Chemical Industry and Engineering Progress ›› 2019, Vol. 38 ›› Issue (03): 1561-1566.DOI: 10.16085/j.issn.1000-6613.2018-1089
Previous Articles Next Articles
Process simulation and evaluation of CO2 removal from flue gas by binary compound solutions
Tongbo FANG1,Bingtao ZHAO1( ),Daqi WANG1,Yaxin SU2
),Daqi WANG1,Yaxin SU2
- 1. School of Energy and Power Engineering, University of Shanghai for Science and Technology, Shanghai 200093, China
2. School of Environmental Science and Engineering, Donghua University, Shanghai 201620, China
-
Received:2018-05-28Revised:2018-07-03Online:2019-03-05Published:2019-03-05 -
Contact:Bingtao ZHAO
二元复合溶液脱除烟气中CO2 的过程模拟与评价
- 1. 上海理工大学能源与动力工程学院,上海 200093
2. 东华大学环境科学与工程学院,上海 201620
-
通讯作者:赵兵涛 -
作者简介:方童波 (1993—),男,硕士研究生,研究方向为燃烧污染控制。|赵兵涛,教授,硕士生导师,研究方向为CO2捕获及燃烧源大气污染控制。E-mail:zhaobingtao@usst.edu.cn 。 -
基金资助:上海市自然科学基金(17ZR1419300)
CLC Number:
Cite this article
Tongbo FANG,Bingtao ZHAO,Daqi WANG,Yaxin SU. Process simulation and evaluation of CO2 removal from flue gas by binary compound solutions[J]. Chemical Industry and Engineering Progress, 2019, 38(03): 1561-1566.
方童波,赵兵涛,王大淇,苏亚欣. 二元复合溶液脱除烟气中CO2 的过程模拟与评价[J]. 化工进展, 2019, 38(03): 1561-1566.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2018-1089
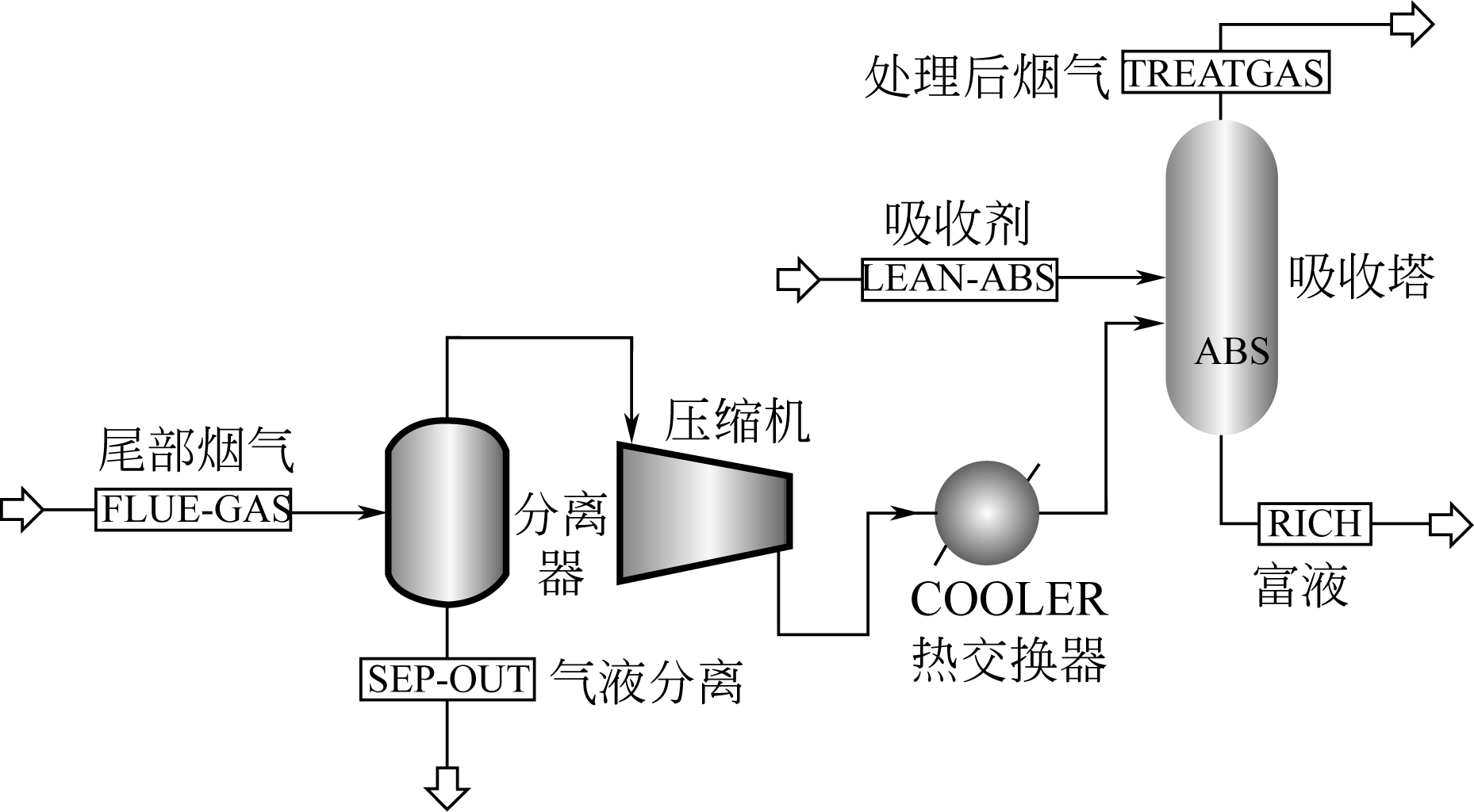
| 烟气参数 | 数值 | 吸收剂参数 | 数值 |
|---|---|---|---|
| 流量/kmol·h-1 | 69738.9 | 流量/kmol·h-1 | 250000 |
| 压力/MPa | 0.1 | 压力/MPa | 0.12 |
| 温度/℃ | 57.8 | 温度/℃ | 40 |
| CO2体积分数/% | 12.8 | 总摩尔浓度/mol·L-1 | 2 |
| O2体积分数/% | 2.9 | 摩尔分数配比 | 90%∶10% |
| N2体积分数/% | 68.3 | CO2负荷/molCO2·(mol吸附剂-1) | 0.1 |
| H2O体积分数/% | 16 |
| 烟气参数 | 数值 | 吸收剂参数 | 数值 |
|---|---|---|---|
| 流量/kmol·h-1 | 69738.9 | 流量/kmol·h-1 | 250000 |
| 压力/MPa | 0.1 | 压力/MPa | 0.12 |
| 温度/℃ | 57.8 | 温度/℃ | 40 |
| CO2体积分数/% | 12.8 | 总摩尔浓度/mol·L-1 | 2 |
