Chemical Industry and Engineering Progress ›› 2021, Vol. 40 ›› Issue (6): 3421-3433.DOI: 10.16085/j.issn.1000-6613.2020-1446
• Fine chemicals • Previous Articles Next Articles
Research advances on the carboxylation of terminal alkynes with CO2
LI Minkang1,2( ), ZHANG Lina2, ZHANG Afang1, ZHAO Yonghui2, SUN Nannan2(
), ZHANG Lina2, ZHANG Afang1, ZHAO Yonghui2, SUN Nannan2( ), WEI Wei2(
), WEI Wei2( )
)
- 1.School of Materials Science & Engineering, Shanghai University, Shanghai 200444, China
2.CAS Key Laboratory of Low-Carbon Conversion Science and Engineering, Shanghai Advanced Research Institute, Chinese Academy of Sciences, Shanghai 201210, China
-
Received:2020-07-27Revised:2020-09-05Online:2021-06-22Published:2021-06-06 -
Contact:SUN Nannan,WEI Wei
CO2插入C—H(sp)键制备丙炔酸衍生物的研究进展
李民康1,2( ), 张莉娜2, 张阿方1, 赵永慧2, 孙楠楠2(
), 张莉娜2, 张阿方1, 赵永慧2, 孙楠楠2( ), 魏伟2(
), 魏伟2( )
)
- 1.上海大学材料科学与工程学院,上海 200444
2.中国科学院上海高等研究院低碳转化科学与工程重点实验室,上海 201210
-
通讯作者:孙楠楠,魏伟 -
作者简介:李民康(1995—),男,硕士研究生,研究方向为CO2气体催化转化。E-mail:liminkang2018@sari.ac.cn 。
CLC Number:
Cite this article
LI Minkang, ZHANG Lina, ZHANG Afang, ZHAO Yonghui, SUN Nannan, WEI Wei. Research advances on the carboxylation of terminal alkynes with CO2[J]. Chemical Industry and Engineering Progress, 2021, 40(6): 3421-3433.
李民康, 张莉娜, 张阿方, 赵永慧, 孙楠楠, 魏伟. CO2插入C—H(sp)键制备丙炔酸衍生物的研究进展[J]. 化工进展, 2021, 40(6): 3421-3433.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2020-1446
| 催化剂 | 温度/℃ | 压力/MPa | 时间/h | 收率/% | TON | TOF/h-1 | CTA-D/CTA-E | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| (IPr)CuCl | 60 | 1.5 | 24 | 91 | 9 | 0.38 | CTA-E | [ |
| Cu-NHC | 25 | 0.1 | 16 | 90 | 45 | 2.81 | CTA-D | [ |
| bis-(NHC)-Ag | 室温 | 0.1 | 16 | 85 | 85 | 5.31 | CTA-D | [ |
| L3/Ag | 35 | 0.1 | 24 | 98 | 392 | 16.33 | CTA-D | [ |
| Ag-NHC化合物 | 室温 | 0.1 | 16 | 82 | 82 | 5.13 | CTA-D | [ |
| Ag-NHC化合物 | 40 | 0.1 | 48 | 92 | 46 | 0.96 | CTA-E | [ |
| Ag-NHC化合物 | 60 | 0.2 | 12 | 47 | 5 | 0.39 | CTA-D | [ |
| CuI+PEt3 | 室温 | 0.1 | 24 | 90 | 11 | 0.47 | CTA-E | [ |
| [CuI(dtbpf)] | 25 | 0.1 | 24 | 96 | 48 | 2.00 | CTA-D | [ |
| [Cu2(μ-CN)2(k2-P,P-dppet)2] | 25 | 0.1 | 12 | 97 | 97 | 8.08 | CTA-D | [ |
| AgI | 50 | 0.2 | 12 | 94 | 94 | 7.83 | CTA-D | [ |
| AgI | 60 | 1.5 | 24 | 91 | 910 | 37.92 | CTA-E | [ |
| CuI | 50 | 8 | 12 | 92 | 46 | 3.83 | CTA-E | [ |
| AgBF4 | 50 | 0.1 | 16 | 99 | 1980 | 123.75 | CTA-D | [ |
| CuI | 80 | 0.1 | 18 | 99 | 10 | 0.55 | CTA-E | [ |
| AgI | 40 | 0.1 | 48 | 88 | 18 | 0.37 | CTA-E | [ |
| CuCl | 室温 | 0.1 | 16 | 90 | 18 | 1.13 | CTA-D | [ |
| Ag2WO4 | 室温 | 0.1 | 24 | 99 | 40 | 1.65 | CTA-E | [ |
| Ag(O2CNMe2) | 50 | 0.1 | 24 | 77 | 39 | 1.60 | CTA-E | [ |
| 催化剂 | 温度/℃ | 压力/MPa | 时间/h | 收率/% | TON | TOF/h-1 | CTA-D/CTA-E | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| (IPr)CuCl | 60 | 1.5 | 24 | 91 | 9 | 0.38 | CTA-E | [ |
| Cu-NHC | 25 | 0.1 | 16 | 90 | 45 | 2.81 | CTA-D | [ |
| bis-(NHC)-Ag | 室温 | 0.1 | 16 | 85 | 85 | 5.31 | CTA-D | [ |
| L3/Ag | 35 | 0.1 | 24 | 98 | 392 | 16.33 | CTA-D | [ |
| Ag-NHC化合物 | 室温 | 0.1 | 16 | 82 | 82 | 5.13 | CTA-D | [ |
| Ag-NHC化合物 | 40 | 0.1 | 48 | 92 | 46 | 0.96 | CTA-E | [ |
