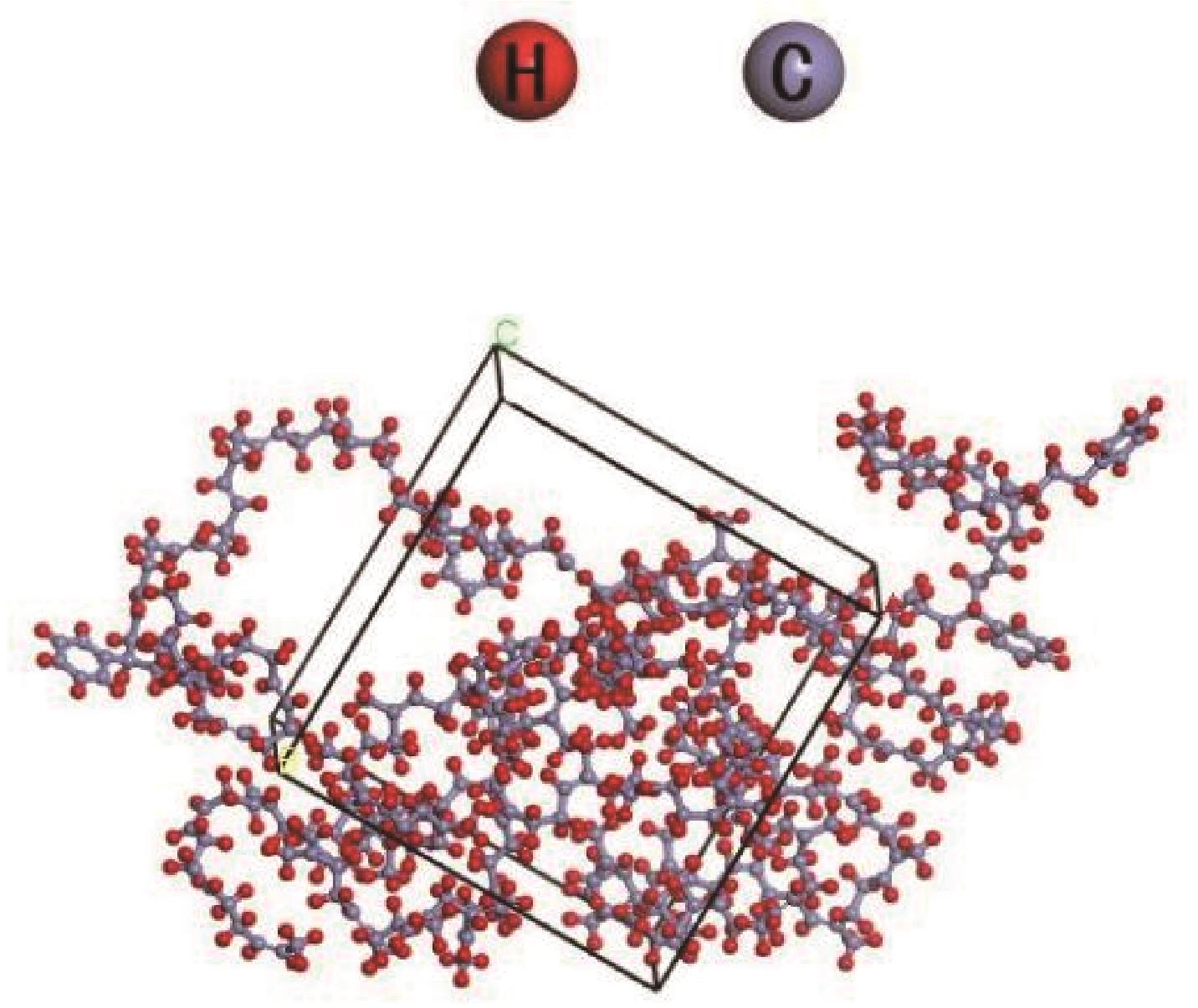
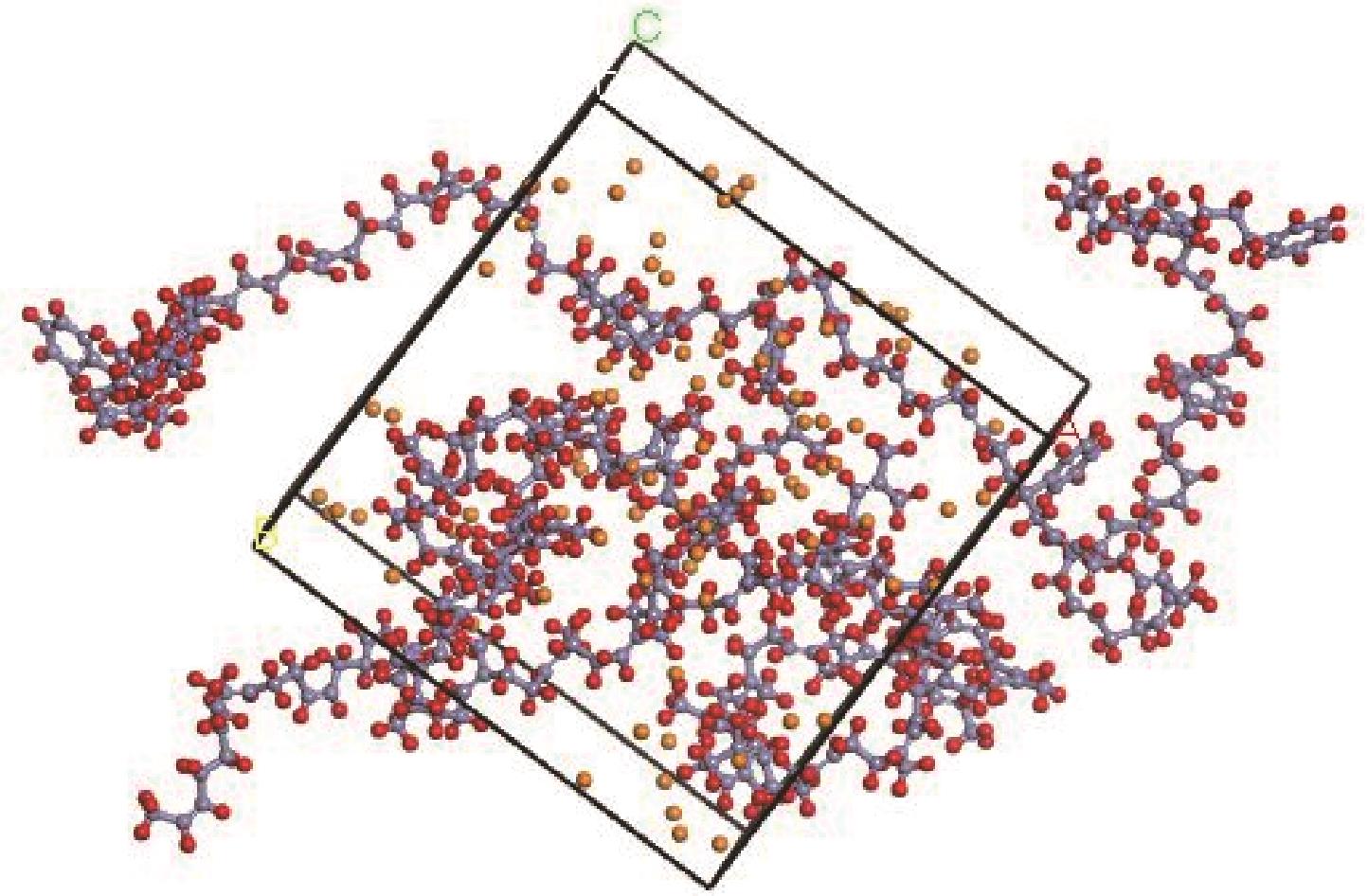
Chemical Industry and Engineering Progress ›› 2022, Vol. 41 ›› Issue (6): 3010-3021.DOI: 10.16085/j.issn.1000-6613.2021-1339
• Industrial catalysis • Previous Articles Next Articles
Mechanism of hydrogen production by catalytic pyrolysis of tire rubber based on molecular dynamics simulation
TAO Li( ), YANG Qirong(
), YANG Qirong( ), LI Zhaoying(
), LI Zhaoying( ), QI Hao, WANG Liwei, MA Xinru
), QI Hao, WANG Liwei, MA Xinru
- College of Mechanical and Electrical Engineering, Qingdao University, Qingdao 266071, Shandong, China
-
Received:2021-06-25Revised:2021-09-01Online:2022-06-21Published:2022-06-10 -
Contact:YANG Qirong,LI Zhaoying
基于分子动力学模拟的轮胎橡胶催化热解制氢机理
陶礼( ), 杨启容(
), 杨启容( ), 李昭莹(
), 李昭莹( ), 亓昊, 王力伟, 马欣如
), 亓昊, 王力伟, 马欣如
- 青岛大学机电工程学院,山东 青岛 266071
-
通讯作者:杨启容,李昭莹 -
作者简介:陶礼(1997—),女,硕士研究生,研究方向为轮胎橡胶热解制氢。E-mail:2256713985@qq.com 。 -
基金资助:青岛大学青年卓越人才科研启动经费(QDPYHT-5-065)
CLC Number:
Cite this article
TAO Li, YANG Qirong, LI Zhaoying, QI Hao, WANG Liwei, MA Xinru. Mechanism of hydrogen production by catalytic pyrolysis of tire rubber based on molecular dynamics simulation[J]. Chemical Industry and Engineering Progress, 2022, 41(6): 3010-3021.
陶礼, 杨启容, 李昭莹, 亓昊, 王力伟, 马欣如. 基于分子动力学模拟的轮胎橡胶催化热解制氢机理[J]. 化工进展, 2022, 41(6): 3010-3021.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2021-1339
| 橡胶 | 主要成分分子式 | 单链模型 | 长链模型 |
|---|---|---|---|
天然 橡胶 |
顺-1,4-聚异戊二烯 |
单链 |
长链 |
丁苯 橡胶 |
苯乙烯 1,3-丁二烯 |
单链 |
长链 |
顺丁 橡胶 |
顺-1,4-聚丁二烯 |
单链 |
长链 |
| 橡胶 | 主要成分分子式 | 单链模型 | 长链模型 |
|---|---|---|---|
天然 橡胶 |
顺-1,4-聚异戊二烯 |
单链 |
长链 |
丁苯 橡胶 |
苯乙烯 1,3-丁二烯 |
单链 |
长链 |
顺丁 橡胶 |
顺-1,4-聚丁二烯 |
单链 |
长链 |
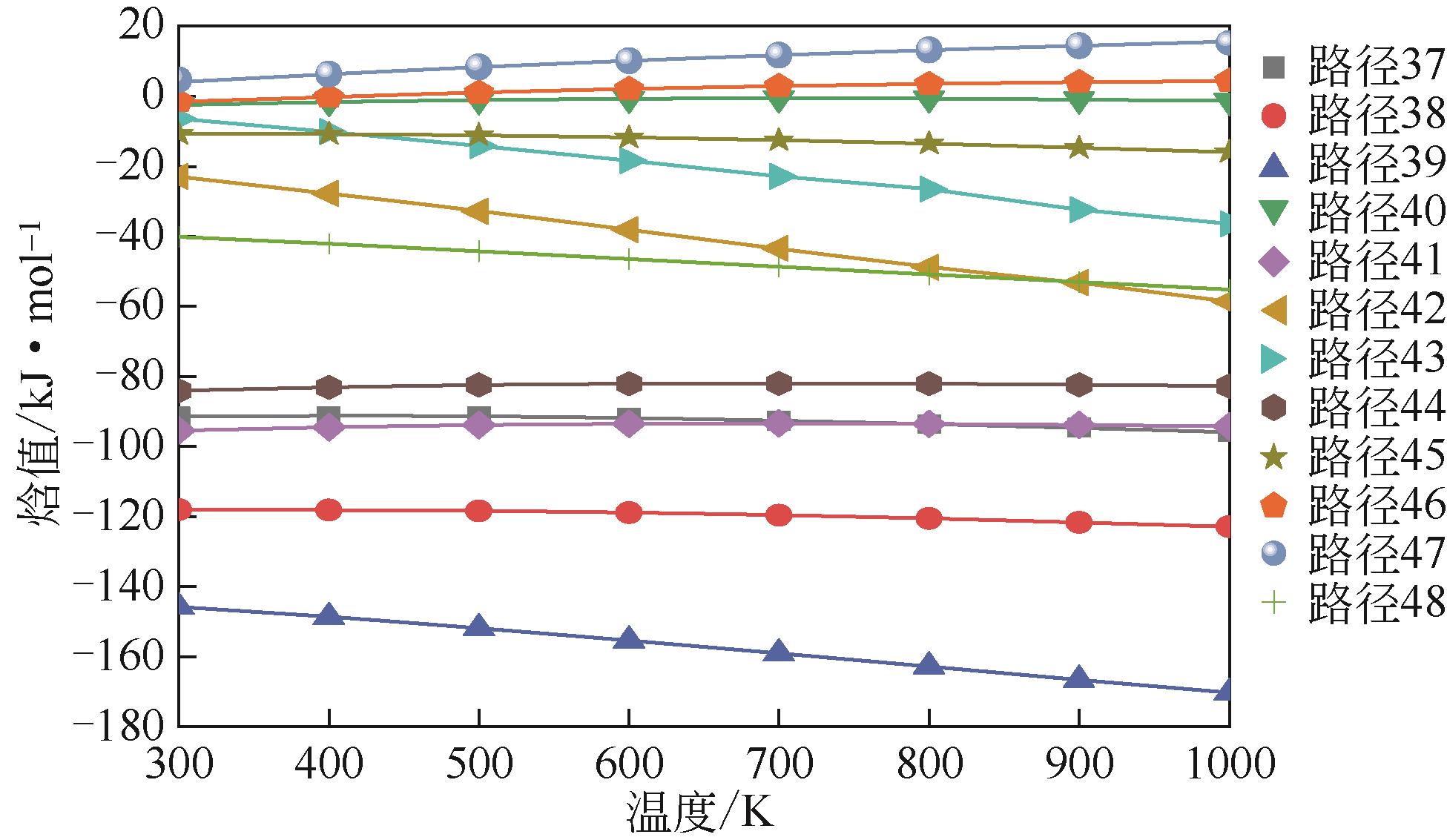
催化前路径→ 催化后路径 | 反应式 | 催化前能垒E /kJ·mol-1 | 催化后能垒E /kJ·mol-1 | 差值(前-后)?E /kJ·mol-1 |
|---|---|---|---|---|
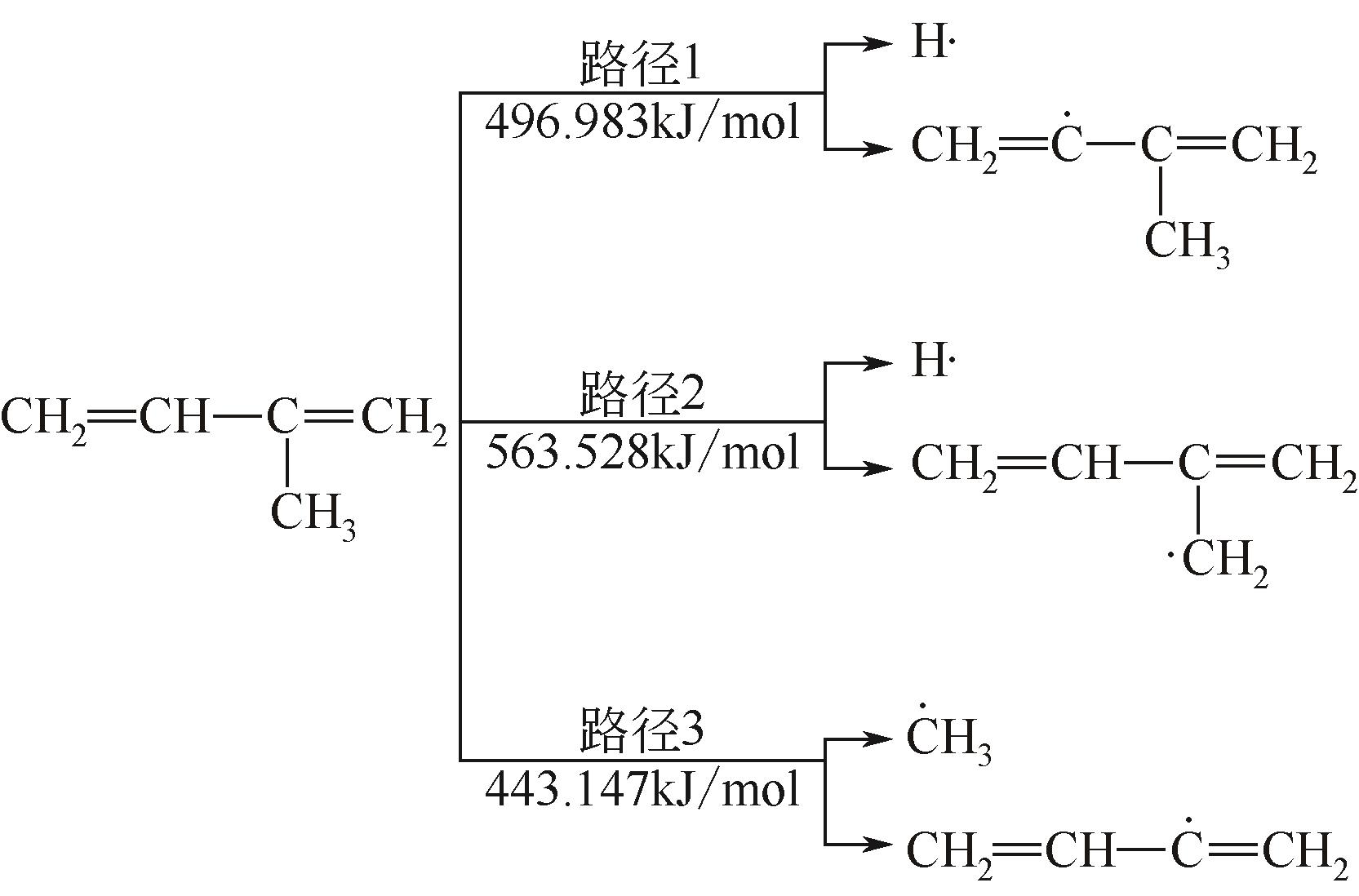
| 1→25 | CH2CHC(CH3)CH2 | 496.983 | 489.913 | 7.070 |
| 2→26 | CH2CHC(CH3)CH2 | 563.528 | 471.695 | 91.833 |
| 3→27 | CH2CHC(CH3)CH2 | 443.147 | 426.989 | 16.158 |
| 4→28 | CH2CHCHCH2 | 557.935 | 540.672 | 17.263 |
| 5→29 | CH2CHCHCH2 | 472.465 | 422.380 | 50.085 |
| 6→30 | CH2CHCHCH2 | 568.622 | 551.242 | 17.380 |
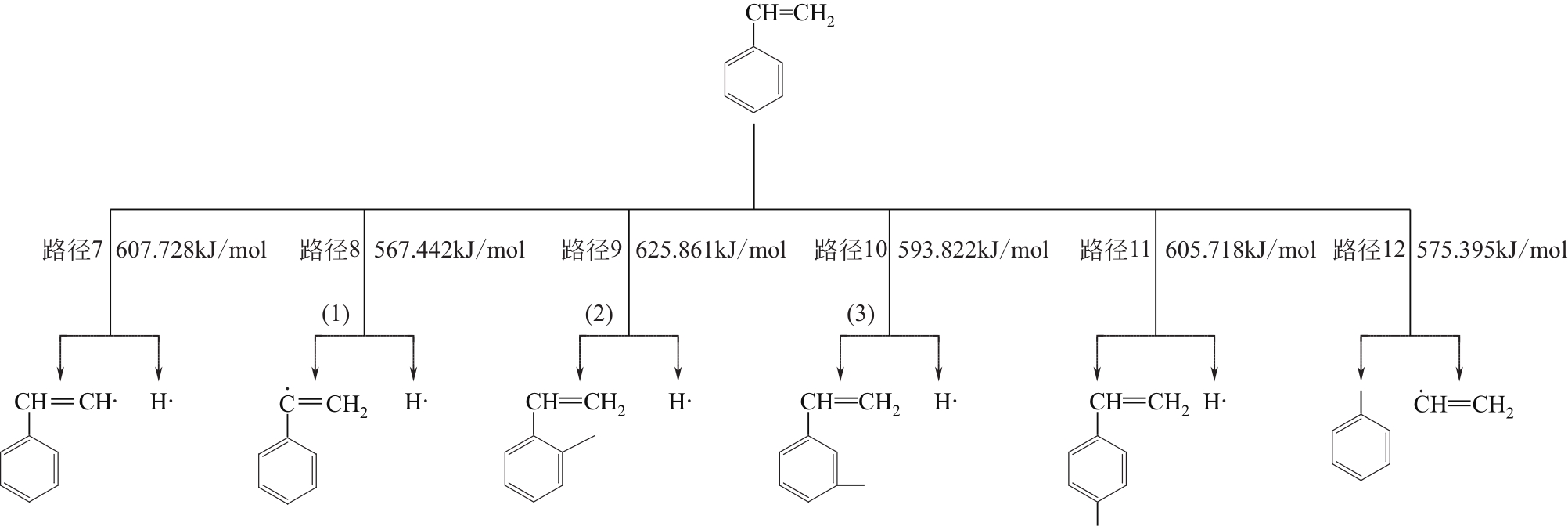
| 7→31 | C6H5CHCH2 | 607.728 | 588.120 | 19.608 |
| 8→32 | C6H5CHCH2 | 567.442 | 548.374 | 19.068 |
