Chemical Industry and Engineering Progress ›› 2024, Vol. 43 ›› Issue (1): 60-75.DOI: 10.16085/j.issn.1000-6613.2023-1069
• Column: Chemical process intensification • Previous Articles Next Articles
Progress in the mechanism of CH4 and CO2co-conversion reactions
CHENG Haolin1( ), NIAN Yao1,2(
), NIAN Yao1,2( ), HAN You1,2(
), HAN You1,2( )
)
- 1.College of Chemical Engineering, Tianjin University, Tianjin 300072, China
2.Haihe Laboratory of Sustainable Chemical Transformations, Tianjin 300192, China
-
Received:2023-06-28Revised:2023-08-15Online:2024-02-05Published:2024-01-20 -
Contact:NIAN Yao, HAN You
CH4和CO2共转化反应机理研究进展
- 1.天津大学化工学院,天津 300072
2.物质绿色创造与制造海河实验室,天津 300192
-
通讯作者:年瑶,韩优 -
作者简介:成昊霖(2000—),男,硕士研究生,研究方向为理论催化。E-mail:chenghaolin@tju.edu.cn。 -
基金资助:国家自然科学基金(21978210);中国博士后科学基金(2022M722360);天津大学自主创新基金(2023XQM-0012)
CLC Number:
Cite this article
CHENG Haolin, NIAN Yao, HAN You. Progress in the mechanism of CH4 and CO2co-conversion reactions[J]. Chemical Industry and Engineering Progress, 2024, 43(1): 60-75.
成昊霖, 年瑶, 韩优. CH4和CO2共转化反应机理研究进展[J]. 化工进展, 2024, 43(1): 60-75.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-1069
| 反应式 | ||
|---|---|---|
| CH4(g)+3CO2(g) | +235.1 | +209.2 |
| CH4(g)+CO2(g) | +247.5 | +170.8 |
| CH4(g)+CO2(g) | +36.4 | +71.1 |
| 2CH4(g)+CO2(g) | +58.8 | +88.0 |
| 2CH4(g)+2CO2(g) | +189.7 | +208.3 |
| 2CH4(g)+CO2(g) | +8.5 | +115.0 |
| 反应式 | ||
|---|---|---|
| CH4(g)+3CO2(g) | +235.1 | +209.2 |
| CH4(g)+CO2(g) | +247.5 | +170.8 |
| CH4(g)+CO2(g) | +36.4 | +71.1 |
| 2CH4(g)+CO2(g) | +58.8 | +88.0 |
| 2CH4(g)+2CO2(g) | +189.7 | +208.3 |
| 2CH4(g)+CO2(g) | +8.5 | +115.0 |
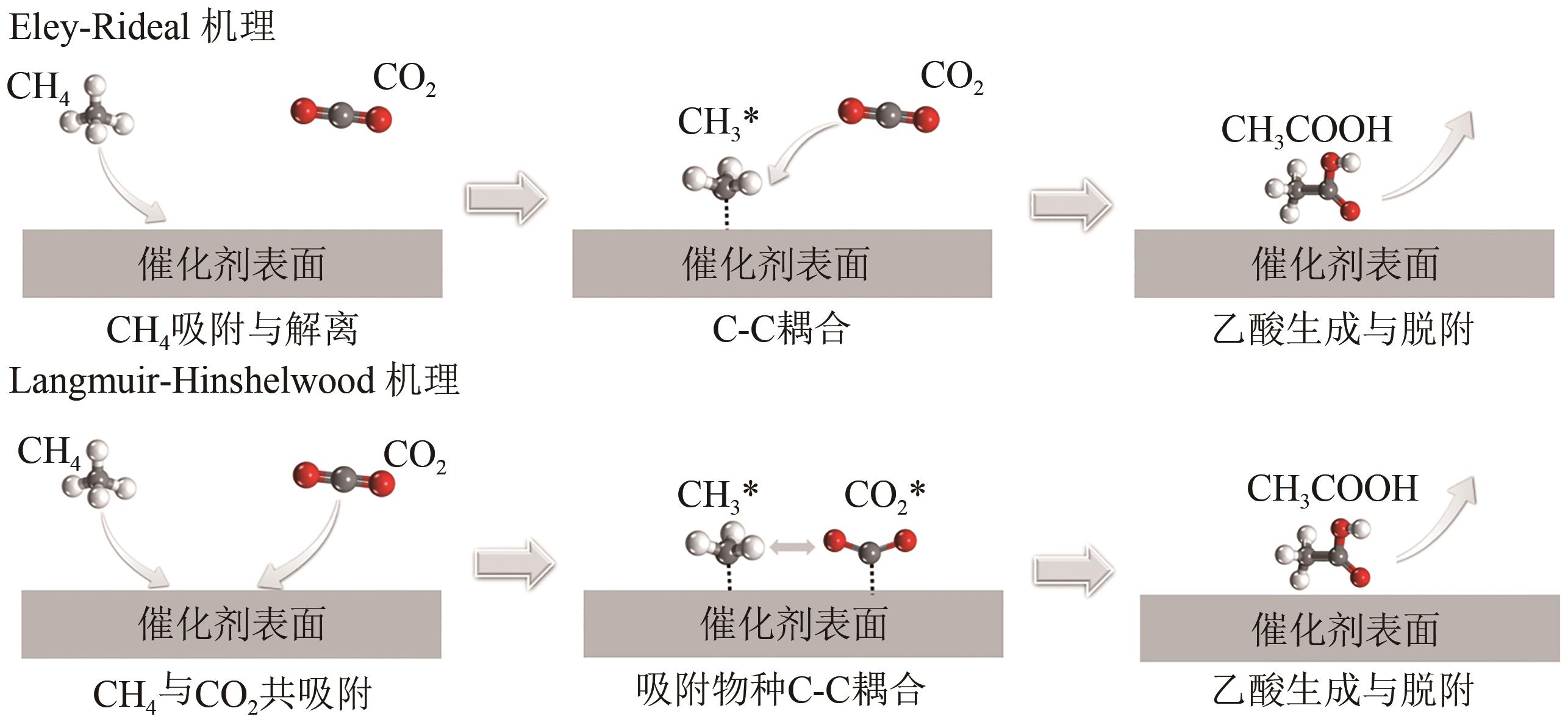
| 解离物种 | Ru | Rh | Pd |
|---|---|---|---|
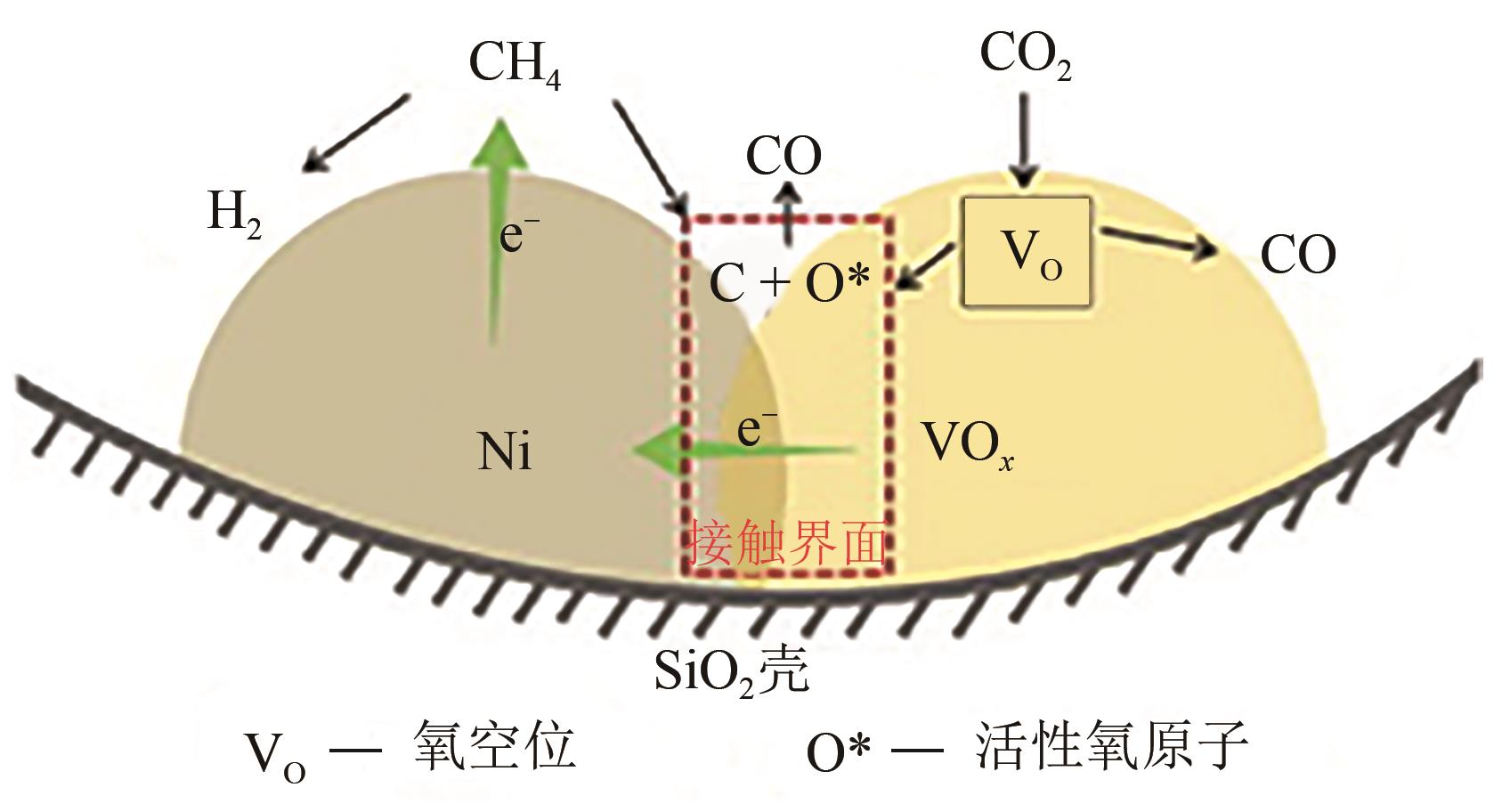
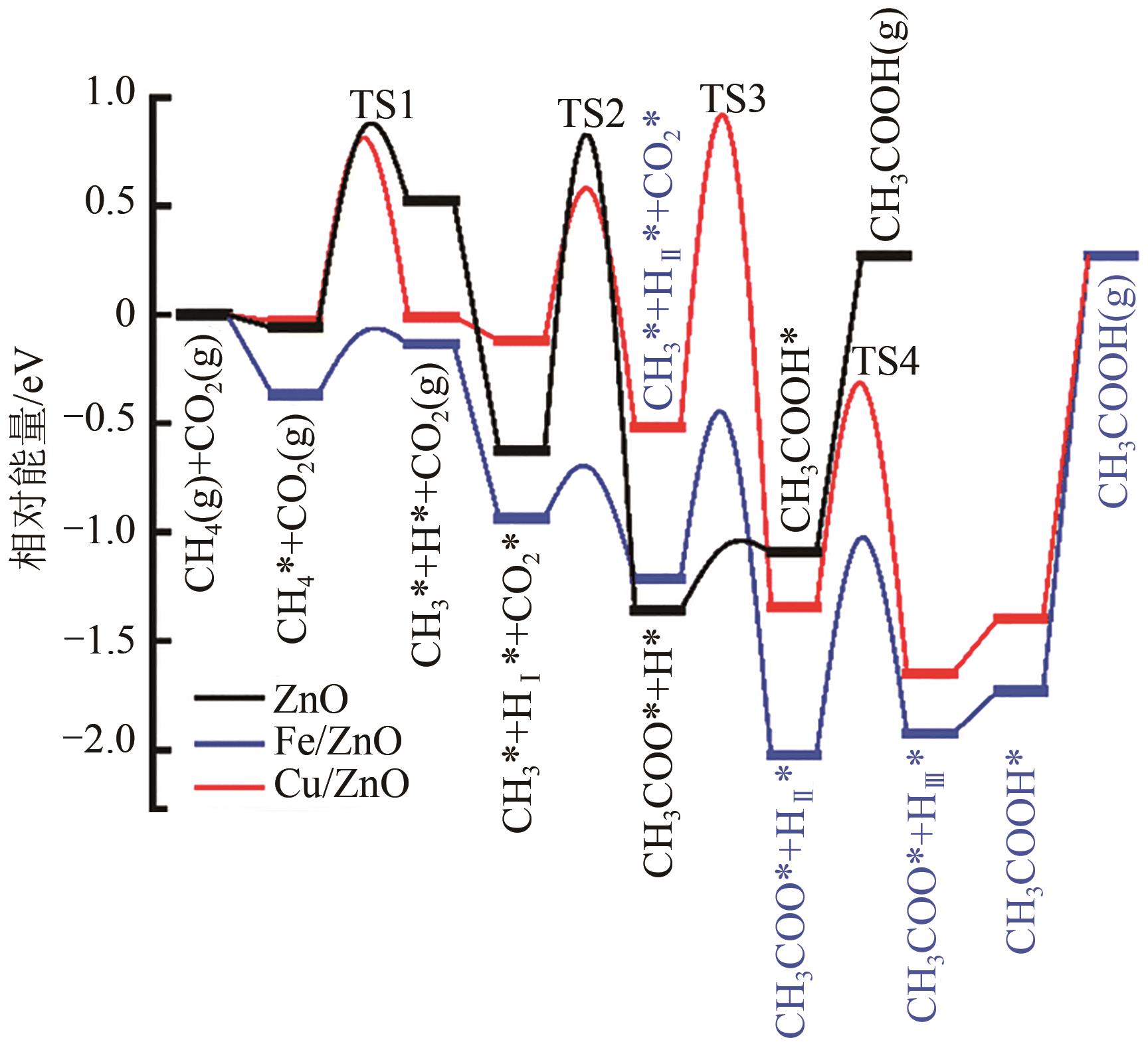
| CH3 | -2.70 | -2.58 | -2.20 |
| CH2 | -4.07 | -3.77 | -2.93 |
| CH | -5.64 | -5.45 | -4.46 |
| 解离物种 | Ru | Rh | Pd |
|---|---|---|---|
| CH3 | -2.70 | -2.58 | -2.20 |
| CH2 | -4.07 | -3.77 | -2.93 |
| CH | -5.64 | -5.45 | -4.46 |
| 催化剂 | 温度/K | V(CO2)/V(CH4) | C2烃选择性/% | C2烃产率/% |
|---|---|---|---|---|
| CaO-ZnO | 1123 | 2.3 | 80.0 | 4.3 |
| La2O3-ZnO | 1123 | 2.0 | 90.6 | 2.8 |
| Cr2O3-CaO | 850 | 2.3 | 63.0 | 4.0 |
| CeO2-CaO | 850 | 1.0 | 62.0 | 3.2 |
| MnO2-SrCO3 | 875 | 2.3 | 51.0 | 4.5 |
| Na2WO4-Mn/SiO2 | 1093.15 | 2.0 | 94.0 | 4.5 |
| 催化剂 | 温度/K | V(CO2)/V(CH4) | C2烃选择性/% | C2烃产率/% |
|---|---|---|---|---|
| CaO-ZnO | 1123 | 2.3 | 80.0 | 4.3 |
| La2O3-ZnO | 1123 | 2.0 | 90.6 | 2.8 |
| Cr2O3-CaO | 850 | 2.3 | 63.0 | 4.0 |
| CeO2-CaO | 850 | 1.0 | 62.0 | 3.2 |
| MnO2-SrCO3 | 875 | 2.3 | 51.0 | 4.5 |
| Na2WO4-Mn/SiO2 | 1093.15 | 2.0 | 94.0 | 4.5 |
| 1 | JIANG Yao, TAN Peng, QI Shichao, et al. Metal-organic frameworks with target-specific active sites switched by photoresponsive motifs: Efficient adsorbents for tailorable CO2 capture[J]. Angewandte Chemie International Edition, 2019, 58(20): 6600-6604. |
