Chemical Industry and Engineering Progress ›› 2022, Vol. 41 ›› Issue (12): 6338-6349.DOI: 10.16085/j.issn.1000-6613.2022-0285
• Industrial catalysis • Previous Articles Next Articles
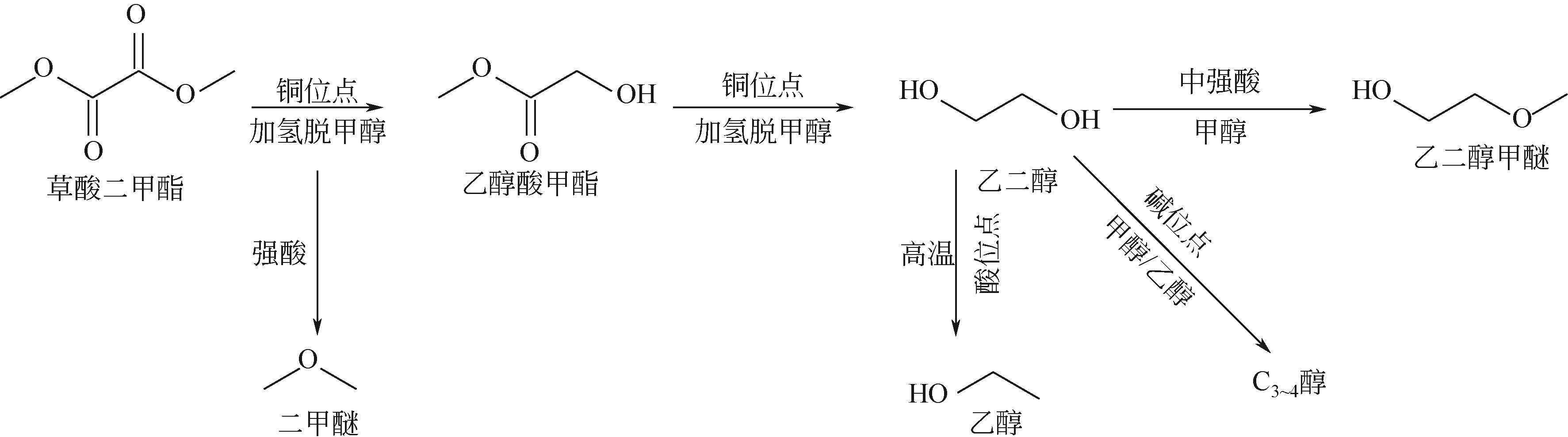
Effect of carrier surface hydroxyl group on performance of Cu/SiO2 catalyst for DMO hydrogenation
YUN Hongfei( ), ZHAO Yu(
), ZHAO Yu( ), LI Guixian
), LI Guixian
- School of Petrochemical Engineering, Lanzhou University of Technology, Lanzhou 730050, Gansu, China
-
Received:2022-02-25Revised:2022-04-24Online:2022-12-29Published:2022-12-20 -
Contact:ZHAO Yu
载体表面羟基种类对DMO加氢用Cu/SiO2催化剂性能的影响
- 兰州理工大学石油化工学院,甘肃 兰州 730050
-
通讯作者:赵鹬 -
作者简介:贠宏飞(1989—),男,博士研究生,研究方向为工业催化。E-mail:yzyeduc@163.com。 -
基金资助:甘肃省重点研发项目(18YF1GA062);国家自然科学基金(21763016);甘肃省高等学校产业支撑引导项目(2020C-06)
CLC Number:
Cite this article
YUN Hongfei, ZHAO Yu, LI Guixian. Effect of carrier surface hydroxyl group on performance of Cu/SiO2 catalyst for DMO hydrogenation[J]. Chemical Industry and Engineering Progress, 2022, 41(12): 6338-6349.
