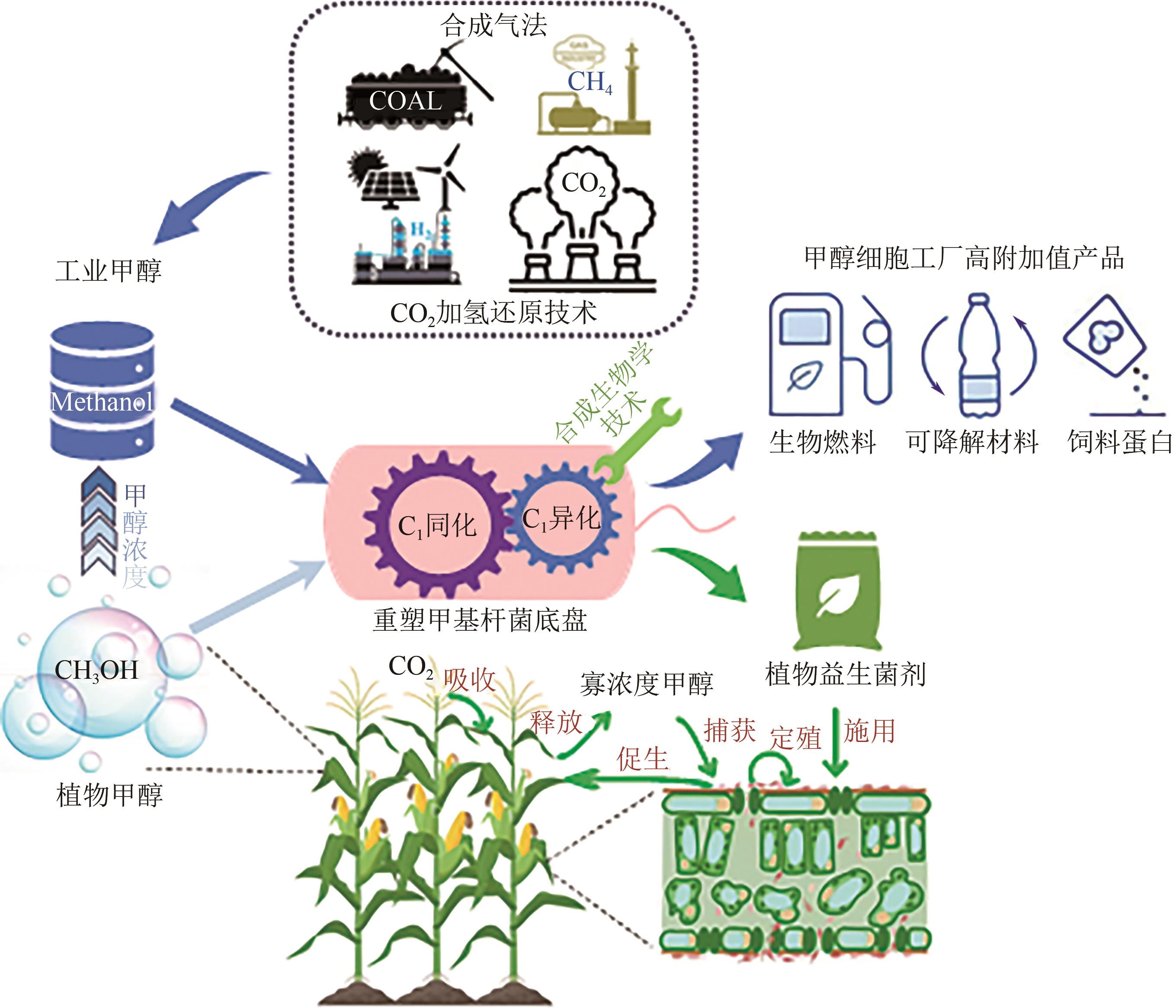
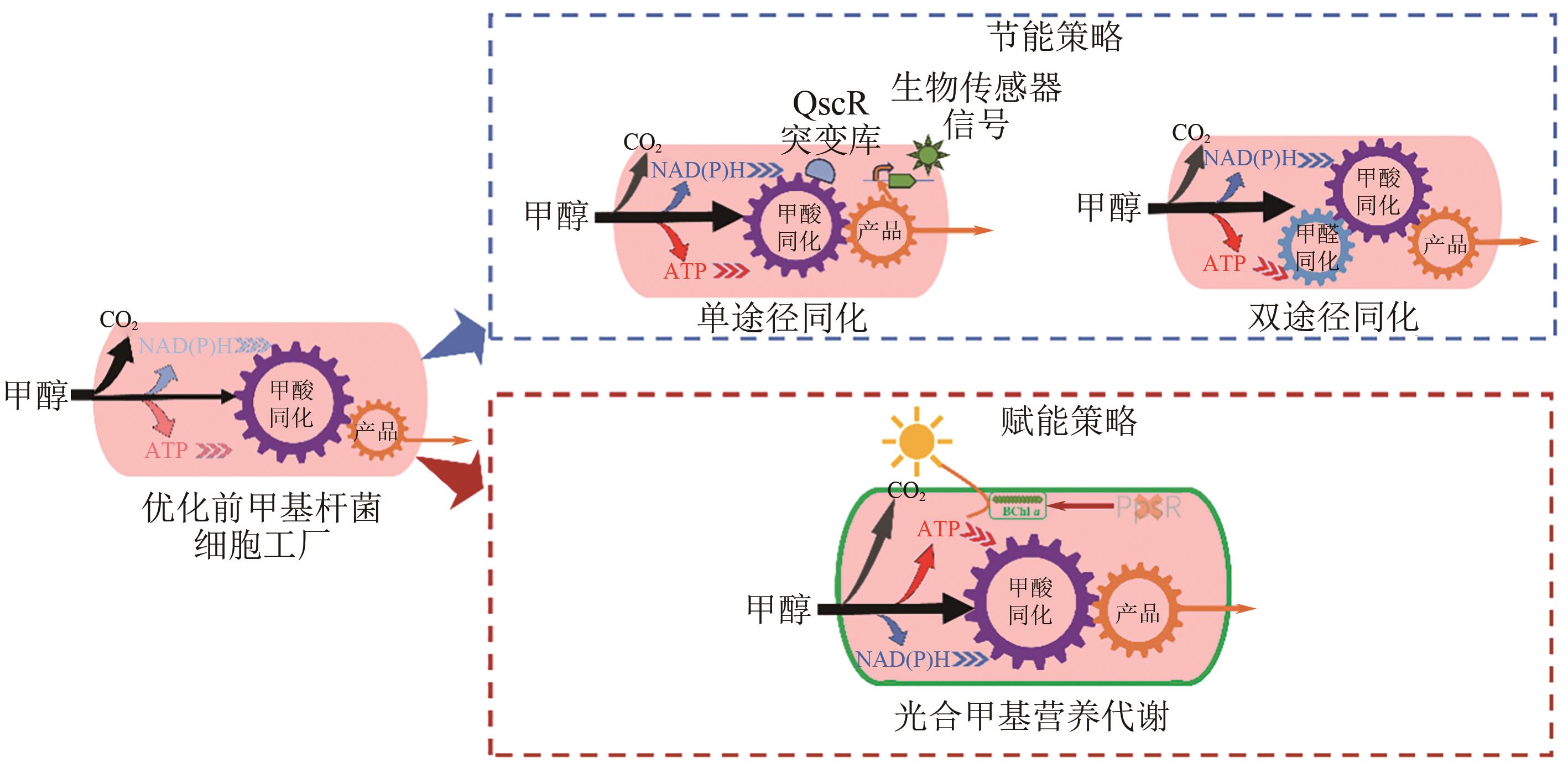
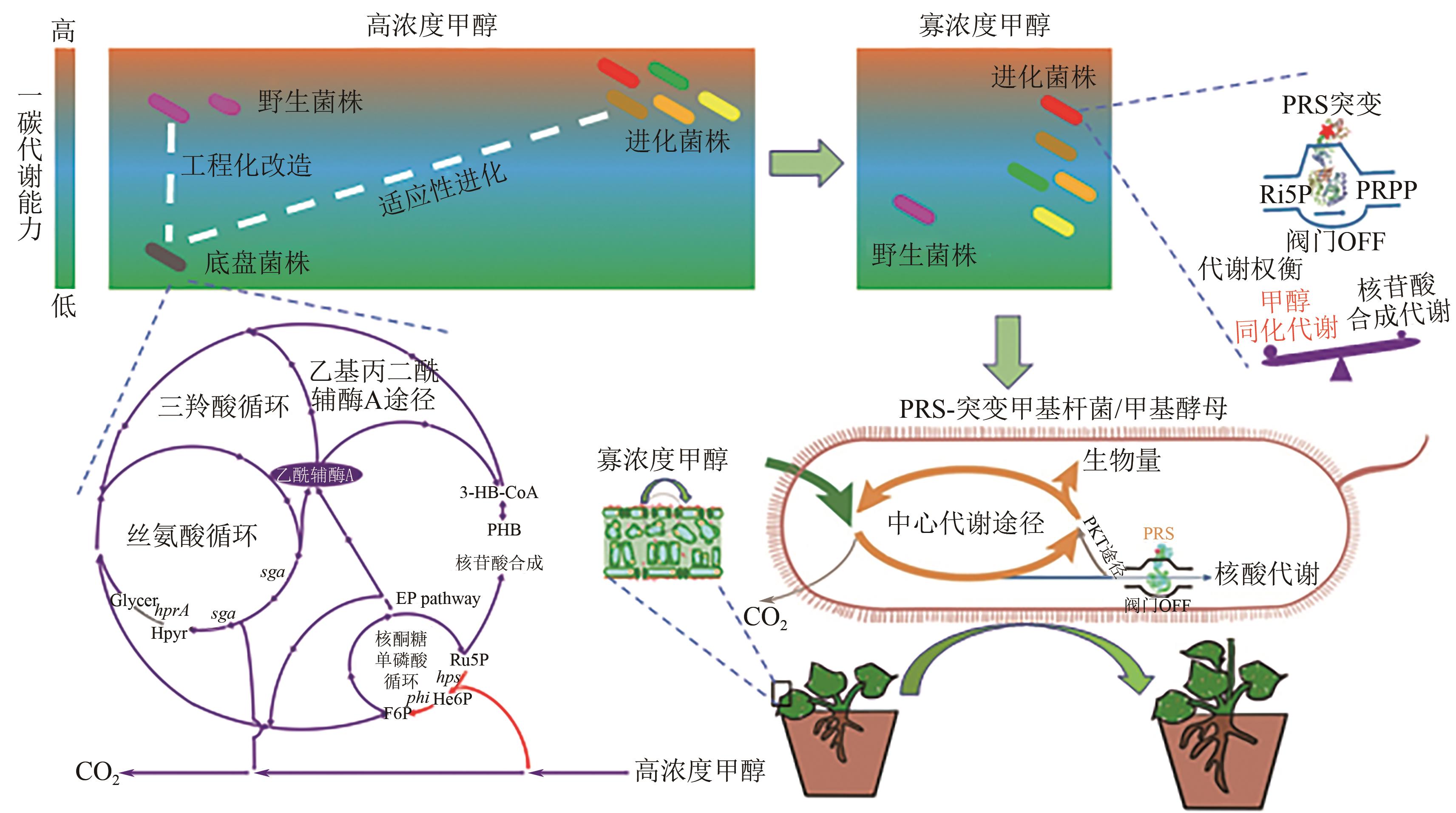
Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (5): 2451-2462.DOI: 10.16085/j.issn.1000-6613.2024-1841
• Synthetic biomanufacturing • Previous Articles
Recent advances in strengthening Methylobacterium chassis for the utilization of methanol from industrial and plant sources
YAO Lu1,2,3( ), MA Zengxin1,2,3(
), MA Zengxin1,2,3( ), ZHANG Cong1,2,3, YANG Song1,2,3(
), ZHANG Cong1,2,3, YANG Song1,2,3( ), XING Xinhui4,5(
), XING Xinhui4,5( )
)
- 1.School of Life Sciences, Qingdao Agricultural University, Qingdao 266109, Shandong, China
2.Shandong Province Key Laboratory of Applied Mycology, Qingdao Agricultural University, Qingdao 266109, Shandong, China
3.Qingdao International Center on Microbes Utilizing Biogas, Qingdao Agricultural University, Qingdao 266109, Shandong, China
4.MOE Key Laboratory for Industrial Biocatalysis, Institute of Biochemical Engineering, Department of Chemical Engineering, Center for Synthetic & Systems Biology, Tsinghua University, Beijing 100084, China
5.Institute of Biopharmaceutical and Health Engineering, Tsinghua Shenzhen International Graduate School, Shenzhen 518055, Guangdong, China
-
Received:2024-11-11Revised:2025-02-10Online:2025-05-20Published:2025-05-25 -
Contact:YANG Song, XING Xinhui
甲基杆菌有效同化工业甲醇和植物甲醇的研究进展
姚陆1,2,3( ), 马增新1,2,3(
), 马增新1,2,3( ), 张聪1,2,3, 杨松1,2,3(
), 张聪1,2,3, 杨松1,2,3( ), 邢新会4,5(
), 邢新会4,5( )
)
- 1.青岛农业大学生命科学学院,山东 青岛 266109
2.青岛农业大学山东省应用真菌重点实验室,山东 青岛 266109
3.青岛农业大学青岛生物沼气环境微生物国际合作基地,山东 青岛 266109
4.清华大学工业生物催化 教育部重点实验室,化工系生物化工研究所,合成与系统生物学中心,北京 100084
5.清华大学深圳国际 研究生院生物医药与健康工程研究院,广东 深圳 518055
-
通讯作者:杨松,邢新会 -
作者简介:姚陆(1990—),男,博士,研究方向为代谢工程和微生物菌剂。E-mail:yaolu113@163.com
马增新(1988—),男,博士,研究方向为代谢工程和合成生物学。E-mail:mazengxin@foxmail.com。 -
基金资助:国家重点研发计划(2021YFC2103500);国家自然科学基金(22078169);国家自然科学基金青年基金(31900004)
CLC Number:
Cite this article
YAO Lu, MA Zengxin, ZHANG Cong, YANG Song, XING Xinhui. Recent advances in strengthening Methylobacterium chassis for the utilization of methanol from industrial and plant sources[J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2451-2462.
姚陆, 马增新, 张聪, 杨松, 邢新会. 甲基杆菌有效同化工业甲醇和植物甲醇的研究进展[J]. 化工进展, 2025, 44(5): 2451-2462.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-1841
| 产品 | 代谢工程策略 | 发酵规模 | 产量 | 参考文献 |
|---|---|---|---|---|
| 单细胞蛋白 | 高密度发酵 | 发酵罐 | 42~72g/L | [ |
| 聚β-羟基丁酸酯 | 限氮两阶段发酵 | 发酵罐 | 46~136g/L | [ |
| 聚β-羟基丁酸-戊酸-己酸酯 | 替换本源phaC,过表达β-酮硫解酶和乙酰乙酰辅酶A还原酶 | 摇瓶 | 43.6% | [ |
| 衣康酸 | 表达顺乌头酸脱羧酶,并解除PhaR调控 | 摇瓶 | 31.6mg/L | [ |
| 异丁醇 | 优化异丁醇合成途径基因及拷贝,减少竞争途径乳酸合成 | 摇瓶 | 19mg/L | [ |
| 3-羟基丙酸 | 表达mcr并优化启动子和拷贝数 | 摇瓶 | 69.8mg/L | [ |
| 3-羟基丙酸 | 构建协同同化途径,强化ATP和还原力供给 | 发酵罐 | 0.86g/L | [ |
| 3-羟基丙酸 | 构建光合甲基营养型菌株,强化能量供给 | 发酵罐 | 1.8g/L | [ |
| 蛇麻烯 | 表达甲羟戊酸途径和蛇麻烯合酶 | 发酵罐 | 1.65g/L | [ |
| 甲羟戊酸 | 构建甲羟戊酸生物传感器对转录因子QscR进行筛选 | 发酵罐 | 2.67g/L | [ |
| 筛选3-羟基丁酰辅酶A变位酶并进行限氮两阶段发酵 | 发酵罐 | 2.1g/L | [ | |
| 类胡萝卜素 | 利用CRISPRi打靶竞争途径 | 摇瓶 | 0.3μg/mg | [ |
| 紫色杆菌素 | 表达紫色杆菌素基因簇vioABCDE并进行随机突变筛选 | 摇瓶 | 118mg/L | [ |
| 产品 | 代谢工程策略 | 发酵规模 | 产量 | 参考文献 |
|---|---|---|---|---|
| 单细胞蛋白 | 高密度发酵 | 发酵罐 | 42~72g/L | [ |
| 聚β-羟基丁酸酯 | 限氮两阶段发酵 | 发酵罐 | 46~136g/L | [ |
| 聚β-羟基丁酸-戊酸-己酸酯 | 替换本源phaC,过表达β-酮硫解酶和乙酰乙酰辅酶A还原酶 | 摇瓶 | 43.6% | [ |
| 衣康酸 | 表达顺乌头酸脱羧酶,并解除PhaR调控 | 摇瓶 | 31.6mg/L | [ |
| 异丁醇 | 优化异丁醇合成途径基因及拷贝,减少竞争途径乳酸合成 | 摇瓶 | 19mg/L | [ |
| 3-羟基丙酸 | 表达mcr并优化启动子和拷贝数 | 摇瓶 | 69.8mg/L | [ |
| 3-羟基丙酸 | 构建协同同化途径,强化ATP和还原力供给 | 发酵罐 | 0.86g/L | [ |
| 3-羟基丙酸 | 构建光合甲基营养型菌株,强化能量供给 | 发酵罐 | 1.8g/L | [ |