| 9→33 | (1) C6H5CHCH2 | 625.861 | 571.079 | 54.782 |
| 10→34 | (2) C6H5CHCH2 | 593.822 | 589.774 | 4.048 |
| 11→35 | (3) C6H5CHCH2 | 605.718 | 597.183 | 8.535 |
| 12→36 | C6H5CHCH2 | 575.395 | 555.164 | 20.231 |
催化前路径→ 催化后路径 | 反应式 | 催化前能垒E /kJ·mol-1 | 催化后能垒E /kJ·mol-1 | 差值(前-后)?E /kJ·mol-1 |
|---|---|---|---|---|
| 1→25 | CH2CHC(CH3)CH2 | 496.983 | 489.913 | 7.070 |
| 2→26 | CH2CHC(CH3)CH2 | 563.528 | 471.695 | 91.833 |
| 3→27 | CH2CHC(CH3)CH2 | 443.147 | 426.989 | 16.158 |
| 4→28 | CH2CHCHCH2 | 557.935 | 540.672 | 17.263 |
| 5→29 | CH2CHCHCH2 | 472.465 | 422.380 | 50.085 |
| 6→30 | CH2CHCHCH2 | 568.622 | 551.242 | 17.380 |
| 7→31 | C6H5CHCH2 | 607.728 | 588.120 | 19.608 |
| 8→32 | C6H5CHCH2 | 567.442 | 548.374 | 19.068 |
| 9→33 | (1) C6H5CHCH2 | 625.861 | 571.079 | 54.782 |
| 10→34 | (2) C6H5CHCH2 | 593.822 | 589.774 | 4.048 |
| 11→35 | (3) C6H5CHCH2 | 605.718 | 597.183 | 8.535 |
| 12→36 | C6H5CHCH2 | 575.395 | 555.164 | 20.231 |
催化前路径→ 催化后路径 | 反应式 | 催化前能垒E /kJ·mol-1 | 催化后能垒E /kJ·mol-1 | 差值(前-后)?E /kJ·mol-1 |
|---|---|---|---|---|
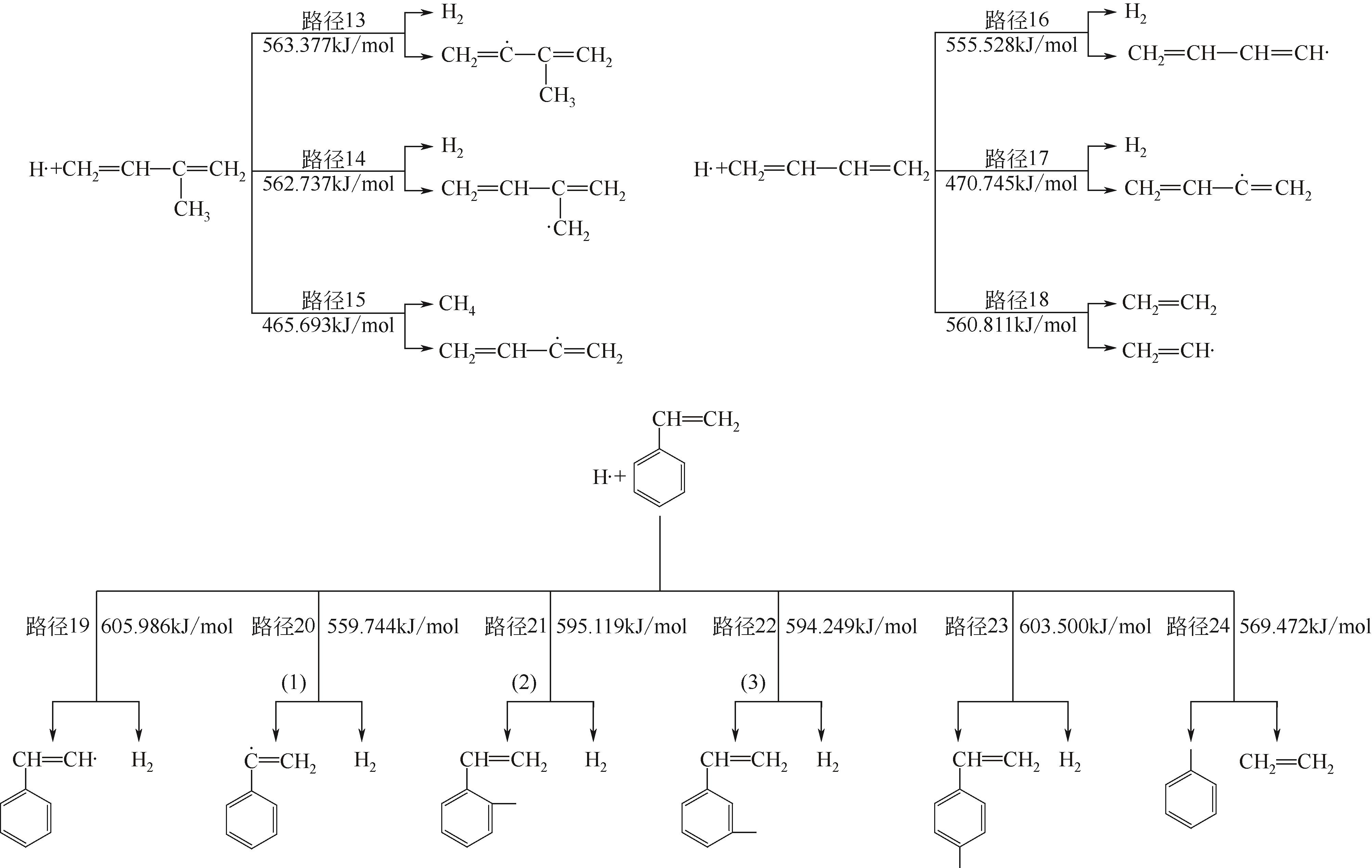
| 13→37 | CH2CHC(CH3)CH2+H· | 563.377 | 558.412 | 4.965 |
| 14→38 | CH2CHC(CH3)CH2+H· | 562.737 | 466.852 | 95.885 |
| 15→39 | CH2CHC(CH3)CH2+H· | 465.693 | 534.293 | -68.600 |
| 16→40 | CH2CHCHCH2+H· | 555.528 | 548.718 | 6.810 |
| 17→41 | CH2CHCHCH2+H· | 470.745 | 424.791 | 45.954 |
| 18→42 | CH2CHCHCH2+H· | 560.811 | 552.665 | 8.146 |
| 19→43 | C6H5CHCH2+H· | 605.986 | 596.668 | 9.318 |
| 20→44 | C6H5CHCH2+H· | 559.744 | 550.074 | 9.670 |
| 21→45 | (1) C6H5CHCH2+H· | 595.119 | 574.616 | 20.503 |
| 22→46 | (2) C6H5CHCH2+H· | 594.249 | 585.621 | 8.628 |
| 23→47 | (3) C6H5CHCH2+H· | 603.500 | 598.502 | 4.998 |
| 24→48 | C6H5CHCH2+H· | 569.472 | 555.126 | 14.346 |
催化前路径→ 催化后路径 | 反应式 | 催化前能垒E /kJ·mol-1 | 催化后能垒E /kJ·mol-1 | 差值(前-后)?E /kJ·mol-1 |
|---|---|---|---|---|
| 13→37 | CH2CHC(CH3)CH2+H· | 563.377 | 558.412 | 4.965 |
| 14→38 | CH2CHC(CH3)CH2+H· | 562.737 | 466.852 | 95.885 |
| 15→39 | CH2CHC(CH3)CH2+H· | 465.693 | 534.293 | -68.600 |
| 16→40 | CH2CHCHCH2+H· | 555.528 | 548.718 | 6.810 |
| 17→41 | CH2CHCHCH2+H· | 470.745 | 424.791 | 45.954 |
| 18→42 | CH2CHCHCH2+H· | 560.811 | 552.665 | 8.146 |
| 19→43 | C6H5CHCH2+H· | 605.986 | 596.668 | 9.318 |
| 20→44 | C6H5CHCH2+H· | 559.744 | 550.074 | 9.670 |
| 21→45 | (1) C6H5CHCH2+H· | 595.119 | 574.616 | 20.503 |