贠宏飞, 赵鹬, 李贵贤. 载体表面羟基种类对DMO加氢用Cu/SiO2催化剂性能的影响[J]. 化工进展, 2022, 41(12): 6338-6349.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-0285
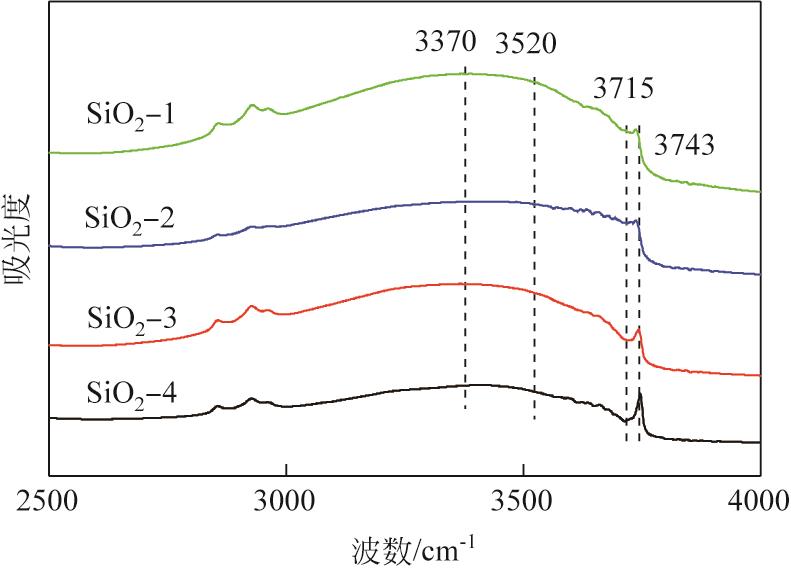
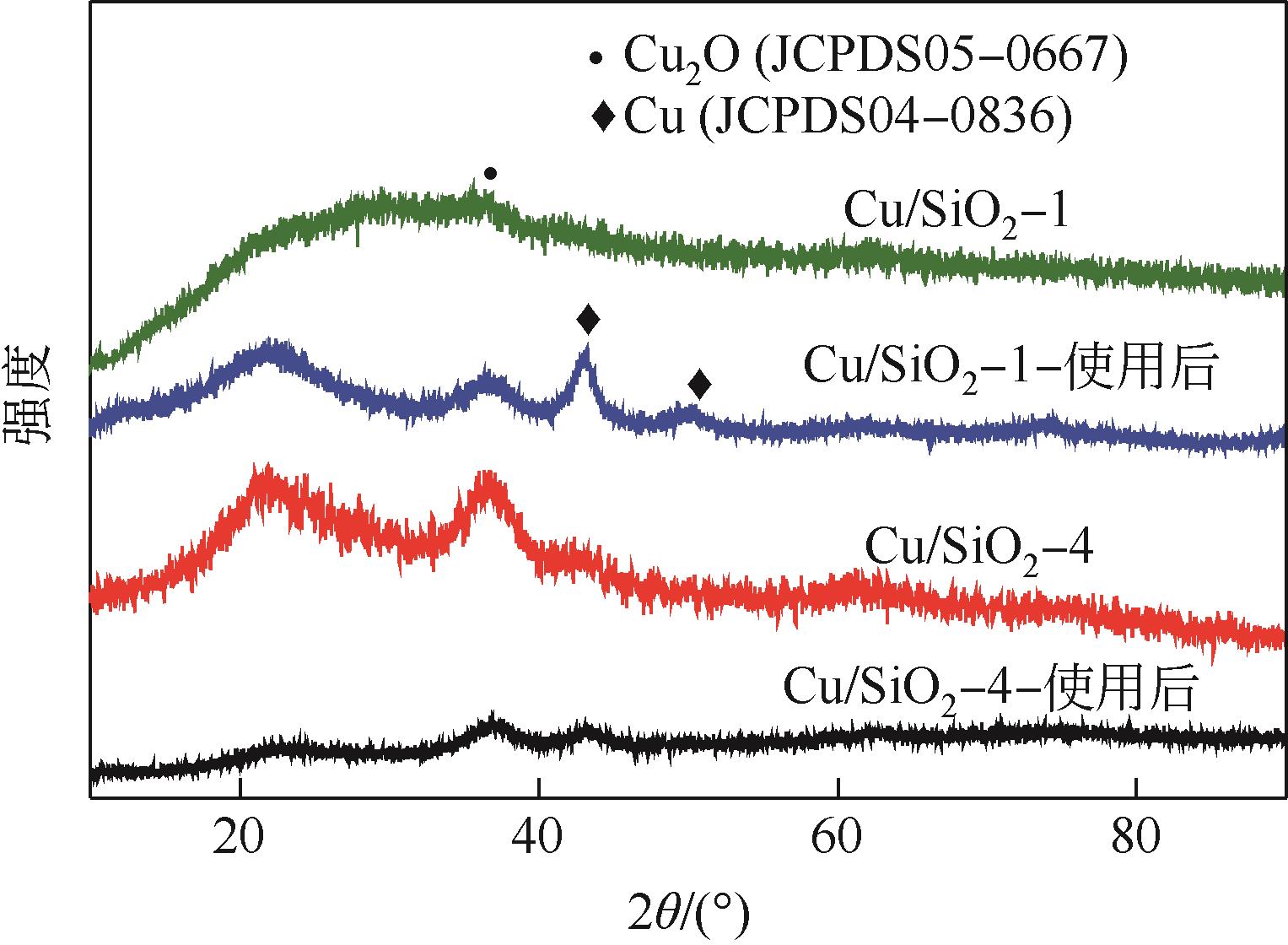
| 样品 | 铜质量分数 /% | 比表面积 /m2·g-1 | 孔体积 /cm3·g-1 | 平均孔径 /nm | I670/I800 |
|---|---|---|---|---|---|
| Cu/SiO2-1 | 23.69 | 484.5 | 1.15 | 7.51 | 0.32 |
| Cu/SiO2-2 | 22.17 | 460.7 | 1.02 | 6.97 | 0.31 |
| Cu/SiO2-3 | 23.07 | 457.2 | 0.98 | 6.78 | 0.28 |
| Cu/SiO2-4 | 22.21 | 440.6 | 1.03 | 9.4 | 0.38 |
| 样品 | 铜质量分数 /% | 比表面积 /m2·g-1 | 孔体积 /cm3·g-1 | 平均孔径 /nm | I670/I800 |
|---|---|---|---|---|---|
| Cu/SiO2-1 | 23.69 | 484.5 | 1.15 | 7.51 | 0.32 |
| Cu/SiO2-2 | 22.17 | 460.7 | 1.02 | 6.97 | 0.31 |
| Cu/SiO2-3 | 23.07 | 457.2 | 0.98 | 6.78 | 0.28 |
| Cu/SiO2-4 | 22.21 | 440.6 | 1.03 | 9.4 | 0.38 |
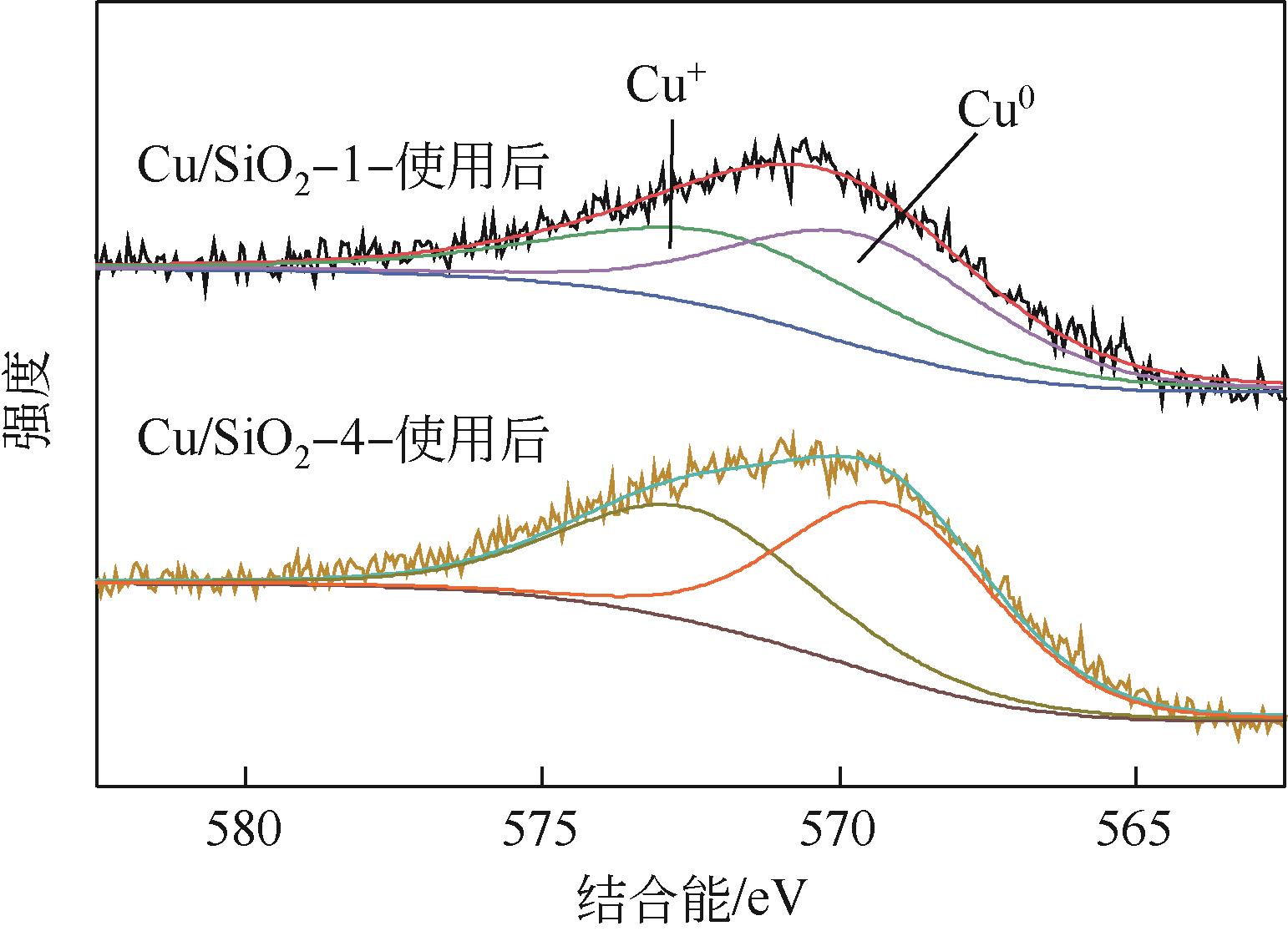
| 样品 | 铜分散度 /% | 铜比表面积 /m2·g-1 | XCu+ /% | XCu+(使用后) /% |
|---|---|---|---|---|
| Cu/SiO2-1 | 58.49 | 16.7 | 52.36 | 48.36 |
| Cu/SiO2-2 | 52.25 | 15.9 | 47.61 | — |
| Cu/SiO2-3 | 52.22 | 15.3 | 44.41 | — |
| Cu/SiO2-4 | 55.01 | 16.5 | 45.86 | 44.42 |
| 样品 | 铜分散度 /% | 铜比表面积 /m2·g-1 | XCu+ /% | XCu+(使用后) /% |
|---|---|---|---|---|
| Cu/SiO2-1 | 58.49 | 16.7 | 52.36 | 48.36 |
| Cu/SiO2-2 | 52.25 | 15.9 | 47.61 | — |
| Cu/SiO2-3 | 52.22 | 15.3 | 44.41 | — |
| Cu/SiO2-4 | 55.01 | 16.5 | 45.86 | 44.42 |
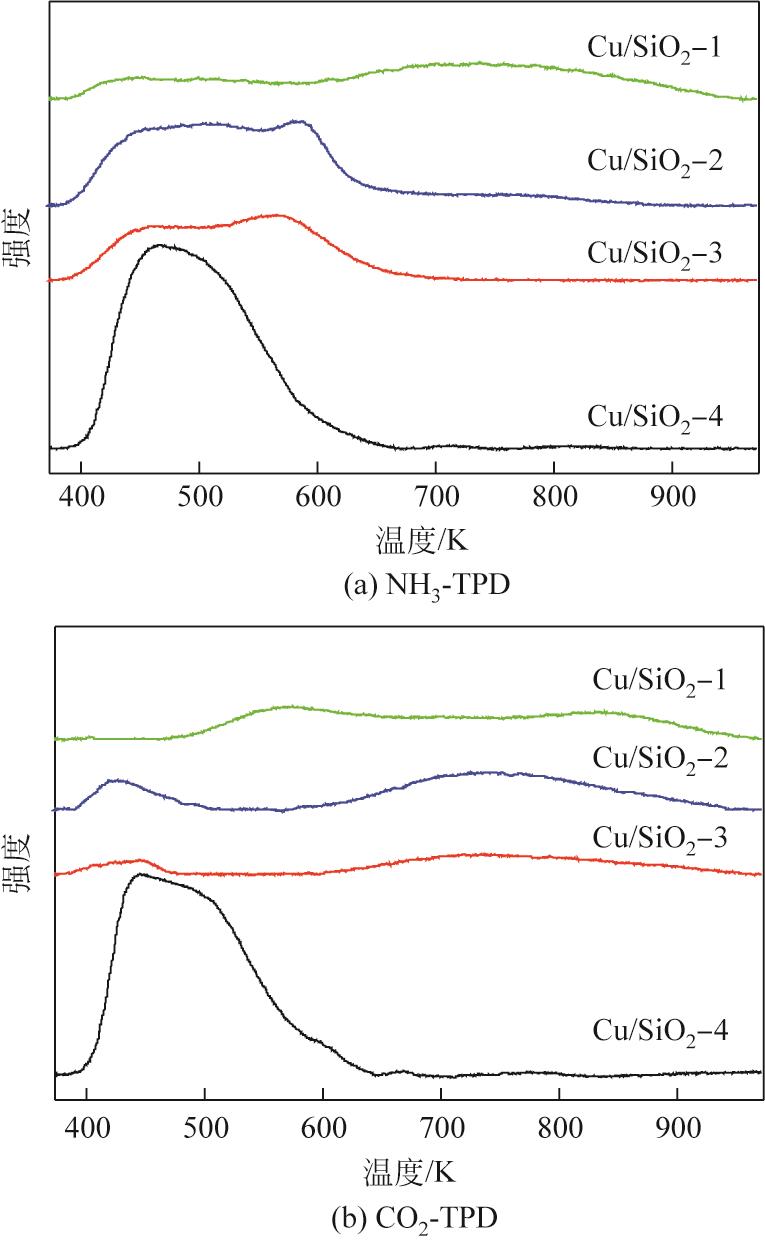
| 样品 | 酸浓度/mmol·g-1 | 碱浓度/mmol·g-1 | ||||
|---|---|---|---|---|---|---|
| 弱酸 | 中强酸 | 强酸 | 弱碱 | 中强碱 | 强碱 | |
| Cu/SiO2-1 | 0.069 | 0.074 | 0.368 | 0.183 | 0.064 | 0.171 |
| Cu/SiO2-2 | 0.474 | 0.243 | 0.066 | 0.077 | 0.325 | 0 |
| Cu/SiO2-3 | 0.188 | 0.305 | 0 | 0.033 | 0.179 | 0 |
| Cu/SiO2-4 | 0.178 | 0 | 0 | 0.174 | 0 | 0 |
| 样品 | 酸浓度/mmol·g-1 | 碱浓度/mmol·g-1 | ||||
|---|---|---|---|---|---|---|
| 弱酸 | 中强酸 | 强酸 | 弱碱 | 中强碱 | 强碱 | |
| Cu/SiO2-1 | 0.069 | 0.074 | 0.368 | 0.183 | 0.064 | 0.171 |
| Cu/SiO2-2 | 0.474 | 0.243 | 0.066 | 0.077 | 0.325 | 0 |
| Cu/SiO2-3 | 0.188 | 0.305 | 0 | 0.033 | 0.179 | 0 |
| Cu/SiO2-4 | 0.178 | 0 | 0 | 0.174 | 0 | 0 |
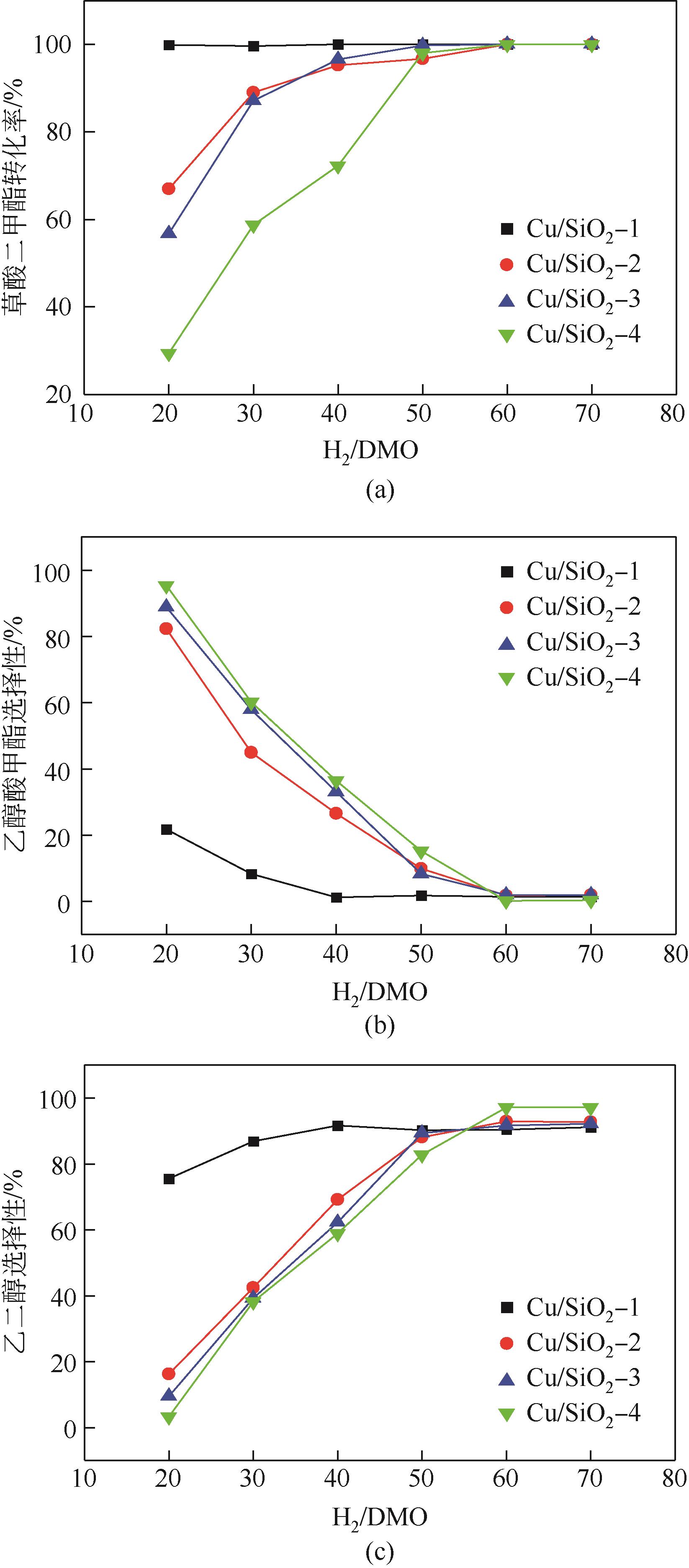
| 样品 | 草酸二甲酯转化率/% | 乙醇含量/% | 乙醇酸甲酯选择性/% | 乙二醇选择性/% | 乙二醇甲醚含量/% | C3~4OH含量/% | 其他产物含量/% |