| 蛇麻烯 | 表达甲羟戊酸途径和蛇麻烯合酶 | 发酵罐 | 1.65g/L | [ |
| 甲羟戊酸 | 构建甲羟戊酸生物传感器对转录因子QscR进行筛选 | 发酵罐 | 2.67g/L | [ |
| 筛选3-羟基丁酰辅酶A变位酶并进行限氮两阶段发酵 | 发酵罐 | 2.1g/L | [ | |
| 类胡萝卜素 | 利用CRISPRi打靶竞争途径 | 摇瓶 | 0.3μg/mg | [ |
| 紫色杆菌素 | 表达紫色杆菌素基因簇vioABCDE并进行随机突变筛选 | 摇瓶 | 118mg/L | [ |
| 1 | GUO Feng, QIAO Yangyi, XIN Fengxue, et al. Bioconversion of C1 feedstocks for chemical production using Pichia pastoris[J]. Trends in Biotechnology, 2023, 41(8): 1066-1079. |
| 2 | 姚伦, 周雍进. 一碳化合物生物利用和转化研究进展[J]. 化工进展, 2023, 42(1): 16-29. |
| YAO Lun, ZHOU Yongjin. Progress in microbial utilization of one-carbon feedstocks for biomanufacturing[J]. Chemical Industry and Engineering Progress, 2023, 42(1): 16-29. | |
| 3 | 李灿. 绿色氢能在“双碳” 战略中的作用[J]. 科技导报, 2024, 42(15): 1-2. |
| LI Can. The role of green hydrogen energy in dual carbon strategy[J]. Science & Technology Review, 2024, 42(15): 1-2. | |
| 4 | CAI Peng, WU Xiaoyan, DENG Jun, et al. Methanol biotransformation toward high-level production of fatty acid derivatives by engineering the industrial yeast Pichia pastoris [J]. Proceedings of the National Academy of Sciences of the United States of America, 2022, 119(29): e2201711119. |
| 5 | GUO Yuanke, ZHANG Rui, WANG Jing, et al. Engineering yeasts to co-utilize methanol or formate coupled with CO2 fixation[J]. Metabolic Engineering, 2024, 84: 1-12. |
| 6 | LIU Zihe, WANG Kai, CHEN Yun, et al. Third-generation biorefineries as the means to produce fuels and chemicals from CO2 [J]. Nature Catalysis, 2020, 3: 274-288. |
| 7 | MILLET D B, JACOB D J, CUSTER T G, et al. New constraints on terrestrial and oceanic sources of atmospheric methanol[J]. Atmospheric Chemistry & Physics, 2008, 8(149): 6887-6905. |
| 8 | KOLB Steffen. Aerobic methanol-oxidizing bacteria in soil[J]. FEMS Microbiology Letters, 2009, 300(1): 1-10. |
| 9 | ZHANG Min, YUAN Xiaojie, ZHANG Cong, et al. Bioconversion of methanol into value-added chemicals in native and synthetic methylotrophs[J]. Current Issues in Molecular Biology, 2019, 33: 225-236. |
| 10 | CUI Lanyu, YANG Jing, LIANG Weifan, et al. Sodium formate redirects carbon flux and enhances heterologous mevalonate production in Methylobacterium extorquens AM1[J]. Biotechnology Journal, 2023, 18(2): e2200402. |
| 11 | MA Zengxin, ZHANG Min, ZHANG Changtai, et al. Metabolomic analysis improves bioconversion of methanol to isobutanol in Methylorubrum extorquens AM1[J]. Biotechnology Journal, 2021, 16(6): e2000413. |
| 12 | YOON Jihee, Jiyun BAE, KANG Seulgi, et al. Poly-3-hydroxybutyrate production in acetate minimal medium using engineered Methylorubrum extorquens AM1[J]. Bioresource Technology, 2022, 353: 127127. |
| 13 | HURT Allison, BIBIK Jacob D, MARTINEZ-GOMEZ Norma Cecilia, et al. Engineering terpene production pathways in Methylobacterium extorquens AM1[J]. Microorganisms, 2024, 12(3): 500. |
| 14 | SALZE Guillaume P, TIBBETTS Sean M. Apparent digestibility coefficients of proximate nutrients and essential amino acids from a single-cell protein meal derived from Methylobacterium extorquens for pre-smolt Atlantic salmon (Salmo salar L.)[J]. Aquaculture Research, 2021, 52(12): 6818-6823. |
| 15 | JONES Shawn W, KARPOL Alon, FRIEDMAN Sivan, et al. Recent advances in single cell protein use as a feed ingredient in aquaculture[J]. Current Opinion in Biotechnology, 2020, 61: 189-197. |
| 16 | BÉLANGER L, FIGUEIRA M M, BOURQUE D, et al. Production of heterologous protein by Methylobacterium extorquens in high cell density fermentation[J]. FEMS Microbiology Letters, 2004, 231(2): 197-204. |
