S2- | 21.03 | 20.19 | 19.26 |
| MnS ⇌ Mn2++S2- | 12.95 | 12.59 | 12.4 |
| PbS ⇌ Pb2++S2- | 28.06 | 26.28 | 23.89 |
| SnS ⇌ Sn2++S2- | 27.53 | 26.04 | 24.08 |
| ZnS ⇌ Zn2++S2- | 23.10 | 22.07 | 20.83 |
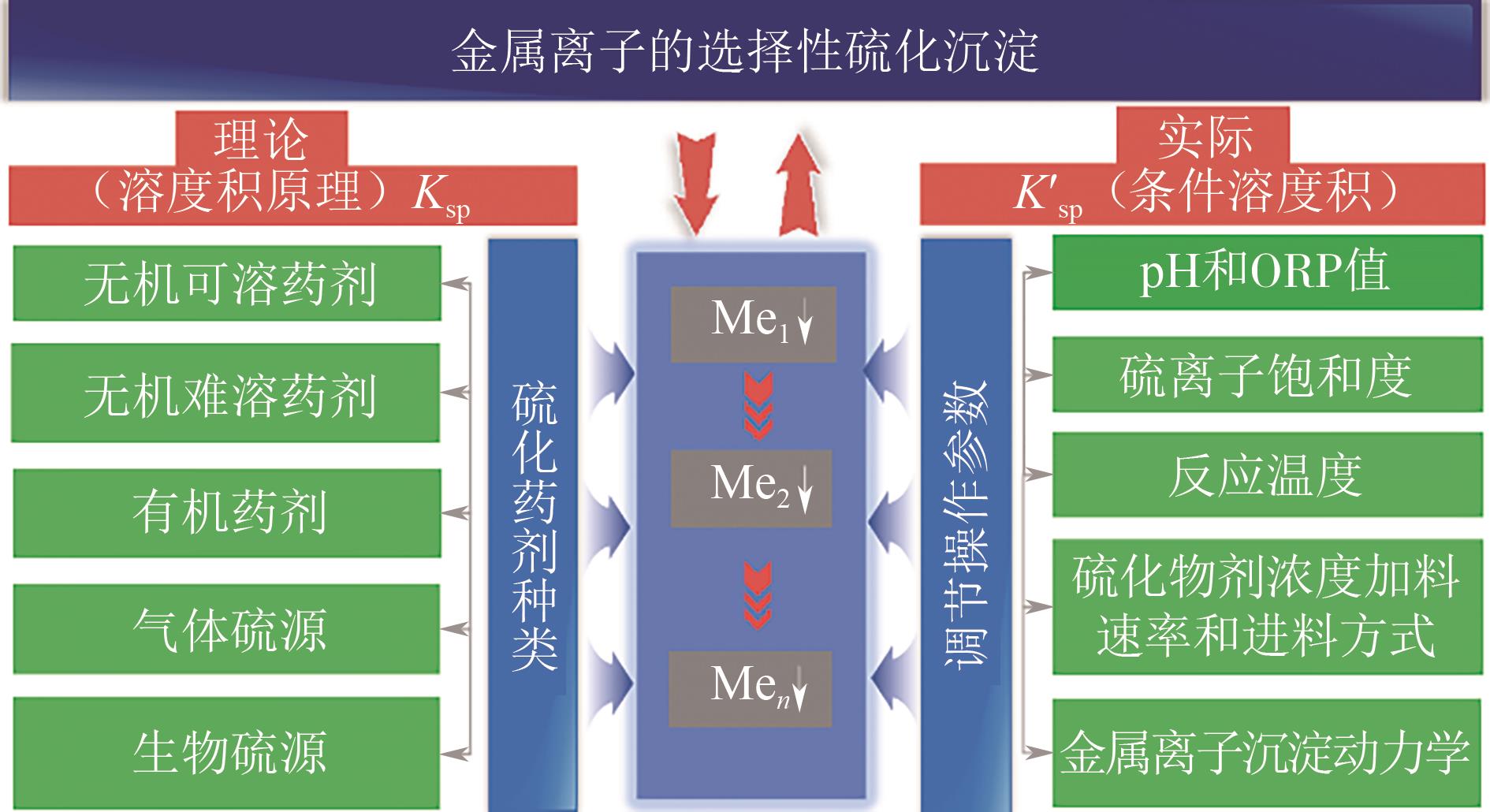
| 硫源类型 | 描述 | 优点 | 缺点 |
|---|---|---|---|
| 无机可溶 | 采用Na2S、NaHS、(NH4)2S和Na2S2O3等化学可溶剂作为水溶沉淀剂连续或间歇进行 | 1.成本低,原料来源广; 2.反应速度快; 3.易于操作 | 1.硫离子局部浓度过高; 2.反应效率低,需要过量使用; 3.形成胶状产物,难以过滤; 4.H2S的二次污染 |
| 无机难溶 | CaS、FeS、ZnS、MnS和P2S5化学难溶性材料以固体或悬浮液的形式引入 | 1.降低溶液的过饱和水平; 2.提高产物颗粒大小; 3.有效控制H2S逸出 | 1.反应速度慢,适用性有限; 2.表面易被涂覆,反应效率低; 3.反应效率靠外场和环境影响; 4.引入其他金属阳离子 |
| 有机可溶 | 有机硫源中含有有机基团中的硫原子,在溶液中可水解或发生氧化反应,释放出硫离子 | 1.与无机难溶剂相比,具有更好的溶解度; 2.不同的官能团提高选择性; 3.反应条件温和,用量低 | 1.成本高,合成工艺复杂; 2.可能会残留危险或有毒的有机物,如CS2; 3.金属-有机配合物在环境中容易分解,金属被重新释放 |
| 气体 | 指能提供硫离子的气体化合物。最常用的是H2S,它能溶解于溶液中,与金属离子形成硫化物沉淀 | 1.高纯度和高效率; 2.不引入其他金属阳离子 | 1.剧毒气体,易造成安全事故; 2.需要特殊的硫化设备; 3.严格的管理制度和储存、运输和使用的限制 |
| 生物硫化 | 通过硫酸盐还原菌利用废水中的单质硫、废硫酸或硫酸盐生产H2S | 1.工艺环保,适应性广; 2.具有高稳定性和可持续性 | 1.需要较长的反应时间; 2.对环境温度、水质、微生物种类的选择要求高; 3.操作难度高 |
| 硫源类型 | 描述 | 优点 | 缺点 |
|---|---|---|---|
| 无机可溶 | 采用Na2S、NaHS、(NH4)2S和Na2S2O3等化学可溶剂作为水溶沉淀剂连续或间歇进行 | 1.成本低,原料来源广; 2.反应速度快; 3.易于操作 | 1.硫离子局部浓度过高; 2.反应效率低,需要过量使用; 3.形成胶状产物,难以过滤; 4.H2S的二次污染 |
| 无机难溶 | CaS、FeS、ZnS、MnS和P2S5化学难溶性材料以固体或悬浮液的形式引入 | 1.降低溶液的过饱和水平; 2.提高产物颗粒大小; 3.有效控制H2S逸出 | 1.反应速度慢,适用性有限; 2.表面易被涂覆,反应效率低; 3.反应效率靠外场和环境影响; 4.引入其他金属阳离子 |
| 有机可溶 | 有机硫源中含有有机基团中的硫原子,在溶液中可水解或发生氧化反应,释放出硫离子 | 1.与无机难溶剂相比,具有更好的溶解度; 2.不同的官能团提高选择性; 3.反应条件温和,用量低 | 1.成本高,合成工艺复杂; 2.可能会残留危险或有毒的有机物,如CS2; 3.金属-有机配合物在环境中容易分解,金属被重新释放 |
| 气体 | 指能提供硫离子的气体化合物。最常用的是H2S,它能溶解于溶液中,与金属离子形成硫化物沉淀 | 1.高纯度和高效率; 2.不引入其他金属阳离子 | 1.剧毒气体,易造成安全事故; 2.需要特殊的硫化设备; 3.严格的管理制度和储存、运输和使用的限制 |
| 生物硫化 | 通过硫酸盐还原菌利用废水中的单质硫、废硫酸或硫酸盐生产H2S | 1.工艺环保,适应性广; 2.具有高稳定性和可持续性 | 1.需要较长的反应时间; 2.对环境温度、水质、微生物种类的选择要求高; 3.操作难度高 |
| [1] | ZHANG Xingfei, FAN Huan, YUAN Jia, et al. The application and mechanism of iron sulfides in arsenic removal from water and wastewater: A critical review[J]. Journal of Environmental Chemical Engineering, 2022, 10(6): 108856. |
| [2] | VIJAYARAGHAVAN K, YUN Yeoung-Sang. Bacterial biosorbents and biosorption[J]. Biotechnology Advances, 2008, 26(3): 266-291. |
| [3] | SALEH Tawfik A, GUPTA Vinod K. Synthesis and characterization of alumina nano-particles polyamide membrane with enhanced flux rejection performance[J]. Separation and Purification Technology, 2012, 89: 245-251. |
| [4] | Imran ALI, JAIN Chakresh Kumar. Advances in arsenic speciation techniques[J]. International Journal of Environmental Analytical Chemistry, 2004, 84(12): 947-964. |
| [5] | 赵洪兴. 碱性溶液中砷萃取分离研究[D]. 赣州: 江西理工大学, 2015. |