| 22→46 | (2) C6H5CHCH2+H· | 594.249 | 585.621 | 8.628 |
| 23→47 | (3) C6H5CHCH2+H· | 603.500 | 598.502 | 4.998 |
| 24→48 | C6H5CHCH2+H· | 569.472 | 555.126 | 14.346 |
| 路径 | ZSM-5催化前 能垒E/kJ·mol-1 | ZSM-5催化后 能垒E/kJ·mol-1 | ZSM-5差值?E(前-后) /kJ·mol-1 | Ni/ZSM-5催化前 能垒E/kJ·mol-1 | Ni/ZSM-5催化后 能垒E/kJ·mol-1 | Ni/ZSM-5差值?E(前-后) /kJ·mol-1 |
|---|---|---|---|---|---|---|
| 1 | 496.983 | 538.039 | -41.056 | 496.983 | 514.535 | -17.552 |
| 2 | 563.528 | 563.264 | 0.264 | 563.528 | 557.316 | 6.212 |
| 3 | 443.147 | 516.820 | -73.673 | 443.147 | 501.211 | -58.064 |
| 4 | 557.935 | 560.108 | -2.173 | 557.935 | 554.662 | 3.273 |
| 5 | 472.465 | 483.454 | -10.988 | 472.465 | 468.531 | 3.934 |
| 6 | 568.622 | 547.395 | 21.227 | 568.622 | 543.058 | 25.564 |
| 7 | 607.728 | 595.726 | 12.002 | 607.728 | 593.713 | 14.015 |
| 8 | 567.442 | 568.036 | -0.594 | 567.442 | 560.853 | 6.589 |
| 9 | 625.861 | 585.312 | 40.549 | 625.861 | 577.266 | 48.595 |
| 10 | 593.822 | 586.593 | 7.229 | 593.822 | 586.341 | 7.481 |
| 11 | 605.718 | 595.927 | 9.791 | 605.718 | 595.153 | 10.565 |
| 12 | 575.395 | 573.566 | 1.829 | 575.395 | 567.689 | 7.706 |
| 路径 | ZSM-5催化前 能垒E/kJ·mol-1 | ZSM-5催化后 能垒E/kJ·mol-1 | ZSM-5差值?E(前-后) /kJ·mol-1 | Ni/ZSM-5催化前 能垒E/kJ·mol-1 | Ni/ZSM-5催化后 能垒E/kJ·mol-1 | Ni/ZSM-5差值?E(前-后) /kJ·mol-1 |
|---|---|---|---|---|---|---|
| 1 | 496.983 | 538.039 | -41.056 | 496.983 | 514.535 | -17.552 |
| 2 | 563.528 | 563.264 | 0.264 | 563.528 | 557.316 | 6.212 |
| 3 | 443.147 | 516.820 | -73.673 | 443.147 | 501.211 | -58.064 |
| 4 | 557.935 | 560.108 | -2.173 | 557.935 | 554.662 | 3.273 |
| 5 | 472.465 | 483.454 | -10.988 | 472.465 | 468.531 | 3.934 |
| 6 | 568.622 | 547.395 | 21.227 | 568.622 | 543.058 | 25.564 |
| 7 | 607.728 | 595.726 | 12.002 | 607.728 | 593.713 | 14.015 |
| 8 | 567.442 | 568.036 | -0.594 | 567.442 | 560.853 | 6.589 |
| 9 | 625.861 | 585.312 | 40.549 | 625.861 | 577.266 | 48.595 |
| 10 | 593.822 | 586.593 | 7.229 | 593.822 | 586.341 | 7.481 |
| 11 | 605.718 | 595.927 | 9.791 | 605.718 | 595.153 | 10.565 |
| 12 | 575.395 | 573.566 | 1.829 | 575.395 | 567.689 | 7.706 |
| 路径 | ZSM-5催化前 能垒E/kJ·mol-1 | ZSM-5催化后 能垒E/kJ·mol-1 | ZSM-5差值?E(前-后) /kJ·mol-1 | Ni/ZSM-5催化前 能垒E /kJ·mol-1 | Ni/ZSM-5催化后 能垒E /kJ·mol-1 | Ni/ZSM-5差值?E(前-后) /kJ·mol-1 |
|---|---|---|---|---|---|---|
