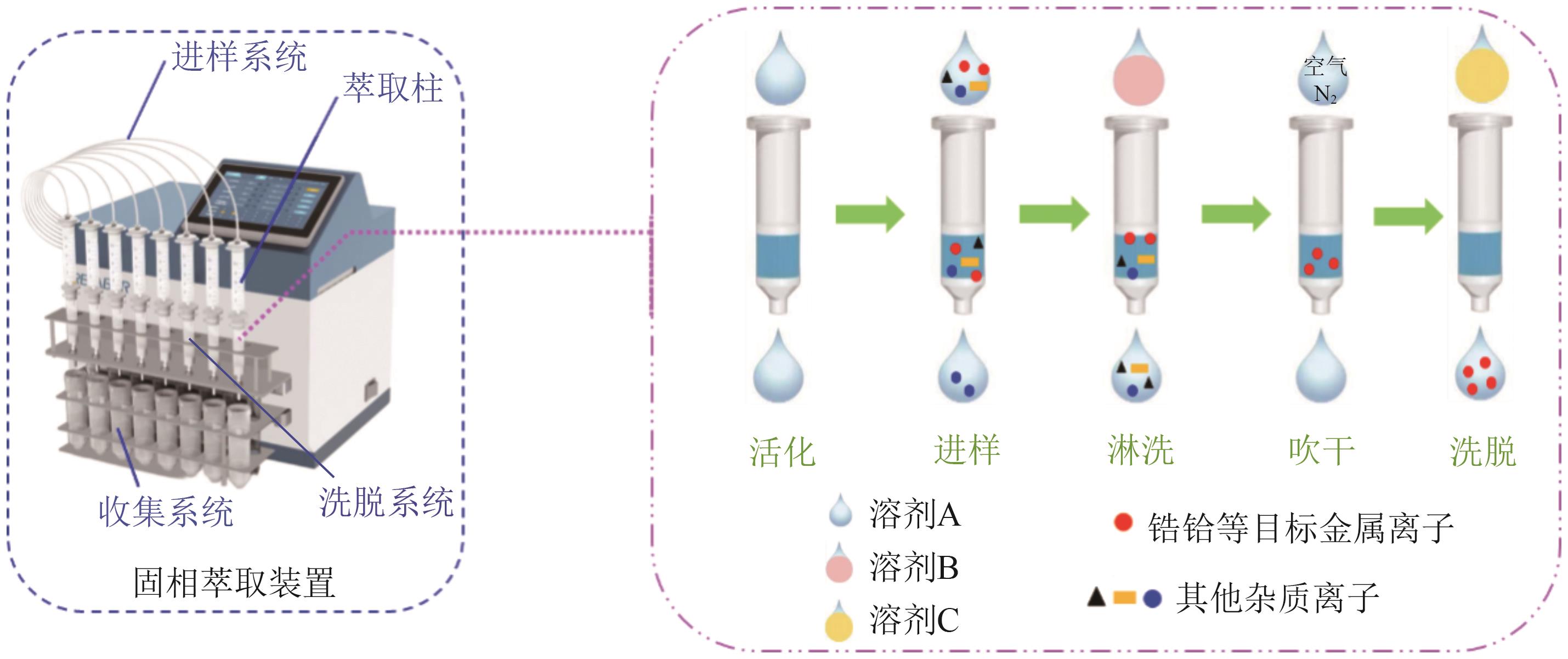
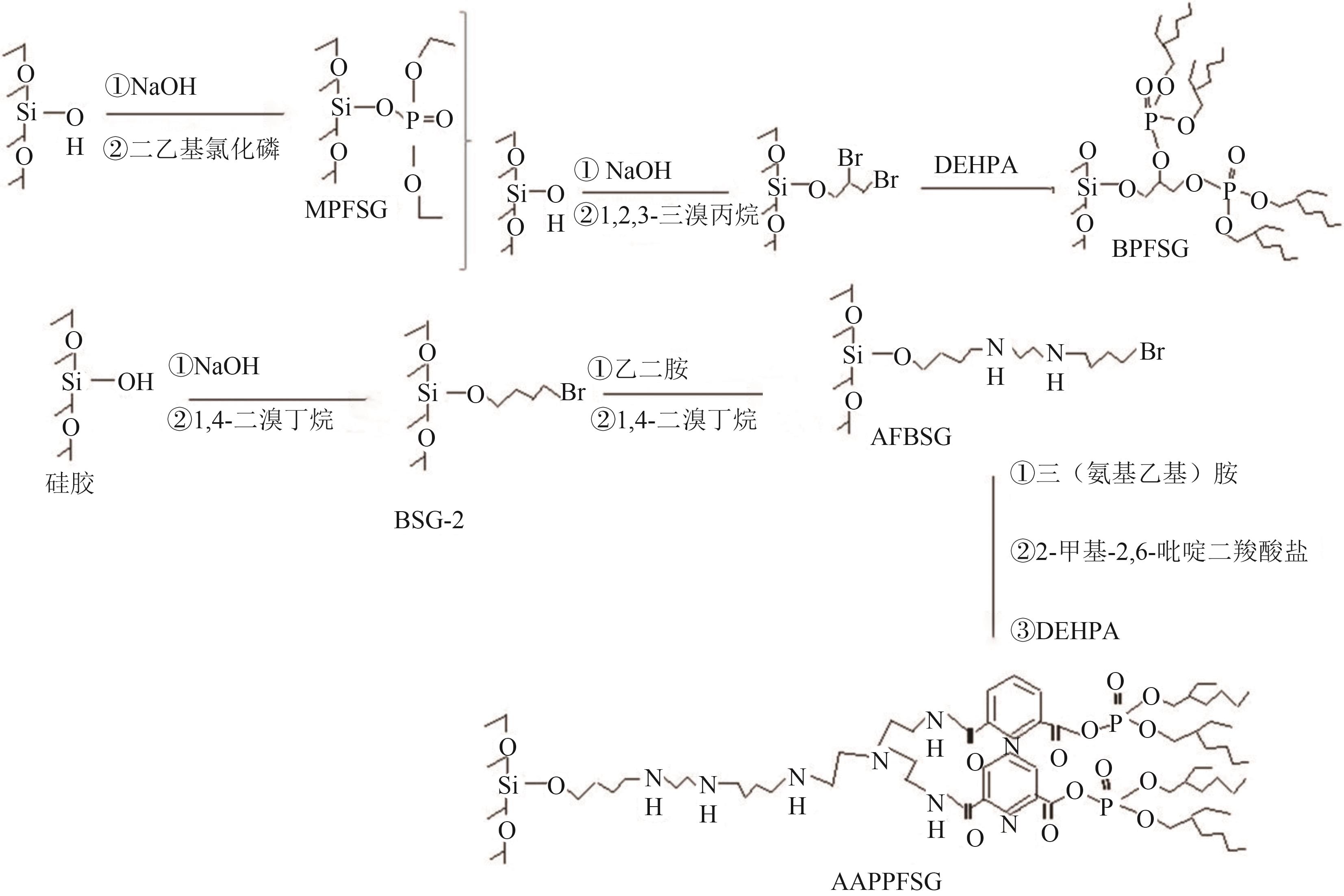
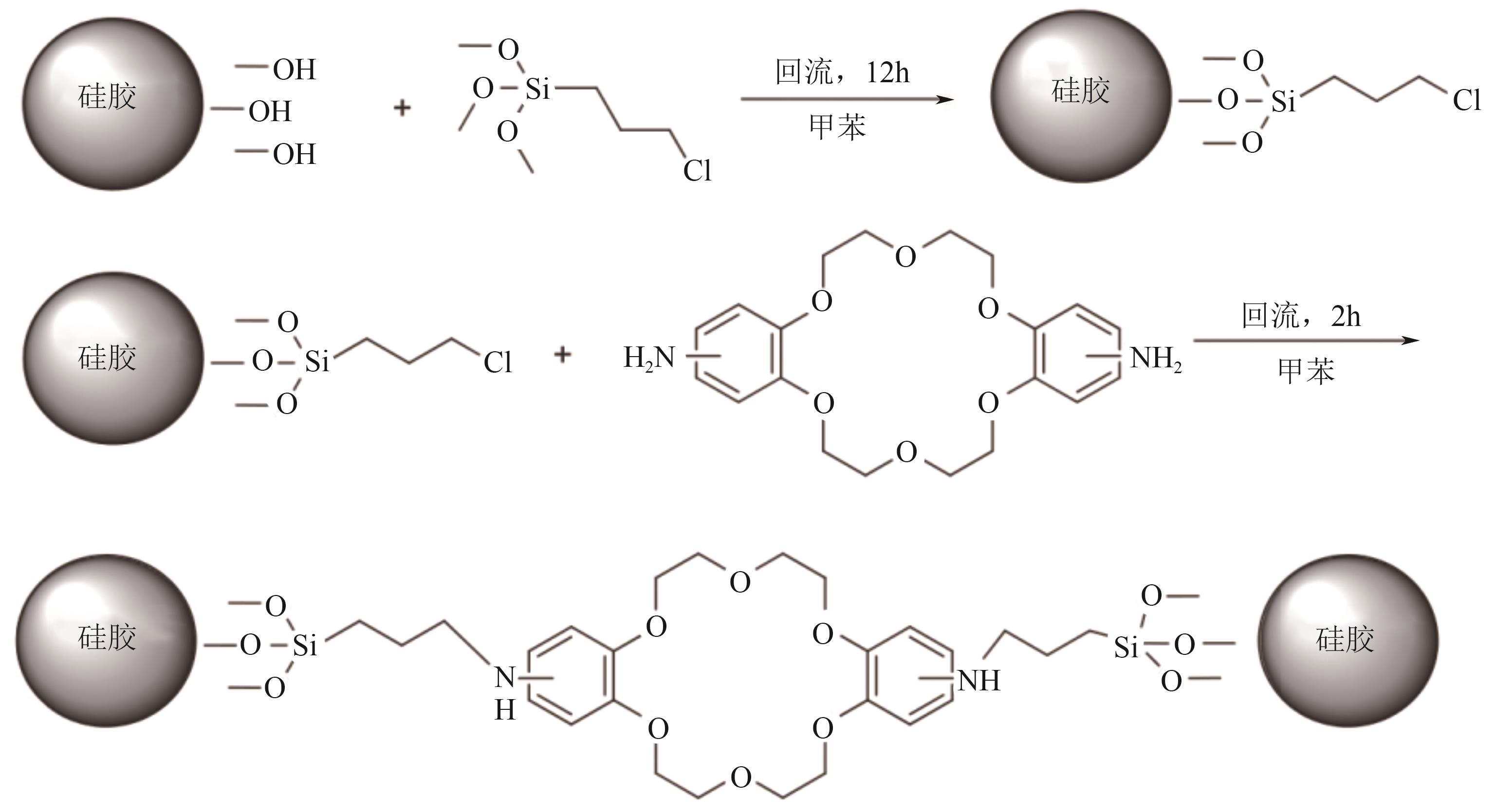
Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (10): 5991-6003.DOI: 10.16085/j.issn.1000-6613.2024-1443
• Resources and environmental engineering • Previous Articles
Preparation, structures and performance of extraction resins based on zirconium and hafnium separation with extraction chromatography
YANG Xiumin1( ), HU Chengqiang1, WANG Junlian1(
), HU Chengqiang1, WANG Junlian1( ), LI Yong2, LIU Xinyu3
), LI Yong2, LIU Xinyu3
- 1.School of Civil and Resource Engineering, University of Science and Technology Beijing, Beijing 100083, China
2.Consulting and Research Center of Ministry of Natural Resources, Beijing 100035, China
3.Norin Mining Co. , Ltd. , Beijing 100053, China
-
Received:2024-09-03Revised:2024-11-18Online:2025-11-10Published:2025-10-25 -
Contact:WANG Junlian
基于萃取色层法分离锆铪的萃淋树脂制备、结构与性能
杨秀敏1( ), 胡成强1, 王俊莲1(
), 胡成强1, 王俊莲1( ), 李勇2, 刘新宇3
), 李勇2, 刘新宇3
- 1.北京科技大学土木与资源工程学院,北京 100083
2.自然资源部咨询研究中心,北京 100035
3.北方矿业有限责任公司,北京 100053
-
通讯作者:王俊莲 -
作者简介:杨秀敏(2000—),女,硕士研究生,研究方向为锆铪萃取分离。E-mail:xiuminyang2022@163.com。 -
基金资助:国家自然科学基金(51974026)
CLC Number:
Cite this article
YANG Xiumin, HU Chengqiang, WANG Junlian, LI Yong, LIU Xinyu. Preparation, structures and performance of extraction resins based on zirconium and hafnium separation with extraction chromatography[J]. Chemical Industry and Engineering Progress, 2025, 44(10): 5991-6003.
杨秀敏, 胡成强, 王俊莲, 李勇, 刘新宇. 基于萃取色层法分离锆铪的萃淋树脂制备、结构与性能[J]. 化工进展, 2025, 44(10): 5991-6003.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-1443
| 树脂 | 官能团 | 基底 | 制备方法 | 体系最佳条件 | 选择性 | Qmax(Hf)/mg·g-1 | Qmax(Zr)/mg·g-1 | 分离系数β | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| SIR-MIBK/HPD100 | 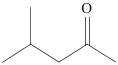 | HPD100 | 浸渍法 | [NH4SCN]=0.5mol/L,[HCl]=1.1mol/L | Hf | 14.33 | 473.80 | — | [ |
| SIR-TBP/PEG-coated-SPIONs | 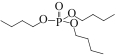 | PEG-coated-γ-Fe2O3 | 浸渍法 | [HNO3]=4.0mol/L | Zr | — | — | βZr/Hf=5 | [ |
| [HCl]=4.0mol/L | Hf | — | — | βHf/Zr=1.4 | |||||
| UTEVA | 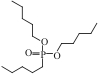 | — | 浸渍法 | [HCl]=5.6mol/L | Zr | — | — | βZr/Hf>9.4 | [ |
| [HNO3]=4.9mol/L | Zr | — | — | βZr/Hf=10±1 | |||||
| [H2SO4]=12.1mol/L | Hf | — | — | βHf/Zr=2.0±0.2 | |||||
| MPFSG | 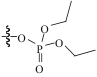 | SiO2 | 接枝法 | HNO3, pH=1.8 | Zr | 11.8 | 12.3 | βZr/Hf=1.1 | [ |
| BPFSG | 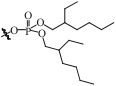 | SiO2 | 接枝法 | HNO3, pH=1.8 | Zr | 15 | 16.7 | βZr/Hf=1.2 | [ |
| AAPPFSG | 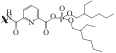 | SiO2 | 接枝法 | HNO3, pH=1.8 | Zr | 24 | 29 | βZr/Hf=11.0 | [ |
| SGN18 | 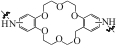 | SiO2 | 接枝法 | HCl, pH=1.5 | Zr | 3.3 | 38.3 | βZr/Hf=5.72 | [ |
| SIR-Calix[ | 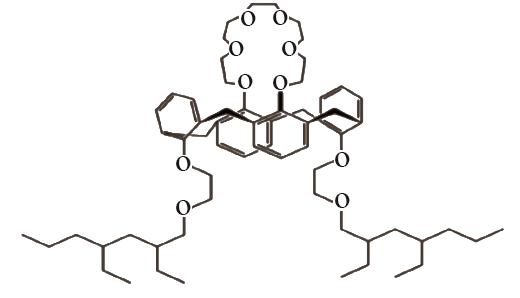 | SiO2-P | 浸渍法 | [HCl]=1mol/L | Zr | — | — | βZr/Hf=1.4 | [ |
| SIR-TODGA/SiO2-P | 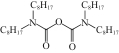 | SiO2-P | 浸渍法 | [HCl]=1.5mol/L | Zr | 75.85 | 44.24 | βZr/Hf=5.2 | [ |
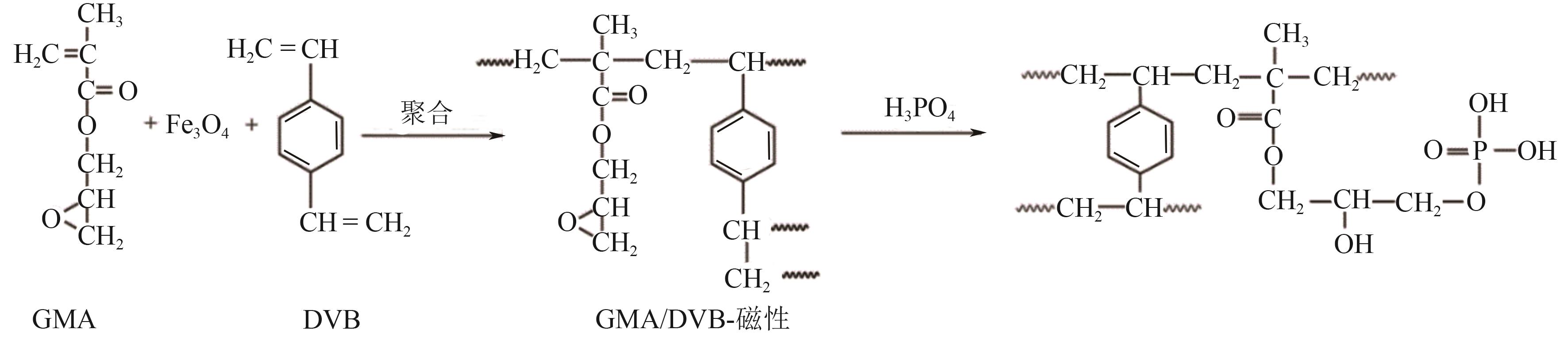
| R-PO4H2 | 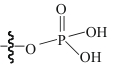 | GMA/DVB 修饰的 Fe3O4 | 接枝法 | HCl, pH=2.5 | Zr | 42.88 | 85.75 | βZr/Hf=6 | [ |
| TVEX-P507 | 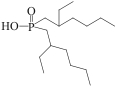 | 苯乙烯-二乙烯基共聚物 | 原位聚合法 | [H2SO4]=0.08mol/L | Hf | 0.21 | — | βHf/Zr=6.81 | [ |
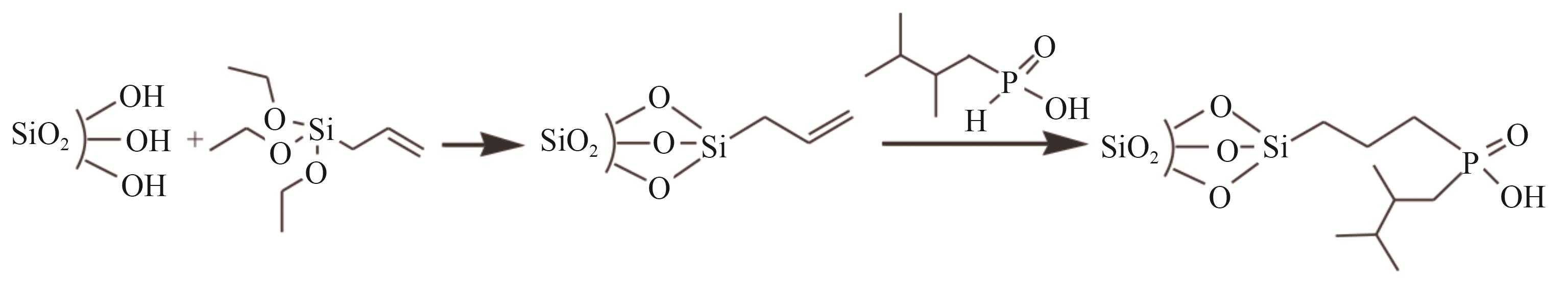
