Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (9): 4947-4956.DOI: 10.16085/j.issn.1000-6613.2022-1862
• Resources and environmental engineering • Previous Articles Next Articles
Characterization of heavy metals during co-pyrolysis of sludge with PVC
LI Zhiyuan1( ), HUANG Yaji1(
), HUANG Yaji1( ), ZHAO Jiaqi1, YU Mengzhu1, ZHU Zhicheng1, CHENG Haoqiang1, SHI Hao1, WANG Sheng2
), ZHAO Jiaqi1, YU Mengzhu1, ZHU Zhicheng1, CHENG Haoqiang1, SHI Hao1, WANG Sheng2
- 1.Key Laboratory of Energy Thermal Conversion and Control of Ministry of Education, Southeast University, Nanjing 210096, Jiangsu, China
2.State Key Laboratory of Clean and Efficient Coal-fired Power Generation and Pollution Control, Nanjing 210023, Jiangsu, China
-
Received:2022-10-08Revised:2022-11-25Online:2023-09-28Published:2023-09-15 -
Contact:HUANG Yaji
污泥与聚氯乙烯共热解重金属特性
李志远1( ), 黄亚继1(
), 黄亚继1( ), 赵佳琪1, 于梦竹1, 朱志成1, 程好强1, 时浩1, 王圣2
), 赵佳琪1, 于梦竹1, 朱志成1, 程好强1, 时浩1, 王圣2
- 1.东南大学能源热转换及其过程测控教育部重点实验室,江苏 南京 210096
2.清洁高效燃煤发电与污染控制国家重点实验室,江苏 南京 210023
-
通讯作者:黄亚继 -
作者简介:李志远(1995—),男,博士研究生,研究方向为固体废弃物处理。E-mail:230218064@seu.edu.cn。 -
基金资助:江苏省科技计划(BE2022604)
CLC Number:
Cite this article
LI Zhiyuan, HUANG Yaji, ZHAO Jiaqi, YU Mengzhu, ZHU Zhicheng, CHENG Haoqiang, SHI Hao, WANG Sheng. Characterization of heavy metals during co-pyrolysis of sludge with PVC[J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4947-4956.
李志远, 黄亚继, 赵佳琪, 于梦竹, 朱志成, 程好强, 时浩, 王圣. 污泥与聚氯乙烯共热解重金属特性[J]. 化工进展, 2023, 42(9): 4947-4956.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-1862
| 样品 | 工业分析(ad)/% | 元素分析(ad)/% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | V | FC | Ash | C | H | O | N | S | Cl | ||
| SS | 8.68 | 44.32 | 5.06 | 41.94 | 18.96 | 3.645 | 22.81 | 2.94 | 0.885 | 0.14 | |
| PVC | 0.25 | 91.96 | 7.24 | 0.55 | 38.85 | 5.69 | 0.39 | 0.03 | 0.75 | 53.49 | |
| 样品 | 工业分析(ad)/% | 元素分析(ad)/% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | V | FC | Ash | C | H | O | N | S | Cl | ||
| SS | 8.68 | 44.32 | 5.06 | 41.94 | 18.96 | 3.645 | 22.81 | 2.94 | 0.885 | 0.14 | |
| PVC | 0.25 | 91.96 | 7.24 | 0.55 | 38.85 | 5.69 | 0.39 | 0.03 | 0.75 | 53.49 | |
| 提取形态 | 样品 | 提取试剂及条件 |
|---|---|---|
| 酸溶态和可交换态(F1) | 0.5g | 20mL、0.1mol/L乙酸,振荡16h后离心,取上清液 |
| 可还原态(F2) | 上一步的固态残渣 | 20mL、0.1mol/L盐酸羟胺,振荡16h后离心,取上清液 |
| 可氧化态(F3) | 上一步的固态残渣 | 首先,5mL、体积分数30%过氧化氢,室温1h;然后,5mL、体积分数30%过氧化氢,水浴(85±5)℃,1h;最后,25mL、1.0mol/L乙酸铵,振荡16h后离心,取上清液 |
| 残渣态(F4) | 上一步的固态残渣 | 消解方法如1.3.1节中所述 |
| 提取形态 | 样品 | 提取试剂及条件 |
|---|---|---|
| 酸溶态和可交换态(F1) | 0.5g | 20mL、0.1mol/L乙酸,振荡16h后离心,取上清液 |
| 可还原态(F2) | 上一步的固态残渣 | 20mL、0.1mol/L盐酸羟胺,振荡16h后离心,取上清液 |