| 13 | 563.377 | 551.610 | 11.767 | 563.377 | 512.982 | 50.395 |
| 14 | 562.737 | 485.404 | 77.333 | 562.737 | 563.570 | -0.833 |
| 15 | 465.693 | 429.513 | 36.180 | 465.693 | 431.711 | 33.982 |
| 16 | 555.528 | 561.711 | -6.183 | 555.528 | 553.184 | 2.344 |
| 17 | 470.745 | 458.099 | 12.646 | 470.745 | 441.238 | 29.507 |
| 18 | 560.811 | 537.675 | 23.136 | 560.811 | 546.240 | 14.571 |
| 19 | 605.986 | 599.984 | 6.002 | 605.986 | 606.070 | -0.084 |
| 20 | 559.744 | 579.891 | -20.147 | 559.744 | 550.765 | 8.979 |
| 21 | 595.119 | 598.029 | -2.910 | 595.119 | 590.540 | 4.579 |
| 22 | 594.249 | 588.556 | 5.693 | 594.249 | 586.053 | 8.196 |
| 23 | 603.500 | 602.583 | 0.917 | 603.500 | 598.740 | 4.760 |
| 24 | 569.472 | 585.647 | -16.175 | 569.472 | 567.295 | 2.177 |
| 路径 | ZSM-5催化前 能垒E/kJ·mol-1 | ZSM-5催化后 能垒E/kJ·mol-1 | ZSM-5差值?E(前-后) /kJ·mol-1 | Ni/ZSM-5催化前 能垒E /kJ·mol-1 | Ni/ZSM-5催化后 能垒E /kJ·mol-1 | Ni/ZSM-5差值?E(前-后) /kJ·mol-1 |
|---|---|---|---|---|---|---|
| 13 | 563.377 | 551.610 | 11.767 | 563.377 | 512.982 | 50.395 |
| 14 | 562.737 | 485.404 | 77.333 | 562.737 | 563.570 | -0.833 |
| 15 | 465.693 | 429.513 | 36.180 | 465.693 | 431.711 | 33.982 |
| 16 | 555.528 | 561.711 | -6.183 | 555.528 | 553.184 | 2.344 |
| 17 | 470.745 | 458.099 | 12.646 | 470.745 | 441.238 | 29.507 |
| 18 | 560.811 | 537.675 | 23.136 | 560.811 | 546.240 | 14.571 |
| 19 | 605.986 | 599.984 | 6.002 | 605.986 | 606.070 | -0.084 |
| 20 | 559.744 | 579.891 | -20.147 | 559.744 | 550.765 | 8.979 |
| 21 | 595.119 | 598.029 | -2.910 | 595.119 | 590.540 | 4.579 |
| 22 | 594.249 | 588.556 | 5.693 | 594.249 | 586.053 | 8.196 |
| 23 | 603.500 | 602.583 | 0.917 | 603.500 | 598.740 | 4.760 |
| 24 | 569.472 | 585.647 | -16.175 | 569.472 | 567.295 | 2.177 |
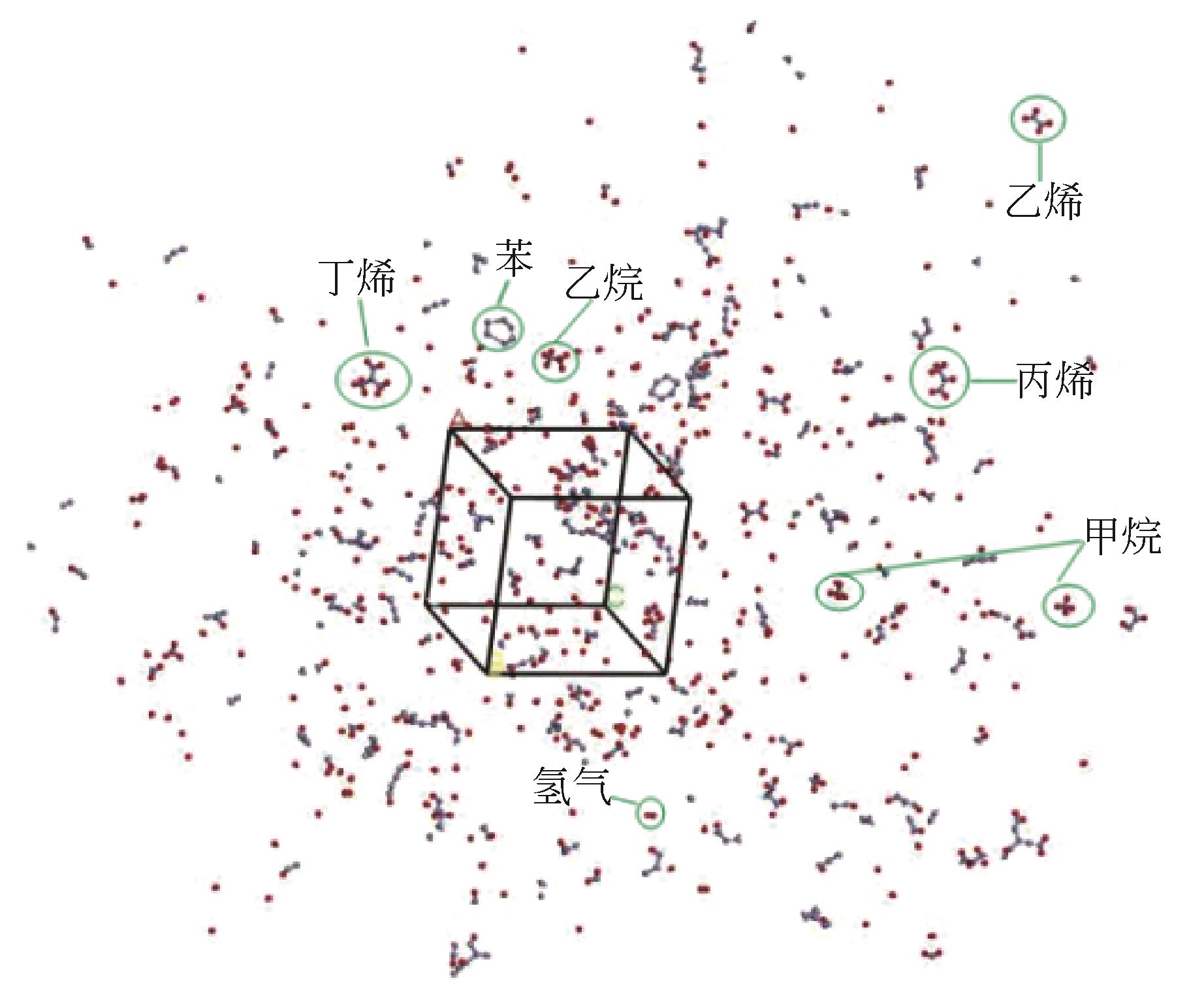
不同比例 (橡胶∶催化剂) | 1100K时热解情况 | 1500K时热解情况 |
|---|---|---|
| 1∶0 |  |  |
| 5∶1 |  |  |
| 3∶1 |  |  |
| 1∶1 |  |  |
不同比例 (橡胶∶催化剂) | 1100K时热解情况 | 1500K时热解情况 |
|---|---|---|
| 1∶0 |  |  |
| 5∶1 |  |  |
| 3∶1 |  |  |
| 1∶1 |  |  |
| 1 | 张立民, 李志学. 我国新能源经济发展现状分析[J]. 现代商业, 2021(7): 25-27. |
| ZHANG Limin, LI Zhixue. Analysis of the present situation of the development of new energy economy in China[J]. Modern Business, 2021 (7): 25-27. | |
| 2 | LI G, ZHANG K, YANG B, et al. Life cycle analysis of a coal to hydrogen process based on ash agglomerating fluidized bed gasification[J]. Energy, 2019, 174: 638-646. |
