Chemical Industry and Engineering Progress ›› 2019, Vol. 38 ›› Issue (03): 1552-1560.DOI: 10.16085/j.issn.1000-6613.2018-1192
Previous Articles Next Articles
Extraction of iron from pyrite cinder via oxalic acid-intensified leaching method
Baojun YANG( ),Xiaoyu FANG,Bainian WANG(
),Xiaoyu FANG,Bainian WANG( ),Yinzhe DONG,Mengmeng YANG
),Yinzhe DONG,Mengmeng YANG
- School of Chemistry and Chemical Engineering, Hefei University of Technology, Hefei 230009, Anhui, China
-
Received:2018-06-07Revised:2018-07-17Online:2019-03-05Published:2019-03-05 -
Contact:Bainian WANG
草酸助剂强化酸浸法提取硫酸烧渣中的铁
- 合肥工业大学化学与化工学院,安徽 合肥 230009
-
通讯作者:王百年 -
作者简介:杨保俊 (1970—),男,博士,教授,研究方向为化学工艺、非金属矿产和大宗城市矿产资源综合利用技术。E-mail:bj_yang@hfut.edu.cn 。|王百年,博士,副教授,研究方向为化学工艺、无机纳米材料的合成与应用。E-mail:284855884@163.com 。
CLC Number:
Cite this article
Baojun YANG,Xiaoyu FANG,Bainian WANG,Yinzhe DONG,Mengmeng YANG. Extraction of iron from pyrite cinder via oxalic acid-intensified leaching method[J]. Chemical Industry and Engineering Progress, 2019, 38(03): 1552-1560.
杨保俊,方晓宇,王百年,董胤喆,杨蒙蒙. 草酸助剂强化酸浸法提取硫酸烧渣中的铁[J]. 化工进展, 2019, 38(03): 1552-1560.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2018-1192
| 主要元素 | 含量/% | 主要元素 | 含量/% |
|---|---|---|---|
| Fe | 56.04 | Cu | 1.05 |
| O | 26.32 | Mg | 0.61 |
| S | 6.25 | C | 0.54 |
| Si | 4.71 | Zn | 0.46 |
| Al | 1.29 | Cr | 0.01 |
| Ca | 1.21 | 其他 | 1.51 |
| 主要元素 | 含量/% | 主要元素 | 含量/% |
|---|---|---|---|
| Fe | 56.04 | Cu | 1.05 |
| O | 26.32 | Mg | 0.61 |
| S | 6.25 | C | 0.54 |
| Si | 4.71 | Zn | 0.46 |
| Al | 1.29 | Cr | 0.01 |
| Ca | 1.21 | 其他 | 1.51 |
| 助剂 | 铁浸取率/% | 助剂 | 铁浸取率/% |
|---|---|---|---|
| 无 | 50.6 | 乙酸 | 47.26 |
| 氯化钠 | 54.0 | 柠檬酸 | 50.8 |
| 硫酸钠 | 54.7 | 硼酸 | 51.3 |
| 硫酸铵 | 48.0 | DL-苹果酸 | 54.6 |
| 三聚磷酸钠 | 51.0 | 琥珀酸 | 48.0 |
| 尿素 | 50.6 | 硬脂酸 | 50.6 |
| 乙二胺四乙酸二钠 | 50.7 | 月桂酸 | 54.0 |
| 苯甲酸钠 | 52.2 | 草酸 | 58.5 |
| 乙酰胺 | 55.4 | Na2S | 58.2 |
| 水杨酸钠 | 52.3 | 硫代硫酸钠 | 57.3 |
| D-葡萄糖酸钠 | 55.6 | 硝酸 | 55.0 |
| 助剂 | 铁浸取率/% | 助剂 | 铁浸取率/% |
|---|---|---|---|