| SiO2-POOH | 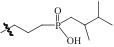 | SiO2 | 接枝法 | [H2SO4]=0.5mol/L | Hf | 31.62 | — | βHf/Zr =4.69 | [ |
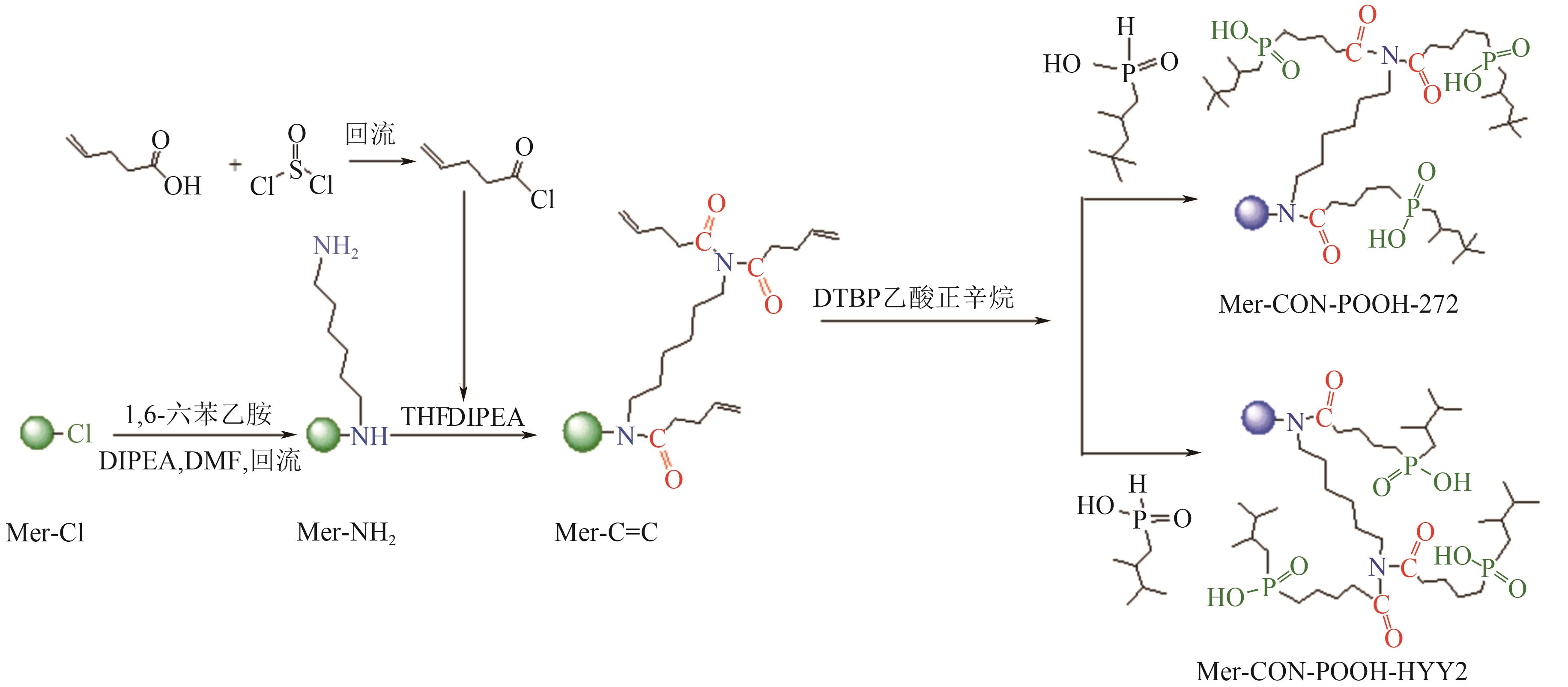
| Mer-CON-POOH-HYY2 | 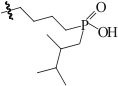 | 氯球 | 接枝法 | [H2SO4]=0.3mol/L | Hf | 13.16 | — | βHf/Zr=4.33 | [ |
| Mer-CON-POOH-272 | 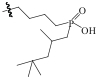 | 氯球 | 接枝法 | [H2SO4]=0.3mol/L | Hf | 11.11 | — | βHf/Zr=2.96 | |
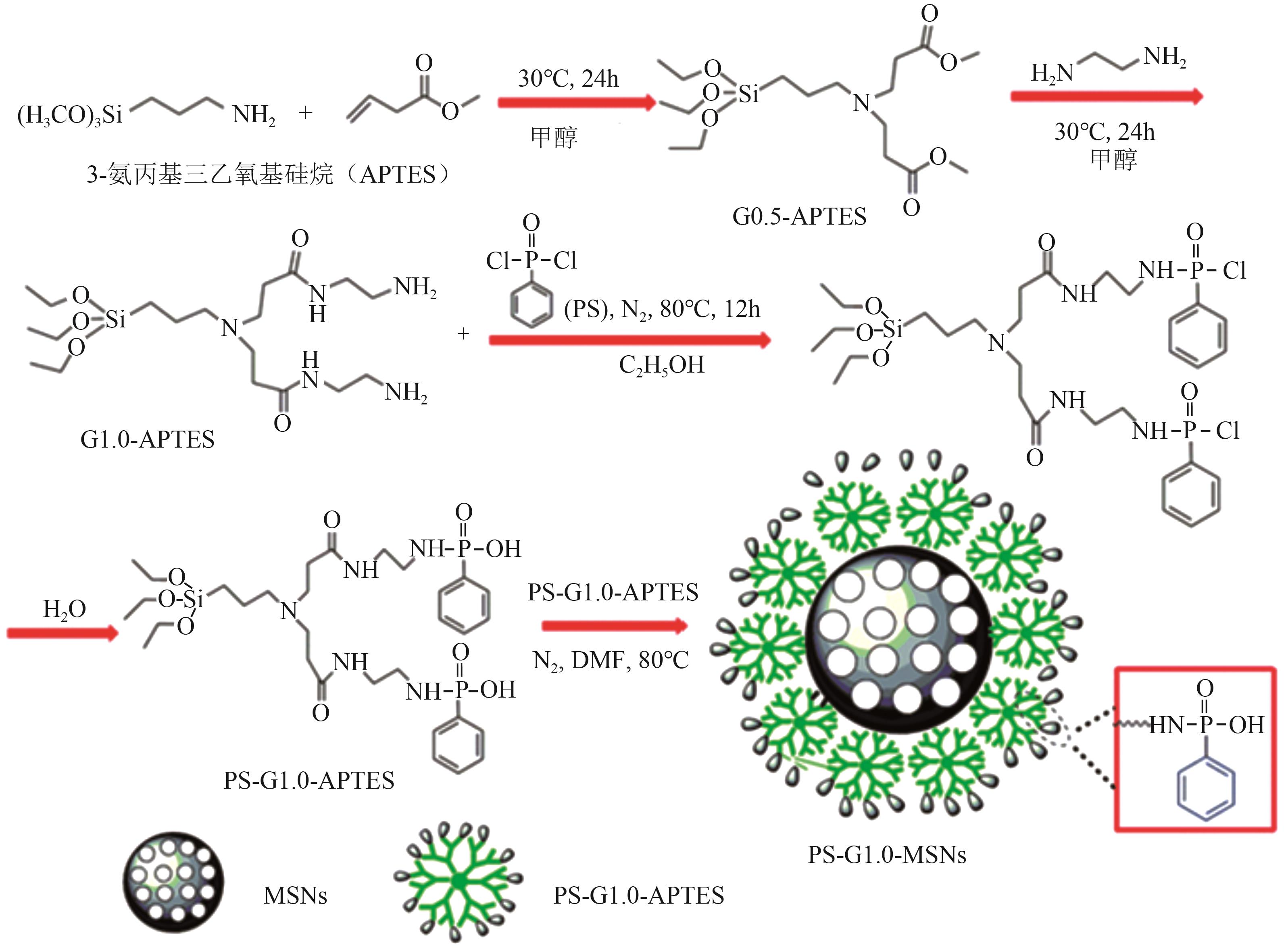
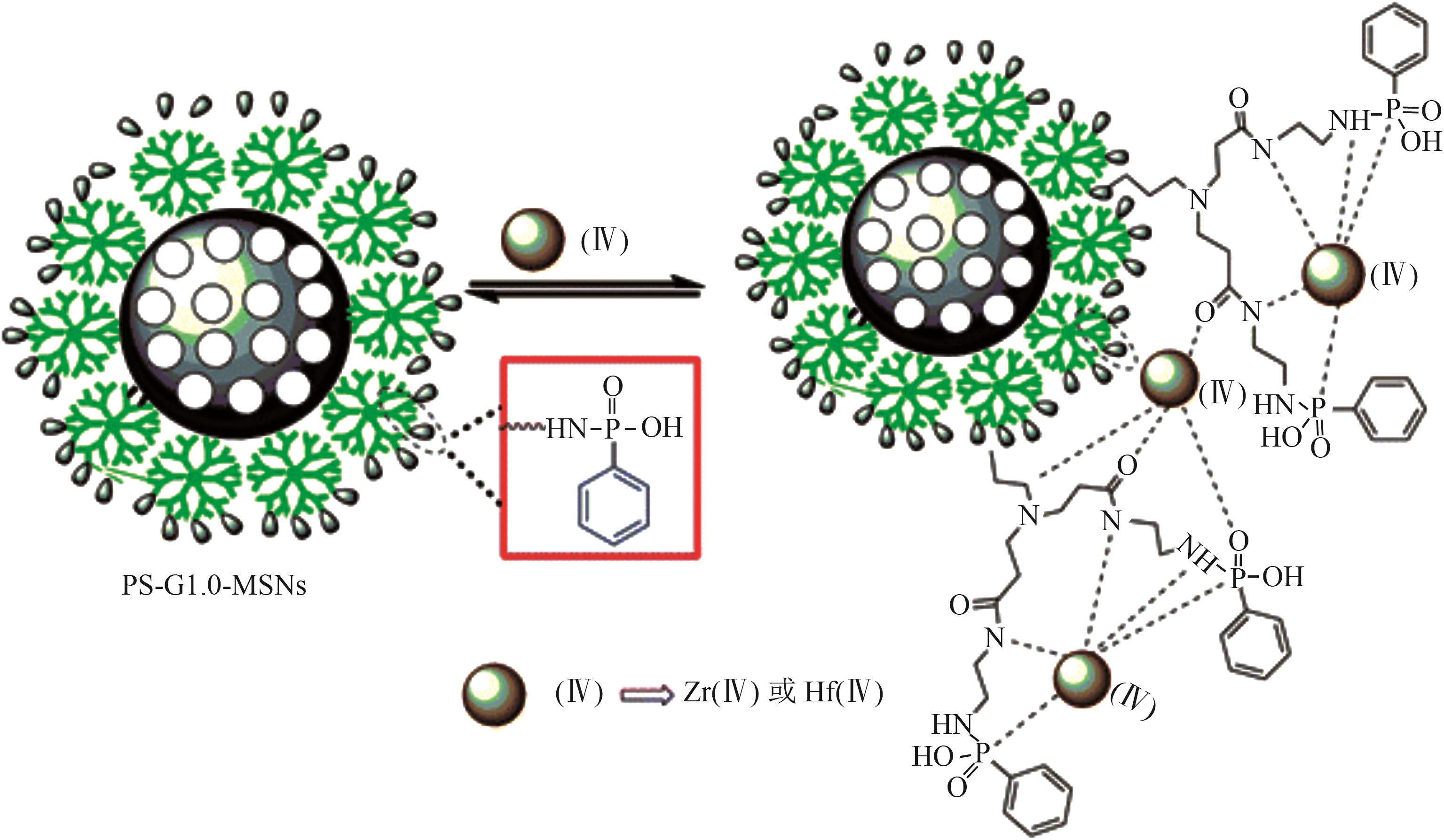
| PS-G1.0-MSNs | 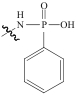 | SiO2 | 接枝法 | HNO3, pH=0.5 | Hf | 5.36 | 25.7 | βHf/Zr=2.0 | [ |
| Marathon C | —SO3H | 苯乙烯-二乙烯基共聚物 | 接枝法 | H2SO4, pH=2.5 | Hf | 24.92 | 103.74 | βHf/Zr=2.63 | [ |
| Diphonix | —PO4H2、—SO3H、—COOH | 苯乙烯-二乙烯基共聚物 | 接枝法 | [H2SO4]=1mol/L | Hf | — | — | βHf/Zr=1.47 | [ |
| Purolite S-957 | —PO4H2、—SO3H | 苯乙烯-二乙烯基共聚物 | 接枝法 | [H2SO4]=0.5mol/L | Hf | — | — | βHf/Zr=2.8 | [ |
| SIR-BTP/ SiO2-P | 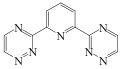 | SiO2-P | 浸渍法 | [HCl]=1mol/L | Hf | — | — | βHf/Zr=1.1 | [ |
| Reillex PVP | 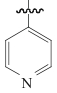 | 苯乙烯-二乙烯基共聚物 | 接枝法 | [HCl]=9.5mol/L | Zr | — | — | βZr/Hf=10.4 | [ |
| TEVA | 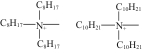 | — | 浸渍法 | [HCl]=8.4mol/L | Zr | — | — | βZr/Hf=18±8 | [ |
| Amberjet 4200 Cl | 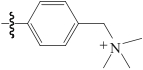 | 苯乙烯-二乙烯基共聚物 | 接枝法 | [HCl]=9.5mol/L | Zr | — | 86 | βZr/Hf=11.4 | [ |
| Reillex HPQ | 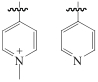 | 聚乙烯基吡咯烷酮-二乙烯基苯 | — | [HCl]=9.5mol/L | Zr | — | — | βZr/Hf=9.7 | [ |
| 树脂 | 官能团 | 基底 | 制备方法 | 体系最佳条件 | 选择性 | Qmax(Hf)/mg·g-1 | Qmax(Zr)/mg·g-1 | 分离系数β | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| SIR-MIBK/HPD100 |  | HPD100 | 浸渍法 | [NH4SCN]=0.5mol/L,[HCl]=1.1mol/L | Hf | 14.33 | 473.80 | — | [ |
| SIR-TBP/PEG-coated-SPIONs |  | PEG-coated-γ-Fe2O3 | 浸渍法 | [HNO3]=4.0mol/L | Zr | — | — | βZr/Hf=5 | [ |
| [HCl]=4.0mol/L | Hf | — | — | βHf/Zr=1.4 | |||||
| UTEVA |  | — | 浸渍法 | [HCl]=5.6mol/L | Zr | — | — | βZr/Hf>9.4 | [ |
| [HNO3]=4.9mol/L | Zr | — | — | βZr/Hf=10±1 | |||||
| [H2SO4]=12.1mol/L | Hf | — | — | βHf/Zr=2.0±0.2 | |||||
| MPFSG |  | SiO2 | 接枝法 | HNO3, pH=1.8 | Zr | 11.8 | 12.3 | βZr/Hf=1.1 | [ |
| BPFSG |  | SiO2 | 接枝法 | HNO3, pH=1.8 | Zr | 15 | 16.7 | βZr/Hf=1.2 | [ |
| AAPPFSG |  | SiO2 | 接枝法 | HNO3, pH=1.8 | Zr | 24 | 29 | βZr/Hf=11.0 | [ |
| SGN18 |  | SiO2 | 接枝法 | HCl, pH=1.5 | Zr | 3.3 | 38.3 | βZr/Hf=5.72 | [ |
| SIR-Calix[ |  | SiO2-P | 浸渍法 | [HCl]=1mol/L | Zr | — | — | βZr/Hf=1.4 | [ |
| SIR-TODGA/SiO2-P |  | SiO2-P | 浸渍法 | [HCl]=1.5mol/L | Zr | 75.85 | 44.24 | βZr/Hf=5.2 | [ |
| R-PO4H2 |  | GMA/DVB 修饰的 Fe3O4 | 接枝法 | HCl, pH=2.5 | Zr | 42.88 | 85.75 | βZr/Hf=6 | [ |
| TVEX-P507 |  | 苯乙烯-二乙烯基共聚物 | 原位聚合法 | [H2SO4]=0.08mol/L | Hf | 0.21 | — | βHf/Zr=6.81 | [ |
| SiO2-POOH |  | SiO2 | 接枝法 | [H2SO4]=0.5mol/L | Hf | 31.62 | — | βHf/Zr =4.69 | [ |
| Mer-CON-POOH-HYY2 |  | 氯球 | 接枝法 | [H2SO4]=0.3mol/L | Hf | 13.16 | — | βHf/Zr=4.33 | [ |
| Mer-CON-POOH-272 |  | 氯球 | 接枝法 | [H2SO4]=0.3mol/L | Hf | 11.11 | — | βHf/Zr=2.96 | |
| PS-G1.0-MSNs |  | SiO2 | 接枝法 | HNO3, pH=0.5 | Hf | 5.36 | 25.7 | βHf/Zr=2.0 | [ |
| Marathon C | —SO3H | 苯乙烯-二乙烯基共聚物 | 接枝法 | H2SO4, pH=2.5 | Hf | 24.92 | 103.74 | βHf/Zr=2.63 | [ |
| Diphonix | —PO4H2、—SO3H、—COOH | 苯乙烯-二乙烯基共聚物 | 接枝法 | [H2SO4]=1mol/L | Hf | — | — | βHf/Zr=1.47 | [ |
| Purolite S-957 | —PO4H2、—SO3H | 苯乙烯-二乙烯基共聚物 | 接枝法 | [H2SO4]=0.5mol/L | Hf | — | — | βHf/Zr=2.8 | [ |