| 可氧化态(F3) | 上一步的固态残渣 | 首先,5mL、体积分数30%过氧化氢,室温1h;然后,5mL、体积分数30%过氧化氢,水浴(85±5)℃,1h;最后,25mL、1.0mol/L乙酸铵,振荡16h后离心,取上清液 |
| 残渣态(F4) | 上一步的固态残渣 | 消解方法如1.3.1节中所述 |
| 样品 | 重金属含量/mg·kg-1 | |||||||
|---|---|---|---|---|---|---|---|---|
| 产率 | As | Cr | Cu | Mn | Ni | Pb | Zn | |
| SS | 63.0±2.0 | 210±8.2 | 270.0±7.1 | 1875.0±72.2 | 92.5±2.4 | 107.5±8.2 | 1570.0±39.8 | |
| PVC | ND | ND | ND | ND | ND | ND | 255.0±11.48 | |
| 500SS | 61.2±0 | 62.5±2.0 | 305.0±1.8 | 393.8±3.8 | 2679.4±156.3 | 138.8±2.8 | 155.6±3.7 | 2265.0±59.9 |
| 500SP1 | 59.8±0.3 | 55.0±4.1 | 293.1±2.7 | 375.6±2.7 | 2656.9±9.7 | 130.6±2.1 | 145.6±3.2 | 2197.5±21.1 |
| 500SP2 | 55.5±0.2 | 45.6±2.1 | 293.8±2.8 | 371.3±2.8 | 2663.8±21.7 | 128.8±2.8 | 143.1±3.2 | 2296.9±22.2 |
| 600SS | 58.8±0 | 58.8±3.8 | 315.6±2.1 | 401.9±3.7 | 2848.1±35.9 | 138.1±2.1 | 155.6±1.1 | 2331.3±17.2 |
| 600SP1 | 57.9±0.5 | 53.8±4.5 | 325.6±9.1 | 400.0±8.1 | 2864.4±104.0 | 145.0±6.8 | 156.3±4.5 | 2331.9±52.0 |
| 600SP2 | 54.5±0.1 | 47.5±1.8 | 299.4±3.7 | 376.3±8.0 | 2686.9±28.2 | 135.6±3.7 | 136.9±3.2 | 2096.3±35.0 |
| 700SS | 58.6±0 | 53.1±5.7 | 343.1±8.7 | 426.3±5.4 | 3018.8±75.3 | 150.6±2.1 | 166.9±2.1 | 2455.6±19.9 |
| 700SP1 | 57.2±0 | 49.4±4.5 | 323.8±4.5 | 412.5±4.0 | 2963.1±55.2 | 145.0±1.8 | 132.5±1.8 | 1931.9±30.7 |
| 700SP2 | 53.9±0.2 | 51.9±4.5 | 316.3±2.2 | 390.6±6.5 | 2836.9±64.4 | 141.3±2.2 | 88.8±2.8 | 1071.9±57.0 |
| 800SS | 56.8±0 | 41.3±3.8 | 346.9±6.0 | 441.9±8.4 | 2790.0±95.9 | 154.4±4.5 | 135.0±1.8 | 2341.9±90.1 |
| 800SP1 | 55.2±0.1 | 38.8±6.3 | 338.1±2.1 | 424.4±8.5 | 2825.0±135.3 | 145.6±2.1 | 61.9±3.7 | 907.5±9.5 |
| 800SP2 | 51.9±0 | 42.5±3.1 | 327.5±7.9 | 403.1±7.2 | 2840.6±93.4 | 143.1±3.7 | 38.8±1.3 | 421.9±20.8 |
| 900SS | 54.0±0 | 57.5±4.1 | 374.4±11.5 | 464.4±16.9 | 3291.9±146.2 | 161.3±7.4 | 46.9±2.7 | 1893.1±15.8 |
| 900SP1 | 52.6±0.1 | 51.3±3.8 | 332.5±12.5 | 407.5±17.5 | 2805.0±75.0 | 142.5±5.0 | 23.8±1.3 | 543.8±8.8 |
| 900SP2 | 50.4±0 | 48.8±2.8 | 338.8±5.2 | 414.4±9.6 | 3009.4±68.2 | 144.4±3.2 | 18.8±2.2 | 247.5±30.4 |
| 样品 | 重金属含量/mg·kg-1 | |||||||
|---|---|---|---|---|---|---|---|---|
| 产率 | As | Cr | Cu | Mn | Ni | Pb | Zn | |
| SS | 63.0±2.0 | 210±8.2 | 270.0±7.1 | 1875.0±72.2 | 92.5±2.4 | 107.5±8.2 | 1570.0±39.8 | |
| PVC | ND | ND | ND | ND | ND | ND | 255.0±11.48 | |
| 500SS | 61.2±0 | 62.5±2.0 | 305.0±1.8 | 393.8±3.8 | 2679.4±156.3 | 138.8±2.8 | 155.6±3.7 | 2265.0±59.9 |
| 500SP1 | 59.8±0.3 | 55.0±4.1 | 293.1±2.7 | 375.6±2.7 | 2656.9±9.7 | 130.6±2.1 | 145.6±3.2 | 2197.5±21.1 |
| 500SP2 | 55.5±0.2 | 45.6±2.1 | 293.8±2.8 | 371.3±2.8 | 2663.8±21.7 | 128.8±2.8 | 143.1±3.2 | 2296.9±22.2 |