| 无 | 50.6 | 乙酸 | 47.26 |
| 氯化钠 | 54.0 | 柠檬酸 | 50.8 |
| 硫酸钠 | 54.7 | 硼酸 | 51.3 |
| 硫酸铵 | 48.0 | DL-苹果酸 | 54.6 |
| 三聚磷酸钠 | 51.0 | 琥珀酸 | 48.0 |
| 尿素 | 50.6 | 硬脂酸 | 50.6 |
| 乙二胺四乙酸二钠 | 50.7 | 月桂酸 | 54.0 |
| 苯甲酸钠 | 52.2 | 草酸 | 58.5 |
| 乙酰胺 | 55.4 | Na2S | 58.2 |
| 水杨酸钠 | 52.3 | 硫代硫酸钠 | 57.3 |
| D-葡萄糖酸钠 | 55.6 | 硝酸 | 55.0 |
| 序号 | 配合基 | 铁离子 | 配位数 | lgβ n |
|---|---|---|---|---|
| 1 | C2O4 2- | Fe2+ | 1,2,3 | 2.9,4.52,5.22 |
| Fe3+ | 1,2,3 | 9.4,16.2,20.2 | ||
| 2 | SO4 2- | Fe3+ | 1,2 | 4.04,5.38 |
| 序号 | 配合基 | 铁离子 | 配位数 | lgβ n |
|---|---|---|---|---|
| 1 | C2O4 2- | Fe2+ | 1,2,3 | 2.9,4.52,5.22 |
| Fe3+ | 1,2,3 | 9.4,16.2,20.2 | ||
| 2 | SO4 2- | Fe3+ | 1,2 | 4.04,5.38 |
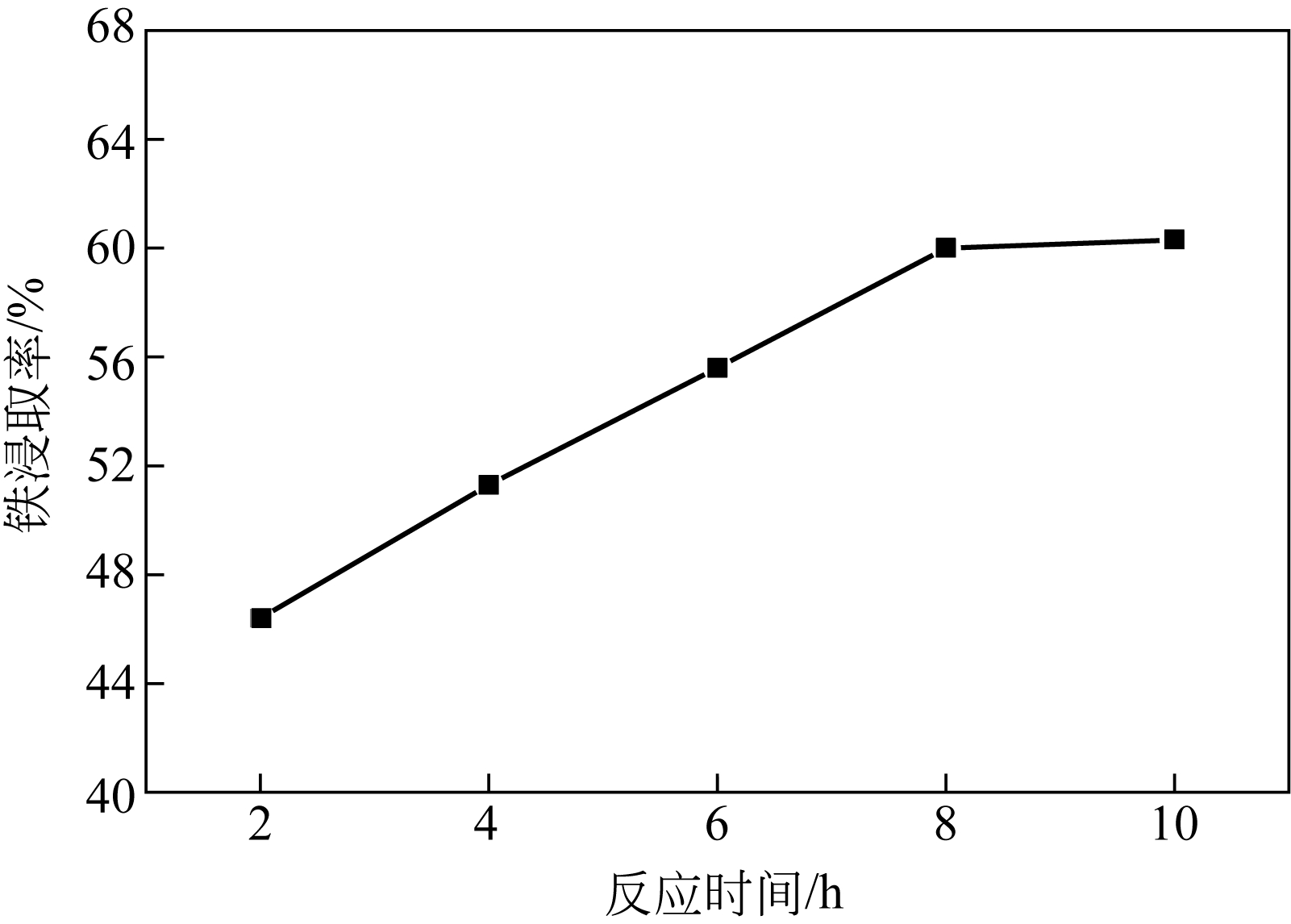
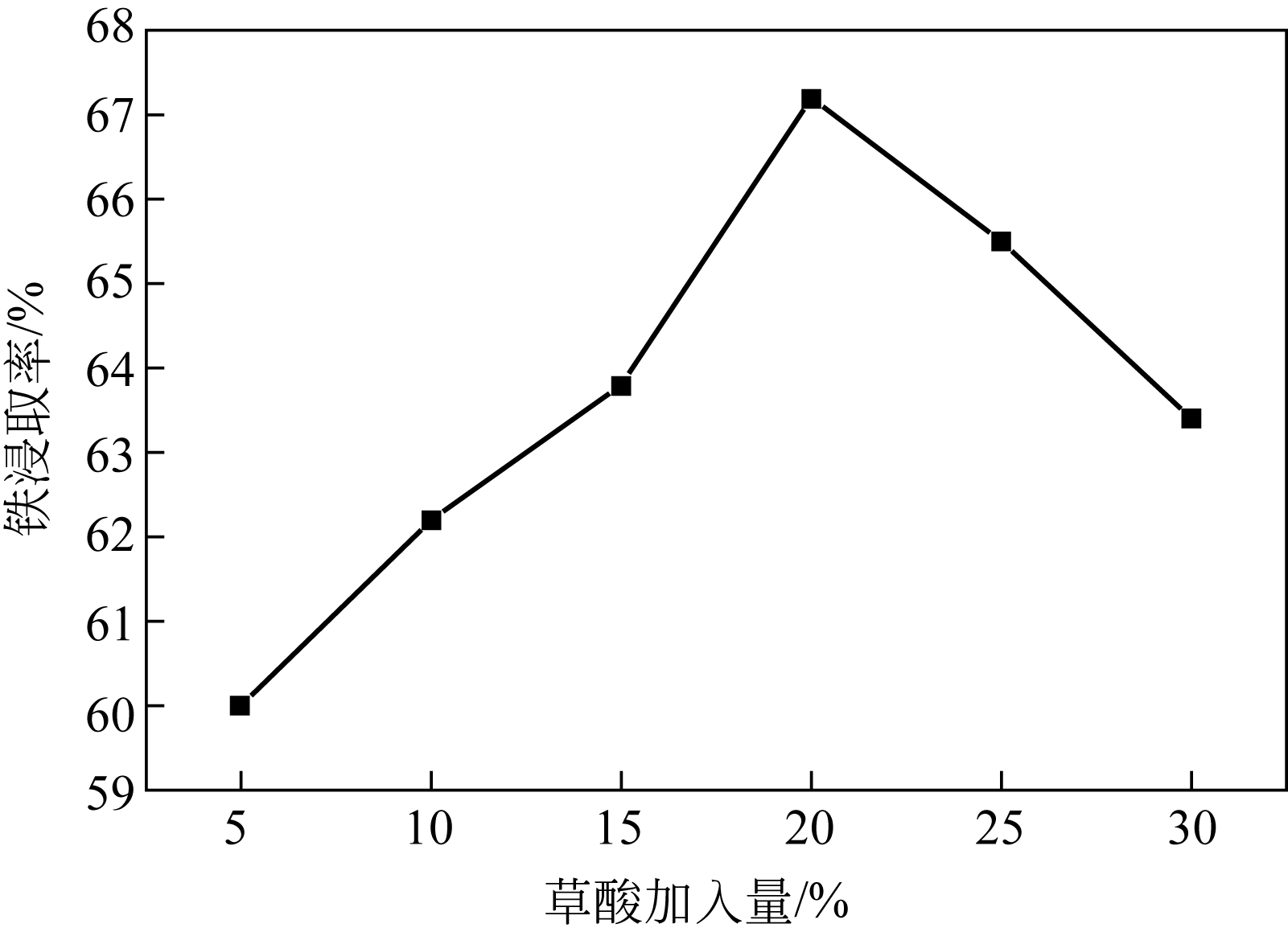
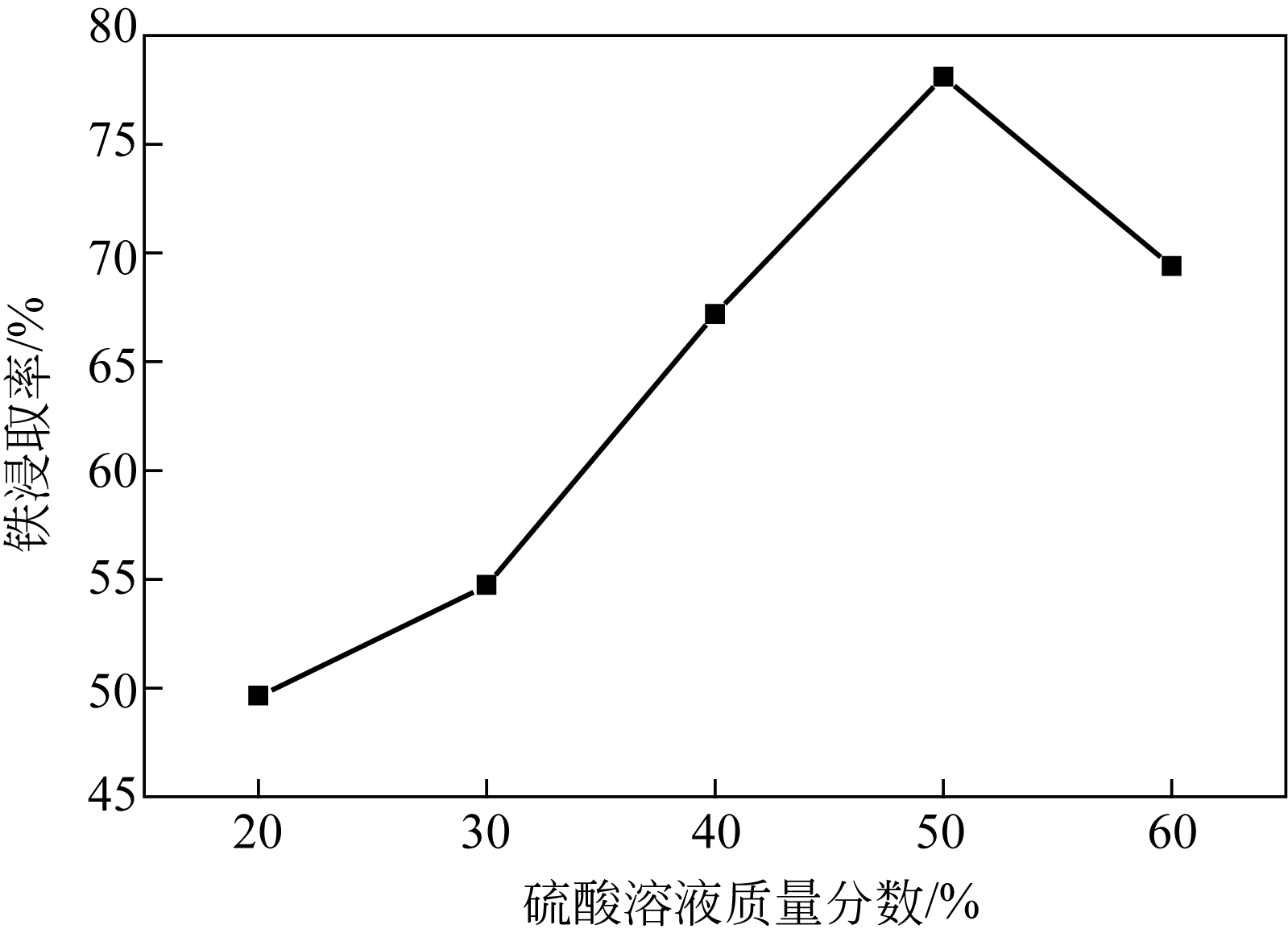
| 水平序号 | 反应时间 A/h | 草酸加入量B/% | 硫酸质量分数C/% | 反应温度D/℃ |
|---|---|---|---|---|
| 1 | 7.0 | 16 | 44 | 89 |
| 2 | 7.5 | 18 | 46 | 92 |
| 3 | 8.0 | 20 | 48 | 95 |
| 4 | 8.5 | 22 | 50 | 98 |
| 水平序号 | 反应时间 A/h | 草酸加入量B/% | 硫酸质量分数C/% | 反应温度D/℃ |
|---|---|---|---|---|
| 1 | 7.0 | 16 | 44 | 89 |
| 2 | 7.5 | 18 | 46 | 92 |
| 3 | 8.0 | 20 | 48 | 95 |
| 4 | 8.5 | 22 | 50 | 98 |
| 实验号 | A | B | C | D | 铁浸取率/% |
|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 67.2 |
| 2 | 1 | 2 | 2 | 2 | 78.1 |
| 3 | 1 | 3 | 3 | 3 | 87.6 |
| 4 | 1 | 4 | 4 | 4 | 94.2 |
| 5 | 2 | 1 | 2 | 3 | 81.5 |
| 6 | 2 | 2 | 1 | 4 | 78.9 |
| 7 | 2 | 3 | 4 | 1 | 89.0 |
| 8 | 2 | 4 | 3 | 2 | 88.7 |
| 9 | 3 | 1 | 3 | 4 | 85.5 |
| 10 | 3 | 2 | 4 | 3 | 90.2 |
| 11 | 3 | 3 | 1 | 2 | 74.2 |
| 12 | 3 | 4 | 2 | 1 | 78.9 |
| 13 | 4 | 1 | 4 | 2 | 88.3 |
| 14 | 4 | 2 | 3 | 1 | 83.6 |
| 15 | 4 | 3 | 2 | 4 | 89.5 |
| 16 | 4 | 4 | 1 | 3 | 76.4 |
| k 1 | 327.1 | 322.5 | 296.7 | 318.7 | |
| k 2 | 338.1 | 330.8 | 328 | 329.3 | |
| k 3 | 328.8 | 340.3 | 345.4 | 335.7 | |
| k 4 | 337.8 | 338.2 | 361.7 | 348.1 | |
| K 1 | 81.8 | 80.6 | 74.2 | 79.7 | |
| K 2 | 84.5 | 82.7 | 82.0 | 82.3 | |
| K 3 | 82.2 | 85.1 | 86.4 | 83.9 | |
| K 4 | 84.2 | 84.6 | 90.4 | 87.0 | |