| SIR-BTP/ SiO2-P |  | SiO2-P | 浸渍法 | [HCl]=1mol/L | Hf | — | — | βHf/Zr=1.1 | [ |
| Reillex PVP |  | 苯乙烯-二乙烯基共聚物 | 接枝法 | [HCl]=9.5mol/L | Zr | — | — | βZr/Hf=10.4 | [ |
| TEVA | 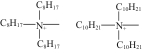 | — | 浸渍法 | [HCl]=8.4mol/L | Zr | — | — | βZr/Hf=18±8 | [ |
| Amberjet 4200 Cl |  | 苯乙烯-二乙烯基共聚物 | 接枝法 | [HCl]=9.5mol/L | Zr | — | 86 | βZr/Hf=11.4 | [ |
| Reillex HPQ |  | 聚乙烯基吡咯烷酮-二乙烯基苯 | — | [HCl]=9.5mol/L | Zr | — | — | βZr/Hf=9.7 | [ |
| [1] | SHATALOV V V, NIKONOV V I, KOTSAR M L. Prospects for zirconium and hafnium supplies for nuclear power in Russia up to 2030[J]. Atomic Energy, 2008, 105(4): 242-247. |
| [2] | XU L, XIAO Y, VAN SANDWIJK A, et al. Production of nuclear grade zirconium: A review[J]. Journal of Nuclear Materials, 2015, 466: 21-28. |
| [3] | 王俊莲, 付家帅, 许文, 等. 基于锆铪溶剂萃取分离的萃取剂性能[J]. 稀有金属, 2020, 44(6): 658-667. |
| WANG Junlian, FU Jiashuai, XU Wen, et al. Performance of various extractants for zirconium and hafnium separation by solvent extraction[J]. Chinese Journal of Rare Metals, 2020, 44(6): 658-667. | |
| [4] | XU L, XIAO Y, VAN SANDWIJK A, et al. Separation of zirconium and hafnium: A review[C]//Energy Materials 2014. Cham: Springer International Publishing, 2014: 451-457. |
| [5] | 郭宁, 俞中华. 钽铌锆铪行业“十三五”前瞻[J]. 中国有色金属, 2016(11): 48-49. |
| GUO Ning, YU Zhonghua. Prospects of the tantalum, niobium, zirconium and hafnium industries during the period of the 13th Five-Years Plan[J]. China Nonferrous Metals, 2016(11): 48-49. | |
| [6] | NISELSON Lev A, EGOROV Egor A, CHUVILINA Elena L, et al. Solid-liquid and liquid-vapor equilibria in the Zr(Hf)Cl4-KalCl4 systems: A basis for the extractive distillation separation of zirconium and hafnium tetrachlorides[J]. Journal of Chemical & Engineering Data, 2009, 54(3): 726-729. |
| [7] | 徐亮. 熔盐萃取锆铪分离和锆在熔盐中的电化学行为[D]. 沈阳: 东北大学, 2017. |
| XU Liang. Separation of zirconium and hafnium with molten salt extraction and electrochemical behavior of zirconium in molten salt[D]. Shenyang: Northeastern University, 2017. | |
| [8] | 柴延全. 熔盐萃取法分离锆铪的研究[D]. 马鞍山: 安徽工业大学, 2017. |
| CHAI Yanquan. Research on the separation of zirconium and hafnium by molten salt extraction[D]. Ma’anshan: Anhui University of Technology, 2017. | |
| [9] | BRANKEN D J, LACHMANN G, KRIEG H M, et al. A density-functional theory approach to the separation of K2ZrF6 and K2HfF6 via fractional crystallization[J]. International Journal of Quantum Chemistry, 2011, 111(3): 682-693. |
| [10] | WANG Junlian, LIU Hui, ZHAO Hongru, et al. Selective extraction of Hf over Zr by a novel extractant (n-octyl)(2,4,4′-trimethylpentyl)phosphinic acid (INET-1) from sulfuric acid media[J]. Journal of Radioanalytical and Nuclear Chemistry, 2023, 332(7): 2473-2485. |
| [11] | ZHAO Hongru, HU Chengqiang, SUI Na, et al. Evaluation of novel solvent extraction systems for Zr/Hf separation with MIBK homologues[J]. Arabian Journal of Chemistry, 2024, 17(1): 105425. |
| [12] | TANG Tingting, YANG Fan, XIE Meiying, et al. Highly efficient separation and enrichment of hafnium from zirconium oxychloride solutions by advanced ion-imprinted membrane separation technology[J]. Journal of Membrane Science, 2023, 668: 121237. |
| [13] | ALFONSO M C, BENNETT M E, FOLDEN C M. Extraction chromatography of the Rf homologs, Zr and Hf, using TEVA and UTEVA resins in HCl, HNO3, and H2SO4 media[J]. Journal of Radioanalytical and Nuclear Chemistry, 2016, 307(2): 1529-1536. |