| 17 | TLUSTY Michael, RHYNE Andrew, SZCZEBAK Joseph T, et al. A transdisciplinary approach to the initial validation of a single cell protein as an alternative protein source for use in aquafeeds[J]. PeerJ, 2017, 5: e3170. |
| 18 | SUZUKI Takahiro, YAMANE Tsuneo, SHIMIZU Shoichi. Mass production of poly-β-hydroxybutyric acid by fully automatic fed-batch culture of methylotroph[J]. Applied Microbiology and Biotechnology, 1986, 23(5): 322-329. |
| 19 | BOURQUE D, POMERLEAU Y, GROLEAU D. High-cell-density production of poly-β-hydroxybutyrate (PHB) from methanol by Methylobacterium extorquens: Production of high-molecular-mass PHB[J]. Applied Microbiology and Biotechnology, 1995, 44(3): 367-376. |
| 20 | ORITA Izumi, NISHIKAWA Kouta, NAKAMURA Satoshi, et al. Biosynthesis of polyhydroxyalkanoate copolymers from methanol by Methylobacterium extorquens AM1 and the engineered strains under cobalt-deficient conditions[J]. Applied Microbiology and Biotechnology, 2014, 98(8): 3715-3725. |
| 21 | Chee Kent LIM, VILLADA Juan C, CHALIFOUR Annie, et al. Designing and engineering Methylorubrum extorquens AM1 for itaconic acid production[J]. Frontiers in Microbiology, 2019, 10: 1027. |
| 22 | YANG Yiming, CHEN Wenjing, YANG Jing, et al. Production of 3-hydroxypropionic acid in engineered Methylobacterium extorquens AM1 and its reassimilation through a reductive route[J]. Microbial Cell Factories, 2017, 16(1): 179. |
| 23 | YUAN Xiaojie, CHEN Wenjing, MA Zengxin, et al. Rewiring the native methanol assimilation metabolism by incorporating the heterologous ribulose monophosphate cycle into Methylorubrum extorquens [J]. Metabolic Engineering, 2021, 64: 95-110. |
| 24 | MA Zengxin, FENG Chenxi, SONG Yazhen, et al. Engineering photo-methylotrophic Methylobacterium for enhanced 3-hydroxypropionic acid production during non-growth stage fermentation[J]. Bioresource Technology, 2024, 393: 130104. |
| 25 | SONNTAG Frank, KRONER Cora, LUBUTA Patrice, et al. Engineering Methylobacterium extorquens for de novo synthesis of the sesquiterpenoid α-humulene from methanol[J]. Metabolic Engineering, 2015, 32: 82-94. |
| 26 | LIANG Weifan, CUI Lanyu, CUI Jinyu, et al. Biosensor-assisted transcriptional regulator engineering for Methylobacterium extorquens AM1 to improve mevalonate synthesis by increasing the acetyl-CoA supply[J]. Metabolic Engineering, 2017, 39: 159-168. |
| 27 | ROHDE Maria-Teresa, TISCHER Sylvi, HARMS Hauke, et al. Production of 2-hydroxyisobutyric acid from methanol by Methylobacterium extorquens AM1 expressing (R)-3-hydroxybutyryl coenzyme A-isomerizing enzymes[J]. Applied and Environmental Microbiology, 2017, 83(3): e02622-16. |
| 28 | MO Xuhua, ZHANG Hui, WANG Tianmin, et al. Establishment of CRISPR interference in Methylorubrum extorquens and application of rapidly mining a new phytoene desaturase involved in carotenoid biosynthesis[J]. Applied Microbiology and Biotechnology, 2020, 104(10): 4515-4532. |
| 29 | QUYNH LE Hoa Thi, Dung Hoang ANH MAI, NA Jeong-Geol, et al. Development of Methylorubrum extorquens AM1 as a promising platform strain for enhanced violacein production from co-utilization of methanol and acetate[J]. Metabolic Engineering, 2022, 72: 150-160. |
| 30 | ZHANG Cong, ZHOU Difei, WANG Mengying, et al. Phosphoribosylpyrophosphate synthetase as a metabolic valve advances Methylobacterium/Methylorubrum phyllosphere colonization and plant growth[J]. Nature Communications, 2024, 15(1): 5969. |