| O2体积分数/% | 2.9 | 摩尔分数配比 | 90%∶10% |
| N2体积分数/% | 68.3 | CO2负荷/molCO2·(mol吸附剂-1) | 0.1 |
| H2O体积分数/% | 16 |
| 水平 | 因素 | ||
|---|---|---|---|
| 温度A/℃ | 摩尔流量B/kmol·h-1 | 摩尔分数C配比 | |
| 1 | 30 | 2.0×105 | 60%∶40% |
| 2 | 40 | 2.5×105 | 70%∶30% |
| 3 | 50 | 3.0×105 | 80%∶20% |
| 4 | 60 | 3.5×105 | 90%∶10% |
| 水平 | 因素 | ||
|---|---|---|---|
| 温度A/℃ | 摩尔流量B/kmol·h-1 | 摩尔分数C配比 | |
| 1 | 30 | 2.0×105 | 60%∶40% |
| 2 | 40 | 2.5×105 | 70%∶30% |
| 3 | 50 | 3.0×105 | 80%∶20% |
| 4 | 60 | 3.5×105 | 90%∶10% |
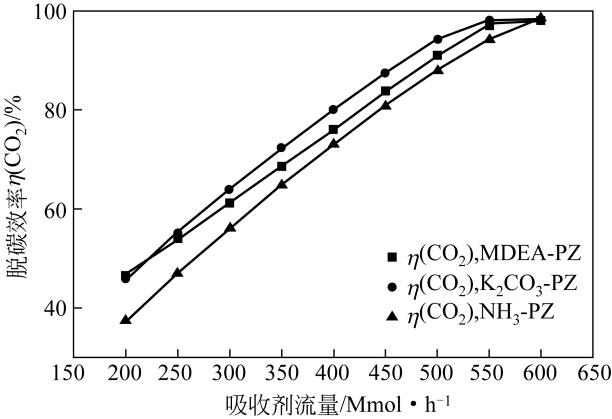
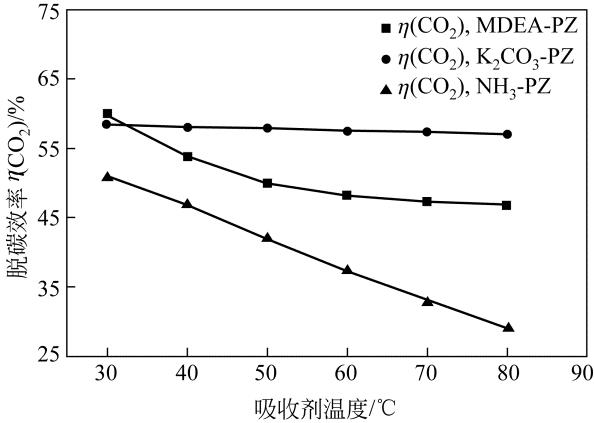
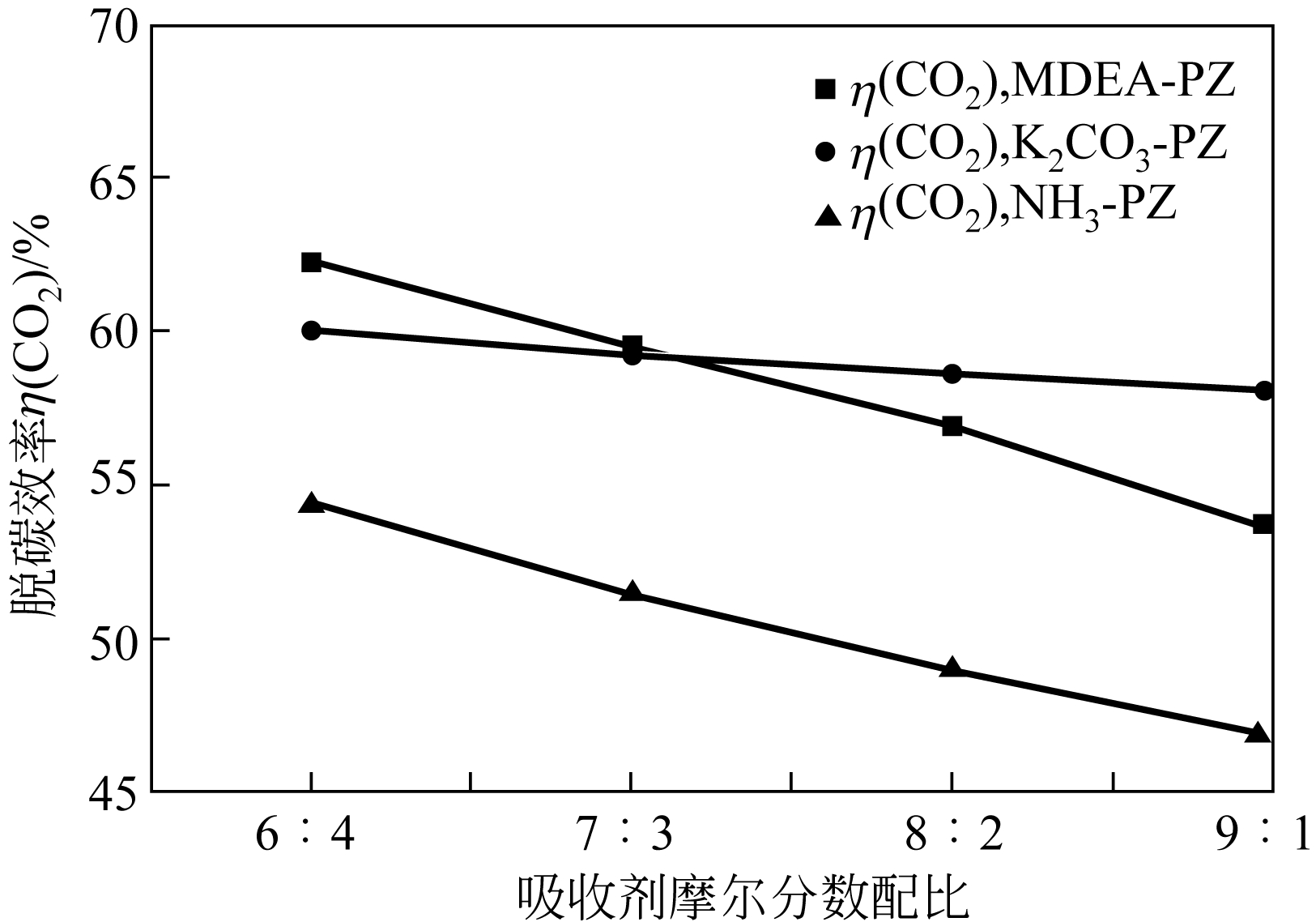
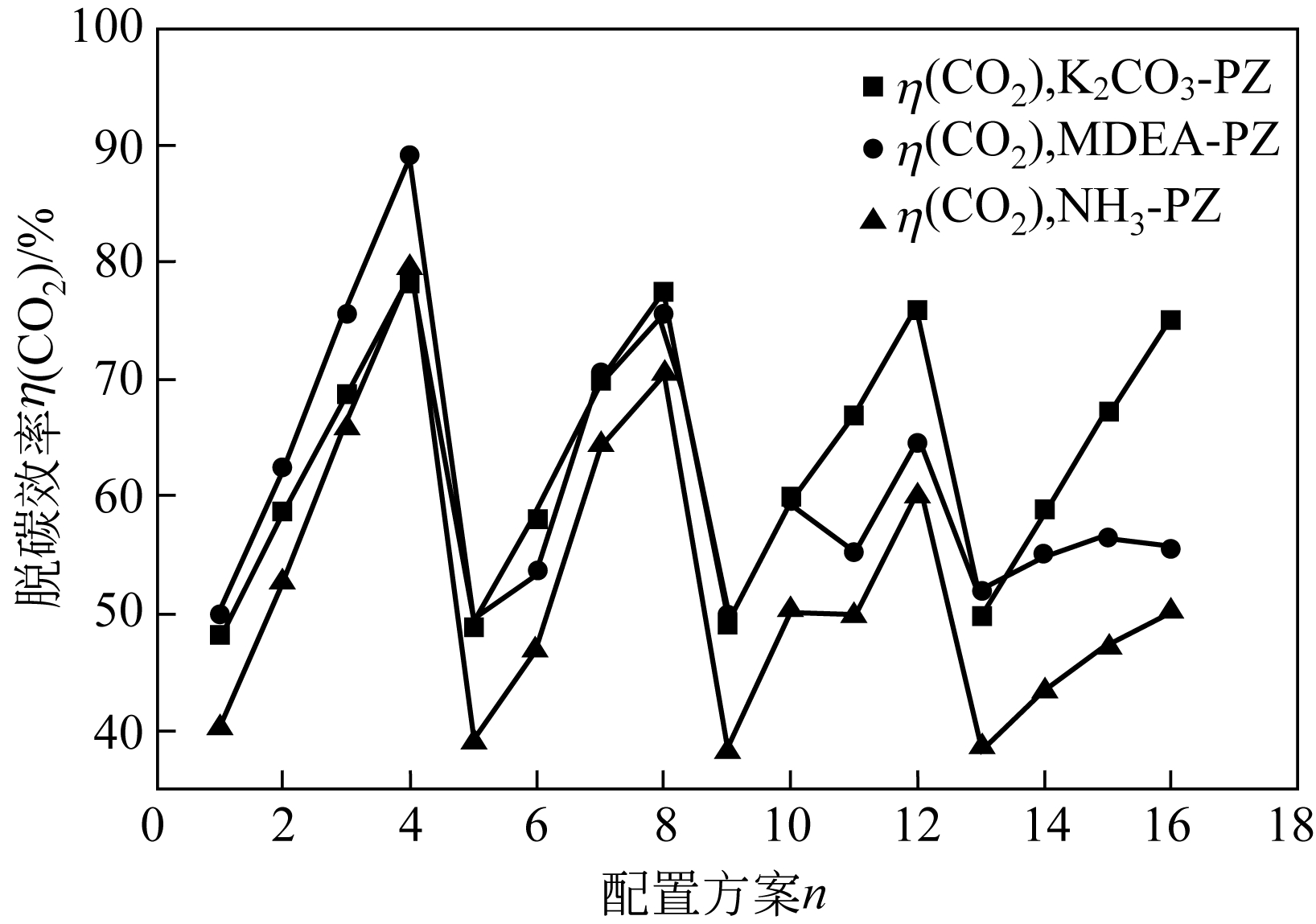
| 参数 | K2CO3-PZ二元复合溶液 | MDEA-PZ二元复合溶液 | NH3-PZ二元复合溶液 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | A | B | C | A | B | C | |
| K 1 | 254.1 | 195.9 | 257.7 | 277.0 | 200.5 | 271.0 | 238.2 | 156.3 | 232.9 |
| K 2 | 254.1 | 235.5 | 254.3 | 248.9 | 230.9 | 256.3 | 220.8 | 193.4 | 218.1 |
| K 3 | 251.9 | 272.6 | 250.6 | 229.0 | 257.8 | 232.5 | 199.0 | 227.3 | 199.3 |
| K 4 | 250.8 | 306.8 | 248.3 | 219 | 284.8 | 214.2 | 179.4 | 260.4 | 187.1 |
| k 1 | 63.51 | 48.99 | 64.42 | 69.24 | 50.1 | 67.74 | 59.55 | 39.07 | 58.23 |
| k 2 | 63.53 | 58.88 | 63.58 | 62.24 | 57.72 | 64.08 | 55.21 | 48.36 | 54.53 |
| k 3 | 62.97 | 68.14 | 62.65 | 57.25 | 64.45 | 58.12 | 49.76 | 56.81 | 49.83 |
| k 4 | 62.7 | 76.7 | 62.07 | 54.75 | 71.19 | 53.54 | 44.84 | 65.11 | 46.77 |
| 极差 | 0.833 | 27.713 | 2.348 | 14.493 | 21.07 | 14.203 | 14.705 | 26.038 | 11.463 |
| 参数 | K2CO3-PZ二元复合溶液 | MDEA-PZ二元复合溶液 | NH3-PZ二元复合溶液 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | A | B | C | A | B | C | |
| K 1 | 254.1 | 195.9 | 257.7 | 277.0 | 200.5 | 271.0 | 238.2 | 156.3 | 232.9 |