| 3 | TOUILI S, ALAMI MERROUNI A, HASSOUANI Y EL, et al. Analysis of the yield and production cost of large-scale electrolytic hydrogen from different solar technologies and under several Moroccan climate zones[J]. International Journal of Hydrogen Energy, 2020, 45(51): 26785-26799. |
| 4 | LI W C, REN X S, DING S M, et al. A multi-criterion decision making for sustainability assessment of hydrogen production technologies based on objective grey relational analysis[J]. International Journal of Hydrogen Energy, 2020, 45(59): 34385-34395. |
| 5 | 郭博文, 罗聃, 周红军. 可再生能源电解制氢技术及催化剂的研究进展[J]. 化工进展, 2021, 40(6): 2933-2951. |
| GUO Bowen, LUO Dan, ZHOU Hongjun. Recent advances in renewable energy electrolysis hydrogen production technology and related electrocatalysts[J]. Chemical Industry and Engineering Progress, 2021, 40(6): 2933-2951. | |
| 6 | 国家统计局. 2019年7—12月我国合成橡胶、轮胎和汽车产量[J]. 橡胶科技, 2020, 18(3): 178. |
| National Bureau of Statistics. Output of synthetic rubber, tires and cars in China from July to December 2019[J]. Rubber Science and Technology, 2020, 18(3): 178. | |
| 7 | CHOI G G, OH S J, KIM J S, et al. Non-catalytic pyrolysis of scrap tires using a newly developed two-stage pyrolyzer for the production of a pyrolysis oil with a low sulfur content[J]. Applied Energy, 2016, 170: 140-147. |
| 8 | 狄伟强. 废轮胎热解炭特性及其对废轮胎催化性能研究[D]. 大连: 大连理工大学, 2019. |
| DI Weiqiang. Study on the characteristics of waste tire pyrolysis char and its catalytic pyrolysis effect on waste tires[D]. Dalian: Dalian University of Technology, 2019. | |
| 9 | LI W P, WEI M M, LIU Y Q, et al. Catalysts evaluation for production of hydrogen gas and carbon nanotubes from the pyrolysis-catalysis of waste tyres[J]. International Journal of Hydrogen Energy, 2019, 44(36): 19563-19572. |
| 10 | Williams P T, Brindle A J.Catalytic pyrolysis of tyres: influence of catalyst temperature[J]. Fuel, 2002, 81(18): 2425-2434. |
| 11 | YU J, LIU S, CARDOSO A, et al. Catalytic pyrolysis of rubbers and vulcanized rubbers using modified zeolites and mesoporous catalysts with Zn and Cu[J]. Energy, 2019, 188: 116117. |
| 12 | 李秉繁, 刘刚, 陈雷. 基于分子动力学模拟的CH4溶解对原油分子间作用的影响机制研究[J]. 化工学报, 2021, 72(3): 1253-1263. |