| Ag-NHC化合物 | 60 | 0.2 | 12 | 47 | 5 | 0.39 | CTA-D | [ |
| CuI+PEt3 | 室温 | 0.1 | 24 | 90 | 11 | 0.47 | CTA-E | [ |
| [CuI(dtbpf)] | 25 | 0.1 | 24 | 96 | 48 | 2.00 | CTA-D | [ |
| [Cu2(μ-CN)2(k2-P,P-dppet)2] | 25 | 0.1 | 12 | 97 | 97 | 8.08 | CTA-D | [ |
| AgI | 50 | 0.2 | 12 | 94 | 94 | 7.83 | CTA-D | [ |
| AgI | 60 | 1.5 | 24 | 91 | 910 | 37.92 | CTA-E | [ |
| CuI | 50 | 8 | 12 | 92 | 46 | 3.83 | CTA-E | [ |
| AgBF4 | 50 | 0.1 | 16 | 99 | 1980 | 123.75 | CTA-D | [ |
| CuI | 80 | 0.1 | 18 | 99 | 10 | 0.55 | CTA-E | [ |
| AgI | 40 | 0.1 | 48 | 88 | 18 | 0.37 | CTA-E | [ |
| CuCl | 室温 | 0.1 | 16 | 90 | 18 | 1.13 | CTA-D | [ |
| Ag2WO4 | 室温 | 0.1 | 24 | 99 | 40 | 1.65 | CTA-E | [ |
| Ag(O2CNMe2) | 50 | 0.1 | 24 | 77 | 39 | 1.60 | CTA-E | [ |
| 催化剂 | 温度 /℃ | 压力 /MPa | 时间 /h | 收率 /% | TON | TOF /h-1 | CTA-D/CTA-E | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| Ag@P-NHC | 25 | 0.1 | 20 | 98 | 327 | 16.35 | CTA-D | [ |
| CuBr@C | 80 | 0.1 | 2 | 90 | 18 | 9.00 | CTA-E | [ |
| Ag/PCNF | 25 | 0.1 | 18 | 98 | 71 | 3.94 | CTA-D | [ |
| Ag@PHNCT | 50 | 0.1 | 20 | 98 | 94 | 4.68 | CTA-D | [ |
| Cu-CN | 80 | 0.1 | 10 | 97 | 97 | 9.70 | CTA-D | [ |
| AgNPs@m-MgO | 70 | 0.1 | 12 | 98 | 47 | 3.89 | CTA-D | [ |
| Ag/M-CeO2 | 60 | 0.5 | 24 | 91 | 26 | 1.09 | CTA-E | [ |
| CuNPs/Al2O3 | 60 | 0.2 | 16 | 92 | 18 | 1.15 | CTA-E | [ |
| Ag/F-Al2O3 | 50 | 6 | 18 | 62 | 12 | 0.67 | CTA-D | [ |
| Ag/Schiff-SiO2 | 60 | 0.1 | 24 | 98 | 705 | 29.38 | CTA-D | [ |
| 催化剂 | 温度 /℃ | 压力 /MPa | 时间 /h | 收率 /% | TON | TOF /h-1 | CTA-D/CTA-E | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| Ag@P-NHC | 25 | 0.1 | 20 | 98 | 327 | 16.35 | CTA-D | [ |
| CuBr@C | 80 | 0.1 | 2 | 90 | 18 | 9.00 | CTA-E | [ |
| Ag/PCNF | 25 | 0.1 | 18 | 98 | 71 | 3.94 | CTA-D | [ |
| Ag@PHNCT | 50 | 0.1 | 20 | 98 | 94 | 4.68 | CTA-D | [ |
| Cu-CN | 80 | 0.1 | 10 | 97 | 97 | 9.70 | CTA-D | [ |
| AgNPs@m-MgO | 70 | 0.1 | 12 | 98 | 47 | 3.89 | CTA-D | [ |
| Ag/M-CeO2 | 60 | 0.5 | 24 | 91 | 26 | 1.09 | CTA-E | [ |
| CuNPs/Al2O3 | 60 | 0.2 | 16 | 92 | 18 | 1.15 | CTA-E | [ |
| Ag/F-Al2O3 | 50 | 6 | 18 | 62 | 12 | 0.67 | CTA-D | [ |
| Ag/Schiff-SiO2 | 60 | 0.1 | 24 | 98 | 705 | 29.38 | CTA-D | [ |
| 催化剂 | 温度/℃ | 压力/MPa | 时间/h | 收率/% | TON | TOF/h-1 | CTA-D/ CTA-E | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| Ag@MIL-101(Fe) | 50 | 0.1 | 15 | 97 | 36 | 2.40 | CTA-D | [ |
| Ag@UIO-66(Zr) | 50 | 0.1 | 15 | 96.5 | 21 | 1.42 | CTA-D | [ |
| AgNPs/Co-MOF | 80 | 0.1 | 14 | 98 | 49 | 3.50 | CTA-D | [ |
| Pd-Cu/MIL-101 | 25 | 0.1 | 24 | 96 | 691 | 28.79 | CTA-D | [ |
| Ag@1 | 60 | 0.1 | 6 | 91 | 18 | 3.03 | CTA-E | [ |
| Ag@ZIF-8 | 40 | 0.1 | 20 | 97 | 86 | 4.31 | CTA-D | [ |
| UiO-66@UiO-67-BPY-Ag | 50 | 0.1 | 24 | 97 | 80 | 3.31 | CTA-D | [ |
| ZIF-8@Au25@ZIF-67 | 50 | 0.1 | 12 | 99 | 4433 | 369.42 | CTA-D | [ |
| Cu-MOFs | 80 | 0.1 | 4 | 80 | 20 | 5.00 | CTA-E | [ |
| Ag/KAPs-P | 60 | 0.1 | 10 | 92 | 9936 | 993.60 | CTA-D | [ |
| Ag@CTFN | 60 | 0.1 | 24 | 97 | 128 | 5.34 | CTA-D | [ |
| CTF-DCE-Ag | 50 | 0.1 | 24 | 90.2 | 226 | 9.42 | CTA-D | [ |
| Ag@NOMP | 50 | 0.1 | 12 | 96 | 960 | 80.00 | CTA-D | [ |
