Chemical Industry and Engineering Progress ›› 2024, Vol. 43 ›› Issue (11): 6233-6245.DOI: 10.16085/j.issn.1000-6613.2023-1813
• Materials science and technology • Previous Articles
Reaction characteristics of organic modifications on the surface of oxide nanoparticles
GAN Yuxin( ), ZHAO Mei, ZHAO Shaolei, XIE Jiuren, YANG Ling, WANG Tingjie(
), ZHAO Mei, ZHAO Shaolei, XIE Jiuren, YANG Ling, WANG Tingjie( )
)
- Department of Chemical Engineering, Tsinghua University, Beijing 100084, China
-
Received:2023-10-16Revised:2023-12-11Online:2024-12-07Published:2024-11-15 -
Contact:WANG Tingjie
氧化物纳米颗粒表面有机修饰反应特性
甘雨欣( ), 赵美, 赵绍磊, 谢就任, 杨令, 王亭杰(
), 赵美, 赵绍磊, 谢就任, 杨令, 王亭杰( )
)
- 清华大学化学工程系,北京 100084
-
通讯作者:王亭杰 -
作者简介:甘雨欣(1999—),女,博士研究生,研究方向为纳米颗粒表面修饰。E-mail:ganyx21@mails.tsinghua.edu.cn。
CLC Number:
Cite this article
GAN Yuxin, ZHAO Mei, ZHAO Shaolei, XIE Jiuren, YANG Ling, WANG Tingjie. Reaction characteristics of organic modifications on the surface of oxide nanoparticles[J]. Chemical Industry and Engineering Progress, 2024, 43(11): 6233-6245.
甘雨欣, 赵美, 赵绍磊, 谢就任, 杨令, 王亭杰. 氧化物纳米颗粒表面有机修饰反应特性[J]. 化工进展, 2024, 43(11): 6233-6245.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-1813
| 溶剂体系 | 修饰剂 | 氧化物颗粒 | 主要反应条件 | 研究目标 | 参考文献 |
|---|---|---|---|---|---|
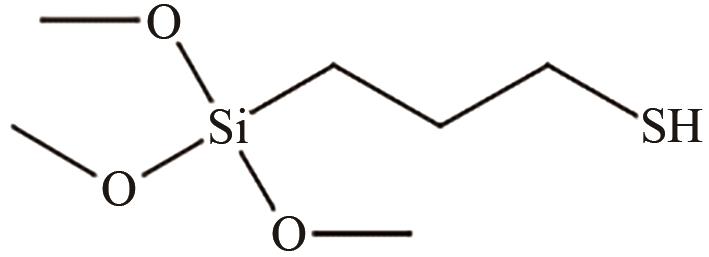
| 有机相 | MPTMS
| SiO2 | 溶剂为甲苯 室温,24h | 巯基功能化 | [ |
DETAS
| SiO2 | 溶剂为乙醇 室温,12h | 氨基功能化 | [ | |
APTES
| AlOOH | 溶剂为乙醇 75℃,12h | 氨基功能化 | [ | |