| LI Bingfan, LIU Gang, CHEN Lei. Study on the influence mechanism of CH4 dissolution on the intermolecular interaction between crude oil molecules based on molecular dynamics simulation[J]. CIESC Journal, 2021, 72(3): 1253-1263. | |
| 13 | LIU Y L, DING J X, HAN K L. Molecular dynamics simulation of the high-temperature pyrolysis of methylcyclohexane[J]. Fuel, 2018, 217: 185-192. |
| 14 | 魏鑫. 天然橡胶热解气相产物生成机理研究[D]. 青岛: 青岛大学, 2019. |
| WEI Xin. Study on the formation mechanism of gas phase products of natural rubber pyrolysis[D]. Qingdao: Qingdao University, 2019. | |
| 15 | YANG Q R, YU S P, ZHONG H W, et al. Gas products generation mechanism during co-pyrolysis of styrene-butadiene rubber and natural rubber[J]. Journal of Hazardous Materials, 2021, 401: 123302. |
| 16 | 于双鹏, 杨启容, 陶礼, 等. 基于分子动力学模拟的轮胎橡胶气相热解产物反应机理[J]. 化工进展, 2021, 40(6): 3119-3131. |
| YU Shuangpeng, YANG Qirong, TAO Li, et al. Gas phase pyrolysis products of tire rubber based on molecular dynamics simulation[J]. Chemical Industry and Engineering Progress, 2021, 40(6): 3119-3131. | |
| 17 | 潘旗. 基于分子动力学模拟的变压器油纸绝缘老化特性基础研究[D]. 徐州: 中国矿业大学, 2020. |
| PAN Qi. Basic research on aging characteristics of transformer oil paper insulation based on molecular dynamics simulation[D]. Xuzhou: China University of Mining and Technology, 2020. | |
| 18 | HU S D, SUN W G, FU J, et al. Initiation mechanisms and kinetic analysis of the isothermal decomposition of poly(α-methylstyrene): a ReaxFF molecular dynamics study[J]. RSC Advances, 2018, 8(7): 3423-3432. |
| 19 | KWON H, LELE A, ZHU J Q, et al. ReaxFF-based molecular dynamics study of bio-derived polycyclic alkanes as potential alternative jet fuels[J]. Fuel, 2020, 279: 118548. |
| 20 | 张贻亮. 原子-键电负性均衡方法及其在理论化学中的应用[D]. 长春: 吉林大学, 2002. |
| ZHANG Yiliang. Atom-bond electronegativity equalization method and its application in theoretical chemistry[D]. Changchun: Jilin University, 2002. | |
| 21 | ZHENG M, WANG Z, LI X X, et al. Initial reaction mechanisms of cellulose pyrolysis revealed by ReaxFF molecular dynamics[J]. Fuel, 2016, 177: 130-141. |
| 22 | 杜鸟锋, 甯红波, 李泽荣, 等. 1,3-丁二烯热裂解的动力学计算与模型研究[J]. 物理化学学报, 2016, 32(2): 453-464. |
| DU Niaofeng, NING Hongbo, LI Zerong, et al. Kinetic calculation and modeling study of 1,3-butadiene pyrolysis[J]. Acta Physico-Chimica Sinica, 2016, 32(2): 453-464. | |
| 23 | 徐宗平, 郭庆民. 废轮胎热解回收中的废气综合利用[J]. 中国轮胎资源综合利用, 2018(11): 36-39. |