| 2 | XIE L, DING J, KONG X, et al. Microwave-assisted synthesis of Mg-gallate for efficient CO2 capture[J]. Materials Today Sustainability, 2023, 22: 100356. |
| 3 | 杨海霞, 吴聃. 国际能源署发布《2022年全球二氧化碳排放》报告[J]. 世界石油工业, 2023, 30(2): 80-81. |
| YANG Haixia, WU Dan. The International Energy Agency releases the report “Global CO2 emissions by 2022”[J]. World Petroleum Industry, 2023, 30(2): 80-81. | |
| 4 | 张彦 著. 谋划甲烷控排双多边国际合作——基于G20成员国甲烷排放结构的差异性[J]. 可持续发展经济导刊, 2023(4): 16-19. |
| ZHANG Yanzhu. Thought of bilateral and multilateral cooperation on methane emission control based on differences in methane emission portfolios among G20 members[J]. China Sustainability Tribune, 2023(4): 16-19. | |
| 5 | 李志勤, 李侨, 黄伟, 等. 酸改性CoPd/TiO2催化CH4-CO2直接合成C2含氧化合物[J]. 化工进展, 2020, 39(3): 1035-1042. |
| LI Zhiqin, LI Qiao, HUANG Wei, et al. Direct synthesis of C2-oxygenates from CH4 and CO2 over acid-modified CoPd/TiO2 catalyst[J]. Chemical Industry and Engineering Progress, 2020, 39(3): 1035-1042. | |
| 6 | 赵云涛. CH4与CO2或其他含氧化合物在金属氧化物表面基于C-C直接耦合的密度泛函理论研究[D]. 天津: 天津大学, 2020. |
| ZHAO Yuntao. Theoretical study of conversion of CH4 with CO2 or other oxygenates through direct C-C coupling on metal oxide surface[D]. Tianjin: Tianjin University, 2020. | |
| 7 | CAI Xiaojiao, HU Yunhang. Advances in catalytic conversion of methane and carbon dioxide to highly valuable products[J]. Energy Science & Engineering, 2019, 7(1): 4-29. |
| 8 | WANG Ye, YAO Lu, WANG Yannan, et al. Low-temperature catalytic CO2 dry reforming of methane on Ni-Si/ZrO2 catalyst[J]. ACS Catalysis, 2018, 8(7): 6495-6506. |
| 9 | YAN Xiaoliang, HU Tong, LIU Peng, et al. Highly efficient and stable Ni/CeO2-SiO2 catalyst for dry reforming of methane: Effect of interfacial structure of Ni/CeO2 on SiO2 [J]. Applied Catalysis B: Environmental, 2019, 246: 221-231. |
| 10 | BODROV N, APELBAUM L, TEMKIN M J K K. Kinetics of the reaction of methane with steam on the surface of nickel[J]. Kinetics and Catalysis, 1964, 5: 696. |
| 11 | ROSTRUPNIELSEN J R, HANSEN J H B. CO2-reforming of methane over transition metals[J]. Journal of Catalysis, 1993, 144(1): 38-49. |
| 12 | 张云飞, 张晓娣, 王影, 等. 镍基CH4-CO2重整催化剂研究综述[J]. 洁净煤技术, 2022, 28(5): 29-50. |
| ZHANG Yunfei, ZHANG Xiaodi, WANG Ying, et al. Review of nickel-based CH4-CO2 reforming catalysts[J]. Clean Coal Technology, 2022, 28(5): 29-50. | |
| 13 | ZHANG Shuangshuang, YING Ming, YU Jun, et al. Ni x Al1O2- δ mesoporous catalysts for dry reforming of methane: The special role of NiAl2O4 spinel phase and its reaction mechanism[J]. Applied Catalysis B: Environmental, 2021, 291: 120074. |
| 14 | DAS S, ASHOK J, BIAN Z F, et al. Silica-ceria sandwiched Ni core-shell catalyst for low temperature dry reforming of biogas: Coke resistance and mechanistic insights[J]. Applied Catalysis B: Environmental, 2018, 230: 220-236. |