| 极差R | 2.4 | 4.5 | 16.2 | 7.3 |
| 实验号 | A | B | C | D | 铁浸取率/% |
|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 67.2 |
| 2 | 1 | 2 | 2 | 2 | 78.1 |
| 3 | 1 | 3 | 3 | 3 | 87.6 |
| 4 | 1 | 4 | 4 | 4 | 94.2 |
| 5 | 2 | 1 | 2 | 3 | 81.5 |
| 6 | 2 | 2 | 1 | 4 | 78.9 |
| 7 | 2 | 3 | 4 | 1 | 89.0 |
| 8 | 2 | 4 | 3 | 2 | 88.7 |
| 9 | 3 | 1 | 3 | 4 | 85.5 |
| 10 | 3 | 2 | 4 | 3 | 90.2 |
| 11 | 3 | 3 | 1 | 2 | 74.2 |
| 12 | 3 | 4 | 2 | 1 | 78.9 |
| 13 | 4 | 1 | 4 | 2 | 88.3 |
| 14 | 4 | 2 | 3 | 1 | 83.6 |
| 15 | 4 | 3 | 2 | 4 | 89.5 |
| 16 | 4 | 4 | 1 | 3 | 76.4 |
| k 1 | 327.1 | 322.5 | 296.7 | 318.7 | |
| k 2 | 338.1 | 330.8 | 328 | 329.3 | |
| k 3 | 328.8 | 340.3 | 345.4 | 335.7 | |
| k 4 | 337.8 | 338.2 | 361.7 | 348.1 | |
| K 1 | 81.8 | 80.6 | 74.2 | 79.7 | |
| K 2 | 84.5 | 82.7 | 82.0 | 82.3 | |
| K 3 | 82.2 | 85.1 | 86.4 | 83.9 | |
| K 4 | 84.2 | 84.6 | 90.4 | 87.0 | |
| 极差R | 2.4 | 4.5 | 16.2 | 7.3 |
| 实验序号 | 加入草酸铁浸取率/% | 未加入草酸铁浸取率/% |
|---|---|---|
| 1 | 95.3 | 81.9 |
| 2 | 96.1 | 82.2 |
| 3 | 95.6 | 82.4 |
| 平均值 | 95.7 | 82.2 |
| 实验序号 | 加入草酸铁浸取率/% | 未加入草酸铁浸取率/% |
|---|---|---|
| 1 | 95.3 | 81.9 |
| 2 | 96.1 | 82.2 |
| 3 | 95.6 | 82.4 |
| 平均值 | 95.7 | 82.2 |
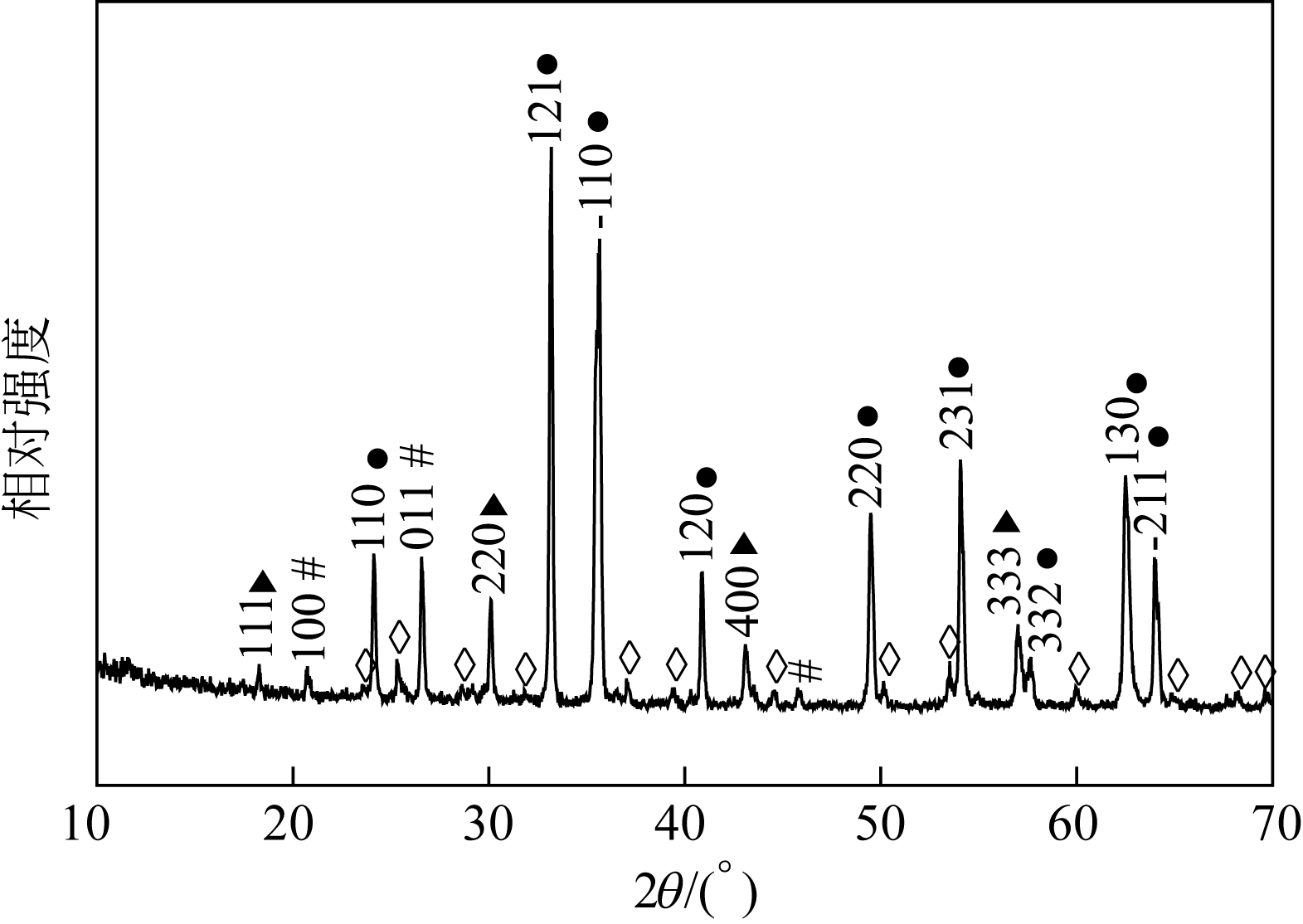
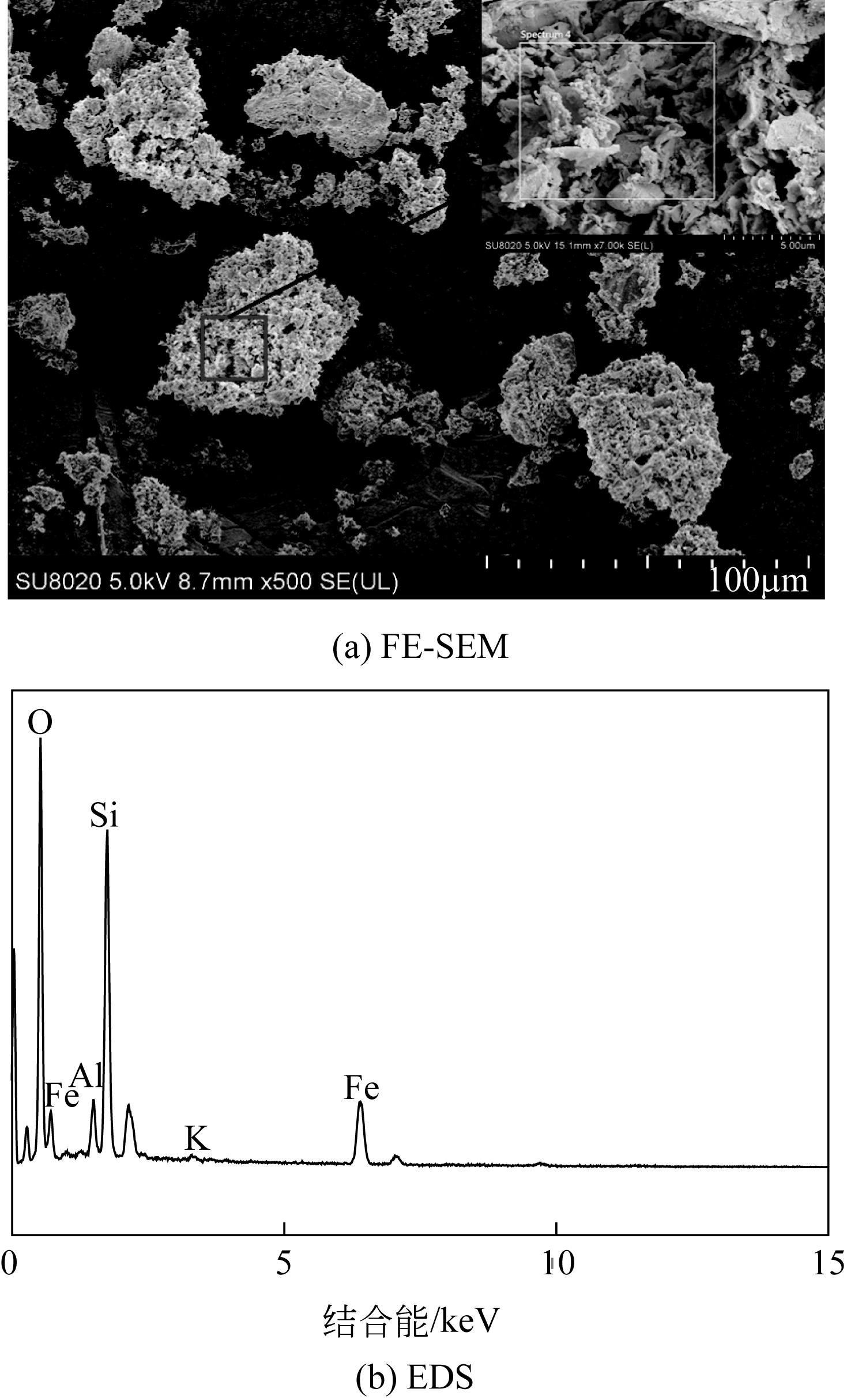
| 主要 元素 | 硫酸烧渣样品的EDS图中主要元素的质量分数/% | 未加入助剂酸浸后残渣样品的EDS图中主要元素的质量分数/% | 加入助剂酸浸后残渣样品的EDS图中主要元素的质量分数/% |
|---|---|---|---|
| Fe | 60.5 | 18.5 | 4.4 |
| O | 31.8 | 44.6 | 40.3 |
| Si | 6.5 | 26.2 | 29.4 |
| Al | 1.0 | 2.4 | 3.1 |
| K | 0.3 | 0.3 | 1.7 |
| 主要 元素 | 硫酸烧渣样品的EDS图中主要元素的质量分数/% | 未加入助剂酸浸后残渣样品的EDS图中主要元素的质量分数/% | 加入助剂酸浸后残渣样品的EDS图中主要元素的质量分数/% |
|---|---|---|---|
| Fe | 60.5 | 18.5 | 4.4 |
| O | 31.8 | 44.6 | 40.3 |
| Si | 6.5 | 26.2 | 29.4 |
| Al | 1.0 | 2.4 | 3.1 |
| K | 0.3 | 0.3 | 1.7 |
| 助剂 | 铁浸取率/% |
|---|---|
| 草酸 | 95.7 |
| 草酸铵 | 95.2 |
| 助剂 | 铁浸取率/% |
|---|---|
| 草酸 | 95.7 |
| 草酸铵 | 95.2 |
| 1 | 张仲伟, 陈吉春, 李旭 . 硫铁矿烧渣制备铁系化工产品研究方法综述[J]. 化工矿产地质, 2004, 26(3): 181-185. |