|---|---|---|---|---|---|---|---|
| Cu/SiO2-1 | 100 | 1.06 | 1.14 | 91.55 | 0.31 | 4.76 | 5.94 |
| Cu/SiO2-2 | 100 | 0.48 | 1.72 | 92.76 | 1.48 | 1.74 | 1.82 |
| Cu/SiO2-3 | 100 | 0.53 | 1.83 | 91.68 | 2.52 | 0.55 | 2.89 |
| Cu/SiO2-4 | 100 | 0.76 | 0.15 | 97.4 | 0.15 | 0.7 | 0.84 |
| 样品 | 草酸二甲酯转化率/% | 乙醇含量/% | 乙醇酸甲酯选择性/% | 乙二醇选择性/% | 乙二醇甲醚含量/% | C3~4OH含量/% | 其他产物含量/% |
|---|---|---|---|---|---|---|---|
| Cu/SiO2-1 | 100 | 1.06 | 1.14 | 91.55 | 0.31 | 4.76 | 5.94 |
| Cu/SiO2-2 | 100 | 0.48 | 1.72 | 92.76 | 1.48 | 1.74 | 1.82 |
| Cu/SiO2-3 | 100 | 0.53 | 1.83 | 91.68 | 2.52 | 0.55 | 2.89 |
| Cu/SiO2-4 | 100 | 0.76 | 0.15 | 97.4 | 0.15 | 0.7 | 0.84 |
| 1 | YUE Hairong, ZHAO Yujun, MA Xinbin, et al. Ethylene glycol: properties, synthesis, and applications[J]. Chemical Society Reviews, 2012, 41(11): 4218-4244. |
| 2 | JIN Erlei, HE Leilei, ZHANG Yulong, et al. A nanostructured CeO2 promoted Pd/α-alumina diethyl oxalate catalyst with high activity and stability[J]. RSC Advances, 2014, 4(90): 48901-48904. |
| 3 | YANG Qing, YANG Qingchun, XU Simin, et al. Technoeconomic and environmental analysis of ethylene glycol production from coal and natural gas compared with oil-based production[J]. Journal of Cleaner Production, 2020, 273: 123120-123135. |
| 4 | WANG Zhiqiao, XU Zhongning, PENG Siyan, et al. New catalysts for coal to ethylene glycol[J]. Chinese Journal of Chemistry, 2017, 35(6): 759-768. |
| 5 | LIN Haiqiang, ZHENG Xinlei, HE Zhe, et al. Cu/SiO2 hybrid catalysts containing HZSM-5 with enhanced activity and stability for selective hydrogenation of dimethyl oxalate to ethylene glycol[J]. Applied Catalysis A: General, 2012, 445: 287-296. |
| 6 | WANG Shurong, LI Xinbao, YIN Qianqian, et al. Highly active and selective Cu/SiO2 catalysts prepared by the urea hydrolysis method in dimethyl oxalate hydrogenation[J]. Catalysis Communications, 2011, 12(13): 1246-1250. |
| 7 | YIN Anyuan, GUO Xiuying, DAI Weilin, et al. The nature of active copper species in Cu-HMS catalyst for hydrogenation of dimethyl oxalate to ethylene glycol: new insights on the synergetic effect between Cu0 and Cu+ [J]. The Journal of Physical Chemistry C, 2009, 113(25): 11003-11013. |