| K 2 | 254.1 | 235.5 | 254.3 | 248.9 | 230.9 | 256.3 | 220.8 | 193.4 | 218.1 |
| K 3 | 251.9 | 272.6 | 250.6 | 229.0 | 257.8 | 232.5 | 199.0 | 227.3 | 199.3 |
| K 4 | 250.8 | 306.8 | 248.3 | 219 | 284.8 | 214.2 | 179.4 | 260.4 | 187.1 |
| k 1 | 63.51 | 48.99 | 64.42 | 69.24 | 50.1 | 67.74 | 59.55 | 39.07 | 58.23 |
| k 2 | 63.53 | 58.88 | 63.58 | 62.24 | 57.72 | 64.08 | 55.21 | 48.36 | 54.53 |
| k 3 | 62.97 | 68.14 | 62.65 | 57.25 | 64.45 | 58.12 | 49.76 | 56.81 | 49.83 |
| k 4 | 62.7 | 76.7 | 62.07 | 54.75 | 71.19 | 53.54 | 44.84 | 65.11 | 46.77 |
| 极差 | 0.833 | 27.713 | 2.348 | 14.493 | 21.07 | 14.203 | 14.705 | 26.038 | 11.463 |
| 1 | 高凤玲,崔国民,黄晓璜 . CO2的温室效应饱和度分析及其大气体积分数预测模型[J]. 上海理工大学学报,2017(4): 323-328. |
| GAO Fengling , CUI Guomin , HUANG Xiaohuang . Greenhouse effect saturation analysis and the atmospheric concentration prediction model of CO2 [J]. Journal of University of Shanghai for Science & Technology, 2017(4): 323-328. | |
| 2 | 何书申,赵兵涛,俞致远 . 基于胺法的旋流喷淋气液吸收烟气CO2的性能[J]. 上海理工大学学报,2016,38(1):25-30. |
| HE Shushen , ZHAO Bingtao , YU Zhiyuan . Performance of CO2 capture from flue gas with amines in vortex flow spraying scrubber [J]. Journal of University of Shanghai for Science & Technology, 2016, 38(1): 25-30. | |
| 3 | 周响球 . 燃煤电厂烟气二氧化碳捕获系统的仿真研究[D]. 重庆:重庆大学, 2008. |
| ZHOU Xiangqiu . Simulation of CO2 capture system for coal-fired power plants [D]. Chongqing: Chongqing University, 2008. | |
| 4 | ARACHCHIGE U S P R , MELAAEN M C . Aspen Plus simulation of CO2 removal from coal and gas fired power plants[J]. Energy Procedia, 2012, 23(2): 391-399. |
| 5 | YU Jingwen , WANG Shujuan , YU Hai . Experimental studies and Rate-based simulations of CO2 absorption with aqueous ammonia and piperazine blended solutions[J]. International Journal of Greenhouse Gas Control, 2016, 50: 135-146. |