| 600SS | 58.8±0 | 58.8±3.8 | 315.6±2.1 | 401.9±3.7 | 2848.1±35.9 | 138.1±2.1 | 155.6±1.1 | 2331.3±17.2 |
| 600SP1 | 57.9±0.5 | 53.8±4.5 | 325.6±9.1 | 400.0±8.1 | 2864.4±104.0 | 145.0±6.8 | 156.3±4.5 | 2331.9±52.0 |
| 600SP2 | 54.5±0.1 | 47.5±1.8 | 299.4±3.7 | 376.3±8.0 | 2686.9±28.2 | 135.6±3.7 | 136.9±3.2 | 2096.3±35.0 |
| 700SS | 58.6±0 | 53.1±5.7 | 343.1±8.7 | 426.3±5.4 | 3018.8±75.3 | 150.6±2.1 | 166.9±2.1 | 2455.6±19.9 |
| 700SP1 | 57.2±0 | 49.4±4.5 | 323.8±4.5 | 412.5±4.0 | 2963.1±55.2 | 145.0±1.8 | 132.5±1.8 | 1931.9±30.7 |
| 700SP2 | 53.9±0.2 | 51.9±4.5 | 316.3±2.2 | 390.6±6.5 | 2836.9±64.4 | 141.3±2.2 | 88.8±2.8 | 1071.9±57.0 |
| 800SS | 56.8±0 | 41.3±3.8 | 346.9±6.0 | 441.9±8.4 | 2790.0±95.9 | 154.4±4.5 | 135.0±1.8 | 2341.9±90.1 |
| 800SP1 | 55.2±0.1 | 38.8±6.3 | 338.1±2.1 | 424.4±8.5 | 2825.0±135.3 | 145.6±2.1 | 61.9±3.7 | 907.5±9.5 |
| 800SP2 | 51.9±0 | 42.5±3.1 | 327.5±7.9 | 403.1±7.2 | 2840.6±93.4 | 143.1±3.7 | 38.8±1.3 | 421.9±20.8 |
| 900SS | 54.0±0 | 57.5±4.1 | 374.4±11.5 | 464.4±16.9 | 3291.9±146.2 | 161.3±7.4 | 46.9±2.7 | 1893.1±15.8 |
| 900SP1 | 52.6±0.1 | 51.3±3.8 | 332.5±12.5 | 407.5±17.5 | 2805.0±75.0 | 142.5±5.0 | 23.8±1.3 | 543.8±8.8 |
| 900SP2 | 50.4±0 | 48.8±2.8 | 338.8±5.2 | 414.4±9.6 | 3009.4±68.2 | 144.4±3.2 | 18.8±2.2 | 247.5±30.4 |
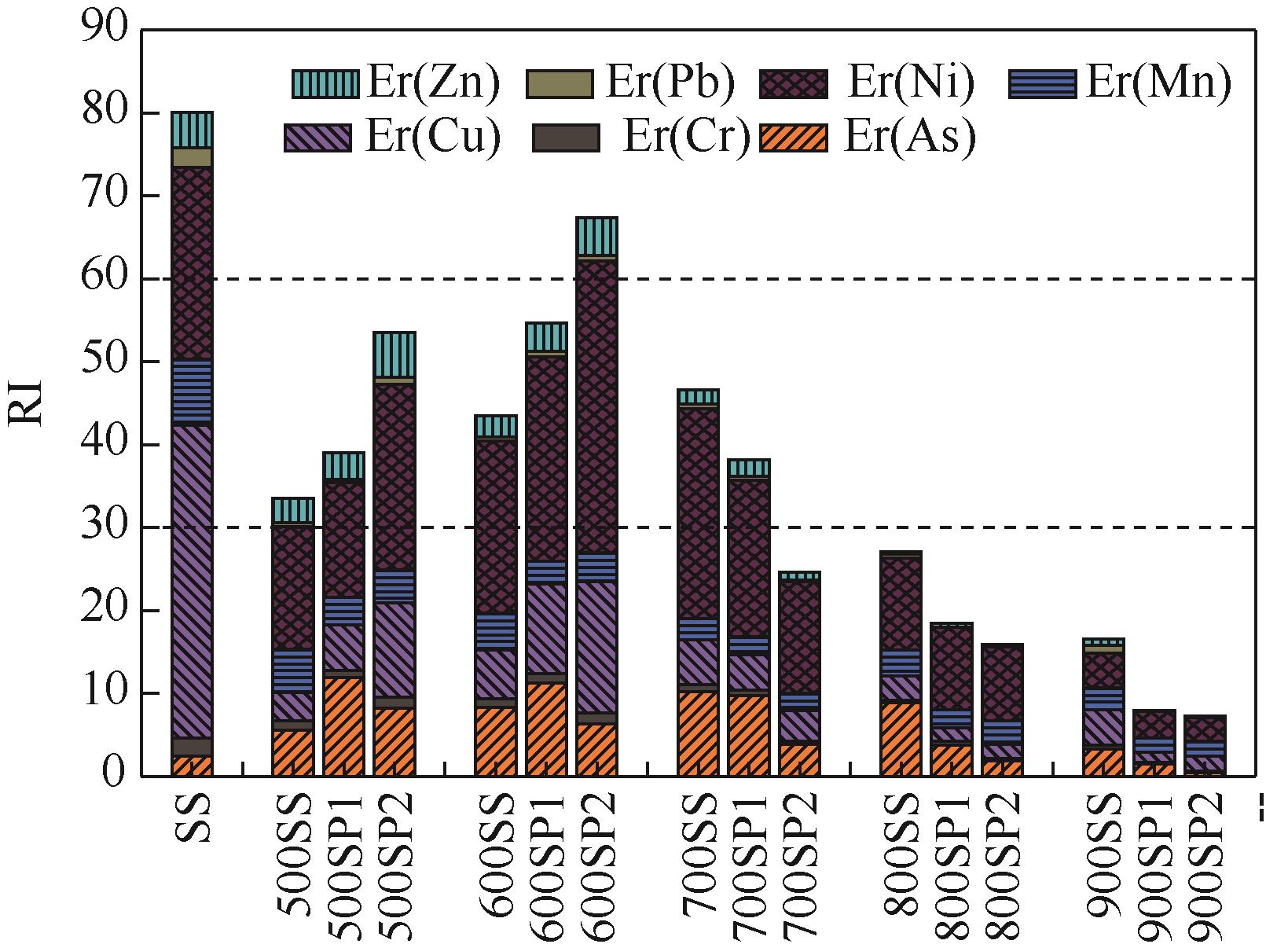
| 样品 | Cf | Er | RI | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| As | Cr | Cu | Mn | Ni | Pb | Zn | As | Cr | Cu | Mn | Ni | Pb | Zn | |||
| SS | 0.24 | 1.11 | 7.54 | 7.93 | 3.86 | 0.48 | 4.18 | 2.44 | 2.22 | 37.72 | 7.93 | 23.16 | 2.39 | 4.18 | 80.04 | |
| 500SS | 0.56 | 0.56 | 0.69 | 5.19 | 2.47 | 0.09 | 3.01 | 5.56 | 1.12 | 3.43 | 5.19 | 14.84 | 0.44 | 3.01 | 33.58 | |
| 500SP1 | 1.19 | 0.41 | 1.10 | 3.37 | 2.30 | 0.07 | 3.20 | 11.94 | 0.82 | 5.50 | 3.37 | 13.80 | 0.35 | 3.20 | 38.98 | |
| 500SP2 | 0.82 | 0.64 | 2.28 | 3.92 | 3.74 | 0.16 | 5.41 | 8.24 | 1.28 | 11.42 | 3.92 | 22.45 | 0.79 | 5.41 | 53.50 | |
| 600SS | 0.83 | 0.54 | 1.19 | 4.37 | 3.47 | 0.08 | 2.61 | 8.26 | 1.08 | 5.95 | 4.37 | 20.82 | 0.42 | 2.61 | 43.50 | |
| 600SP1 | 1.13 | 0.57 | 2.15 | 2.82 | 4.10 | 0.13 | 3.49 | 11.29 | 1.14 | 10.74 | 2.82 | 24.58 | 0.66 | 3.49 | 54.73 | |
| 600SP2 | 0.64 | 0.63 | 3.17 | 3.49 | 5.87 | 0.13 | 4.58 | 6.35 | 1.25 | 15.87 | 3.49 | 35.22 | 0.64 | 4.58 | 67.41 | |
| 700SS | 1.03 | 0.39 | 1.08 | 2.58 | 4.22 | 0.10 | 1.73 | 10.27 | 0.78 | 5.41 | 2.58 | 25.34 | 0.48 | 1.73 | 46.60 | |
| 700SP1 | 0.97 | 0.31 | 0.86 | 2.17 | 3.15 | 0.09 | 1.99 | 9.75 | 0.62 | 4.29 | 2.17 | 18.92 | 0.47 | 1.99 | 38.21 | |