| 8 | ZHANG Yajing, ZHENG Na, WANG Kangjun, et al. Effect of copper nanoparticles dispersion on catalytic performance of Cu/SiO2 catalyst for hydrogenation of dimethyl oxalate to ethylene glycol[J]. Journal of Nanomaterials, 2013(1): 629375-1-629375-8. |
| 9 | ZHANG Shaoyan, LIU Quanyao, FAN Guoli, et al. Highly-dispersed copper-based catalysts from Cu-Zn-Al layered double hydroxide precursor for gas-phase hydrogenation of dimethyl oxalate to ethylene glycol[J]. Catalysis Letters, 2012, 142(9): 1121-1127. |
| 10 | WANG Bin, WEN Chao, CUI Yuanyuan, et al. Remarkable crystal phase effect of Cu/TiO2 catalysts on the selective hydrogenation of dimethyl oxalate[J]. RSC Advances, 2015, 5(37): 29040-29047. |
| 11 | ZHANG Chuancai, WANG Denghao, ZHU Mingyuan, et al. Plasma-enhanced copper dispersion and activity performance of Cu-Ni/ZrO2 catalyst for dimethyl oxalate hydrogenation[J]. Catalysis Communications, 2017, 102: 31-34. |
| 12 | Xiaoguang SAN, ZHAO Guodong, WANG Guosheng, et al. Synthesis of Cu/ZnO flower-like hierarchical porous structures and investigation of their catalytic performance for dimethyl oxalate hydrogenation[J]. China Petroleum Processing& Petrochemical Technology, 2017, 19(2): 40-47. |
| 13 | KONG Xiangpeng, CHEN Zheng, WU Yuehuan, et al. Synthesis of Cu-Mg/ZnO catalysts and catalysis in dimethyl oxalate hydrogenation to ethylene glycol: enhanced catalytic behavior in the presence of a Mg2+ dopant[J]. RSC Advances, 2017, 7(78): 49548-49561. |
| 14 | WANG Yue, SHEN Yongli, ZHAO Yujun, et al. Insight into the balancing effect of active Cu species for hydrogenation of carbon-oxygen bonds[J]. ACS Catalysis, 2015, 5(10): 6200-6208. |
| 15 | LI Siming, WANG Yue, ZHANG Jian, et al. Kinetics study of hydrogenation of dimethyl oxalate over Cu/SiO2 catalyst[J]. Industrial & Engineering Chemistry Research, 2015, 54(4): 1243-1250. |
| 16 | ZHU Yifeng, ZHU Yulei, DING Guoqiang, et al. Highly selective synthesis of ethylene glycol and ethanol via hydrogenation of dimethyl oxalate on Cu catalysts: influence of support[J]. Applied Catalysis A: General, 2013, 468: 296-304. |