| [14] | XU Zhigao, WU Yanke, ZHANG Jiandong, et al. Equilibrium and kinetic data of adsorption and separation for zirconium and hafnium onto MIBK extraction resin[J]. Transactions of Nonferrous Metals Society of China, 2010, 20(8): 1527-1533. |
| [15] | 王俊莲, 孙春宝, 徐盛明. 基于稀土分离的萃淋树脂制备与应用研究[J]. 中国稀土学报, 2015, 33(2): 129-145. |
| WANG Junlian, SUN Chunbao, XU Shengming. Advances in preparation and application of solid-liquid extraction resins based on rare earth separation[J]. Journal of the Chinese Society of Rare Earths, 2015, 33(2): 129-145. | |
| [16] | FAN Jinlong, DUAN Li, WANG Yufeng, et al. Assembly of a polymer-based extraction resin and separation of minor actinides[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 610: 125473. |
| [17] | HUANG Weini, LIN Zian. Recent advances in sample pretreatment techniques for chromatographic analysis[J]. Se Pu=Chinese Journal of Chromatography, 2021, 39(1): 1-3. |
| [18] | Semih ÖTLES, KARTAL Canan. Solid-phase extraction (SPE): Principles and applications in food samples[J]. Acta Scientiarum Polonorum Technologia Alimentaria, 2016, 15(1): 5-15. |
| [19] | 李华昌, 周春山, 符斌. 萃淋树脂技术及其在湿法冶金中的应用[J]. 有色金属, 2001(1): 70-73. |
| LI Huachang, ZHOU Chunshan, FU Bin. Extraction resin technology and its application in wet metallurgy[J]. Nonferrous Metals, 2001(1): 70-73. | |
| [20] | 熊洁, 许云书, 黄玮. 偕胺肟基螯合吸附分离材料研究进展[J]. 材料导报, 2006, 20(7): 102-104, 108. |
| XIONG Jie, XU Yunshu, HUANG Wei. Recent development of absorption and separation materials with amidoxime group[J]. Materials Review, 2006, 20(7): 102-104, 108. | |
| [21] | Nadia A ALI, ABD-ELNAIEM Alaa M, HUSSEIN Seenaa I, et al. Thermal and mechanical properties of epoxy resin functionalized copper and graphene hybrids using in situ polymerization method[J]. Current Nanoscience, 2021, 17(3): 494-502. |
| [22] | LANASA Jacob A, TORRES Vincent M, HICKEY Robert J. In situ polymerization and polymer grafting to stabilize polymer-functionalized nanoparticles in polymer matrices[J]. Journal of Applied Physics,2020, 127(13): 134701. |
| [23] | BORAI Emad, KARESOJA Mikko, HARJULA Risto. Separation of cobalt from europium with mesoporous hybrid silica-poly(styrene) impregnated chelating resin[J]. Mineral Processing and Extractive Metallurgy Review, 2013, 34(1): 57-72. |
| [24] | ZHANG Xiaofeng, Zihao OU, XIANG Jinxin. Fabrication of magnetic activated carbon from waste macroporous resin via Fenton’s reagent impregnation[J]. Journal of Porous Materials, 2021, 28(1): 165-170. |
| [25] | THEBAULT Marion, KUTUZOVA Larysa, JURY Sandra, et al. Effect of phenolation, lignin-type and degree of substitution on the properties of lignin-modified phenol-formaldehyde impregnation resins: Molecular weight distribution, wetting behavior, rheological properties and thermal curing profiles[J]. Journal of Renewable Materials, 2020, 8(6): 603-630. |
| [26] | ZHANG Zhen, ZHOU Yuedi, ZHOU Jingbo, et al. Synthesis of TOPO/XAD-16 impregnated resins and effective adsorption of uranium (Ⅵ) in acidic solution[J]. Journal of Radioanalytical and Nuclear Chemistry, 2023, 332(4): 1149-1162. |
| [27] | MATSUNAGA Hideyuki, ISMAIL Adel ALI, WAKUI Yoshito, et al. Extraction of rare earth elements with 2-ethylhexyl hydrogen 2-ethylhexyl phosphonate impregnated resins having different morphology and reagent content[J]. Reactive and Functional Polymers, 2001, 49(3): 189-195. |