| ZHANG Zhongwei , CHEN Jichun , LI Xu . Summary of studying methods on preparation of iron-based prodcts from greigite cinder[J]. Geology of Chemical Minerals, 2004, 26(3): 181-185. | |
| 2 | 李崇 . 中国硫酸工业现状及“十三五”发展思路[J]. 硫酸工业, 2016(1): 1-6. |
| LI Chong . Status of China sulphuric acid industry and development planning ideas in the thirteenth five-year plan period[J]. Sulphuric Acid Industry, 2016(1): 1-6. | |
| 3 | 李国旺 . 硫酸渣富铁降硫工艺研究[D]. 贵阳: 贵州大学,2015. |
| LI Guowang . Research on enrichment of iron and reduction of sulfur in pyrite cinder[D]. Guiyang: Guizhou University,2015. | |
| 4 | VINALS J , BALART M J , ROCA A . Inertization of pyrite cinders and co-inertization with electric arc furnace flue dusts by pyroconsolidation at solid state[J]. Waste Management, 2002, 22(7): 773-782. |
| 5 | JIAN L , WEN S M , YU C , et al . Process optimization and reaction mechanism of removing copper from an Fe-rich pyrite cinder using chlorination roasting[J]. Journal of Iron and Steel Research International, 2013, 20(8): 20-26. |
| 6 | 徐玉萍 . 硫铁矿烧渣制复合聚铁混凝剂及其性能研究[J]. 化学研究与应用, 2017, 29(9): 1429-1432. |
| XU Yuping . Study on preparation and properties of poly ferric coagulant from pyrite cinder[J]. Chemical Research and Application, 2017, 29(9): 1429-1432. | |
| 7 | 傅开彬, 焦宇, 徐信, 等 . 山东某硫铁矿烧渣硫酸浸取液制备铁红工艺研究[J]. 应用化工, 2018, 47(2): 293-295. |
| FU Kaibin , JIAO Yu , XU Xin , et al . Preparation of iron oxide red from sulfuric acid solution of a pyrite cinder from Shandong province [J]. Applied Chemical Industry, 2018, 47(2): 293-295. | |
| 8 | ZHENG Y J , GONG Z Q , CHEN B Z , et al . Preparation of solid polyferric sulfate from pyrite cinders and its structure feature[J]. Trans. Nonferrous Met. Soc. China, 2003, 13(3): 690-694. |
| 9 | ZHENG Y J , GONG Z Q , LIU L H , et al . Comparisons of species and coagulation effects of PFS solution and solid PFS from pyrite cinders[J]. Trans. Nonferrous Met. Soc. China, 2002, 12(5): 983-986. |