| 17 | CHEN Liangfeng, GUO Pingjun, QIAO Minghua, et al. Cu/SiO2 catalysts prepared by the ammonia-evaporation method: texture, structure, and catalytic performance in hydrogenation of dimethyl oxalate to ethylene glycol[J]. Journal of Catalysis, 2008, 257(1): 172-180. |
| 18 | KOHLER M A, CURRY-HYDE H E, HUGHES A E, et al. The structure of CuSiO2 catalysts prepared by the ion-exchange technique[J]. Journal of Catalysis, 1987, 108(2): 323-333. |
| 19 | TOUPANCE T, KERMAREC M, LAMBERT J F, et al. Conditions of formation of copper phyllosilicates in silica-supported copper catalysts prepared by selective adsorption[J]. Journal of Physical Chemistry B, 2002, 106(9): 2277-2286. |
| 20 | YIN Anyuan, GUO Xiuying, FAN Kangnian, et al. Ion-exchange temperature effect on Cu/HMS catalysts for the hydrogenation of dimethyl oxalate to ethylene glycol[J]. ChemCatChem, 2010, 2(2): 206-213. |
| 21 | WANG Meilin, YAO Dawei, LI Antai, et al. Enhanced selectivity and stability of Cu/SiO2 catalysts for dimethyl oxalate hydrogenation to ethylene glycol by using silane coupling agents for surface modification[J]. Industrial & Engineering Chemistry Research, 2020, 59(20): 9414-9422. |
| 22 | CRÉPEAU G, Montouillout V, VIMONT A, et al. Nature, structure and strength of the acidic sites of amorphous silica alumina: an IR and NMR study[J]. The Journal of Physical Chemistry B, 2006, 110(31): 15172-15185. |
| 23 | SONG Yanbo, ZHANG Jian, Jing LYU, et al. Hydrogenation of dimethyl oxalate over copper-based catalysts: acid-base properties and reaction paths[J]. Industrial & Engineering Chemistry Research, 2015, 54(40): 9699-9707. |
| 24 | BURNEAU André, CARTERET Cédric. Near infrared and ab initio study of the vibrational modes of isolated silanol on silica[J]. Physical Chemistry Chemical Physics, 2000, 2(14): 3217-3226. |
| 25 | BUSH S G, JORGENSON J W. Confirmation and application of transmission near infrared absorption technique for absolute quantitation of functional groups on silica gel[J]. Journal of Chromatography A, 1990, 503(1): 69-91. |
| 26 | CHRISTY A A. New insights into the surface functionalities and adsorption evolution of water molecules on silica gel surface: a study by second derivative near infrared spectroscopy[J]. Vibrational Spectroscopy, 2010, 54(1): 42-49. |