| ZHAO Hongxing. Study on extraction and separation of arsenic from alkaline solution[D]. Ganzhou: Jiangxi University of Science and Technology, 2015. | |
| [6] | BARAKAT M A. New trends in removing heavy metals from industrial wastewater[J]. Arabian Journal of Chemistry, 2011, 4(4): 361-377. |
| [7] | TIAN Jia, ZHANG Xingfei, WANG Yufeng, et al. Alkali circulating leaching of arsenic from copper smelter dust based on arsenic-alkali efficient separation[J]. Journal of Environmental Management, 2021, 287: 112348. |
| [8] | 潘国强, 李红. 纳滤膜法与化学沉淀耦合法处理实际矿山废水工艺研究[J]. 广东化工, 2023, 50(3): 150-152. |
| PAN Guoqiang, LI Hong. Study on the treatment of actual mine wastewater by nanofiltration membrane coupled with chemical precipitation[J]. Guangdong Chemical Industry, 2023, 50(3): 150-152. | |
| [9] | 张候文, 杨大锦, 代龙果, 等. 从酸性高浓度含砷溶液中脱砷研究进展[J]. 湿法冶金, 2024, 43(4): 370-379. |
| ZHANG Houwen, YANG Dajin, DAI Longguo, et al. Research progress on removal of arsenic from acidic high-concentration arsenic solution [J]. Hydrometallurgy, 2024, 43(4): 370-379. | |
| [10] | LEWIS A. Precipitation of heavy metals[M]//RENE Eldon R, SAHINKAYA Erkan, LEWIS Alison, et al. Sustainable Heavy Metal Remediation: Volume 1: Principles and Processes. Springer, 2017: 101-120. |
| [11] | ESTAY Humberto, BARROS Lorena, TRONCOSO Elizabeth. Metal sulfide precipitation: Recent breakthroughs and future outlooks[J]. Minerals, 2021, 11(12): 1385. |
| [12] | LIANG Qian, JIANG Linhua, DUAN Ning, et al. Exploration of the arsenic removal performance fluctuation using H2S from highly acidic wastewater in copper smelting[J]. Journal of Cleaner Production, 2022, 376: 134311. |
| [13] | 李栋, 袁乐乐, 张勇兵, 等. 有色冶金污酸废水资源化研究进展[J]. 有色金属科学与工程, 2024, 15(6): 822-830. |
| LI Dong, YUAN Lele, ZHANG Yongbing, et al. Research progress on recycling of acid wastewater from nonferrous metallurgy [J]. Nonferrous Metals Science and Engineering, 2024, 15(6): 822-830. | |
| [14] | 李玉虎. 有色冶金含砷烟尘中砷的脱除与固化[D]. 长沙: 中南大学, 2012. |
| LI Yuhu. Removal and solidification of arsenic from arsenic-containing dust in nonferrous metallurgy[D]. Changsha: Central South University, 2012. | |
| [15] | CALDERON April Rose Malagum, ALORRO Richard Diaz, TADESSE Bogale, et al. Repurposing of nickeliferous pyrrhotite from mine tailings as magnetic adsorbent for the recovery of gold from chloride solution[J]. Resources, Conservation and Recycling, 2020, 161: 104971. |
| [16] | Gülay BULUT, Ünzile YENIAL, Emrecan EMIROĞLU, et al. Arsenic removal from aqueous solution using pyrite[J]. Journal of Cleaner Production, 2014, 84: 526-532. |