| Ag-HMP | 80 | 0.1 | 12 | 98 | 82 | 6.86 | CTA-D | [ |
| AgNPs@m-PS-PC | 70 | 0.1 | 10 | 91 | 779 | 77.93 | CTA-D | [ |
| [Cu(Im12)2][CuBr2] | 25 | 0.1 | 12 | 96 | 10 | 0.83 | CTA-E | [ |
| CuCl2@poly-GLY(1-vim)3(OMs)3 | 40 | 4 | 12 | 96 | 5 | 0.40 | CTA-E | [ |
| 催化剂 | 温度/℃ | 压力/MPa | 时间/h | 收率/% | TON | TOF/h-1 | CTA-D/ CTA-E | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| Ag@MIL-101(Fe) | 50 | 0.1 | 15 | 97 | 36 | 2.40 | CTA-D | [ |
| Ag@UIO-66(Zr) | 50 | 0.1 | 15 | 96.5 | 21 | 1.42 | CTA-D | [ |
| AgNPs/Co-MOF | 80 | 0.1 | 14 | 98 | 49 | 3.50 | CTA-D | [ |
| Pd-Cu/MIL-101 | 25 | 0.1 | 24 | 96 | 691 | 28.79 | CTA-D | [ |
| Ag@1 | 60 | 0.1 | 6 | 91 | 18 | 3.03 | CTA-E | [ |
| Ag@ZIF-8 | 40 | 0.1 | 20 | 97 | 86 | 4.31 | CTA-D | [ |
| UiO-66@UiO-67-BPY-Ag | 50 | 0.1 | 24 | 97 | 80 | 3.31 | CTA-D | [ |
| ZIF-8@Au25@ZIF-67 | 50 | 0.1 | 12 | 99 | 4433 | 369.42 | CTA-D | [ |
| Cu-MOFs | 80 | 0.1 | 4 | 80 | 20 | 5.00 | CTA-E | [ |
| Ag/KAPs-P | 60 | 0.1 | 10 | 92 | 9936 | 993.60 | CTA-D | [ |
| Ag@CTFN | 60 | 0.1 | 24 | 97 | 128 | 5.34 | CTA-D | [ |
| CTF-DCE-Ag | 50 | 0.1 | 24 | 90.2 | 226 | 9.42 | CTA-D | [ |
| Ag@NOMP | 50 | 0.1 | 12 | 96 | 960 | 80.00 | CTA-D | [ |
| Ag-HMP | 80 | 0.1 | 12 | 98 | 82 | 6.86 | CTA-D | [ |
| AgNPs@m-PS-PC | 70 | 0.1 | 10 | 91 | 779 | 77.93 | CTA-D | [ |
| [Cu(Im12)2][CuBr2] | 25 | 0.1 | 12 | 96 | 10 | 0.83 | CTA-E | [ |
| CuCl2@poly-GLY(1-vim)3(OMs)3 | 40 | 4 | 12 | 96 | 5 | 0.40 | CTA-E | [ |
| 1 | DECONTO R M, POLLARD D. Contribution of Antarctica to past and future sea-level rise[J]. Nature, 2016, 531(7596): 591-597. |
| 2 | CARLETON T A, HSIANG S M. Social and economic impacts of climate[J]. Science, 2016, 353(6304): add9837. |
| 3 | IPCC. Climate change 2013: the physical science basis. working group Ⅰ contribution to the fifth assessment report of the intergovernmental panel on climate change[R]. Cambridge: Cambridge University Press, 2013. |
| 4 | IEA. World energy outlook 2019[R]. Paris: IEA, 2019. |
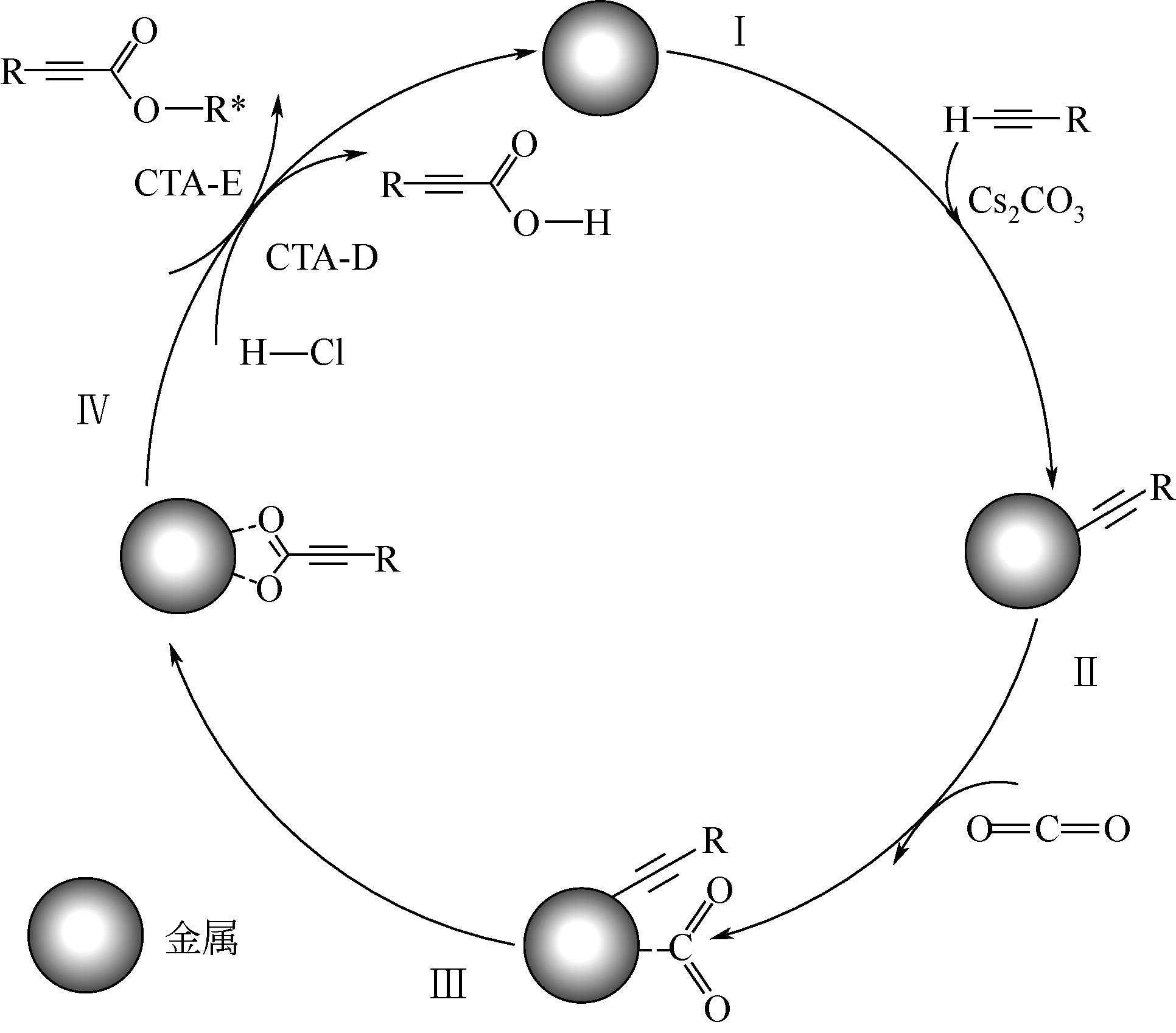
| 5 | MANJOLINHOHO F, ARANT M, GOOßEN K, et al. Catalytic C—H carboxylation of terminal alkynes with carbon dioxide[J]. ChemInform, 2012, 43(44): 2014-2021. |
| 6 | QIAO C, CAO Y, HE L N. Transition metal-catalyzed carboxylation of terminal alkynes with CO2[J]. Mini-Reviews in Organic Chemistry, 2018, 15: 283-290. |
| 7 | YU B, DIAO Z F, GUO C X, et al. Carboxylation of terminal alkynes at ambient CO2 pressure in ethylene carbonate[J]. Green Chemistry, 2013, 15(9): 2401-2407. |
| 8 | GOOßEN L J, RODRÍGUEZ N, MANJOLINHO F, et al. Synthesis of propiolic acids via copper-catalyzed insertion of carbon dioxide into the C—H bond of terminal alkynes[J]. Advanced Synthesis & Catalysis, 2010, 352(17): 2913-2917. |
| 9 | LIU C, LUO Y, ZHANG W Z, et al. DFT studies on the silver-catalyzed carboxylation of terminal alkynes with CO2: an insight into the catalytically activespecies [J]. Organometallics, 2014, 33(12): 2984-2989. |
| 10 | YASUO FUKUE S O, INOUE YOSHIO. Direct synthesis of alkyl 2-alkynoates from alklynes, CO2, and bromoalkanes catalysed by copper(Ⅰ) or silver(Ⅰ) salt[J]. Journal of the Chemical Society, Chemical Communications, 1994, 18: 2091. |