| 10 | ZOU Z , XUAN A G , YAN Z G , et al . Preparation of Fe3O4, particles from copper/iron ore cinder and their microwave absorption properties[J]. Chemical Engineering Science, 2010, 65(1): 160-164. |
| 11 | 徐杰, 李书营, 王瑞 . 硫铁矿烧渣的综合利用研究[J]. 环境科学与管理, 2010, 35(2): 153-157. |
| XU Jie , LI Shuying , WANG Rui . Utilization of pyrite cinder[J]. Environmental Science and Management, 2010, 35(2): 153-157. | |
| 12 | 陈永亮, 陈吉春 . 以硫铁矿烧渣制备聚合硫酸铁的新工艺[J]. 资源环境与工程, 2006, 20(4): 467-481. |
| CHEN Yongliang , CHEN Jichun . A new preparation process of polyferric sulfate from pyrite cinders[J]. Resources Environment & Engineering, 2006, 20(4): 467-481. | |
| 13 | ALP I, DEVECI H , YAZICI E Y , et al . Potential use of pyrite cinders as raw material in cement production: results of industrial scale trial operations[J]. Journal of Hazardous Materials, 2009, 166(1): 144-149. |
| 14 | 占寿祥, 郑雅杰 . 硫铁矿烧渣酸溶反应动力学研究[J]. 化学工程, 2006, 34(11): 36-39. |
| ZHAN Shouxiang , ZHENG Yajie . Study on leaching kinetics of pyrite cinder[J]. Chemical Engineering, 2006, 34(11): 36-39. | |
| 15 | 左豪恩, 温建康, 崔兴兰, 等 . 硫酸渣脱硫制备高品质铁精矿研究进展[J]. 工程科学学报, 2018, 40(1):1-8. |
| ZUO Haoen , WEN Jiankang , CUI Xinglan , et al . Review of research process on preparation of high-quality iron concentrate from pyrite cinder by desulphurization[J]. Chinese Journal of Engineering, 2018, 40(1):1-8. | |
| 16 | 董风芝 . 硫铁矿烧渣用作炼铁原料研究进展[J]. 化工矿物与加工, 2012, 41(8): 41-44. |
| DONG Fengzhi . Progress on research of utilizing pyrite cinder as raw materials for iron-making[J]. Industrial Minerals & Processing, 2012, 41(8): 41-44. | |
| 17 | 郑晓虹, 陈玉峰, 黄升光, 等 . 硫酸浸取法从硫铁矿烧渣中提取铁的研究[J]. 化工进展, 2003, 22(2): 165-168. |
| ZHENG Xiaohong , CHEN Yufeng , HUANG Shengguang , et al . Investigation on extracting iron from pyrite cinder by sulfuric acid leaching [J]. Chemical Industry and Engineering Progress, 2003, 22(2): 165-168. | |
| 18 | 李威, 华涛, 周启星 . 盐酸酸溶硫铁矿烧渣的最佳工艺条件[J]. 应用基础与工程科学学报, 2008, 16(6): 795-801. |
| LI Wei , HUA Tao , ZHOU Qixing . Optimum technical conditions study of dissolving pyrite cinders by hydrochloric acid[J]. Journal of Basic Science and Engineering, 2008, 16(6): 795-801. | |