| 27 | ZHAO Yujun, ZHANG Yaqing, WANG Yue, et al. Structure evolution of mesoporous silica supported copper catalyst for dimethyl oxalate hydrogenation[J]. Applied Catalysis A: General, 2017, 539: 59-69. |
| 28 | D'SOUZA A S, PANTANO C G. Mechanisms for silanol formation on amorphous silica fracture surfaces[J]. Journal of the American Ceramic Society, 1999, 82(5): 1289-1293. |
| 29 | WANG Rongwei, WUNDER S L. Effects of silanol density, distribution, and hydration state of fumed silica on the formation of self-assembled monolayers of n-octadecyltrichlorosilane[J]. Langmuir: the ACS Journal of Surfaces and Colloids, 2000, 16(11): 5008-5016. |
| 30 | SAUER J, HILL J R. The acidity of surface silanol groups: a theoretical estimate based on ab initio calculations on a model surface[J]. Chemical Physics Letters, 1994, 218(4): 333-337. |
| 31 | SINDORF D W, MACIEL G E. 29Si NMR study of dehydrated/rehydrated silica-gel using cross-polarization and magic-angle spinning[J]. Journal of the American Chemical Society, 1983, 105(6): 1487-1493. |
| 32 | YOUNG G J. Interaction of water vapor with silica surfaces[J]. Journal of Colloid Science, 1958, 13(1): 67-85. |
| 33 | WANG Yue, YANG Wenlong, YAO Dawei, et al. Effect of surface hydroxyl group of ultra-small silica on the chemical states of copper catalyst for dimethyl oxalate hydrogenation[J]. Catalysis Today, 2020, 350: 127-135. |
| 34 | MUSTER T H, PRESTIDGE C A, HAYES R A. Water adsorption kinetics and contact angles of silica particles[J]. Colloids & Surfaces A: Physicochemical & Engineering Aspects, 2001, 176(2/3): 253-266. |
| 35 | DING Jie, POPA Tiberiu, TANG Jinke, et al. Highly selective and stable Cu/SiO2 catalysts prepared with a green method for hydrogenation of diethyl oxalate into ethylene glycol[J]. Applied Catalysis B: Environmental, 2017, 209: 530-542. |
| 36 | ZHENG Jianwei, ZHOU Junfu, LIN Haiqiang, et al. CO-mediated deactivation mechanism of SiO2-supported copper catalysts during dimethyl oxalate hydrogenation to ethylene glycol[J]. The Journal of Physical Chemistry C, 2015, 119(24): 13758-13766. |