| 11 | ZHANG W Z, LI W J, ZHANG X, et al. Cu(Ⅰ)-catalyzed carboxylative coupling of terminal alkynes, allylic chlorides, and CO2[J]. Organic Letters, 2010, 12(21): 4748-4751. |
| 12 | YU D, ZHANG Y. Copper-and copper-N-heterocyclic carbene-catalyzed C—H activating carboxylation of terminal alkynes with CO2 at ambient conditions[J]. Proceeding of the Royal Society of Sciences of the UnitedStates of America, 2010, 107(47): 20184-20189. |
| 13 | DÍAZ VELÁZQUEZ H, WU Z X, VANDICHEL M, et al. Inserting CO2 into terminal alkynes via bis-(NHC)-metal complexes [J]. Catalysis Letters, 2017, 147(2): 463-471. |
| 14 | YUAN Y, CHEN C, ZENG C, et al. Carboxylation of terminal alkynes with carbon dioxide catalyzed by an in situ Ag2O/N-heterocyclic carbene precursor system [J]. ChemCatChem, 2017, 9(5): 882-887. |
| 15 | LI S S, SUN J, ZHANG Z Z, et al. Carboxylation of terminal alkynes with CO2 using novel silver N-heterocyclic carbene complexes[J]. Dalton Transacitions, 2016, 45(26): 10577-10584. |
| 16 | ZHANG Z Z, MI R J, GUO F J, et al. 1,3-bis(4-methylbenzyl)imidazol-2-ylidene silver(Ⅰ) chloride catalyzed carboxylative coupling of terminal alkynes, butyl iodide and carbon dioxide[J]. Journal of Saudi Chemical Society, 2017, 21(6): 685-690. |
| 17 | WANG W, ZHANG G, LANG R, et al. pH-responsive N-heterocyclic carbene copper(Ⅰ) complexes: syntheses and recoverable applications in the carboxylation of arylboronic esters and benzoxazole with carbon dioxide[J]. Green Chemistry, 2013, 15(3): 635-640. |
| 18 | PAPASTAVROU A T, PAUZE M, GÓMEZ BENGOA E, et al. Unprecedented multicomponent organocatalytic synthesis of propargylic esters via CO2 activation[J]. ChemCatChem, 2019, 11(21): 5379-5386. |
| 19 | INAMOTO K, ASANO N, KOBAYASHI K, et al. A copper-based catalytic system for carboxylation of terminal alkynes: synthesis of alkyl 2-alkynoates[J]. Organic & Biomolecular Chemistry, 2012, 10(8): 1514-1516. |
| 20 | TRIVEDI M, SINGH G, KUMAR A, et al. 1,1'-bis(di-tert-butylphosphino) ferrocene copper(Ⅰ) complex catalyzed C—H activation and carboxylation of terminal alkynes[J]. Dalton Transactions, 2015, 44(48): 20874-20882. |
| 21 | TRIVEDI M, SMREKER J R, SINGH G, et al. Cis-1,2-bis(diphenylphosphino)ethylene copper(Ⅰ) catalyzed C—H activation and carboxylation of terminal alkynes[J]. New Journal of Chemistry, 2017, 41(23): 14145-14151. |
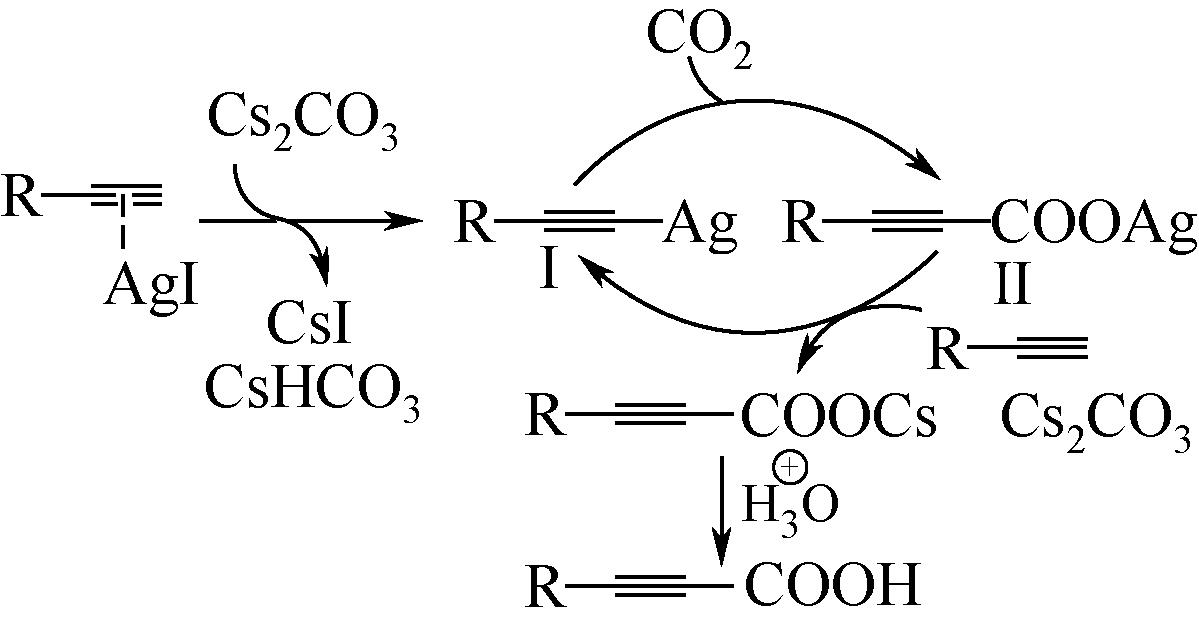
| 22 | ZHANG X, ZHANG W Z, REN X, et al. Ligand-free Ag(Ⅰ)-catalyzed carboxylation of terminal alkynes with CO2[J]. Organic Letters, 2011, 40(5): 2435-2452. |
| 23 | ZHANG X, ZHANG W Z, SHI L L, et al. Ligand-free Ag(Ⅰ)-catalyzed carboxylative coupling of terminal alkynes, chloride compounds, and CO2[J]. Tetrahedron, 2012, 68(44): 9085-9089. |
| 24 | LI F W, SUO Q L, HONG H L, et al. DBU and copper(Ⅰ) mediated carboxylation of terminal alkynes using supercritical CO2 as a reactant and solvent[J]. Tetrahedron Letters, 2014, 55(29): 3878-3880. |