| 31 | VERA Rocío Torres, GARCÍA Antonio José Bernabé, ÁLVAREZ Francisco José Carmona, et al. Application and effectiveness of Methylobacterium symbioticum as a biological inoculant in maize and strawberry crops[J]. Folia Microbiologica, 2024, 69(1): 121-131. |
| 32 | GROSSI Cecilia E M, TANI Akio, MORI Izumi C, et al. Plant growth-promoting abilities of Methylobacterium sp. 2A involve auxin-mediated regulation of the root architecture[J]. Plant, Cell & Environment, 2024, 47(12): 5343-5357. |
| 33 | VADIVUKKARASI Ponnusamy, SUSEELA BHAI R. Phyllosphere-associated Methylobacterium: A potential biostimulant for ginger (Zingiber officinale Rosc.) cultivation[J]. Archives of Microbiology, 2020, 202(2): 369-375. |
| 34 | SENTHILKUMAR M, PUSHPAKANTH P, Arul JOSE P, et al. Diversity and functional characterization of endophytic Methylobacterium isolated from banana cultivars of South India and its impact on early growth of tissue culture banana plantlets[J]. Journal of Applied Microbiology, 2021, 131(5): 2448-2465. |
| 35 | ZHU Liping, SONG Yazhen, MA Shunan, et al. Heterologous production of 3-hydroxypropionic acid in Methylorubrum extorquens by introducing the MCR gene via a multi-round chromosomal integration system based on cre-lox71/lox66 and transposon[J]. Microbial Cell Factories, 2024, 23(1): 5. |
| 36 | ZHU Wenliang, CUI Jinyu, CUI Lanyu, et al. Bioconversion of methanol to value-added mevalonate by engineered Methylobacterium extorquens AM1 containing an optimized mevalonate pathway[J]. Applied Microbiology and Biotechnology, 2016, 100(5): 2171-2182. |
| 37 | PEYRAUD Rémi, SCHNEIDER Kathrin, KIEFER Patrick, et al. Genome-scale reconstruction and system level investigation of the metabolic network of Methylobacterium extorquens AM1[J]. BMC Systems Biology, 2011, 5: 189. |
| 38 | YANG Jing, ZHANG Changtai, YUAN Xiaojie, et al. Metabolic engineering of Methylobacterium extorquens AM1 for the production of butadiene precursor[J]. Microbial Cell Factories, 2018, 17(1): 194. |
| 39 | KRÜSEMANN Jan L, RAINALDI Vittorio, COTTON Charles AR, et al. The cofactor challenge in synthetic methylotrophy: Bioengineering and industrial applications[J]. Current Opinion in Biotechnology, 2023, 82: 102953. |
| 40 | ZHAN Chunjun, LI Xiaowei, YANG Yankun, et al. Strategies and challenges with the microbial conversion of methanol to high-value chemicals[J]. Biotechnology and Bioengineering, 2021, 118(10): 3655-3668. |
| 41 | SINGH Hawaibam Birla, KANG Min-Kyoung, KWON Moonhyuk, et al. Developing methylotrophic microbial platforms for a methanol-based bioindustry[J]. Frontiers in Bioengineering and Biotechnology, 2022, 10: 1050740. |
| 42 | CUI Jinyu, GOOD Nathan M, HU Bo, et al. Metabolomics revealed an association of metabolite changes and defective growth in Methylobacterium extorquens AM1 overexpressing ecm during growth on methanol[J]. PLoS One, 2016, 11(4): e0154043. |
| 43 | DENG Chen, WU Yaokang, Xueqin Lyu, et al. Refactoring transcription factors for metabolic engineering[J]. Biotechnology Advances, 2022, 57: 107935. |
| 44 | KALYUZHNAYA Marina G, LIDSTROM Mary E. QscR-mediated transcriptional activation of serine cycle genes in Methylobacterium extorquens AM1[J]. Journal of Bacteriology, 2005, 187(21): 7511-7517. |
| 45 | YU Hong, LIAO James C. A modified serine cycle in Escherichia coli coverts methanol and CO2 to two-carbon compounds[J]. Nature Communications, 2018, 9(1): 3992. |
| 46 | HE Hai, Rune HÖPER, Moritz DODENHÖFT, et al. An optimized methanol assimilation pathway relying on promiscuous formaldehyde-condensing aldolases in E. coli [J]. Metabolic Engineering, 2020, 60: 1-13. |