| 37 | VAN DER GRIFT C J G, WIELERS A F H, MULDER A, et al. The reduction behaviour of silica-supported copper catalysts prepared by deposition-precipitation[J]. Thermochimica Acta, 1990, 171: 95-113. |
| 38 | SULPIZI M, GAIGEOT M P, SPRIK M. The silica-water interface: how the silanols determine the surface acidity and modulate the water properties[J]. Journal of Chemical Theory and Computation, 2012, 8(3): 1037-1047. |
| 39 | MA Xiangang, YANG Zhiqiang, LIU Xuebin, et al. Dynamic redox cycle of Cu0 and Cu+ over Cu/SiO2 catalyst in ester hydrogenation[J]. RSC Advances, 2015, 5(47): 37581-37584. |
| 40 | LI Feng, LU Chunshan, LI Xiaonian. The effect of the amount of ammonia on the Cu0/Cu+ ratio of Cu/SiO2 catalyst for the hydrogenation of dimethyl oxalate to ethylene glycol[J]. Chinese Chemical Letters, 2014, 25(11): 1461-1465. |
| 41 | YIN Anyuan, GUO Xiuying, FAN Kangnian, et al. Influence of copper precursors on the structure evolution and catalytic performance of Cu/HMS catalysts in the hydrogenation of dimethyl oxalate to ethylene glycol[J]. Applied Catalysis A: General, 2010, 377(1/2): 128-133. |
| [1] | ZHAO Chen, MIAO Tianze, ZHANG Chaoyang, HONG Fangjun, WANG Dahai. Heat transfer characteristics of ethylene glycol aqueous solution in slit channel under negative pressure [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 148-157. |
| [2] | ZHANG Mingyan, LIU Yan, ZHANG Xueting, LIU Yake, LI Congju, ZHANG Xiuling. Research progress of non-noble metal bifunctional catalysts in zinc-air batteries [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 276-286. |
| [3] | SHI Yongxing, LIN Gang, SUN Xiaohang, JIANG Weigeng, QIAO Dawei, YAN Binhang. Research progress on active sites in Cu-based catalysts for CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 287-298. |
| [4] | XIE Luyao, CHEN Songzhe, WANG Laijun, ZHANG Ping. Platinum-based catalysts for SO2 depolarized electrolysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 299-309. |
| [5] | YANG Xiazhen, PENG Yifan, LIU Huazhang, HUO Chao. Regulation of active phase of fused iron catalyst and its catalytic performance of Fischer-Tropsch synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 310-318. |