| 6 | 张亚萍,刘建周,季芹芹,等 . 醇胺法捕集燃煤烟气CO2工艺模拟及优化[J]. 化工进展,2013, 32(4): 930-935. |
| ZHANG Yaping , LIU Jianzhou , JI Qinqin , et al . Process simulation and optimization of flue gas CO2 capture by the alkanolamine solutions [J]. Chemical Industry and Engineering Progress, 2013, 32(4): 930-935. | |
| 7 | ZHANG Minkai , GUO Yincheng . A novel process for NH3-based CO2 capture by integrating flow-by capacitive ion separation [J]. International Journal of Greenhouse Gas Control, 2016, 54: 50-58. |
| 8 | ZHAO Bin , LIU Fangzheng , CUI Zheng , et al . Enhancing the energetic efficiency of MDEA/PZ-based CO2 capture technology for a 650 MW power plant: Process improvement[J]. Applied Energy, 2017, 185: 362-375. |
| 9 | AROONWILAS A , VEAWAB A . Integration of CO2 capture unit using blended MEA-AMP solution into coal-fired power plants[J]. Energy Procedia, 2009, 1(1): 4315-4321. |
| 10 | 张克舫,刘中良,王远亚,等 . 化学吸收法CO2捕集解吸能耗的分析计算[J]. 化工进展,2013,32(12):3008-3014. |
| ZHANG Kefang , LIU Zhongliang , WANG Yuanya , et al . Analysis and calculation of the desorption energy consumption of CO2 capture process by chemical absorption method[J]. Chemical Industry and Engineering Progress, 2013, 32(12): 3008-3014. | |
| 11 | DEY A, AROONWILAS A . CO2 absorption into MEA-AMP blend: mass transfer and absorber height index[J]. Energy Procedia, 2009, 1(1): 211-215. |
| 12 | 骆培成,焦真,张志炳 . 填料塔中碳酸钾/哌嗪混合吸收液脱除CO2的体积传质系数[J]. 化工学报, 2005, 56(1): 53-57. |
| LUO Peicheng , JIAO Zhen , ZHANG Zhibing . Volumetric mass transfer coefficients of dilute CO2 absorption into mixtures of potassium carbonate and piperazine in packed column[J]. Journal of Chemical Industry & Engineering (China), 2005, 56(1): 53-57. | |