| 15 | YENTEKAKIS I V, GOULA G, HATZISYMEON M, et al. Effect of support oxygen storage capacity on the catalytic performance of Rh nanoparticles for CO2 reforming of methane[J]. Applied Catalysis B: Environmental, 2019, 243: 490-501. |
| 16 | ZHOU Wei, WANG Binghao, TANG Long, et al. Photocatalytic dry reforming of methane enhanced by “dual-path” strategy with excellent low-temperature catalytic performance[J]. Advanced Functional Materials, 2023, 33(27): 2214068. |
| 17 | AZANCOT L, BOBADILLA L F, CENTENO M A, et al. IR spectroscopic insights into the coking-resistance effect of potassium on nickel-based catalyst during dry reforming of methane[J]. Applied Catalysis B: Environmental, 2021, 285: 119822. |
| 18 | BARAŃSKI A. On the usefulness of Campbell’s concept of the rate-determining step[J]. Solid State Ionics, 1999, 117(1/2): 123-128. |
| 19 | CHEN Shuyue, ZAFFRAN J, YANG Bo. Descriptor design in the computational screening of Ni-based catalysts with balanced activity and stability for dry reforming of methane reaction[J]. ACS Catalysis, 2020, 10(5): 3074-3083. |
| 20 | WEI Junmei, IGLESIA E. Isotopic and kinetic assessment of the mechanism of reactions of CH4 with CO2 or H2O to form synthesis gas and carbon on nickel catalysts[J]. Journal of Catalysis, 2004, 224(2): 370-383. |
| 21 | BRADFORD M C J, VANNICE M A. Catalytic reforming of methane with carbon dioxide over nickel catalysts Ⅱ. Reaction kinetics[J]. Applied Catalysis A: General, 1996, 142(1): 97-122. |
| 22 | OSAKI T. Effect of nickel diameter on the rates of elementary steps involved in CO2 reforming of CH4 over Ni/Al2O3 catalysts[J]. Catalysis Letters, 2015, 145(11): 1931-1940. |
| 23 | 余长春, 李然家, 王伟, 等. CO2/CH4干重整转化催化剂的积炭控制研究[J]. 石油化工, 2020, 49(10): 925-930. |
| YU Changchun, LI Ranjia, WANG Wei, et al. Study of carbon deposition controlling over CO2/CH4 dry reforming catalyst[J]. Petrochemical Technology, 2020, 49(10): 925-930. | |
| 24 | WANG Baochuan, CHEN Shuyue, ZHANG Jiaming, et al. Propagating DFT uncertainty to mechanism determination, degree of rate control, and coverage analysis: The kinetics of dry reforming of methane[J]. The Journal of Physical Chemistry C, 2019, 123(50): 30389-30397. |
| 25 | BIAN Zhoufeng, DAS S, Minghui WAI, et al. A review on bimetallic nickel-based catalysts for CO2 reforming of methane[J]. ChemPhysChem, 2017, 18(22): 3117-3134. |
| 26 | KAWI S, KATHIRASER Y, NI Jun, et al. Progress in synthesis of highly active and stable nickel-based catalysts for carbon dioxide reforming of methane[J]. ChemSusChem, 2015, 8(21): 3556-3575. |
| 27 | MORTENSEN P M, DYBKJÆR I. Industrial scale experience on steam reforming of CO2-rich gas[J]. Applied Catalysis A: General, 2015, 495: 141-151. |