| [17] | GARG Srinath, JUDD Kurtis, MAHADEVAN Radhakrishnan, et al. Leaching characteristics of nickeliferous pyrrhotite tailings from the Sudbury, Ontario area[J]. Canadian Metallurgical Quarterly, 2017, 56(4): 372-381. |
| [18] | 范帅. 电镀废水中铜的电沉积回收及其工艺设计[D]. 衡阳: 南华大学, 2015. |
| FAN Shuai. Recovery of copper from electroplating wastewater by electrodeposition and its process design[D]. Hengyang: University of South China, 2015. | |
| [19] | SONG Shaole, SUN Wei, WANG Li, et al. Recovery of cobalt and zinc from the leaching solution of zinc smelting slag[J]. Journal of Environmental Chemical Engineering, 2019, 7(1): 102777. |
| [20] | ETTEL V A, MOSOIU M A, DEVUYST E A. A novel oxidant for nickel hydrometallurgy[J]. Hydrometallurgy, 1979, 4(3): 247-257. |
| [21] | TOKUDA H, KUCHAR D, MIHARA N, et al. Study on reaction kinetics and selective precipitation of Cu, Zn, Ni and Sn with H2S in single-metal and multi-metal systems[J]. Chemosphere, 2008, 73(9): 1448-1452. |
| [22] | BIJMANS Martijn F M, VAN HELVOORT Pieter-Jan, Shabir A DAR, et al. Selective recovery of nickel over iron from a nickel-iron solution using microbial sulfate reduction in a gas-lift bioreactor[J]. Water Research, 2009, 43(3): 853-861. |
| [23] | SAMPAIO R M M, TIMMERS R A, KOCKS N, et al. Zn-Ni sulfide selective precipitation: The role of supersaturation[J]. Separation and Purification Technology, 2010, 74(1): 108-118. |
| [24] | SETHURAJAN Manivannan, HUGUENOT David, LENS Piet N L, et al. Leaching and selective copper recovery from acidic leachates of Três Marias zinc plant (MG, Brazil) metallurgical purification residues[J]. Journal of Environmental Management, 2016, 177: 26-35. |
| [25] | POTT Britt-Marie, MATTIASSON Bo. Separation of heavy metals from water solutions at the laboratory scale[J]. Biotechnology Letters, 2004, 26(5): 451-456. |
| [26] | LIU Weifeng, SUN Baiqi, ZHANG Duchao, et al. Selective separation of similar metals in chloride solution by sulfide precipitation under controlled potential[J]. JOM, 2017, 69(11): 2358-2363. |
| [27] | YANG Tianzu, HU Bin, LIU Weifeng, et al. Selective separation of copper from copper-smelting waste acid by potential control[J]. Metallurgical Research & Technology, 2019, 116(4): 409. |
| [28] | LEWIS Alison Emslie. Review of metal sulphide precipitation[J]. Hydrometallurgy, 2010, 104(2): 222-234. |
| [29] | MOKONE T P, VAN HILLE R P, LEWIS A E. Metal sulphides from wastewater: Assessing the impact of supersaturation control strategies[J]. Water Research, 2012, 46(7): 2088-2100. |