| 700SP2 | 0.38 | 0.21 | 0.73 | 2.07 | 2.25 | 0.03 | 1.02 | 3.85 | 0.42 | 3.67 | 2.07 | 13.48 | 0.16 | 1.02 | 24.66 | |
| 800SS | 0.89 | 0.12 | 0.59 | 3.14 | 1.85 | 0.10 | 0.32 | 8.90 | 0.24 | 2.97 | 3.14 | 11.08 | 0.48 | 0.32 | 27.14 | |
| 800SP1 | 0.38 | 0.22 | 0.32 | 2.26 | 1.65 | 0.00 | 0.56 | 3.75 | 0.45 | 1.62 | 2.26 | 9.87 | 0.00 | 0.56 | 18.51 | |
| 800SP2 | 0.19 | 0.16 | 0.33 | 2.88 | 1.48 | 0.00 | 0.30 | 1.87 | 0.31 | 1.67 | 2.88 | 8.86 | 0.00 | 0.30 | 15.89 | |
| 900SS | 0.33 | 0.23 | 0.85 | 2.60 | 0.70 | 0.19 | 0.77 | 3.33 | 0.46 | 4.27 | 2.60 | 4.22 | 0.93 | 0.77 | 16.58 | |
| 900SP1 | 0.15 | 0.08 | 0.25 | 1.73 | 0.53 | 0.00 | 0.15 | 1.49 | 0.17 | 1.24 | 1.73 | 3.19 | 0.00 | 0.15 | 7.97 | |
| 900SP2 | 0.04 | 0.12 | 0.36 | 1.75 | 0.48 | 0.00 | 0.20 | 0.42 | 0.24 | 1.81 | 1.75 | 2.88 | 0.00 | 0.20 | 7.29 | |
| 样品 | Cf | Er | RI | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| As | Cr | Cu | Mn | Ni | Pb | Zn | As | Cr | Cu | Mn | Ni | Pb | Zn | |||
| SS | 0.24 | 1.11 | 7.54 | 7.93 | 3.86 | 0.48 | 4.18 | 2.44 | 2.22 | 37.72 | 7.93 | 23.16 | 2.39 | 4.18 | 80.04 | |
| 500SS | 0.56 | 0.56 | 0.69 | 5.19 | 2.47 | 0.09 | 3.01 | 5.56 | 1.12 | 3.43 | 5.19 | 14.84 | 0.44 | 3.01 | 33.58 | |
| 500SP1 | 1.19 | 0.41 | 1.10 | 3.37 | 2.30 | 0.07 | 3.20 | 11.94 | 0.82 | 5.50 | 3.37 | 13.80 | 0.35 | 3.20 | 38.98 | |
| 500SP2 | 0.82 | 0.64 | 2.28 | 3.92 | 3.74 | 0.16 | 5.41 | 8.24 | 1.28 | 11.42 | 3.92 | 22.45 | 0.79 | 5.41 | 53.50 | |
| 600SS | 0.83 | 0.54 | 1.19 | 4.37 | 3.47 | 0.08 | 2.61 | 8.26 | 1.08 | 5.95 | 4.37 | 20.82 | 0.42 | 2.61 | 43.50 | |
| 600SP1 | 1.13 | 0.57 | 2.15 | 2.82 | 4.10 | 0.13 | 3.49 | 11.29 | 1.14 | 10.74 | 2.82 | 24.58 | 0.66 | 3.49 | 54.73 | |
| 600SP2 | 0.64 | 0.63 | 3.17 | 3.49 | 5.87 | 0.13 | 4.58 | 6.35 | 1.25 | 15.87 | 3.49 | 35.22 | 0.64 | 4.58 | 67.41 | |
| 700SS | 1.03 | 0.39 | 1.08 | 2.58 | 4.22 | 0.10 | 1.73 | 10.27 | 0.78 | 5.41 | 2.58 | 25.34 | 0.48 | 1.73 | 46.60 | |
| 700SP1 | 0.97 | 0.31 | 0.86 | 2.17 | 3.15 | 0.09 | 1.99 | 9.75 | 0.62 | 4.29 | 2.17 | 18.92 | 0.47 | 1.99 | 38.21 | |
| 700SP2 | 0.38 | 0.21 | 0.73 | 2.07 | 2.25 | 0.03 | 1.02 | 3.85 | 0.42 | 3.67 | 2.07 | 13.48 | 0.16 | 1.02 | 24.66 | |
| 800SS | 0.89 | 0.12 | 0.59 | 3.14 | 1.85 | 0.10 | 0.32 | 8.90 | 0.24 | 2.97 | 3.14 | 11.08 | 0.48 | 0.32 | 27.14 | |
| 800SP1 | 0.38 | 0.22 | 0.32 | 2.26 | 1.65 | 0.00 | 0.56 | 3.75 | 0.45 | 1.62 | 2.26 | 9.87 | 0.00 | 0.56 | 18.51 | |
| 800SP2 | 0.19 | 0.16 | 0.33 | 2.88 | 1.48 | 0.00 | 0.30 | 1.87 | 0.31 | 1.67 | 2.88 | 8.86 | 0.00 | 0.30 | 15.89 | |
| 900SS | 0.33 | 0.23 | 0.85 | 2.60 | 0.70 | 0.19 | 0.77 | 3.33 | 0.46 | 4.27 | 2.60 | 4.22 | 0.93 | 0.77 | 16.58 | |