| [6] | WANG Lele, YANG Wanrong, YAO Yan, LIU Tao, HE Chuan, LIU Xiao, SU Sheng, KONG Fanhai, ZHU Canghai, XIANG Jun. Influence of spent SCR catalyst blending on the characteristics and deNO x performance for new SCR catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 489-497. |
| [7] | DENG Liping, SHI Haoyu, LIU Xiaolong, CHEN Yaoji, YAN Jingying. Non-noble metal modified vanadium titanium-based catalyst for NH3-SCR denitrification simultaneous control VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 542-548. |
| [8] | CHENG Tao, CUI Ruili, SONG Junnan, ZHANG Tianqi, ZHANG Yunhe, LIANG Shijie, PU Shi. Analysis of impurity deposition and pressure drop increase mechanisms in residue hydrotreating unit [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4616-4627. |
| [9] | WANG Peng, SHI Huibing, ZHAO Deming, FENG Baolin, CHEN Qian, YANG Da. Recent advances on transition metal catalyzed carbonylation of chlorinated compounds [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4649-4666. |
| [10] | ZHANG Qi, ZHAO Hong, RONG Junfeng. Research progress of anti-toxicity electrocatalysts for oxygen reduction reaction in PEMFC [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4677-4691. |
| [11] | GE Quanqian, XU Mai, LIANG Xian, WANG Fengwu. Research progress on the application of MOFs in photoelectrocatalysis [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4692-4705. |
| [12] | WANG Weitao, BAO Tingyu, JIANG Xulu, HE Zhenhong, WANG Kuan, YANG Yang, LIU Zhaotie. Oxidation of benzene to phenol over aldehyde-ketone resin based metal-free catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4706-4715. |
| [13] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| [14] | WU Haibo, WANG Xilun, FANG Yanxiong, JI Hongbing. Progress of the development and application of 3D printing catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 3956-3964. |
| [15] | MAO Shanjun, WANG Zhe, WANG Yong. Group recognition hydrogenation: From concept to application [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 3917-3922. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||