| [30] | Jakub ZIELIŃSKI, Marta HUCULAK-MĄCZKA, KANIEWSKI Maciej, et al. Kinetic modelling of cadmium removal from wet phosphoric acid by precipitation method[J]. Hydrometallurgy, 2019, 190: 105157. |
| [31] | VEEKEN Adrie H M, AKOTO Lawrence, HULSHOFF POL Look W, et al. Control of the sulfide (S2-) concentration for optimal zinc removal by sulfide precipitation in a continuously stirred tank reactor[J]. Water Research, 2003, 37(15): 3709-3717. |
| [32] | VEEKEN A H M, DE VRIES S, VAN DER MARK A, et al. Selective precipitation of heavy metals as controlled by a sulfide-selective electrode[J]. Separation Science and Technology, 2003, 38(1): 1-19. |
| [33] | SAMPAIO R M M, TIMMERS R A, XU Y, et al. Selective precipitation of Cu from Zn in a pS controlled continuously stirred tank reactor[J]. Journal of Hazardous Materials, 2009, 165(1/2/3): 256-265. |
| [34] | TAKANO Masatoshi, ASANO Satoshi, GOTO Masahiro. Recovery of nickel, cobalt and rare-earth elements from spent nickel-metal-hydride battery: Laboratory tests and pilot trials[J]. Hydrometallurgy, 2022, 209: 105826. |
| [35] | HU Shaohua, XIE Minyan, HSIEH Ya-Min, et al. Resource recycling of gallium arsenide scrap using leaching-selective precipitation[J]. Environmental Progress & Sustainable Energy, 2015, 34(2): 471-475. |
| [36] | GUO Li, DU Yaguang, YI Qiushi, et al. Efficient removal of arsenic from “dirty acid” wastewater by using a novel immersed multi-start distributor for sulphide feeding[J]. Separation and Purification Technology, 2015, 142: 209-214. |
| [37] | ZHANG Erjun, ZHOU Kanggen, ZHANG Xuekai, et al. Selective separation of copper and zinc from high acid leaching solution of copper dust using a sulfide precipitation-pickling approach[J]. Process Safety and Environmental Protection, 2021, 156: 100-108. |
| [38] | ZHANG Xingfei, ZENG Liqiang, WANG Yufeng, et al. Selective separation of metals from wastewater using sulfide precipitation: A critical review in agents, operational factors and particle aggregation[J]. Journal of Environmental Management, 2023, 344: 118462. |
| [39] | CAO Junya, ZHANG Guangji, MAO Zaisha, et al. Precipitation of valuable metals from bioleaching solution by biogenic sulfides[J]. Minerals Engineering, 2009, 22(3): 289-295. |
| [40] | ZHANG Yuhui, FENG Xiaoyan, JIN Bingjie. An effective separation process of arsenic, lead, and zinc from high arsenic-containing copper smelting ashes by alkali leaching followed by sulfide precipitation[J]. Waste Management & Research, 2020, 38(11): 1214-1221. |