| 900SP1 | 0.15 | 0.08 | 0.25 | 1.73 | 0.53 | 0.00 | 0.15 | 1.49 | 0.17 | 1.24 | 1.73 | 3.19 | 0.00 | 0.15 | 7.97 | |
| 900SP2 | 0.04 | 0.12 | 0.36 | 1.75 | 0.48 | 0.00 | 0.20 | 0.42 | 0.24 | 1.81 | 1.75 | 2.88 | 0.00 | 0.20 | 7.29 | |
| 1 | GAO Ningbo, KAMRAN Kamran, QUAN Cui, et al. Thermochemical conversion of sewage sludge: A critical review[J]. Progress in Energy and Combustion Science, 2020, 79: 100843. |
| 2 | ZAKER Ali, CHEN Zhi, WANG Xiaolei, et al. Microwave-assisted pyrolysis of sewage sludge: A review[J]. Fuel Processing Technology, 2019, 187: 84-104. |
| 3 | RAHEEM Abdul, SIKARWAR Vineet Singh, HE Jun, et al. Opportunities and challenges in sustainable treatment and resource reuse of sewage sludge: A review[J]. Chemical Engineering Journal, 2018, 337: 616-641. |
| 4 | CHEN Dezhen, YIN Lijie, WANG Huan, et al. Pyrolysis technologies for municipal solid waste: A review[J]. Waste Management, 2014, 34(12): 2466-2486. |
| 5 | SMITH K M, FOWLER G D, PULLKET S, et al. Sewage sludge-based adsorbents: A review of their production, properties and use in water treatment applications[J]. Water Research, 2009, 43(10): 2569-2594. |
| 6 | LI Rundong, TENG Wenchao, LI Yanlong, et al. Coenrichment characteristics of phosphorus and heavy metals in incinerated sewage sludge ash[J]. Environmental Progress & Sustainable Energy, 2016, 35(4): 1027-1034. |
| 7 | AHMAD Mahtab, RAJAPAKSHA Anushka Upamali, Jung Eun LIM, et al. Biochar as a sorbent for contaminant management in soil and water: A review[J]. Chemosphere, 2014, 99: 19-33. |
| 8 | WANG Jianlong, WANG Shizong. Preparation, modification and environmental application of biochar: A review[J]. Journal of Cleaner Production, 2019, 227: 1002-1022. |
| 9 | LU Peng, HUANG Qunxing, BOURTSALAS A C, et al. Review on fate of chlorine during thermal processing of solid wastes[J]. Journal of Environmental Sciences, 2019, 78: 13-28. |
| 10 | GALEY B, GAUTIER M, KIM B, et al. Trace metal elements vaporization and phosphorus recovery during sewage sludge thermochemical treatment—A review[J]. Journal of Hazardous Materials, 2022, 424: 127360. |
| 11 | TANG P, ZHAO Y C, CHEN D Z, et al. Volatility of heavy metals during incineration of tannery sludge in the presence of chlorides and phosphoric acid[J]. Waste Management & Research, 2008, 26(4): 369-376. |
| 12 | LI Rundong, ZHAO Weiwei, LI Yanlong, et al. Heavy metal removal and speciation transformation through the calcination treatment of phosphorus-enriched sewage sludge ash[J]. Journal of Hazardous Materials, 2015, 283: 423-431. |