| 47 | VON BORZYSKOWSKI Lennart Schada, SONNTAG Frank, Laura PÖSCHEL, et al. Replacing the ethylmalonyl-CoA pathway with the glyoxylate shunt provides metabolic flexibility in the central carbon metabolism of Methylobacterium extorquens AM1[J]. ACS Synthetic Biology, 2018, 7(1): 86-97. |
| 48 | NIEH Liang-Yu, CHEN Frederic Y-H, JUNG Hsin-Wei, et al. Evolutionary engineering of methylotrophic E. coli enables fast growth on methanol[J]. Nature Communications, 2024, 15(1): 8840. |
| 49 | REITER Michael A, BRADLEY Timothy, BÜCHEL Lars A, et al. A synthetic methylotrophic Escherichia coli as a chassis for bioproduction from methanol[J]. Nature Catalysis, 2024, 7(5): 560-573. |
| 50 | WANG Guokun, Mattis OLOFSSON-DOLK, HANSSON Frederik Gleerup, et al. Engineering yeast Yarrowia lipolytica for methanol assimilation[J]. ACS Synthetic Biology, 2021, 10(12): 3537-3550. |
| 51 | WANG Weiting, GUO Shuqi, HOU Qianzi, et al. Bioupcycling methane and CO2 for succinate production by an engineered type Ⅰ methanotrophic bacterium[J]. Journal of Agricultural and Food Chemistry, 2024, 72(44): 24237-24245. |
| 52 | ZHAN Chunjun, LI Xiaowei, LAN Guangxu, et al. Reprogramming methanol utilization pathways to convert Saccharomyces cerevisiae to a synthetic methylotroph[J]. Nature Catalysis, 2023, 6(5): 435-450. |
| 53 | PETERSON Autumn, BASKETT Carina, RATCLIFF William C, et al. Transforming yeast into a facultative photoheterotroph via expression of vacuolar rhodopsin[J]. Current Biology, 2024, 34(3): 648-654.e3. |
| 54 | HU Guipeng, LI Zehong, MA Danlei, et al. Light-driven CO2 sequestration in Escherichia coli to achieve theoretical yield of chemicals[J]. Nature Catalysis, 2021, 4: 395-406. |
| 55 | DAHLIN Lukas R, MEYERS Alex W, STEFANI Skylar W, et al. Heterologous expression of formate dehydrogenase enables photoformatotrophy in the emerging model microalga, Picochlorum renovo [J]. Frontiers in Bioengineering and Biotechnology, 2023, 11: 1162745. |
| 56 | ALESSA Ola, OGURA Yoshitoshi, FUJITANI Yoshiko, et al. Comprehensive comparative genomics and phenotyping of Methylobacterium species[J]. Frontiers in Microbiology, 2021, 12: 740610. |
| 57 | KUZYK Steven B, MESSNER Katia, PLOUFFE Jocelyn, et al. Diverse aerobic anoxygenic phototrophs synthesize bacteriochlorophyll in oligotrophic rather than copiotrophic conditions, suggesting ecological niche[J]. Environmental Microbiology, 2023, 25(11): 2653-2665. |
| 58 | STIEFEL Philipp, ZAMBELLI Tomaso, VORHOLT Julia A. Isolation of optically targeted single bacteria by application of fluidic force microscopy to aerobic anoxygenic phototrophs from the phyllosphere[J]. Applied and Environmental Microbiology, 2013, 79(16): 4895-4905. |
| 59 | YURIMOTO Hiroya, SAKAI Yasuyoshi. Interaction between C1-microorganisms and plants: Contribution to the global carbon cycle and microbial survival strategies in the phyllosphere[J]. Bioscience, Biotechnology, and Biochemistry, 2022, 87(1): 1-6. |
| 60 | YURIMOTO Hiroya, SHIRAISHI Kosuke, SAKAI Yasuyoshi. Physiology of methylotrophs living in the phyllosphere[J]. Microorganisms, 2021, 9(4): 809. |
| 61 | GOURION Benjamin, ROSSIGNOL Michel, VORHOLT Julia A. A proteomic study of Methylobacterium extorquens reveals a response regulator essential for epiphytic growth[J]. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(35): 13186-13191. |
| 62 | TORGONSKAYA M L, FIRSOVA Y E, EKIMOVA G A, et al. Distribution and potential ecophysiological roles of multiple GroEL chaperonins in pink-pigmented facultative methylotrophs[J]. Microbiology, 2024, 93(1): 14-27. |