| [41] | GHARABAGHI Mahdi, IRANNAJAD Mehdi, AZADMEHR Amir Reza. Selective sulphide precipitation of heavy metals from acidic polymetallic aqueous solution by thioacetamide[J]. Industrial & Engineering Chemistry Research, 2012, 51(2): 954-963. |
| [42] | SHI Meiqing, MIN Xiaobo, SHEN Chen, et al. Separation and recovery of copper in Cu-As-bearing copper electrorefining black slime by oxidation acid leaching and sulfide precipitation[J]. Transactions of Nonferrous Metals Society of China, 2021, 31(4): 1103-1112. |
| [43] | KOUZBOUR Sanaa, GOURICH Bouchaib, GROS Fabrice, et al. Purification of industrial wet phosphoric acid solution by sulfide precipitation in batch and continuous modes: Performance analysis, kinetic modeling, and precipitate characterization[J]. Journal of Cleaner Production, 2022, 380: 135072. |
| [44] | VEEKEN A H M, RULKENS W H. Innovative developments in the selective removal and reuse of heavy metals from wastewaters[J]. Water Science and Technology, 2003, 47(10): 9-16. |
| [45] | MISHRA K K, KAPOOR M L. Kinetics of liquid-gas reactions through bubbles[J]. Hydrometallurgy, 1978, 3(1): 75-83. |
| [46] | TANG Lei, TANG Chaobo, XIAO Jin, et al. A cleaner process for valuable metals recovery from hydrometallurgical zinc residue[J]. Journal of Cleaner Production, 2018, 201: 764-773. |
| [47] | ROCHETTE Elizabeth A, BOSTICK Benjamin C, LI Guangchao, et al. Kinetics of arsenate reduction by dissolved sulfide[J]. Environmental Science & Technology, 2000, 34(22): 4714-4720. |
| [48] | ZHAO Youcai, STANFORTH Robert. Selective separation of lead from alkaline zinc solution by sulfide precipitation[J]. Separation Science and Technology, 2001, 36(11): 2561-2570. |
| [49] | REIS Flávia D, SILVA Adarlene M, CUNHA Emanoelle C, et al. Application of sodium-and biogenic sulfide to the precipitation of nickel in a continuous reactor[J]. Separation and Purification Technology, 2013, 120: 346-353. |
| [50] | WANG Li Pang, PONOU Josiane, MATSUO Seiji, et al. Integrating sulfidization with neutralization treatment for selective recovery of copper and zinc over iron from acid mine drainage[J]. Minerals Engineering, 2013, 45: 100-107. |
| [51] | WANG Li Pang, CHEN Yan Jhang. Sequential precipitation of iron, copper, and zinc from wastewater for metal recovery[J]. Journal of Environmental Engineering, 2019, 145(1): 04018130. |
| [52] | KOUZBOUR Sanaa, GOURICH Bouchaib, GROS Fabrice, et al. A novel approach for removing cadmium from synthetic wet phosphoric acid using sulfide precipitation process operating in batch and continuous modes[J]. Minerals Engineering, 2022, 187: 107809. |