| [28] | GENG Shiyu, WEI Jiayuan, Yvonne AITOMÄKI, et al. Well-dispersed cellulose nanocrystals in hydrophobic polymers by in situ polymerization for synthesizing highly reinforced bio-nanocomposites[J]. Nanoscale, 2018, 10(25): 11797-11807. |
| [29] | 柏雨婷, 严岑琪, 李祯, 等. 采用原位成孔法制备热闭孔特性的高强度聚酰亚胺多孔薄膜[J]. 物理化学学报, 2024, 40(9): 83-89. |
| BAI Yuting, YAN Cenqi, LI Zhen, et al. Preparation of high strength polyimide porous films with thermal closed pore characteristics by in situ porosity method [J]. Journal of Physical Chemistry, 2024, 40(9): 83-89. | |
| [30] | WANG Jinhui, YANG Shaowei, MA Fubin, et al. RuCo alloy nanoparticles embedded within N-doped porous two-dimensional carbon nanosheets: A high-performance hydrogen evolution reaction catalyst[J]. Tungsten, 2024, 6(1): 114-123. |
| [31] | 郎倩, 竹筱歆, 张丽新, 等. 原位聚合法制备可降解纤维基地膜及机理分析[J]. 农业工程学报, 2023, 39(15): 249-258. |
| LANG Qian, ZHU Xiaoxin, ZHANG Lixin, et al. Fabrication and mechanism analysis of degradable fiber membrane by in situ polymerization[J]. Transactions of the Chinese Society of Agricultural Engineering, 2023, 39(15): 249-258. | |
| [32] | LIU Yisi, CHEN Zhicheng, LI Zongxu, et al. CoNi nanoalloy-Co-N4 composite active sites embedded in hierarchical porous carbon as bi-functional catalysts for flexible Zn-air battery[J]. Nano Energy, 2022, 99: 107325. |
| [33] | FU Yihan, PENG Yuanyou, ZHAO Lei, et al. Vanadium nitride quantum dots@carbon skeleton anode material synthesized via in situ oxidation initiation strategy[J]. Tungsten, 2024, 6(3): 561-573. |
| [34] | KAUCZOR H W, MEYER A. Structure and properties of levextrel resins[J]. Hydrometallurgy, 1978, 3(1): 65-73. |
| [35] | TAMANG Aditya Moktan, SINGH Nitesh, CHANDRAKER Sandip Kumar, et al. Solvent impregnated resin a potential alternative material for separation dyes, metal and phenolic compounds: A review[J]. Current Research in Green and Sustainable Chemistry, 2022, 5: 100232. |
| [36] | 吴明. DIBK-TBP体系萃取分离锆和铪的动力学研究[D]. 武汉: 武汉工程大学, 2012. |
| WU Ming. Kinetic study on extraction and separation of zirconium and hafnium by DIBK-TBP system[D]. Wuhan: Wuhan Institute of Technology, 2012. | |
| [37] | 林振汉. 用甲基异丁基酮萃取分离锆铪的工艺评价[J]. 稀有金属快报, 2007, 26(1): 93-96. |
| LIN Zhenhan. Extraction separation of zirconium and hafnium with methyl isobutyl ketone (MIBK)[J]. 2007, 26(1): 93-96. | |
| [38] | 徐志高, 王力军, 池汝安, 等. DIBK溶剂萃取法分离锆铪[J]. 有色金属(冶炼部分), 2012(3): 35-38, 42. |
| XU Zhigao, WANG Lijun, CHI Ruan, et al. Solvent extraction and separation of hafnium from zirconium with DIBK[J]. Nonferrous Metals (Extractive Metallurgy), 2012(3): 35-38, 42. | |
| [39] | 林振汉. 用TBP萃取分离和的工艺研究[J]. 稀有金属快报, 2004, 23(11): 21-25. |
| LIN Zhenhan. Study of the technological process for extractive separation of zirconium-hafnium with tributyl phosphate[J]. Rare Metals Letters, 2004, 23(11): 21-25. | |
| [40] | ALIAKBARI Mohsen, SABERYAN Kamal, NOAPARAST Mohammad, et al. Separation of hafnium and zirconium using TBP modified ferromagnetic nanoparticles: Effects of acid and metals concentrations[J]. Hydrometallurgy, 2014, 146: 72-75. |
| [41] | Amrita DAS, CHANDRAKUMAR K R S, PAUL Bhaskar, et al. Enhanced adsorption and separation of zirconium and hafnium under mild conditions by phosphoric acid based ligand functionalized silica gels: Insights from experimental and theoretical investigations[J]. Separation and Purification Technology, 2020, 239: 116518. |