| 63 | IGUCHI Hiroyuki, YOSHIDA Yusuke, FUJISAWA Kento, et al. KaiC family proteins integratively control temperature-dependent UV resistance in Methylobacterium extorquens AM1[J]. Environmental Microbiology Reports, 2018, 10(6): 634-643. |
| 64 | TOLA Yosef Hamba, FUJITANI Yoshiko, TANI Akio. Bacteria with natural chemotaxis towards methanol revealed by chemotaxis fishing technique[J]. Bioscience, Biotechnology, and Biochemistry, 2019, 83(11): 2163-2171. |
| 65 | TANI Akio, MASUDA Sachiko, FUJITANI Yoshiko, et al. Metabolism-linked methylotaxis sensors responsible for plant colonization in Methylobacterium aquaticum strain 22A[J]. Frontiers in Microbiology, 2023, 14: 1258452. |
| 66 | OCHSNER Andrea M, HEMMERLE Lucas, VONDERACH Thomas, et al. Use of rare-earth elements in the phyllosphere colonizer Methylobacterium extorquens PA1[J]. Molecular Microbiology, 2019, 111(5): 1152-1166. |
| 67 | JUMA Patrick Otieno, FUJITANI Yoshiko, ALESSA Ola, et al. Siderophore for lanthanide and iron uptake for methylotrophy and plant growth promotion in Methylobacterium aquaticum strain 22A[J]. Frontiers in Microbiology, 2022, 13: 921635. |
| 68 | FEATHERSTON Emily R, COTRUVO Joseph A. The biochemistry of lanthanide acquisition, trafficking, and utilization[J]. Biochimica et Biophysica Acta (BBA): Molecular Cell Research, 2021, 1868(1): 118864. |
| 69 | GROOM Joseph D, FORD Stephanie M, PESESKY Mitchell W, et al. A mutagenic screen identifies a TonB-dependent receptor required for the lanthanide metal switch in the type Ⅰ methanotroph “Methylotuvimicrobium buryatense” 5GB1C[J]. Journal of Bacteriology, 2019, 201(15): e00120-19. |
| 70 | CHU Frances, LIDSTROM Mary E. XoxF acts as the predominant methanol dehydrogenase in the type Ⅰ methanotroph Methylomicrobium buryatense [J]. Journal of Bacteriology, 2016, 198(8): 1317-1325. |
| 71 | HE Chenyi, FENG Yiping, DENG Yirong, et al. A systematic review and meta-analysis on the root effects and toxic mechanisms of rare earth elements[J]. Chemosphere, 2024, 363: 142951. |
| 72 | JIAO Yunlong, HE Ding, ZHANG Shuya, et al. Lanthanum interferes with the fundamental rhythms of stomatal opening, expression of related genes, and evapotranspiration in Arabidopsis thaliana [J]. Ecotoxicology and Environmental Safety, 2024, 281: 116576. |
| 73 | BAZURTO Jannell V, RIAZI Siavash, Simon D’ALTON, et al. Global transcriptional response of Methylorubrum extorquens to formaldehyde stress expands the role of EfgA and is distinct from antibiotic translational inhibition[J]. Microorganisms, 2021, 9(2): 347. |
| 74 | BAZURTO Jannell V, NAYAK Dipti D, TICAK Tomislav, et al. EfgA is a conserved formaldehyde sensor that leads to bacterial growth arrest in response to elevated formaldehyde[J]. PLoS Biology, 2021, 19(5): e3001208. |
| 75 | LEE Jessica A, RIAZI Siavash, NEMATI Shahla, et al. Microbial phenotypic heterogeneity in response to a metabolic toxin: Continuous, dynamically shifting distribution of formaldehyde tolerance in Methylobacterium extorquens populations[J]. PLoS Genetics, 2019, 15(11): e1008458. |
| 76 | BAZURTO Jannell V, BRUGER Eric L, LEE Jessica A, et al. Formaldehyde-responsive proteins, TtmR and EfgA, reveal a tradeoff between formaldehyde resistance and efficient transition to methylotrophy in Methylorubrum extorquens [J]. Journal of Bacteriology, 2021, 203(9): e00589-20. |