| 28 | ÁLVAREZ MORENO A, RAMIREZ-REINA T, IVANOVA S, et al. Bimetallic Ni-Ru and Ni-Re catalysts for dry reforming of methane: Understanding the synergies of the selected promoters[J]. Frontiers in Chemistry, 2021, 9: 694976. |
| 29 | LU Yao, GUO Dan, ZHAO Yifan, et al. Enhanced catalytic performance of Ni x -V@HSS catalysts for the DRM reaction: The study of interfacial effects on Ni-VO x structure with a unique yolk-shell structure[J]. Journal of Catalysis, 2021, 396: 65-80. |
| 30 | LOVELL E C, FULLER A, SCOTT J, et al. Enhancing Ni-SiO2 catalysts for the carbon dioxide reforming of methane: Reduction-oxidation-reduction pre-treatment[J]. Applied Catalysis B: Environmental, 2016, 199: 155-165. |
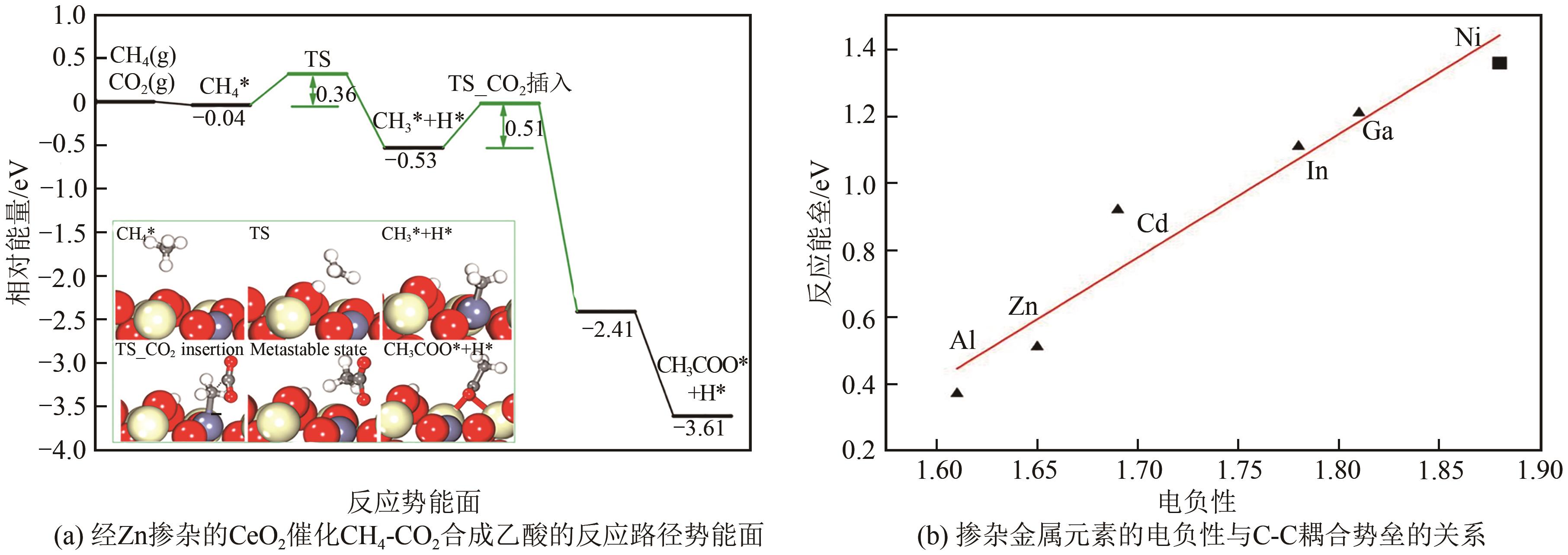
| 31 | BAN Tao, YU Xiyang, KANG Haozhe, et al. Design of single-atom and Frustrated-Lewis-Pair dual active sites for direct conversion of CH4 and CO2 to acetic acid[J]. Journal of Catalysis, 2022, 408: 206-215. |
| 32 | KURIOKA M, NAKATA K, JINTOKU T, et al. Palladium-catalyzed acetic acid synthesis from methane and carbon monoxide or dioxide[J]. Chemistry Letters, 1995, 24(3): 244. |
| 33 | TANIGUCHI Y, HAYASHIDA T, KITAMURA T, et al. Vanadium-catalyzed acetic acid synthesis from methane and carbon dioxide[M]// Studies in Surface Science and Catalysis. Amsterdam: Elsevier, 1998: 439-442. |
| 34 | ZERELLA M, MUKHOPADHYAY S, BELL A T. Synthesis of mixed acid anhydrides from methane and carbon dioxide in acid solvents[J]. Organic Letters, 2003, 5(18): 3193-3196. |
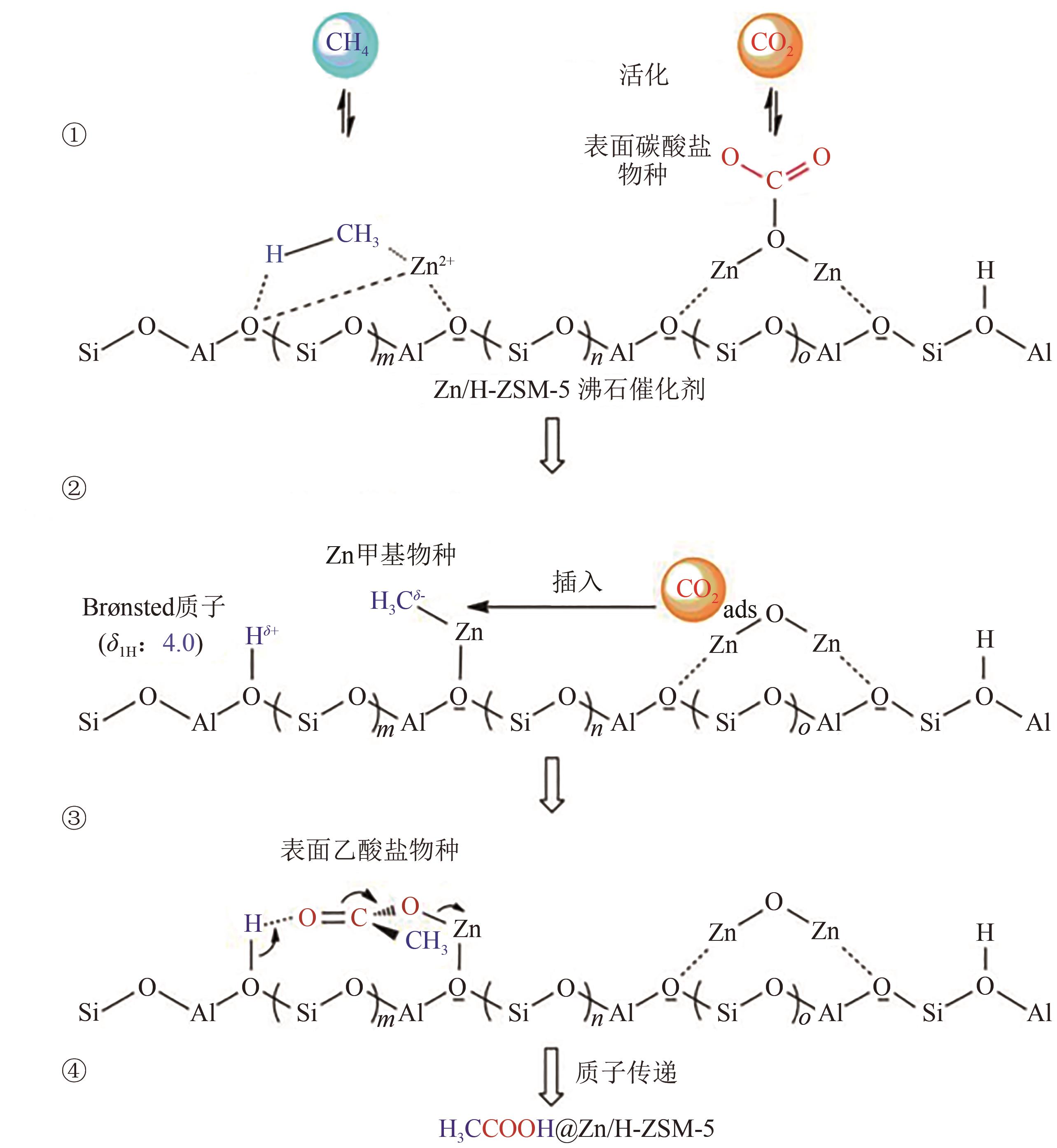
| 35 | WU Jianfeng, YU Simin, WANG Wei D, et al. Mechanistic insight into the formation of acetic acid from the direct conversion of methane and carbon dioxide on zinc-modified H-ZSM-5 zeolite[J]. Journal of the American Chemical Society, 2013, 135(36): 13567-13573. |
| 36 | WANG Sen, GUO Shujia, LUO Yaoya, et al. Direct synthesis of acetic acid from carbon dioxide and methane over Cu-modulated BEA, MFI, MOR and TON zeolites: A density functional theory study[J]. Catalysis Science & Technology, 2019, 9(23): 6613-6626. |
| 37 | ZHANG Riguang, SONG Luzhi, LIU Hongyan, et al. The interaction mechanism of CO2 with CH3 and H on Cu (111) surface in synthesis of acetic acid from CH4/CO2: A DFT study[J]. Applied Catalysis A: General, 2012, 443/444: 50-58. |
| 38 | ZHOU Lei, LI Wei, HU Tongliang. Computational study of Zn single-atom catalysts on In2O3 nanomaterials for direct synthesis of acetic acid from CH4 and CO2 [J]. ACS Applied Nano Materials, 2022, 5(7): 10015-10025. |
| 39 | MAHYUDDIN M H, TANAKA S, SHIOTA Y, et al. Room-temperature activation of methane and direct formations of acetic acid and methanol on Zn-ZSM-5 zeolite: A mechanistic DFT study[J]. Bulletin of the Chemical Society of Japan, 2020, 93(3): 345-354. |