| 13 | XIA Yunxue, TANG Yuanyuan, SHIH Kaimin, et al. Enhanced phosphorus availability and heavy metal removal by chlorination during sewage sludge pyrolysis[J]. Journal of Hazardous Materials, 2020, 382: 121110. |
| 14 | LI Wenjin, MENG Jun, ZHANG Yule, et al. Co-pyrolysis of sewage sludge and metal-free/metal-loaded polyvinyl chloride (PVC) microplastics improved biochar properties and reduced environmental risk of heavy metals[J]. Environmental Pollution, 2022, 302: 119092. |
| 15 | 赵佳琪, 黄亚继, 李志远, 等. 污泥和聚氯乙烯共热解三相产物特性研究[J]. 化工进展, 2023, 42(4): 2122-2129. |
| ZHAO Jiaqi, HUANG Yaji, LI Zhiyuan, et al. Characteristics of three-phase products from co-pyrolysis of sewage sludge and PVC[J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2122-2129. | |
| 16 | QUEVAUVILLER Ph, URE A, MUNTAU H, et al. Improvement of analytical measurements within the BCR-programme: Single and sequential extraction procedures applied to soil and sediment analysis[J]. International Journal of Environmental Analytical Chemistry, 1993, 51(1/2/3/4): 129-134. |
| 17 | HAKANSON Lars. An ecological risk index for aquatic pollution control.a sedimentological approach[J]. Water Research, 1980, 14(8): 975-1001. |
| 18 | LIU Tingting, LIU Zhengang, ZHENG Qingfu, et al. Effect of hydrothermal carbonization on migration and environmental risk of heavy metals in sewage sludge during pyrolysis[J]. Bioresource Technology, 2018, 247: 282-290. |
| 19 | 任浩华, 王帅, 王芳杰, 等. PVC热解过程中HCl的生成及其影响因素[J]. 中国环境科学, 2015, 35(8): 2460-2469. |
| REN Haohua, WANG Shuai, WANG Fangjie, et al. HCl generation reaction and its influence factors during PVC pyrolysis[J]. China Environmental Science, 2015, 35(8): 2460-2469. | |
| 20 | 汪刚, 余广炜, 谢胜禹, 等. 添加不同塑料与污泥混合热解对生物炭中重金属的影响[J]. 燃料化学学报, 2019, 47(5): 611-620. |
| WANG Gang, YU Guangwei, XIE Shengyu, et al. Effect of co-pyrolysis of different plastics with sewage sludge on heavy metals in the biochar[J]. Journal of Fuel Chemistry and Technology, 2019, 47(5): 611-620. | |
| 21 | CHEN Yiwei, LIU Guijian, WANG Lei, et al. Occurrence and fate of some trace elements during pyrolysis of Yima coal, China[J]. Energy & Fuels, 2008, 22(6): 3877-3882. |
| 22 | UDAYANGA W D C, VEKSHA Andrei, GIANNIS A, et al. Fate and distribution of heavy metals during thermal processing of sewage sludge[J]. Fuel, 2018, 226: 721-744. |
| 23 | FRAISSLER G, JOLLER M, MATTENBERGER H, et al. Thermodynamic equilibrium calculations concerning the removal of heavy metals from sewage sludge ash by chlorination[J]. Chemical Engineering and Processing: Process Intensification, 2009, 48(1): 152-164. |