| 13 | MORES P , RODRÍGUEZ N , SCENNA N , et al . CO2 capture in power plants: minimization of the investment and operating cost of the post-combustion process using MEA aqueous solution[J]. International Journal of Greenhouse Gas Control, 2012, 10(1): 148-163. |
| 14 | ZHANG Minkai , GUO Yincheng . Rate-based modeling of absorption and regeneration for CO2 capture by aqueous ammonia solution[J]. Applied Energy, 2013, 111(4): 142-152. |
| 15 | HUAMÁN R N E . Optimized simulation of CO2 removal process from coal fired power plants with MEA by sensitivity analysis in Aspen plus [J]. Labor & Engenho, 2017, 11(2): 191. |
| 16 | BRAVO J L . Mass transfer in gauze packings[J]. Hydrocarbon Processing, 1985, 64(1): 91-95. |
| 17 | CHILTON T H , COLBURN A P . Mass transfer (absorption) coefficients prediction from data on heat transfer and fluid friction[J]. Ind. Eng. Chem., 1934: 26. |
| 18 | ARSHAD M , WUKOVITS W , FRIEDL A . Simulation of CO2 absorption using the system K2CO3-piperazine[J]. Chemical Engineering Transactions, 2014, 39(2): 577-582. |
| 19 | BISHNOI S , ROCHELLE G T . Absorption of carbon dioxide into aqueous piperazine: reaction kinetics, mass transfer and solubility [J]. Chemical Engineering Science, 2000, 55(22): 5531-5543. |
| 20 | ALIE C , BACKHAM L , CROISET E , et al . Simulation of CO2 capture using MEA scrubbing: a flowsheet decomposition method [J]. Energy Conversion & Management, 2005, 46(3): 475-487. |
| 21 | 江文敏 . 化学吸收法捕集二氧化碳工艺的模拟及实验研究[D]. 杭州: 浙江大学, 2015. |
| JIANG Wenmin . Simulation and experimental research of CO2 chemical absorption system [D]. Hangzhou: Zhejiang University, 2015. |
| [1] | DAI Huantao, CAO Lingyu, YOU Xinxiu, XU Haoliang, WANG Tao, XIANG Wei, ZHANG Xueyang. Adsorption properties of CO2 on pomelo peel biochar impregnated by lignin [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 356-363. |