| 19 | 金程, 李登新 . 硫酸烧渣还原浸取铁[J]. 有色金属(冶炼部分), 2012(1): 9-12. |
| JIN Cheng , LI Dengxin . Reductive leaching of iron from sulfate slag[J]. Nonferrous Metals(Extractive Metallurgy), 2012(1): 9-12. | |
| 20 | 方亮 . 药剂学[M]. 3版. 北京: 中国医药科技出版社, 2016:84. |
| FANG Liang . Pharmaceutics[M]. 3th ed. Beijing: China Medical Science Press, 2016:84. | |
| 21 | 李志金,李登新,张武学,等 . 酸浸渣制取硫酸亚铁的研究[J]. 再生资源研究, 2006(6):32-35. |
| LI Zhijin , LI Dengxin , ZHANG Wuxue , et al . The research on preparation ferrous sulfate from acid leaching tailings[J]. Recyclable Resources and Circular Economy, 2006(6):32-35. | |
| 22 | 金程 . 强化酸浸法回收硫酸烧渣中铁的研究[D].上海:东华大学,2012. |
| JIN Cheng . Study on reinforced acid leaching of ferric iron in pyrite cinder[D]. Shanghai: Donghua University, 2012. | |
| 23 | 詹咏, 张焕焕, 疏醒, 等 . 硫铁矿烧渣铁系氧化物的酸溶特性[J].上海理工大学学报, 2017, 39(1): 90-96. |
| ZHAN Yong , ZHANG Huanhuan , SHU Xing , et al . Acid dissolution properties of ferric oxide in pyrite cinder[J]. Journal of University of Shanghai for Science and Technology, 2017, 39(1): 90-96. | |
| 24 | 刘培, 江健, 刘宗宽, 等 . 双酸法提取硫铁矿烧渣中铁[J]. 化工学报, 2013, 64(7): 2619-2624. |
| LIU P , JIANG J , LIU Z K , et al . Iron extraction from pyrite cinder by mixed acid[J]. Journal of Chemical Industry and Engineering, 2013, 64(7): 2619-2624. | |
| 25 | 陆俭洁 . 铁矿石中全铁含量测定方法的改进——重铬酸钾法测定铁矿石中铁含量实验的改进[J]. 中南民族学院学报(自然科学版), 2000, 19(3): 81-83. |
| LU Jianjie . Improvement of the determination of iron in ferrous ore by K2Cr2O7 [J]. Journal of South-Central University for Nationalities(Natural Science Edition), 2000, 19(3): 81-83. | |
| 26 | 刘自亮 . 粉煤灰制备铝硅合金过程中预处理除铁工艺研究[D]. 昆明: 昆明理工大学, 2016. |
| LIU Ziliang . Study on the removal process of iron in the preparation of aluminum silicon alloy from fly ash[D]. Kunming: Kunming University of Science and Technology, 2016. | |
| 27 | 钱红辉 . 氧化铁脱硫剂的制备、改性及回收利用研究[D]. 武汉: 武汉科技大学, 2010. |