| 24 | ADAM C, PEPLINSKI B, MICHAELIS M, et al. Thermochemical treatment of sewage sludge ashes for phosphorus recovery[J]. Waste Management, 2009, 29(3): 1122-1128. |
| 25 | KISTLER R C, WIDMER F, BRUNNER P H. Behavior of chromium, nickel, copper, zinc, cadmium, mercury, and lead during the pyrolysis of sewage sludge[J]. Environmental Science & Technology, 1987, 21(7): 704-708. |
| 26 | WANG Xingdong, CHANG Vitor Wei-Chung, LI Zhiwei, et al. Co-pyrolysis of sewage sludge and organic fractions of municipal solid waste: Synergistic effects on biochar properties and the environmental risk of heavy metals[J]. Journal of Hazardous Materials, 2021, 412: 125200. |
| 27 | ABANADES S, FLAMANT G, GAUTHIER D. Modelling of heavy metal vaporisation from a mineral matrix[J]. Journal of Hazardous Materials, 2001, 88(1): 75-94. |
| 28 | YUAN Xingzhong, HUANG Huajun, ZENG Guangming, et al. Total concentrations and chemical speciation of heavy metals in liquefaction residues of sewage sludge[J]. Bioresource Technology, 2011, 102(5): 4104-4110. |
| 29 | CHEN Ming, LI Xiaoming, YANG Qi, et al. Total concentrations and speciation of heavy metals in municipal sludge from Changsha, Zhuzhou and Xiangtan in middle-south region of China[J]. Journal of Hazardous Materials, 2008, 160(2/3): 324-329. |
| 30 | 任福民, 周玉松, 牛牧晨, 等. 污泥中的重金属特性分析和生态风险评价[J]. 北京交通大学学报, 2007, 31(1): 102-105. |
| REN Fumin, ZHOU Yusong, NIU Muchen, et al. Characteristics analysis and environmental assessment on heavy metals in the sludge of sewage[J]. Journal of Beijing Jiaotong University, 2007, 31(1): 102-105. |
| [1] | LI Shilin, HU Jingze, WANG Yilin, WANG Qingji, SHAO Lei. Research progress in separation and extraction of high value components by electrodialysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 420-429. |
| [2] | SHI Tianxi, SHI Yonghui, WU Xinying, ZHANG Yihao, QIN Zhe, ZHAO Chunxia, LU Da. Effects of Fe2+ on the performance of Anammox EGSB reactor [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 5003-5010. |
| [3] | YANG Ziyu, ZHU Ling, WANG Wenlong, YU Chaofan, SANG Yimin. Research and application progress of smoldering combustion technology for oily sludge [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3760-3769. |
| [4] | ZHENG Xin, JIA Li, WANG Yanlin, ZHANG Jingchao, CHEN Shihu, QIAO Xiaolei, FAN Baoguo. Effect of sewage sludge mixed with coal slime on heavy metal retention characteristics [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3233-3241. |
| [5] | XIU Haoran, WANG Yungang, BAI Yanyuan, ZOU Li, LIU Yang. Combustion characteristics and ash melting behavior of Zhundong coal/municipal sludge blended combustion [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3242-3252. |