| 25 | ARNDT M, RISTO E, KRAUSE T, et al. C—H carboxylation of terminal alkynescatalyzed by low loadings of silver(Ⅰ)/DMSO at ambient CO2 pressure[J]. ChemCatChem, 2012, 4(4): 484-487. |
| 26 | GUO F J, ZHANG Z Z, WANG J Y, et al. Silver-catalyzed one-pot synthesis of benzyl 2-alkynoates under ambient pressure of CO2 and ligand-free conditions[J]. Tetrahedron, 2017, 73(7): 900-906. |
| 27 | WANG W H, JIA L H, FENG X J, et al. Efficient carboxylation of terminal alkynes with carbon dioxide catalyzed by ligand-free copper catalyst under ambient conditions[J]. Asian Journal of Organic Chemistry, 2019, 8(8): 1501-1505. |
| 28 | GUO C X, YU B, XIE J N, et al. Silver tungstate: a single-component bifunctional catalyst for carboxylation of terminal alkynes with CO2 in ambient conditions[J]. Green Chemistry, 2015, 17(1): 474-479. |
| 29 | BRESCIANI G, MARCHETTI F, PAMPALONI G. Carboxylation of terminal alkynes promoted by silver carbamate at ambient pressure[J]. New Journal of Chemistry, 2019, 43(27): 10821-10825. |
| 30 | YU D Y, TAN M X, ZHANG Y G. Carboxylation of terminal alkynes with carbon dioxide catalyzed by poly(N-heterocyclic carbene)-supported silver nanoparticles[J]. Advanced Synthesis & Catalysis, 2012, 354(6): 969-974. |
| 31 |
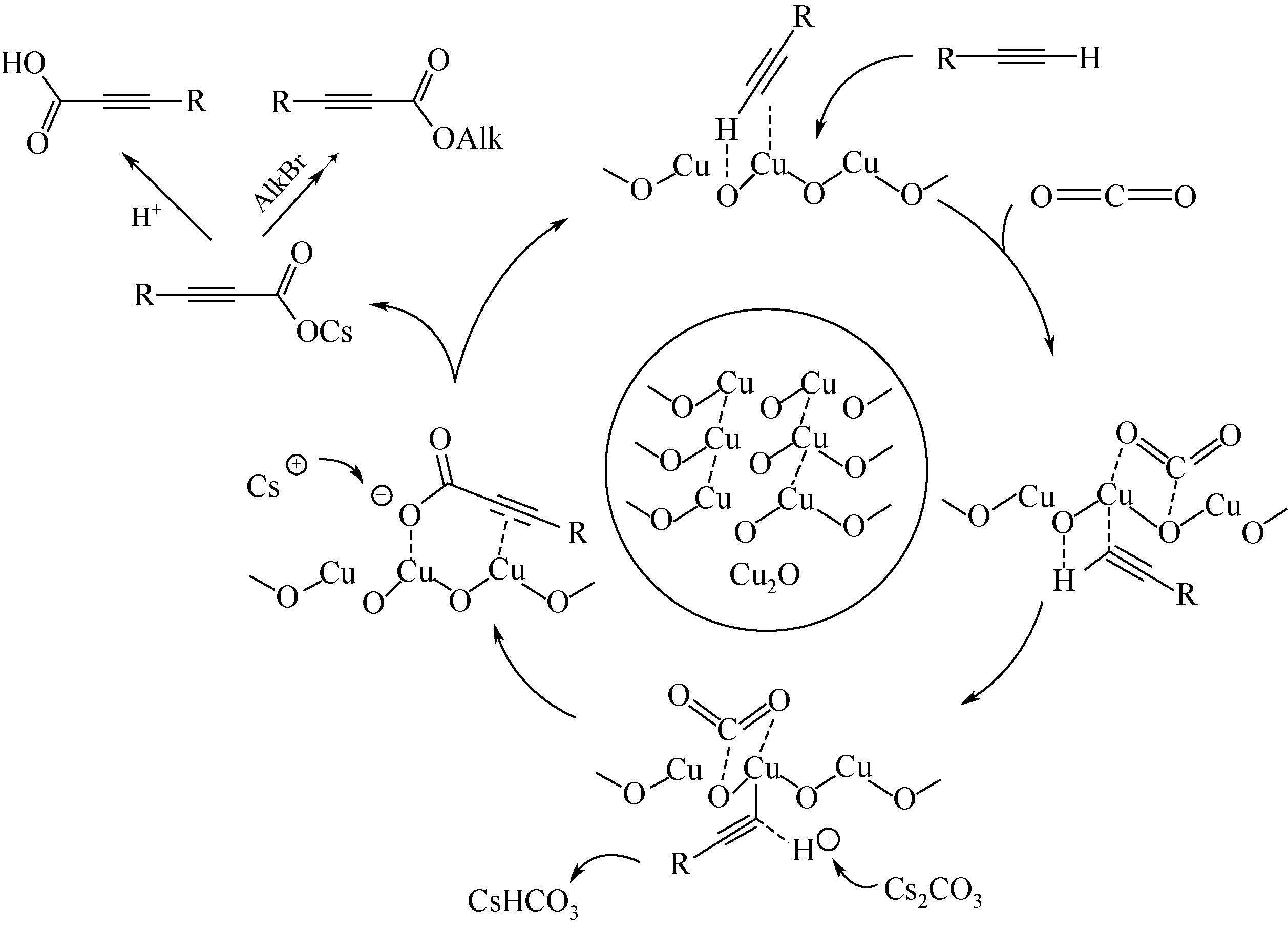
YU B, XIE J N, ZHONG C L, et al. Copper( )@carbon-catalyzed carboxylation of terminal alkynes with CO2 at atmospheric pressure[J]. ACS Catalysis, 2015, 5(7): 3940-3944. )@carbon-catalyzed carboxylation of terminal alkynes with CO2 at atmospheric pressure[J]. ACS Catalysis, 2015, 5(7): 3940-3944.
|
| 32 | LAN X W, LI Y M, DU C, et al. Porous carbon nitride frameworks derived from covalent triazine framework anchored Ag nanoparticles for catalytic CO2 conversion[J]. Chemistry, 2019, 25(36): 8560-8569. |
| 33 | LAN X W, LI Q, CAO L L, et al. Rebuilding supramolecular aggregates to porous hollow N-doped carbon tube inlaid with ultrasmall Ag nanoparticles: a highly efficient catalyst for CO2 conversion[J]. Applied Surface Science, 2020, 508: 45220-45229. |
| 34 | YANG P, ZUO S W, ZHANG F T, et al. Carbon nitride-based single-atom Cu catalysts for highly efficient carboxylation of alkynes with atmospheric CO2[J]. Industrial & Engineering Chemistry Research, 2020, 59(16): 7327-7335. |
| 35 | CHOWDHURY A H, GHOSH S, ISLAM S M. Flower-like AgNPs@ m-MgO as an excellent catalyst for CO2 fixation and acylation reactions under ambient conditions[J]. New Journal of Chemistry, 2018, 42(17): 14194-14202. |
| 36 | ZHANG X, WANG D K, JING M Z, et al. Ordered mesoporous CeO2-supported Ag as an effective catalyst for carboxylative coupling reaction using CO2[J]. ChemCatChem, 2019, 11(8): 2089-2098. |
| 37 | CHOWDHURY A H, KAYAL U, CHOWDHURY I H, et al. Nanoporous ZnO supported CuBr (CuBr/ZnO): an efficient catalyst for CO2 fixation reactions[J]. ChemistrySelect, 2019, 4(3): 1069-1077. |
| 38 | BONDARENKO G N, DVURECHENSKAYA E G, MAGOMMEDOV E S, et al. Copper(0) nanoparticles supported on Al2O3 as catalyst for carboxylation of terminal alkynes[J]. Catalysis Letters, 2017, 147(10): 2570-2580. |