| [42] | JAL P K, PATEL S, MISHRA B K. Chemical modification of silica surface by immobilization of functional groups for extractive concentration of metal ions[J]. Talanta, 2004, 62(5): 1005-1028. |
| [43] | SMITH Jacob B, KERR Stewart H, WHITE Peter S, et al. Thermodynamic studies of cation-macrocycle interactions in nickel pincer-crown ether complexes enable switchable ligation[J]. Organometallics, 2017, 36(16): 3094-3103. |
| [44] | YIN Xiangbiao, WEI Yuezhou, ZU Jianhua. Adsorption behavior of Zr(Ⅳ) and Hf(Ⅳ) on a silica-based macroporous TODGA adsorbent[J]. Nuclear Science and Techniques, 2013, 24(4): 11-18. |
| [45] | QIN Wei, XU Shengming, XU Gang, et al. Preparation of silica gel bound crown ether and its extraction performance towards zirconium and hafnium[J]. Chemical Engineering Journal, 2013, 225: 528-534. |
| [46] | KUMARI Shikha, CARMONA Angelica V, TIWARI Amit K, et al. Amide bond bioisosteres: Strategies, synthesis, and successes[J]. Journal of Medicinal Chemistry, 2020, 63(21): 12290-12358. |
| [47] | Sandra AGUDO-ÁLVAREZ, DÍAZ-MÍNGUEZ Sandra S, Raúl BENITO-ARENAS. The amide group and its preparation methods by acid-amine coupling reactions: An overview[J]. Pure and Applied Chemistry, 2024, 96(5): 691-707. |
| [48] | WANG Ling yun, LEE Man Seung. A review on the aqueous chemistry of Zr(Ⅳ) and Hf(Ⅳ) and their separation by solvent extraction[J]. Journal of Industrial and Engineering Chemistry, 2016, 39: 1-9. |
| [49] | BANDA Raju, LEE Man Seung. Solvent extraction for the separation of Zr and Hf from aqueous solutions[J]. Separation & Purification Reviews, 2015, 44(3): 199-215. |
| [50] | DONIA A M, ATIA A A, DAHER A M, et al. Extraction and separation of zirconium(Ⅳ) and hafnium(Ⅳ) from chloride media using magnetic resin with phosphoric acid functionality[J]. Journal of Dispersion Science and Technology, 2011, 32(2): 193-202. |
| [51] | 周新木, 董雪平, 陈慧勤, 等. 改进的P507在H2SO4体系中对锆铪的静态吸附实验[J]. 有色金属(冶炼部分), 2009(4): 26-29. |
| ZHOU Xinmu, DONG Xueping, CHEN Huiqin, et al. Captive adsorption of zirconium and hafnium in H2SO4 with improved P507[J]. Nonferrous Metals (Extractive Metallurgy), 2009(4): 26-29. | |
| [52] | WANG Junlian, LIU Hui, XU Wen, et al. Selective extraction of hafnium over zirconium with dialkylphosphinic acids from H2SO4 media[J]. Journal of the Brazilian Chemical Society, 2023: 34(7): 1003-1012. |
| [53] | BANDA Raju, MIN Soo Hwan, LEE Man Seung. Selective extraction of Hf(Ⅳ) over Zr(Ⅳ) from aqueous H2SO4 solutions by solvent extraction with acidic organophosphorous based extractants[J]. Journal of Chemical Technology & Biotechnology, 2014, 89(11): 1712-1719. |
| [54] | 刘辉, 王俊莲, 徐国栋, 等. 二烷基次膦酸改性的SiO2基萃淋树脂的制备及吸附分离锆铪的性能[J]. 离子交换与吸附, 2023, 39(1): 1-16. |
| LIU Hui, WANG Junlian, XU Guodong, et al. Dialkylphosphinic acid modified SiO2-based extraction resin: Preparation and its adsorption and separation performance for zirconium and hafnium[J]. Ion Exchange and Adsorption, 2023, 39(1): 1-16. | |
| [55] | HU Chengqiang, ZHAO Hongru, SUN Ruiyi, et al. Novel dialkylphosphinic acid modified Merrifield resins: Synthesis, characterization, and their adsorption and separation behaviors for zirconium and hafnium[J]. Separation and Purification Technology, 2025, 353: 128539. |
| [56] | WANG Junlian, CHEN Guang, XU Shengming, et al. Synthesis of novel nonsymmetric dialkylphosphinic acid extractants and studies on their extraction-separation performance for heavy rare earths[J]. Hydrometallurgy, 2015, 154: 129-136. |