| 77 | OCHSNER Andrea M, CHRISTEN Matthias, HEMMERLE Lucas, et al. Transposon sequencing uncovers an essential regulatory function of phosphoribulokinase for methylotrophy[J]. Current Biology, 2017, 27(17): 2579-2588.e6. |
| 78 | HENARD Calvin A, SMITH Holly K, GUARNIERI Michael T. Phosphoketolase overexpression increases biomass and lipid yield from methane in an obligate methanotrophic biocatalyst[J]. Metabolic Engineering, 2017, 41: 152-158. |
| 79 | CARLSTRÖM Charlotte I, FIELD Christopher M, Miriam BORTFELD-MILLER, et al. Synthetic microbiota reveal priority effects and keystone strains in the Arabidopsis phyllosphere[J]. Nature Ecology & Evolution, 2019, 3(10): 1445-1454. |
| 80 | HOYT Kathryn O, WOOLSTON Benjamin M. Adapting isotopic tracer and metabolic flux analysis approaches to study C1 metabolism[J]. Current Opinion in Biotechnology, 2022, 75: 102695. |
| 81 | HE Lian, FU Yanfen, LIDSTROM Mary E. Quantifying methane and methanol metabolism of “Methylotuvimicrobium buryatense” 5GB1C under substrate limitation[J]. mSystems, 2019, 4(6): 10.1128/msystems.00748-10.1128/msystems.00719. |
| 82 | SONG Yazhen, FENG Chenxi, ZHOU Difei, et al. Constructing efficient bacterial cell factories to enable one-carbon utilization based on quantitative biology: A review[J]. Quantitative Biology, 2024, 12(1): 1-14. |
| 83 | JIAN Xingjin, GUO Xiaojie, WANG Jia, et al. Microbial microdroplet culture system (MMC): An integrated platform for automated, high-throughput microbial cultivation and adaptive evolution[J]. Biotechnology and Bioengineering, 2020, 117(6): 1724-1737. |
| 84 | WANG Jia, JIAN Xingjin, XING Xinhui, et al. Empowering a methanol-dependent Escherichia coli via adaptive evolution using a high-throughput microbial microdroplet culture system[J]. Frontiers in Bioengineering and Biotechnology, 2020, 8: 570. |
| 85 | TIAN Jinzhong, DENG Wangshuying, ZHANG Ziwen, et al. Discovery and remodeling of Vibrio natriegens as a microbial platform for efficient formic acid biorefinery[J]. Nature Communications, 2023, 14(1): 7758. |
| 86 | YANG Xue, ZHANG Yanfei, ZHAO Guoping. Artificial carbon assimilation: From unnatural reactions and pathways to synthetic autotrophic systems[J]. Biotechnology Advances, 2024, 70: 108294. |
| 87 | WANG Xueying, FENG Yanbin, GUO Xiaojia, et al. Creating enzymes and self-sufficient cells for biosynthesis of the non-natural cofactor nicotinamide cytosine dinucleotide[J]. Nature Communications, 2021, 12(1): 2116. |
| 88 | BLACK William B, ZHANG Linyue, Wai Shun MAK, et al. Engineering a nicotinamide mononucleotide redox cofactor system for biocatalysis[J]. Nature Chemical Biology, 2020, 16(1): 87-94. |
| 89 | SELYANIN Vadim, HAURUSEU Dzmitry, Michal KOBLÍŽEK. The variability of light-harvesting complexes in aerobic anoxygenic phototrophs[J]. Photosynthesis Research, 2016, 128(1): 35-43. |
| 90 | CHEN Haihong, WANG Yaohong, WANG Weishan, et al. High-yield porphyrin production through metabolic engineering and biocatalysis[J]. Nature Biotechnology, 2024. |
| 91 | FOONG Lian Chee, Carmen Wai Leng LOH, Hui Suan NG, et al. Recent development in the production strategies of microbial carotenoids[J]. World Journal of Microbiology and Biotechnology, 2021, 37⑴: 12. |
| 92 | MO Xuhua, SUN Yuman, BI Yuxing, et al. Characterization of C30 carotenoid and identification of its biosynthetic gene cluster in Methylobacterium extorquens AM1[J]. Synthetic and Systems Biotechnology, 2023, 8(3): 527-535. |
| 93 | MENG Yujie, LI Shuang, ZHANG Chong, et al. Strain-level profiling with picodroplet microfluidic cultivation reveals host-specific adaption of honeybee gut symbionts[J]. Microbiome, 2022, 10(1): 140. |