| 39 | FINASHINA E D, KUSTOV L M, TKACHENKO O P, et al. Carboxylation of phenylacetylene by carbon dioxide on heterogeneous Ag-containing catalysts[J]. Russian Chemical Bulletin: International Edition, 2014, 63(12): 2652-2656. |
| 40 | WU Z L, SUN L, LIU Q G, et al. A Schiff base-modified silver catalyst for efficient fixation of CO2 as carboxylic acid at ambient pressure[J]. Green Chemistry, 2017, 19(9): 2080-2085. |
| 41 | LIU X H, MA J G, NIU Z, et al. An efficient nanoscale heterogeneous catalyst for the capture and conversion of carbon dioxide at ambient pressure[J]. Angewandte Chemie: International Edition, 2015, 54(3): 988-991. |
| 42 | ZHU N N, LIU X H, LI T, et al. Composite system of Ag nanoparticles and metal-organic frameworks for the capture and conversion of carbon dioxide under mild conditions[J]. Inorganic Chemistry, 2017, 56(6): 3414-3420. |
| 43 | MOLLA R A, GHOSH K, BANERJEE B, et al. Silver nanoparticles embedded over porous metal organic frameworks for carbon dioxide fixation via carboxylation of terminal alkynes at ambient pressure[J]. Journal of Colloid and Interface Science, 2016, 477: 20-29. |
| 44 | TRIVEDI M, BHASKARAN B, KUMAR A, et al. Metal-organic framework MIL-101 supported bimetallic Pd-Cu nanocrystals as efficient catalysts for chromium reduction and conversion of carbon dioxide at room temperature[J]. New Journal of Chemistry, 2016, 40(4): 3109-3118. |
| 45 | DUTTA G, JANA A K, SINGH D K, et al. Encapsulation of silver nanoparticles in an amine-functionalized porphyrin metal-organic framework and its use as a heterogeneous catalyst for CO2 fixation under atmospheric pressure[J]. Chemistry—An Asian Journal, 2018, 13(18): 2677-2684. |
| 46 | SHI J L, ZHANG L N, SUN N N, et al. Facile and rapid preparation of Ag@ZIF-8 for carboxylation of terminal alkynes with CO2 in mild conditions[J]. ACS Applied Materials & Interfaces, 2019, 11(32): 28858-28867. |
| 47 | GONG Y Y, YUAN Y, CHEN C, et al. Core-shell metal-organic frameworks and metal functionalization to access highest efficiency in catalytic carboxylation[J]. Journal of Catalysis, 2019, 371: 106-115. |
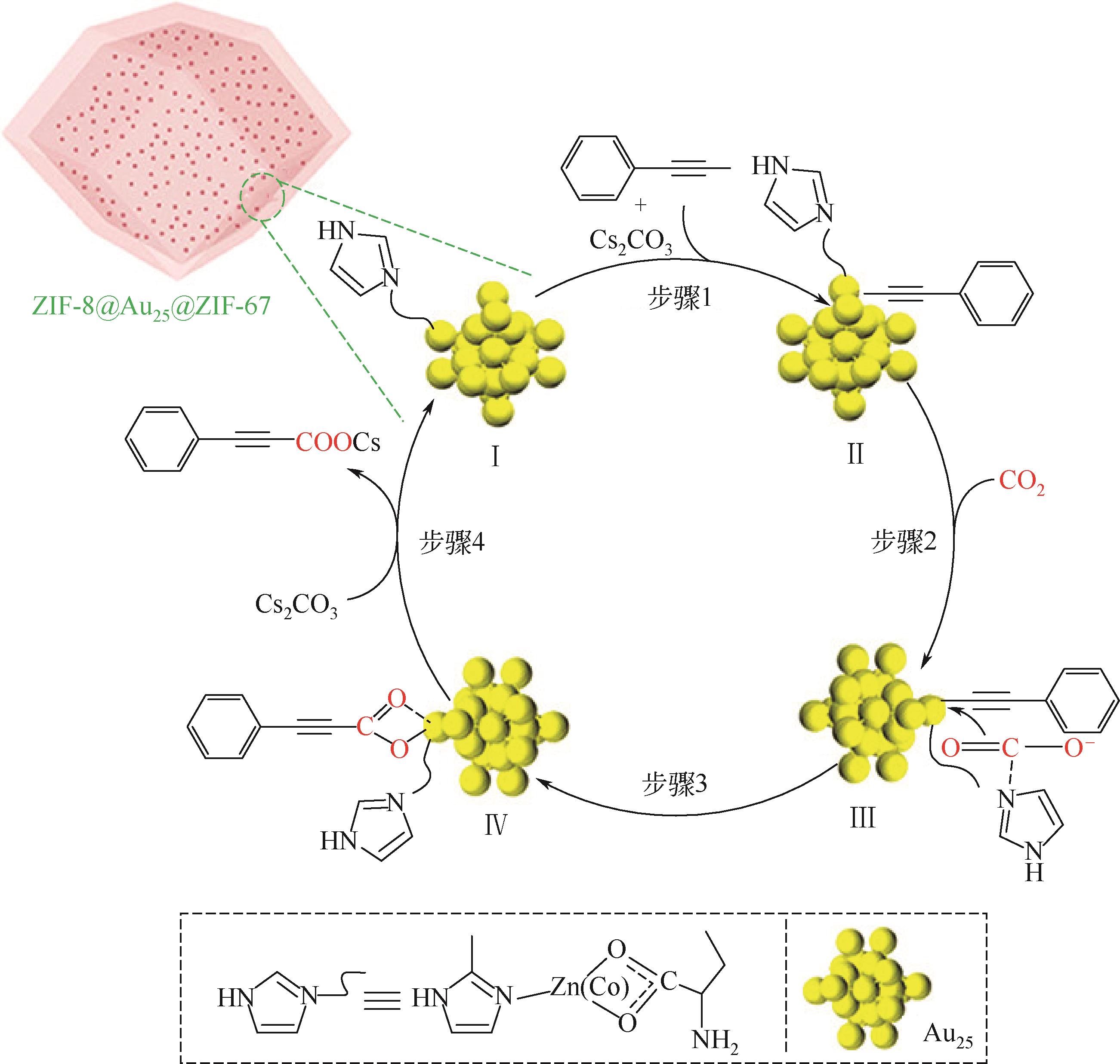
| 48 | YUN Y P, SHENG H T, BAO K, et al. Design and remarkable efficiency of the robust sandwich cluster composite nanocatalysts ZIF-8@Au25@ZIF-67[J]. Journal of the American Chemical Society, 2020, 142(9): 4126-4130. |
| 49 | XIONG G, YU B, DONG J, et al. Cluster-based MOFs with accelerated chemical conversion of CO2 through C—C bond formation[J]. Chemical Communications, 2017, 53(44): 6013-6016. |
| 50 | GANINA O G, BONDARENKO G N, ISAEVA V I, et al. Cu-MOF-catalyzed carboxylation of alkynes and epoxides[J]. Russian Journal of Organic Chemistry, 2019, 55(12): 1813-1820. |
| 51 | WU Z L, LIU Q G, YANG X F, et al. Knitting aryl network polymers-incorporated Ag nanoparticles: a mild and efficient catalyst for the fixation of CO2 as carboxylic acid[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(11): 9634-9639. |
| 52 | LAN X W, DU C, CAO L L, et al. Ultrafine Ag nanoparticles encapsulated by covalent triazine framework nanosheets for CO2 conversion[J]. ACS Applied Materials & Interfaces, 2018, 10(45): 38953-38962. |