| [6] | ZHAN Yong, WANG Hui, WEI Tingting, ZHU Xingyu, WANG Xiankai, CHEN Sisi, DONG Bin. In situ reduction effect of Mn2+ enhanced ozone conditioning on sludge in biological treatment process [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3253-3260. |
| [7] | WANG Zhiwei, GUO Shuaihua, WU Mengge, CHEN Yan, ZHAO Junting, LI Hui, LEI Tingzhou. Recent advances on catalytic co-pyrolysis of biomass and plastic [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2655-2665. |
| [8] | CHANG Zhankun, ZHANG Chi, SU Bingqin, ZHANG Congzheng, WANG Jian, QUAN Xiaohui. Effect of H2S gaseous substrate on sludge bioleaching treatment efficiency [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2733-2743. |
| [9] | YANG Ziqiang, LI Fenghai, GUO Weijie, MA Mingjie, ZHAO Wei. Review on phosphorus migration and transformation during municipal sewage sludge heat treatment [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2081-2090. |
| [10] | ZHAO Jiaqi, HUANG Yaji, LI Zhiyuan, DING Xueyu, QI Shuaijie, ZHANG Yuyao, LIU Jun, GAO Jiawei. Characteristics of three-phase products from co-pyrolysis of sewage sludge and PVC [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2122-2129. |
| [11] | WANG Yu, YU Guangwei, LIN Jiajia, LI Changjiang, JIANG Ruqing, XING Zhenjiao, YU Cheng. Preparation of building ceramsite from food waste digestate residues, incineration fly ash and sludge biochar [J]. Chemical Industry and Engineering Progress, 2023, 42(2): 1039-1050. |
| [12] | ZHANG Han, ZHANG Xiaojing, MA Bingbing, NAI Can, LIU Shuoshuo, MA Yongpeng, SONG Yali. Feasibility of starting anammox process with municipal waste sludge as seed sludge [J]. Chemical Industry and Engineering Progress, 2023, 42(2): 1080-1088. |
| [13] | GUO Yuchen, LIU Qinglin, JIANG Jinyang, ZONG Yongzhong, WANG Jinwei, LI Zhen, LYU Shuxiang. Research progress on recycling methods of chromium-containing sludge [J]. Chemical Industry and Engineering Progress, 2023, 42(2): 575-584. |
| [14] | SUN Qianqian, LIU Zhen, LI Rui, ZHANG Xi, YANG Mingde, WU Yulong. Low temperature hydrothermal coupling of ferrous ion activated persulfate to improve the dewatering performance of waste activated sludge [J]. Chemical Industry and Engineering Progress, 2023, 42(2): 595-602. |
| [15] | DUAN Yihang, GAO Ningbo, QUAN Cui. Effect of hydrothermal treatment on pyrolysis characteristics and kinetics of oily sludge [J]. Chemical Industry and Engineering Progress, 2023, 42(2): 603-613. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||