| [53] | LIU Ruiping, YANG Zhongchao, HE Ziliang, et al. Treatment of strongly acidic wastewater with high arsenic concentrations by ferrous sulfide (FeS): Inhibitive effects of S(0)-enriched surfaces[J]. Chemical Engineering Journal, 2016, 304: 986-992. |
| [54] | LI Yongkui, QI Xianjin, LI Guohua, et al. Double-pathway arsenic removal and immobilization from high arsenic-bearing wastewater by using nature pyrite as in situ Fe and S donator[J]. Chemical Engineering Journal, 2021, 410: 128303. |
| [55] | ZHANG Xingfei, TIAN Jia, HU Yuehua, et al. Selective sulfide precipitation of copper ions from arsenic wastewater using monoclinic pyrrhotite[J]. Science of the Total Environment, 2020, 705: 135816. |
| [56] | WANG Zhao, LIU Chao, SHI Gaofeng, et al. Preparation and electrochemical properties of electrospun FeS/carbon nanofiber composites[J]. Ionics, 2020, 26(6): 3051-3060. |
| [57] | LI Lin, CHEN Xingyu, LIU Xuheng, et al. Removal of Cu from the nickel electrolysis anolyte using amorphous MnS[J]. Hydrometallurgy, 2014, 146: 149-153. |
| [58] | ZHANG Yu, JIA Yun, HUANG Yaoguo, et al. Selective separation of heavy metal ions in sequence by TAA[J]. Separation Science and Technology, 2022, 57(13): 2116-2126. |
| [59] | YANG Yue, LIU Fanhan, SONG Shaole, et al. Recovering valuable metals from the leaching liquor of blended cathode material of spent lithium-ion battery[J]. Journal of Environmental Chemical Engineering, 2020, 8(5): 104358. |
| [60] | YANG Xin, PENG Xianjia, KONG Linghao, et al. Removal of Ni(Ⅱ) from strongly acidic wastewater by chelating precipitation and recovery of NiO from the precipitates[J]. Journal of Environmental Sciences, 2021, 104: 365-375. |
| [61] | YANG Xin, HU Xingyun, KONG Linghao, et al. Selective recovery of Cu(Ⅱ) from strongly acidic wastewater by zinc dimethyldithiocarbamate: Affecting factors, efficiency and mechanism[J]. Journal of Environmental Sciences, 2023, 129: 115-127. |
| [62] | POHL Alina. Removal of heavy metal ions from water and wastewaters by sulfur-containing precipitation agents[J]. Water, Air, & Soil Pollution, 2020, 231(10): 503. |
| [63] | KONDO Hideyuki, FUJITA Takafumi, KUCHAR Dalibor, et al. Separation of metal sulfides from plating wastewater containing Cu, Zn and Ni by selective sulfuration with hydrogen sulfide[J]. Journal of The Surface Finishing Society of Japan, 2006, 57(12): 901. |
| [64] | LEWIS A, SWARTBOOI A. Factors affecting metal removal in mixed sulfide precipitation[J]. Chemical Engineering & Technology, 2006, 29(2): 277-280. |