| XU Zongping, GUO Qingmin. The comprehensive utilization of waste gas in waste tire pyrolysis recycling[J]. China Tire Resources Recycling, 2018(11): 36-39. | |
| 24 | 张会亮, 范晓旭, 刘彦丰, 等. 块状废轮胎固定床热解特性实验研究[J]. 可再生能源, 2015, 33(1): 149-153. |
| ZHANG Huiliang, FAN Xiaoxu, LIU Yanfeng, et al. Experimental study on pyrolysis of blocky tires in a fixed-bed reactor[J]. Renewable Energy Resources, 2015, 33(1): 149-153. | |
| 25 | 张冰, 付琦, 梁畅. 废轮胎橡胶热解技术研究进展[J]. 橡塑技术与装备, 2018, 44(15): 19-23. |
| ZHANG Bing, FU Qi, LIANG Chang. Research progress on waste tire rubber pyrolysis technology[J]. China Rubber/Plastics Technology and Equipment, 2018, 44(15): 19-23. | |
| 26 | ELBABA I F, WILLIAMS P T. Two stage pyrolysis-catalytic gasification of waste tyres: influence of process parameters[J]. Applied Catalysis B: Environmental, 2012, 125: 136-143. |
| 27 | ELBABA I F, WU C F, WILLIAMS P T. Hydrogen production from the pyrolysis-gasification of waste tyres with a nickel/cerium catalyst[J]. International Journal of Hydrogen Energy, 2011, 36(11): 6628-6637. |
| 28 | ZHANG Y S, WU C F, NAHIL M A, et al. Pyrolysis-catalytic reforming/gasification of waste tires for production of carbon nanotubes and hydrogen[J]. Energy & Fuels, 2015, 29(5): 3328-3334. |
| [1] | XIE Luyao, CHEN Songzhe, WANG Laijun, ZHANG Ping. Platinum-based catalysts for SO2 depolarized electrolysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 299-309. |
| [2] | WANG Jiaqing, SONG Guangwei, LI Qiang, GUO Shuaicheng, DAI Qingli. Rubber-concrete interface modification method and performance enhancement path [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 328-343. |
| [3] | GE Quanqian, XU Mai, LIANG Xian, WANG Fengwu. Research progress on the application of MOFs in photoelectrocatalysis [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4692-4705. |
| [4] | ZHANG Yajuan, XU Hui, HU Bei, SHI Xingwei. Preparation of NiCoP/rGO/NF electrocatalyst by eletroless plating for efficient hydrogen evolution reaction [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4275-4282. |
| [5] | WANG Yunqing, YANG Guorui, YAN Wei. Transition metal phosphide modification and its applications in electrochemical hydrogen evolution reaction [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3532-3549. |