| 40 | WU Bo, LIN Tiejun, LU Zhengxing, et al. Fe binuclear sites convert methane to acetic acid with ultrahigh selectivity[J]. Chem, 2022, 8(6): 1658-1672. |
| 41 | DING Yihui, HUANG Wei, WANG Yonggang. Direct synthesis of acetic acid from CH4 and CO2 by a step-wise route over Pd/SiO2 and Rh/SiO2 catalysts[J]. Fuel Processing Technology, 2007, 88(4): 319-324. |
| 42 | SHAVI R, KO J, CHO A, et al. Mechanistic insight into the quantitative synthesis of acetic acid by direct conversion of CH4 and CO2: An experimental and theoretical approach[J]. Applied Catalysis B: Environmental, 2018, 229: 237-248. |
| 43 | 章日光, 黄伟, 王宝俊. Co-Pd催化剂上CH4/CO2合成乙酸反应中CO2与表面金属物种作用的密度泛函理论研究[J]. 高等学校化学学报, 2009, 30(11): 2252-2257. |
| ZHANG Riguang, HUANG Wei, WANG Baojun. Density functional theory study on interaction of CO2 with metal surface carbon species in synthesis of acetic acid from CH4/CO2 on Co-Pd catalysts[J]. Chemical Journal of Chinese Universities, 2009, 30(11): 2252-2257. | |
| 44 | NIE Xiaowa, REN Xianxuan, TU Chunyan, et al. Computational and experimental identification of strong synergy of the Fe/ZnO catalyst in promoting acetic acid synthesis from CH4 and CO2 [J]. Chemical Communications, 2020, 56(28): 3983-3986. |
| 45 | ZHAO Yuntao, CUI Chaonan, HAN Jinyu, et al. Direct C-C coupling of CO2 and the methyl group from CH4 activation through facile insertion of CO2 into Zn-CH3 σ-bond[J]. Journal of the American Chemical Society, 2016, 138(32): 10191-10198. |
| 46 | ORTIZ-BRAVO C A, CHAGAS C A, TONIOLO F S. Oxidative coupling of methane (OCM): An overview of the challenges and opportunities for developing new technologies[J]. Journal of Natural Gas Science and Engineering, 2021, 96: 104254. |
| 47 | ZOU Shihui, LI Zhinian, ZHOU Qiuyue, et al. Surface coupling of methyl radicals for efficient low-temperature oxidative coupling of methane[J]. Chinese Journal of Catalysis, 2021, 42(7): 1117-1125. |
| 48 | KIANI D, SOURAV S, BALTRUSAITIS J, et al. Oxidative coupling of methane (OCM) by SiO2-supported tungsten oxide catalysts promoted with Mn and Na[J]. ACS Catalysis, 2019, 9(7): 5912-5928. |
| 49 | NGUYEN T N, NHAT T T P, TAKIMOTO K, et al. High-throughput experimentation and catalyst informatics for oxidative coupling of methane[J]. ACS Catalysis, 2020, 10(2): 921-932. |
| 50 | LIU Wenchi, RALSTON W T, MELAET G, et al. Oxidative coupling of methane (OCM): Effect of noble metal (M=Pt, Ir, Rh) doping on the performance of mesoporous silica MCF-17 supported Mn x O y -Na2WO4 catalysts[J]. Applied Catalysis A: General, 2017, 545: 17-23. |
| 51 | LIU Zichen, XU Shuman, HAO Jing, et al. Bifunctional catalysts composed of low silicon-content SAPO-34 nanosheets and In2O3/ZrO2 with improved performance for CO2 hydrogenation[J]. Greenhouse Gases: Science and Technology, 2022, 12(2): 305-320. |