| [65] | ZHANG Mingliang, WANG Haixia. Preparation of immobilized sulfate reducing bacteria (SRB) granules for effective bioremediation of acid mine drainage and bacterial community analysis[J]. Minerals Engineering, 2016, 92: 63-71. |
| [66] | FOUCHER S, BATTAGLIA-BRUNET F, IGNATIADIS I, et al. Treatment by sulfate-reducing bacteria of Chessy acid-mine drainage and metals recovery[J]. Chemical Engineering Science, 2001, 56(4): 1639-1645. |
| [67] | LIU Yun, SERRANO Antonio, WYMAN Valentina, et al. Nickel complexation as an innovative approach for nickel-cobalt selective recovery using sulfate-reducing bacteria[J]. Journal of Hazardous Materials, 2021, 402: 123506. |
| [68] | ZOUBOULIS A I, KYDROS K A, MATIS K A. Arsenic(Ⅲ) and arsenic(Ⅴ) removal from solutions by pyrite fines[J]. Separation Science and Technology, 1993, 28(15/16): 2449-2463. |
| [69] | WANG Ying, WU Xi, WANG Shaofeng, et al. The adsorption behavior of thioarsenite on magnetite and ferrous sulfide[J]. Chemical Geology, 2018, 492: 1-11. |
| [70] | DU Meimei, ZHANG Yongqing, HUSSAIN Imtyaz, et al. Effect of pyrite on enhancement of zero-valent iron corrosion for arsenic removal in water: A mechanistic study[J]. Chemosphere, 2019, 233: 744-753. |
| [71] | AN Xueliang, HUANG Fugen, REN Haitao, et al. Oxidative dissolution of amorphous FeS and speciation of secondary Fe minerals: Effects of pH and As(Ⅲ) concentration[J]. Chemical Geology, 2017, 462: 44-54. |
| [1] | XIONG Xiaohe, ZHANG Yinan, ZHANG Jingjing, YANG Fuxin, TAN Houzhang. Retired wind turbine blade coupled with coal fired boiler co-firing technology based on the drop tube experiments [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 555-563. |
| [2] | QUAN Cui, GAO Ningbo, ZHANG Guangtao, SUO Haojie. Leaching characteristics of heavy metals and polycyclic aromatic hydrocarbons from permeable bricks prepared by pyrolysis residue of oily sludge [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5226-5233. |
| [3] | SHA Li, SU Yingjia, LING Zichen, YU Xiaoyan, LI Shupeng, GUO Lili, XIONG Jing, FANG Lianhu, ZHANG Ran, ZHANG Shuting. Effect of bituminous coal mixing on the electro-dewatering performance of sludge [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 2144-2152. |
| [4] | YANG Shuangxia, HOU Jianjun, LI Tianjin, CHEN Lei, SUN Laizhi, HUA Dongliang. Current research and prospect for pyrolysis treatment of antibiotic mycelial residue [J]. Chemical Industry and Engineering Progress, 2024, 43(12): 6933-6943. |
| [5] | WANG Yujie, ZHANG Yanmei, LUAN Jinyi, ZHAO Zhiping. Enzyme-catalyzed carbon sequestration processes and enhancement technologies [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 232-245. |