| [57] | WANG Junlian, XU Shengming, LI Linyan, et al. Synthesis of organic phosphinic acids and studies on the relationship between their structure and extraction-separation performance of heavy rare earths from HNO3 solutions[J]. Hydrometallurgy, 2013, 137: 108-114. |
| [58] | QIN Wei, XU Kaixuan, WANG Junwei, et al. Phosphorous-functionalized PAMAM dendrimers supported on mesoporous silica for Zr(Ⅳ) and Hf(Ⅳ) separation[J]. RSC Advances, 2021, 11(55): 34754-34765. |
| [59] | FELIPE Elaine C B, LADEIRA Ana Claudia Q. Separation of zirconium from hafnium by ion exchange[J]. Separation Science and Technology, 2018, 53(2): 330-336. |
| [60] | SMOLIK M, JAKÓBIK-KOLON A, PORAŃSKI M. Separation of zirconium and hafnium using Diphonix® chelating ion-exchange resin[J]. Hydrometallurgy, 2009, 95(3/4): 350-353. |
| [61] | SMOLIK Marek, Łukasz SIEPIETOWSKI, Agata JAKÓBIK-KOLON. The effects of concentrations of zirconium(Ⅳ) sulphate and sulphuric acid on sorption of zirconium(Ⅳ) and hafnium(Ⅳ) on purolite S-957 resin. adsorption isotherms of zirconium(Ⅳ) and hafnium(Ⅳ) ions[J]. Solvent Extraction and Ion Exchange, 2014, 32(4): 437-446. |
| [62] | KANG Jingu, KIM Yung-Uk, Sung-Ho JOO, et al. Behavior of extraction, stripping, and separation possibilities of rhenium and molybdenum from molybdenite roasting dust leaching solution using amine based extractant tri-otyl-amine (TOA)[J]. Materials Transactions, 2013, 54(7): 1209-1212. |
| [63] | DU PREEZ Jan G H. Recent advances in amines as separating agents for metal ions[J]. Solvent Extraction and Ion Exchange, 2000, 18(4): 679-701. |
| [64] | TEZCAN Fatma, DONAT Ramazan. Single and selective transport of Zr(Ⅳ) ions with trioctyl amine dissolved in kerosene using a multidropped liquid membrane technique[J]. Turkish Journal of Chemistry, 2022, 46(5): 1594-1606. |
| [65] | KRAMER Jurjen, DRIESSEN Willem L, KOCH Klaus R, et al. Highly selective extraction of platinum group metals with silica-based (poly)amine ion exchangers applied to industrial metal refinery effluents[J]. Hydrometallurgy, 2002, 64(1): 59-68. |
| [66] | MAITY Tarun, AGGARWAL Abhishek, DASGUPTA Subhadeep, et al. Efficient removal of uranyl ions using PAMAM dendrimer: Simulation and experiment[J]. Langmuir, 2023, 39(19): 6794-6802. |
| [67] | PORIEL L, PELLET-ROSTAING S, LAMOTTE V, et al. Zirconium and hafnium separation, part 2. solid/liquid extraction in hydrochloric acid aqueous solution with anion exchange resins[J]. Separation Science and Technology, 2006, 41(12): 2711-2722. |
| [68] | FAVRE-RÉGUILLON A, FIATY K, LAURENT P, et al. Solid/liquid extraction of zirconium and hafnium in hydrochloric acid aqueous solution with anion exchange ResinKinetic study and equilibrium analyses[J]. Industrial & Engineering Chemistry Research, 2007, 46(4): 1286-1291. |
| [69] | PEARSON Ralph G. Absolute electronegativity and hardness: Application to inorganic chemistry[J]. Inorganic Chemistry, 1988, 27(4): 734-740. |
| [1] | FU Hongmei, LIU Dinghua, LIU Xiaoqin. Research progress on the separation of aromatic isomers using MOF materials [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5006-5017. |
| [2] | ZHANG Bo, MA Jun, ZHANG Weilong, JIA Shichuan, ZHANG Zhifei, DING Yu, PAN Youhua, WANG Junyu, ZHANG Lanhe. Preparation of α-ZrP/PDMS superhydrophobic anti-corrosion coating and corrosion resistance performance [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5130-5139. |