| [2] | CHEN Chongming, CHEN Qiu, GONG Yunqian, CHE Kai, YU Jinxing, SUN Nannan. Research progresses on zeolite-based CO2 adsorbents [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 411-419. |
| [3] | WANG Shengyan, DENG Shuai, ZHAO Ruikai. Research progress on carbon dioxide capture technology based on electric swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 233-245. |
| [4] | YANG Ying, HOU Haojie, HUANG Rui, CUI Yu, WANG Bing, LIU Jian, BAO Weiren, CHANG Liping, WANG Jiancheng, HAN Lina. Coal tar phenol-based carbon nanosphere prepared by Stöber method for adsorption of CO2 [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 5011-5018. |
| [5] | WANG Yungang, JIAO Jian, DENG Shifeng, ZHAO Qinxin, SHAO Huaishuang. Experimental analysis of condensation heat transfer and synergistic desulfurization [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4230-4237. |
| [6] | BAI Yadi, DENG Shuai, ZHAO Ruikai, ZHAO Li, YANG Yingxia. Exploration on standardized test scheme and experimental performance of temperature swing adsorption carbon capture unit [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3834-3846. |
| [7] | GU Shiya, DONG Yachao, LIU Linlin, ZHANG Lei, ZHUANG Yu, DU Jian. Design and optimization of pipeline system for carbon capture considering intermediate nodes [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2799-2808. |
| [8] | WANG Baowen, LIU Tongqing, ZHANG Gang, LI Weiguang, LIN Deshun, WANG Mengjia, MA Jingjing. Reaction characteristics of CuFe2O4 modified desulfurization slag oxygen carrier with lignite [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2884-2894. |