| [6] | FU Shurong, WANG Lina, WANG Dongwei, LIU Rui, ZHANG Xiaohui, MA Zhanwei. Oxygen evolution cocatalyst enhancing the photoanode performances for photoelectrochemical water splitting [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2353-2370. |
| [7] | ZHENG Yunwu, PEI Tao, LI Donghua, WANG Jida, LI Jirong, ZHENG Zhifeng. Production of hydrocarbon-rich bio-oil by catalytic biomass pyrolysis over metal oxide improved P/HZSM-5 catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1353-1364. |
| [8] | ZHAO Yi, YANG Zhen, WANG Jia, LI Jingwen, ZHENG Yu. Research progress on molecular dynamics simulation of self-healing behavior of asphalt binder [J]. Chemical Industry and Engineering Progress, 2023, 42(2): 803-813. |
| [9] | XIAO Zhourong, LI Guozhu, WANG Li, ZHANG Xiangwen, GU Jianmin, WANG Desong. Research progress of the catalysts for hydrogen production via liquid hydrocarbon fuels steam reforming [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 97-107. |
| [10] | SUN Xianhang, REN Zhu, ZHANG Guojun, SUN Yuan, FAN Kaifeng, HUANG Weiqiu. Study on the desorption mechanism of toluene in activated carbon under supercritical CO2 [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 631-636. |
| [11] | HU Bing, XU Lijun, HE Shan, SU Xin, WANG Jiwei. Researching progress of hydrogen production by PEM water electrolysis under the goal of carbon peak and carbon neutrality [J]. Chemical Industry and Engineering Progress, 2022, 41(9): 4595-4604. |
| [12] | LI Yufeng, WANG Shaoqing, ZHANG Andong, BI Dongmei, LI Zhihe, GAO Liang, WAN Zhen. Preparation of catalytic porous ceramic balls and catalytic pyrolysis of corn stover [J]. Chemical Industry and Engineering Progress, 2022, 41(7): 3597-3607. |
| [13] | YAN Peng, CHENG Yi. Numerical simulation of membrane reactor of methane steam reforming for distributed hydrogen production [J]. Chemical Industry and Engineering Progress, 2022, 41(7): 3446-3454. |
| [14] | ZHANG Xuan, FAN Xinye, WU Zhenyu, ZHENG Lijun. Hydrogen energy supply chain cost analysis and suggestions [J]. Chemical Industry and Engineering Progress, 2022, 41(5): 2364-2371. |
| [15] | ZHANG Shizhong, CHEN Zhanxiu, LIU Fengrui, PANG Runyu, WANG Qing. Molecular dynamics simulation of liquid boiling on nanostructured surfaces [J]. Chemical Industry and Engineering Progress, 2022, 41(5): 2311-2321. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||