| 52 | ASAMI K, FUJITA T, KUSAKABE K, et al. Conversion of methane with carbon dioxide into C2 hydrocarbons over metal oxides[J]. Applied Catalysis A: General, 1995, 126(2): 245-255. |
| 53 | LIU Jingwei, HUO Minfeng, ZHU Yan. Surface basic sites and lattice oxygen sensitive Mn-Na2WO4/SiO2 catalysts for oxidative coupling of methane[J]. Journal of Nanoscience and Nanotechnology, 2017, 17(12): 8818-8826. |
| 54 | ASAMI K, KUSAKABE K, ASHI N, et al. Synthesis of ethane and ethylene from methane and carbon dioxide over praseodymium oxide catalysts[J]. Applied Catalysis A: General, 1997, 156(1): 43-56. |
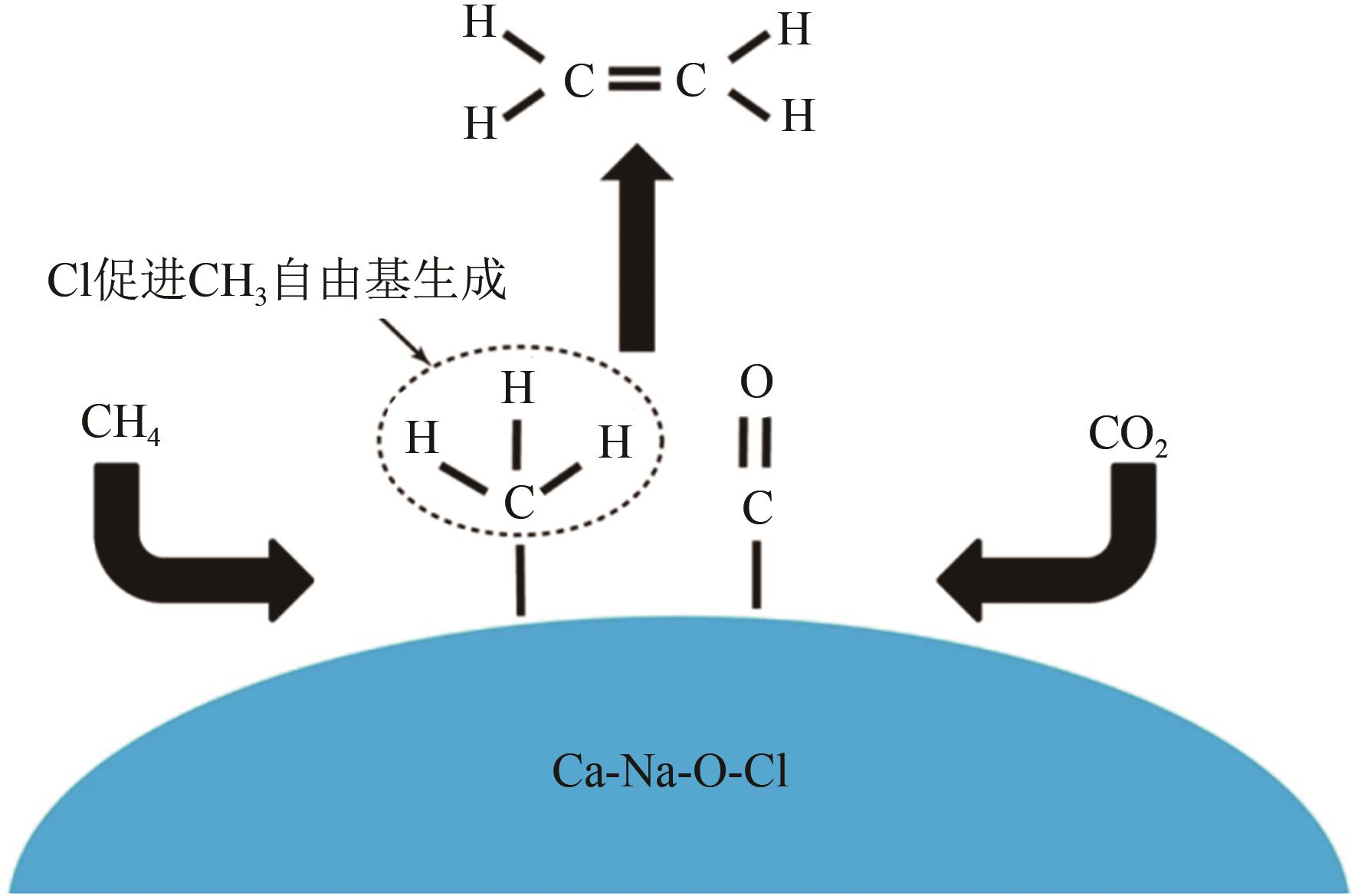
| 55 | 蔡迎春, 丑凌军, 张兵, 等. Mn-CaO催化剂上甲烷-二氧化碳共活化制C2烃研究II.催化剂表征及反应机理研究[J]. 分子催化, 2005, 19(5): 327-331. |
| CAI Yingchun, CHOU Lingjun, ZHANG Bing, et al. Selective conversion of CH4 and CO2 to C2 hydrocarbons over Mn-CaO catalysts Ⅱ. Catalysts characterization and mechanism deduce[J]. Journal of Molecular Catalysis, 2005, 19(5): 327-331. | |
| 56 | ZHANG Xianhua, PEI Chunlei, CHANG Xin, et al. FeO6 octahedral distortion activates lattice oxygen in perovskite ferrite for methane partial oxidation coupled with CO2 splitting[J]. Journal of the American Chemical Society, 2020, 142(26): 11540-11549. |
| 57 | VAN ALPHEN S, SLAETS J, CEULEMANS S, et al. Effect of N2 on CO2-CH4 conversion in a gliding arc plasmatron: Can this major component in industrial emissions improve the energy efficiency?[J]. Journal of CO2 Utilization, 2021, 54: 101767. |
| 58 | SAVINOV S Y, LEE H, SONG H K, et al. Decomposition of methane and carbon dioxide in a radio-frequency discharge[J]. Industrial & Engineering Chemistry Research, 1999, 38(7): 2540-2547. |
| 59 | CHEN Changlin, XU Yide, LI Guangjin, et al. Oxidative coupling of methane by carbon dioxide: A highly C2 selective La2O3/ZnO catalyst[J]. Catalysis Letters, 1996, 42(3): 149-153. |
| 60 | ZHANG Yongzheng, CHO Yohei, YAMAGUCHI A, et al. CO2 oxidative coupling of methane using an earth-abundant CaO-based catalyst[J]. Scientific Reports, 2019, 9(1): 15454. |
| 61 | WANG Ye, TAKAHASHI Y, OHTSUKA Y. Carbon dioxide-induced selective conversion of methane to C2 hydrocarbons on CeO2 modified with CaO[J]. Applied Catalysis A: General, 1998, 172(2): L203-L206. |
| 62 | ARINAGA A M, ZIEGELSKI M C, MARKS T J. Alternative oxidants for the catalytic oxidative coupling of methane[J]. Angewandte Chemie International Edition, 2021, 60(19): 10502-10515. |
| 63 | YOON Suji, Seoyeon LIM, CHOI Jae-Wook, et al. Study on the unsteady state oxidative coupling of methane: Effects of oxygen species from O2, surface lattice oxygen, and CO2 on the C2+ selectivity[J]. RSC Advances, 2020, 10(59): 35889-35897. |
| 64 | SHI Jia, YAO Lu, HU Changwei. Effect of CO2 on the structural variation of Na2WO4/Mn/SiO2 catalyst for oxidative coupling of methane to ethylene[J]. Journal of Energy Chemistry, 2015, 24(4): 394-400. |