| [1] | WU Mengqin, WANG Jiayao, XU Youqiang, WANG Yu. Progress in cascade conversion of CO2 to single cell protein through chemical and biological catalysis [J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2429-2440. |
| [2] | WANG Yuanyuan, ZHANG Chong, HAN Shuangyan, XING Xinhui. Research progress on bioproduction of recombinant proteins by Pichia pastoris utilizing methanol [J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2441-2450. |
| [3] | GAO Jiangang, JIANG Yapeng, BAO Baoqing, WANG Shuqi, CUI Shuming. Green methanol and green ammonia synthesis by green hydrogen [J]. Chemical Industry and Engineering Progress, 2025, 44(4): 1987-1997. |
| [4] | ZHU Guoyu, GE Qi, FU Mingli. Durability testing and life prediction of methanol reforming catalysts for hydrogen production [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1338-1346. |
| [5] | ZUO Ji, LUO Li, XIE Yongkai, CHEN Wenyao, QIAN Gang, ZHOU Xinggui, DUAN Xuezhi. Effect of Cu catalyst particle size on methanol nonoxidative dehydrogenation to formaldehyde [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1347-1354. |
| [6] | HAN Yingna, LI Li, ZHANG Linzi, AN Jinze, LI Wenxiu, ZHANG Tao. Separation of methanol-acetonitrile azeotrope by ionic liquid extractive distillation [J]. Chemical Industry and Engineering Progress, 2025, 44(2): 660-668. |
| [7] | ZHOU Yu, TANG Tian, XIONG Ziyou, WEI Qi. Methanol to olefin wastewater treatment based on a two-stage microchannel separation process [J]. Chemical Industry and Engineering Progress, 2025, 44(1): 100-108. |
| [8] | HU Yang, HAN Chuanjun, HU Qiang, LI Wenying, AN Quancheng, SU Yang, WU Hongsong, YUAN Guo. Research progress on methanol steam reforming reactors for SOFC [J]. Chemical Industry and Engineering Progress, 2025, 44(1): 169-183. |
| [9] | ZHOU Yu, XIA Taiyang, WEI Qi, TANG Tian, TIAN Lei. Optimization of micro-channel coupled reverse osmosis membrane series treatment of methanol to olefin wastewater [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 43-51. |
| [10] | LONG Tao, ZHOU Feng, ZHANG Wei, WU Hong, WANG Jian, CHEN Lin. Synthesis and modification of deuterated methanol catalyst used in CO-CO2 system [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4411-4420. |
| [11] | GUO Peng, LI Hongwei, LI Guixian, JI Dong, WANG Dongliang, ZHAO Xinhong. Mechanisms and coping strategies on deactivation of anode catalysts for direct methanol fuel cells [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 3812-3823. |
| [12] | FANG Yao, LIU Lei, GAO Zhihua, HUANG Wei, ZUO Zhijun. Advances in anode catalysts for photo-assisted direct methanol fuel cells [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2611-2628. |
| [13] | ZHOU Yuntao, WANG Hongxing, LI Xingang, CUI Lifeng. Application and research progress of CeO2 support in CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2723-2738. |
| [14] | ZHOU Qiuming, NIU Congcong, LYU Shuaishuai, LI Hongwei, WEN Fuli, XU Run, LI Mingfeng. Promoting CO2 hydrogenation to methanol through product transformation and separation [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2776-2785. |
| [15] | LI Haipeng, WU Tong, WANG Qi, GAO Shiwang, WANG Xiaolong, LI Xu, GAO Xinhua, NIAN Pei, WEI Yibin. Effective methanol production by CO2 hydrogenation using water-permeable NaA zeolite membrane [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2834-2842. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||