| 53 | DANG Q Q, LIU C Y, WANG X M, et al. Novel covalent triazine framework for high-performance CO2 capture and alkyne carboxylation reaction[J]. ACS Applied Materials & Interfaces, 2018, 10(33): 27972-27978. |
| 54 | ZHANG W, MEI Y, HUANG X, et al. Size-controlled growth of silver nanoparticles onto functionalized ordered mesoporous polymers for efficient CO2 upgrading[J]. ACS Applied Materials & Interfaces, 2019, 11(47): 44241-44248. |
| 55 | GHOSH S, GHOSH A, RIYAJUDDIN S, et al. Silver nanoparticles architectured HMP as a recyclable catalyst for tetramic acid and propiolic acid synthesis through CO2 capture at atmospheric pressure[J]. ChemCatChem, 2020, 12(4): 1055-1067. |
| 56 | SALAM N, PAUL P, GHOSH S, et al. AgNPs encapsulated by an amine-functionalized polymer nanocatalyst for CO2 fixation as a carboxylic acid and the oxidation of cyclohexane under ambient conditions[J]. New Journal of Chemistry, 2020, 44(14): 5448-5456. |
| 57 | XIE J N, YU B, ZHOU Z H, et al. Copper(Ⅰ)-based ionic liquid-catalyzed carboxylation of terminal alkynes with CO2 at atmospheric pressure[J]. Tetrahedron Letters, 2015, 56(50): 7059-7062. |
| 58 | CHAUGULE A A, TAMBOLI A H, KIM H. CuCl2@poly-IL catalyzed carboxylation of terminal alkynes through CO2 utilization[J]. Chemical Engineering Journal, 2017, 326: 1009-1019. |
| 59 | WANG X, LIM Y N, LEE C, et al. 1,5,7-triazabicyclo[4.4.0]dec-1-ene-mediated acetylene dicarboxylation and alkyne carboxylation using carbon dioxide[J]. European Journal of Organic Chemistry, 2013(10): 1867-1871. |
| 60 | TONIOLO D, BOBBINK F D, DYSON P J, et al. Anhydrous conditions enable the catalyst-free carboxylation of aromatic alkynes with CO2 under mild conditions[J]. Helvetica Chimica Acta, 2020, 103(2): e1900258. |
| 61 | YU D Y, ZHANG Y G. The direct carboxylation of terminal alkynes with carbon dioxide[J]. Green Chemistry, 2011, 13(5): 1275-1279. |
| 62 | WANG W H, FENG X J, SUI K, et al. Transition metal-free carboxylation of terminal alkynes with carbon dioxide through dual activation: synthesis of propiolic acids[J]. Journal of CO2 Utilization, 2019, 32: 140-145. |
| 63 | YU D Y, ZHOU F, LIM D S, et al. NHC-Ag/Pd-catalyzed reductive carboxylation of terminal alkynes with CO2 and H2: a combined experimental and computational study for fine-tuned selectivity [J]. ChemSusChem, 2017, 10(5): 836-841. |
| 64 | SONG B, HE B Z, QIN A J, et al. Direct polymerization of carbon dioxide, diynes, and alkyl dihalides under mild reaction conditions[J]. Macromolecules, 2017, 51(1): 42-48. |
| 65 | KUGE K, LUO Y, FUJITA Y, et al. Copper-catalyzed stereodefined construction of acrylic acid derivatives from terminal alkynes via CO2 insertion[J]. Organic Letters, 2017, 19(4): 854-857. |
| 66 | WENDLING T, RISTO E, KRAUSE T, et al. Salt-free strategy for the insertion of CO2 into C—H bonds: catalytic hydroxymethylation of alkynes[J]. Chemistry, 2018, 24(23): 6019-6024. |
| [1] | YANG Hanyue, KONG Lingzhen, CHEN Jiaqing, SUN Huan, SONG Jiakai, WANG Sicheng, KONG Biao. Decarbonization performance of downflow tubular gas-liquid contactor of microbubble-type [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 197-204. |