| 65 | WANG Ye, OHTSUKA Y. CaO-ZnO catalyst for selective conversion of methane to C2 hydrocarbons using carbon dioxide as the oxidant[J]. Journal of Catalysis, 2000, 192(1): 252-255. |
| 66 | 翟林燕, 于淼, 周倩, 等. 常压等离子体作用下CO2氧化CH4转化反应的光谱分析[J]. 光谱学与光谱分析, 2012, 32(3): 734-738. |
| ZHAI Linyan, YU Miao, ZHOU Qian, et al. Spectral analysis of the reaction of CH4 with CO2 as oxidant under plasma at atmospheric pressure[J]. Spectroscopy and Spectral Analysis, 2012, 32(3): 734-738. | |
| 67 | CAI Yingchun, CHOU Lingjun, LI Shuben, et al. Selective conversion of methane to C2 hydrocarbons using carbon dioxide over Mn-SrCO3 catalysts[J]. Catalysis Letters, 2003, 86(4): 191-195. |
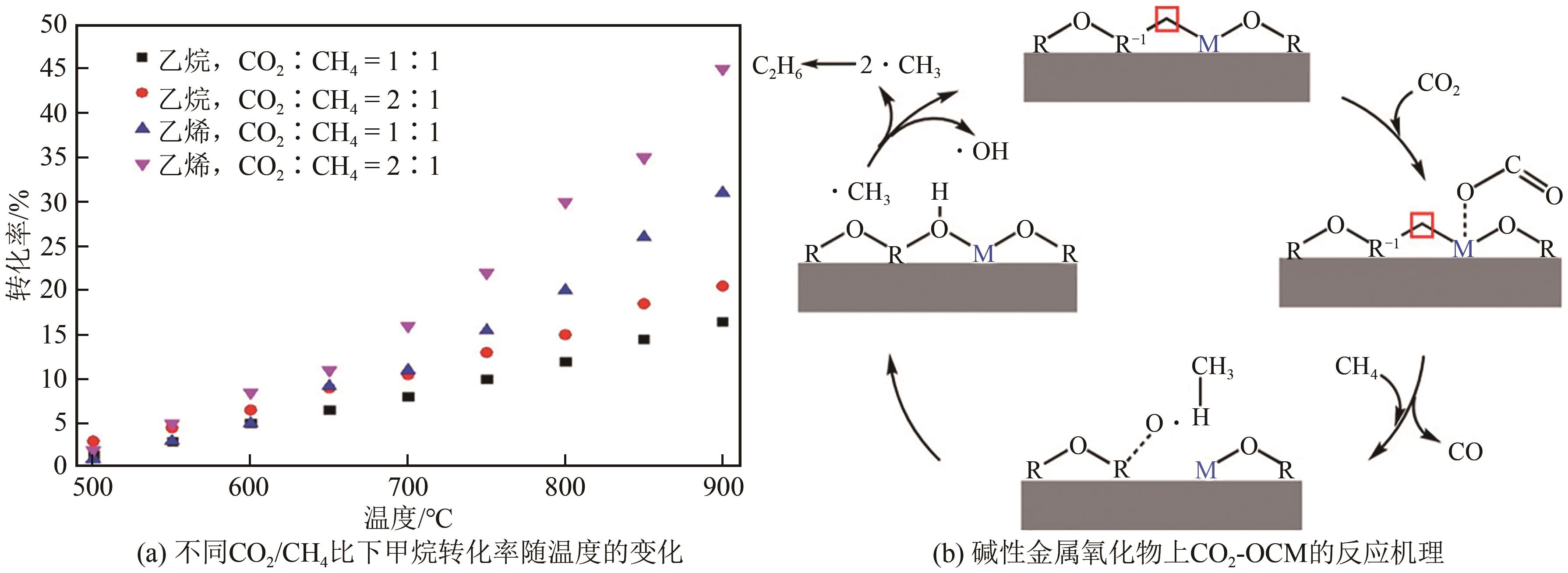
| 68 | FILARDI L R, YANG Feipeng, GUO Jinghua, et al. Surface basicity controls C-C coupling rates during carbon dioxide-assisted methane coupling over bifunctional Ca/ZnO catalysts[J]. Physical Chemistry Chemical Physics, 2023, 25(14): 9859-9867. |
| 69 | LIU Yu, HOU Ruiling, LIU Xuxia, et al. Performance of Na2WO4-Mn/SiO2 catalyst for conversion of CH4 with CO2 into C2 hydrocarbons and its mechanism[M]// Natural Gas Conversion V, Proceedings of the 5th International Natural Gas Conversion Symposium, Amsterdam: Elsevier, 1998: 307-311. |
| 70 | KIANI D, SOURAV S, WACHS I E, et al. Synthesis and molecular structure of model silica-supported tungsten oxide catalysts for oxidative coupling of methane (OCM)[J]. Catalysis Science & Technology, 2020, 10(10): 3334-3345. |
| 71 | WANG Jiyang, FU Yu, KONG Wenbo, et al. Investigation of atom-level reaction kinetics of carbon-resistant bimetallic NiCo-reforming catalysts: Combining microkinetic modeling and density functional theory[J]. ACS Catalysis, 2022, 12(8): 4382-4393. |
| 72 | 中国工程科技知识中心. CO2与甲烷重整转化制备合成气关键技术与工程示范[EB/OL]. (2015-11-24)[2023-07-26]. . |
| China Knowledge Center for Engineering Science and Technology. Key technology and engineering demonstration of CO2 and methane reforming conversion for syngas preparation[EB/OL]. (2015-11-24)[2023-07-26]. . |
| [1] | YU Xiaoxiao, CHAO Yanhong, LIU Haiyan, ZHU Wenshuai, LIU Zhichang. Enhanced photoelectric properties and photocatalytic CO2 conversion by D-A conjugated polymerization [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 292-301. |
| [2] | YANG Mengru, PENG Qin, CHANG Yulong, QIU Shuxing, ZHANG Jianbo, JIANG Xia. Research progress of carbon emission reduction technology with biochar replacing pulverized coal/coke for blast furnace ironmaking [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 490-500. |
| [3] | YANG Xiazhen, PENG Yifan, LIU Huazhang, HUO Chao. Regulation of active phase of fused iron catalyst and its catalytic performance of Fischer-Tropsch synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 310-318. |