| QIAN Honghui . The research on preparation, modification and recycling of iron oxide desulfurizer[D]. Wuhan: Wuhan University of Science and Technology, 2010. | |
| 28 | 石志霞, 余章龙, 彭程, 等 . 低品位铝土矿草酸光催化除铁研究[J]. 材料导报, 2015, 29(s2): 125-128. |
| SHI Zhixia , YU Zhanglong , PENG Cheng , et al . Sunlight irondiation-assisted removal of iron oxide from low grade bauxite using oxalic acid[J]. Material Review, 2015, 29(s2): 125-128. |
| [1] | YU Zhengwei, ZHANG Xiaoxia, LEI Jie, LI Ao, WANG Guangying, DING Xiang, LONG Hongming. Comprehensive recovery of cerium and manganese from waste CeO x -MnO x -based SCR denitrification catalysts by reductive acid leaching [J]. Chemical Industry and Engineering Progress, 2022, 41(9): 5122-5131. |
| [2] | LYU Peng, HE Changfan, HE Lin, LI Xingang, SUI Hong. Degradation characteristics and enhancement mechanism of heavy oily sludge by heterogeneous oxidation [J]. Chemical Industry and Engineering Progress, 2022, 41(11): 6149-6157. |
| [3] | JI Feng, ZHENG Bowen, LUO Ruoyin, DU Wei, DENG Chengwei, YANG Sheng, LIU Zhiqiang. Analysis and optimization of a HT-PEMFC stack [J]. Chemical Industry and Engineering Progress, 2022, 41(10): 5325-5331. |
| [4] | LI Haolin, ZENG Dehui, ZHENG Guangya, XIA Jupei. Extraction of aluminum and iron from coal gangue by low temperature neutralization-pressure acid leaching [J]. Chemical Industry and Engineering Progress, 2021, 40(7): 4011-4020. |
| [5] | MA Zhibin, ZHANG Xueli, GUO Yanxia, CHENG Fangqin. Research progress on characteristics and element dissolution behaviors of circulating gluidized bed-derived fly ash [J]. Chemical Industry and Engineering Progress, 2021, 40(6): 3058-3071. |
| [6] | Guangzhu LI, Shangjing ZENG, Shuhai SUN, Kaicheng XU, Dejun BIAN. Preparation of biochar supported iron oxides composites and its application in water treatment [J]. Chemical Industry and Engineering Progress, 2021, 40(2): 917-931. |
| [7] | Huiyao WANG, Yonggang WEI, Shiwei ZHOU, Bo LI, Yu SHI. Extraction of nickel and cobalt by microwave pretreatment-acid leaching of limonite high manganese laterite ore [J]. Chemical Industry and Engineering Progress, 2020, 39(5): 1907-1914. |