| [2] | ZHANG Mingyan, LIU Yan, ZHANG Xueting, LIU Yake, LI Congju, ZHANG Xiuling. Research progress of non-noble metal bifunctional catalysts in zinc-air batteries [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 276-286. |
| [3] | SHI Yongxing, LIN Gang, SUN Xiaohang, JIANG Weigeng, QIAO Dawei, YAN Binhang. Research progress on active sites in Cu-based catalysts for CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 287-298. |
| [4] | XIE Luyao, CHEN Songzhe, WANG Laijun, ZHANG Ping. Platinum-based catalysts for SO2 depolarized electrolysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 299-309. |
| [5] | YANG Xiazhen, PENG Yifan, LIU Huazhang, HUO Chao. Regulation of active phase of fused iron catalyst and its catalytic performance of Fischer-Tropsch synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 310-318. |
| [6] | ZHENG Qian, GUAN Xiushuai, JIN Shanbiao, ZHANG Changming, ZHANG Xiaochao. Photothermal catalysis synthesis of DMC from CO2 and methanol over Ce0.25Zr0.75O2 solid solution [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 319-327. |
| [7] | WANG Lele, YANG Wanrong, YAO Yan, LIU Tao, HE Chuan, LIU Xiao, SU Sheng, KONG Fanhai, ZHU Canghai, XIANG Jun. Influence of spent SCR catalyst blending on the characteristics and deNO x performance for new SCR catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 489-497. |
| [8] | DENG Liping, SHI Haoyu, LIU Xiaolong, CHEN Yaoji, YAN Jingying. Non-noble metal modified vanadium titanium-based catalyst for NH3-SCR denitrification simultaneous control VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 542-548. |
| [9] | SUN Yuyu, CAI Xinlei, TANG Jihai, HUANG Jingjing, HUANG Yiping, LIU Jie. Optimization and energy-saving of a reactive distillation process for the synthesis of methyl methacrylate [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 56-63. |
| [10] | CHENG Tao, CUI Ruili, SONG Junnan, ZHANG Tianqi, ZHANG Yunhe, LIANG Shijie, PU Shi. Analysis of impurity deposition and pressure drop increase mechanisms in residue hydrotreating unit [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4616-4627. |
| [11] | WANG Peng, SHI Huibing, ZHAO Deming, FENG Baolin, CHEN Qian, YANG Da. Recent advances on transition metal catalyzed carbonylation of chlorinated compounds [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4649-4666. |
| [12] | ZHANG Qi, ZHAO Hong, RONG Junfeng. Research progress of anti-toxicity electrocatalysts for oxygen reduction reaction in PEMFC [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4677-4691. |
| [13] | GE Quanqian, XU Mai, LIANG Xian, WANG Fengwu. Research progress on the application of MOFs in photoelectrocatalysis [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4692-4705. |
| [14] | WANG Weitao, BAO Tingyu, JIANG Xulu, HE Zhenhong, WANG Kuan, YANG Yang, LIU Zhaotie. Oxidation of benzene to phenol over aldehyde-ketone resin based metal-free catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4706-4715. |
| [15] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||