| [9] | LU Shijian, ZHANG Yuanyuan, WU Wenhua, YANG Fei, LIU Ling, KANG Guojun, LI Qingfang, CHEN Hongfu, WANG Ning, WANG Feng, ZHANG Juanjuan. Health risk assessment of nitrosamine pollutant diffusion in a million ton CO2 capture project [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3209-3216. |
| [10] | WANG Jiuheng, RONG Nai, LIU Kaiwei, HAN Long, SHUI Taotao, WU Yan, MU Zhengyong, LIAO Xuqing, MENG Wenjia. Enhanced CO2 capture performance and strength of cellulose-templated CaO-based pellets with steam reactivation [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3217-3225. |
| [11] | HE Chuan, WU Guoxun, LI Ang, ZHANG Fajie, BIAN Zijun, LU Chengzheng, WANG Lipeng, ZHAO Min. Characteristics of calcium and magnesium deactivation and regeneration of waste incineration SCR catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2413-2420. |
| [12] | FU Le, YANG Yang, XU Wenqing, GENG Zanbu, ZHU Tingyu, HAO Runlong. Research progress in CO2 capture technology using novel biphasic organic amine absorbent [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2068-2080. |
| [13] | SANG Wei, TANG Jianfeng, HUA Yihuai, CHEN Jie, SUN Peiyuan, XU Yifei. Effects of physical solvent and amine properties on the performance of biphasic solvent [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2151-2159. |
| [14] | SHANG Yu, XIAO Man, CUI Qiufang, TU Te, YAN Shuiping. Recovery characteristics of PVDF/BN-OH flat composite membrane for waste heat of hot stripped gas in CO2 capture process [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1618-1628. |
| [15] | KONG Xiangru, ZHANG Xiaoyang, SUN Pengxiang, CUI Lin, DONG Yong. Research progress of solid porous materials for direct CO2 capture from air [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1471-1483. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||