| [4] | ZHENG Qian, GUAN Xiushuai, JIN Shanbiao, ZHANG Changming, ZHANG Xiaochao. Photothermal catalysis synthesis of DMC from CO2 and methanol over Ce0.25Zr0.75O2 solid solution [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 319-327. |
| [5] | SUN Yuyu, CAI Xinlei, TANG Jihai, HUANG Jingjing, HUANG Yiping, LIU Jie. Optimization and energy-saving of a reactive distillation process for the synthesis of methyl methacrylate [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 56-63. |
| [6] | YANG Hanyue, KONG Lingzhen, CHEN Jiaqing, SUN Huan, SONG Jiakai, WANG Sicheng, KONG Biao. Decarbonization performance of downflow tubular gas-liquid contactor of microbubble-type [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 197-204. |
| [7] | LAI Shini, JIANG Lixia, LI Jun, HUANG Hongyu, KOBAYASHI Noriyuki. Research progress of ammonia blended fossil fuel [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4603-4615. |
| [8] | WANG Yaogang, HAN Zishan, GAO Jiachen, WANG Xinyu, LI Siqi, YANG Quanhong, WENG Zhe. Strategies for regulating product selectivity of copper-based catalysts in electrochemical CO2 reduction [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4043-4057. |
| [9] | LIU Yi, FANG Qiang, ZHONG Dazhong, ZHAO Qiang, LI Jinping. Cu facets regulation of Ag/Cu coupled catalysts for electrocatalytic reduction of carbon dioxide [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4136-4142. |
| [10] | HUANG Yufei, LI Ziyi, HUANG Yangqiang, JIN Bo, LUO Xiao, LIANG Zhiwu. Research progress on catalysts for photocatalytic CO2 and CH4 reforming [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4247-4263. |
| [11] | XI Yonglan, WANG Chengcheng, YE Xiaomei, LIU Yang, JIA Zhaoyan, CAO Chunhui, HAN Ting, ZHANG Yingpeng, TIAN Yu. Research progress on the application of micro/nano bubbles in anaerobic digestion [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4414-4423. |
| [12] | LOU Baohui, WU Xianhao, ZHANG Chi, CHEN Zhen, FENG Xiangdong. Advances in nanofluid for CO2 absorption and separation [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3802-3815. |
| [13] | LIU Yang, YE Xiaomei, MIAO Xiao, WANG Chengcheng, JIA Zhaoyan, CAO Chunhui, XI Yonglan. Pilot-scale process research on dry digestion of rural organic household waste under ammonia stress [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3847-3854. |
| [14] | ZHANG Kai, LYU Qiunan, LI Gang, LI Xiaosen, MO Jiamei. Morphology and occurrence characteristics of methane hydrates in the mud of the South China Sea [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3865-3874. |
| [15] | LU Yang, ZHOU Jinsong, ZHOU Qixin, WANG Tang, LIU Zhuang, LI Bohao, ZHOU Lingtao. Leaching mechanism of Hg-absorption products on CeO2/TiO2 sorbentsin syngas [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3875-3883. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||