| [3] | HONG Kai, FAN Huan, TIAN Jia, ZHANG Xingfei. Treatment of copper-arsenic polymetallic acidic wastewater by sulfide precipitation: A review [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5301-5314. |
| [4] | DU Xuan, WANG Zhanhong, ZHENG Bin, XU Wei, WANG Shuo, SHI Peng, GAO Guo. Progress on separation of cobalt-iron acid leaching solution and battery grade iron phosphate recovery technology [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5327-5338. |
| [5] | WANG Xiaoguang, DONG Qing, LANG Wenli, HONG Xiangxin, HUANG Zhenxiang, TAN Fengyu, LEI Yizhu, YU Ziyi. Progress on emission reduction and resource utilization of ultra-low concentration methane [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5363-5376. |
| [6] | YANG Zhenglu, YANG Lifeng, LU Xiaofei, SUO Xian, ZHANG Anyun, CUI Xili, XING Huabin. Advances in machine learning accelerating the screening and discovery of porous adsorbents [J]. Chemical Industry and Engineering Progress, 2025, 44(8): 4288-4301. |
| [7] | YANG Ao, DENG Wei, LI Yong, LUO Jing, WANG Zilin, ZHANG Jun, SHEN Weifeng. Multi-objective optimization design of triple-column pressure-swing distillation for separating ternary azeotropic mixture tetrahydrofuran/methanol/ethanol by thermodynamic topology theory [J]. Chemical Industry and Engineering Progress, 2025, 44(8): 4582-4593. |
| [8] | GAO Yan, LI Yongshuai, LI Gaoyang, PAN Hui, LING Hao. Dynamic control for Agrawal divided-wall column [J]. Chemical Industry and Engineering Progress, 2025, 44(8): 4594-4605. |
| [9] | WANG Guochao, DING Huidian, SHI Li, LI Qiang, XIA Tao, YUAN Yang. Temperature inferential control of compound distillation sequences [J]. Chemical Industry and Engineering Progress, 2025, 44(8): 4720-4731. |
| [10] | TANG Xuan, BAI Xiaowei, ZHANG Feifei, LI Jinping, YANG Jiangfeng. Research progress on zeolite for CO2-N2-CH4 sieving separation [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 3938-3949. |
| [11] | CHEN Qian, TONG Kun, XIE Jiacai, SHAO Zhiguo, NIE Fan, LI Chentao. Research progress on the treatment technology of polymer-containing oil sludge [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 4158-4168. |
| [12] | LI Peiyi, SUN Bolong, LIU Ruiyan, ZHOU Xinyao, LIU Ruilin, HU Yuanyuan, XU Gongtao, LI Xinping. Preparation of sodium alginate/titanium dioxide composite porous material and its application in oil-water separation [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3053-3061. |
| [13] | FU Jiang, SUN Jiaoxia, FU Junjie, ZHU Min, SONG Pinxue, ZHOU Yining, FAN Jianxin. Self-cleaning effect and oil-water separation performance of hydrophobic modified polyester fiber fabric [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3121-3131. |
| [14] | WANG Heng, LU Chunxi. Structural optimization and operational performance analysis of cyclone separators in a 3.6Mt/a catalytic cracking unit [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3238-3246. |
| [15] | ZHANG Lei, ZHANG Xinru, WANG Yonghong, LI Jinping, LIU Chunbo. Research progress of two-dimensional nanomaterial-based mixed matrix membranes in organic pervaporation separation [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3324-3335. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||