| [8] | Qing LIU, Zhenning DENG, Yilong HUA, Guodong ZHAO. Green synthesis of Fe nanoparticles and their environmental applications [J]. Chemical Industry and Engineering Progress, 2020, 39(5): 1950-1963. |
| [9] | Jiayu LYU, Lilin HU, Tianxing SONG, Yang ZHANG, Hai ZHANG, Suxia MA, Junfu LYU. Occurrence transformation of iron oxides and their catalytic reduction of NO under fluidized bed temperature and CO [J]. Chemical Industry and Engineering Progress, 2020, 39(11): 4474-4479. |
| [10] | Feigang YUAN. Intensification of the stripping characteristics of iron (Ⅲ) using oxalic acid [J]. Chemical Industry and Engineering Progress, 2019, 38(10): 4437-4443. |
| [11] | Meixue JIANG, Hongjuan SUN, Tongjiang PENG. Extraction of iron oxide from titanium gypsum by sulfuric acid leaching and phase transform of gypsum [J]. Chemical Industry and Engineering Progress, 2019, 38(04): 2030-2036. |
| [12] | Haijun FENG, Guanning WANG, Libao WANG, Yanan SUN, Biao ZHANG, Tao CHEN, Wei ZHANG. Process for preparing magnetic iron oxide pigment by pickling waste liquid and sludge [J]. Chemical Industry and Engineering Progress, 2019, 38(02): 1129-1133. |
| [13] | DENG Jun, ZHU Xuejun, ZHANG Yi. Experimental study on extracting titanium from high titanium slag recovery dust leached in hydrochloric acid assisted by ultrasonic wave [J]. Chemical Industry and Engineering Progree, 2016, 35(S2): 376-380. |
| [14] | RAO Bing, DAI Huixin, GAO Likun. Metallurgical progress of recovery indium from smelt residues [J]. Chemical Industry and Engineering Progree, 2016, 35(12): 4042-4052. |
| [15] | ZHANG Yanan, FAN Tianbo, GUO Hongfan, LI Xue, HU Bin, LIU Yuanyuan, ZHOU Yonghong, LIU Yunyi. Refining quartz sand tailings and mechanisms by hydrofluoric acid associated with oxalic acid [J]. Chemical Industry and Engineering Progree, 2016, 35(03): 957-962. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||