| [6] | ZHANG Lihong, JIN Yaoru, CHENG Fangqin. Resource utilization of coal gasification slag [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4447-4457. |
| [7] | LI Wenxiu, YANG Yuhang, HUANG Yan, WANG Tao, WANG Lei, FANG Mengxiang. Preparation of ultrafine calcium carbonate by CO2 mineralization using high calcium-based solid waste [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2047-2057. |
| [8] | GAO Ningbo, HU Yadi, QUAN Cui. Research progress on thermochemical transformation and biological treatment of food waste [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 507-515. |
| [9] | YU Zhengwei, ZHANG Xiaoxia, LEI Jie, LI Ao, WANG Guangying, DING Xiang, LONG Hongming. Comprehensive recovery of cerium and manganese from waste CeO x -MnO x -based SCR denitrification catalysts by reductive acid leaching [J]. Chemical Industry and Engineering Progress, 2022, 41(9): 5122-5131. |
| [10] | HUANG Xia, HE Yingying, ZHANG Yidie, YANG Dianhai, DAI Xiaohu, XIE Li. Research progress on enhancing resource utilization of organic solid waste aerobic composting based on biochar [J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4544-4554. |
| [11] | CAI Sichao, ZHOU Jing, DU Jinze, LI Fangzhou, LI Yuansen, HE Lin, LI Xingang, WANG Chengyang. Process analysis of resource utilization of phenol-based distillation residue from coal chemical industry [J]. Chemical Industry and Engineering Progress, 2022, 41(6): 3360-3371. |
| [12] | WANG Jianbin, CHEN Yun, WANG Kehua, YU Xuepeng, CHEN Cong, LIU Jianzhong. Co-processing of solid waste in industrial kilns: a review [J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1494-1502. |
| [13] | XIANG Hongwei, YANG Yong, LI Yongwang. Transformation and development of coal chemical industry under the goal of carbon neutralization [J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1399-1408. |
| [14] | NIE Zimeng, YANG Dian, XIONG Yulu, LI Yingjie, TIAN Senlin, NING Ping. Performance and mechanism of electrolytic manganese slag slurry for flue gas desulfurization [J]. Chemical Industry and Engineering Progress, 2022, 41(2): 1063-1072. |
| [15] | LIU Ancang, CHEN Chuan, CHEN Jianzhong, CHEN Yuzhong, ZHU Chenliang, JIANG Yong, LU Fushen, WANG Shuangxi, ZHONG Ziyi, SONG Yibing. Application of catalytic reaction for CO2 resource utilization and marine antifouling in coastal power plants [J]. Chemical Industry and Engineering Progress, 2021, 40(9): 5145-5155. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||