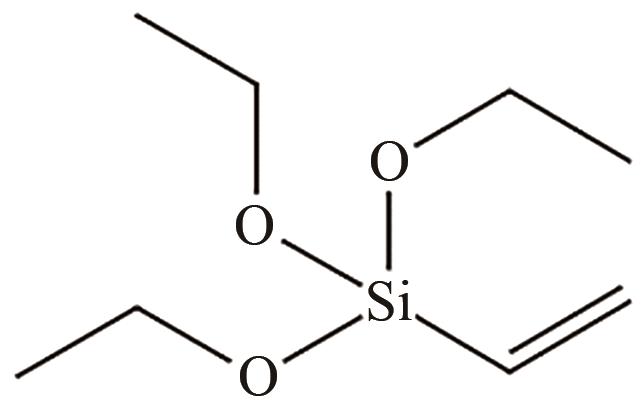
VTES
| ZnO | 溶剂为甲苯 80℃,3h | 提高分散性 | [ | |
KH570
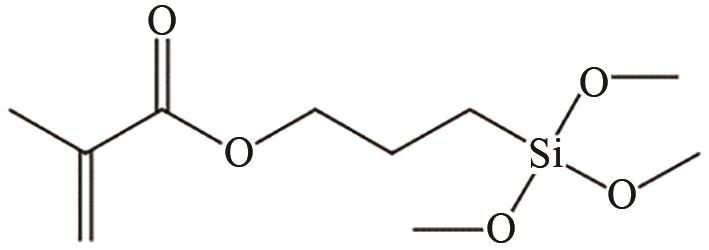
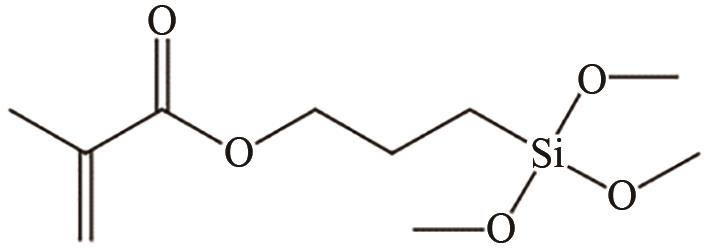
| TiO2 | 溶剂为乙醇 40℃,1h | 提高相容性 | [ | |
KH570
| ZrO2 | 溶剂为乙醇 50℃,2h | 提高分散性 | [ | |
| 醇水溶液 | MPTMS
| SiO2 | 60℃,1.5h | 巯基功能化 | [ |
VTES
| SiO2 | 室温,19h | 双键功能化 | [ | |
APTMS
| SiO2 | 60,3h | 氨基功能化 | [ | |
GPTMS
| SiO2 | 乙醇∶水=95∶5 70℃,2h | 提高分散性 | [ | |
MTMS
| SiO2 | 室温,24h | 提高分散性 | [ | |
DMDCS
| SiO2 | 50℃,16h | 表面润湿性调节 | [ | |
APTES
| TiO2 | 乙醇∶水=95∶5 pH=5,氮气气氛、室温,24h | 氨基功能化 | [ | |
| 水相 | APTMS
IPTMS
| TiO2 | 20~80℃,2~16h | 氨基功能化 交联网络 | [ |
| 溶剂体系 | 修饰剂 | 氧化物颗粒 | 主要反应条件 | 研究目标 | 参考文献 |
|---|---|---|---|---|---|
| 有机相 | MPTMS
| SiO2 | 溶剂为甲苯 室温,24h | 巯基功能化 | [ |
DETAS
| SiO2 | 溶剂为乙醇 室温,12h | 氨基功能化 | [ | |
APTES
| AlOOH | 溶剂为乙醇 75℃,12h | 氨基功能化 | [ | |
VTES
| ZnO | 溶剂为甲苯 80℃,3h | 提高分散性 | [ | |
KH570
| TiO2 | 溶剂为乙醇 40℃,1h | 提高相容性 | [ | |
KH570
| ZrO2 | 溶剂为乙醇 50℃,2h | 提高分散性 | [ | |
| 醇水溶液 | MPTMS
| SiO2 | 60℃,1.5h | 巯基功能化 | [ |
VTES
| SiO2 | 室温,19h | 双键功能化 | [ | |
APTMS
| SiO2 | 60,3h | 氨基功能化 | [ | |
GPTMS
| SiO2 | 乙醇∶水=95∶5 70℃,2h | 提高分散性 | [ | |
MTMS
| SiO2 | 室温,24h | 提高分散性 | [ | |
DMDCS
| SiO2 | 50℃,16h | 表面润湿性调节 | [ | |
APTES
| TiO2 | 乙醇∶水=95∶5 pH=5,氮气气氛、室温,24h | 氨基功能化 | [ | |
| 水相 | APTMS
IPTMS
| TiO2 | 20~80℃,2~16h | 氨基功能化 交联网络 | [ |
| 颗粒 | 修饰机制 |
|---|---|
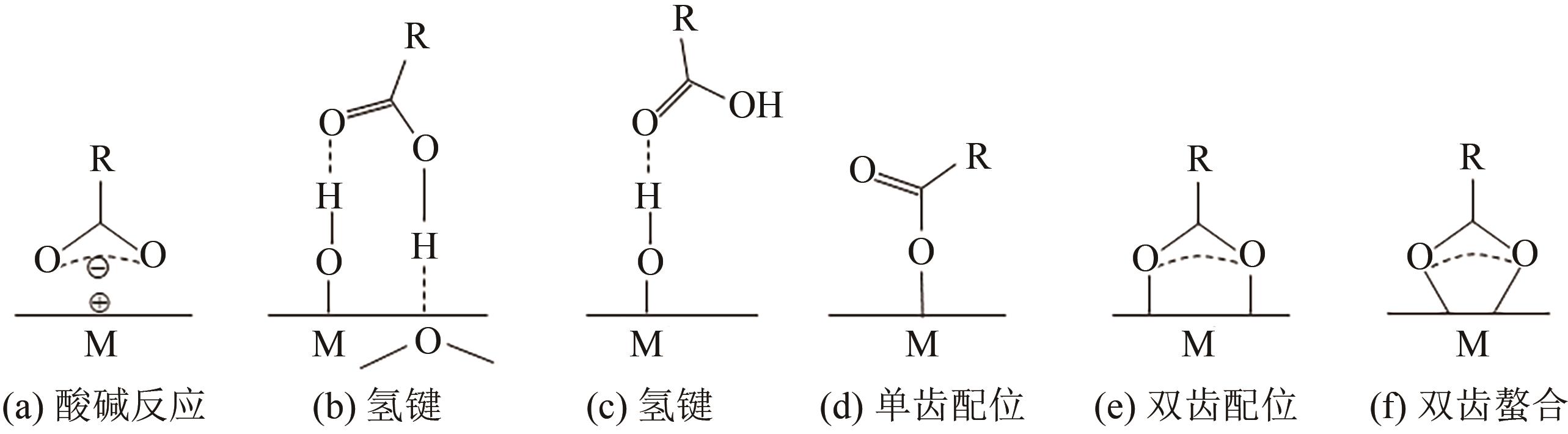
| SiO2 | 氢键[ |
| TiO2 | 双齿接枝[ |
| γ-Al2O3 | 单核单(双)齿接枝[ |
| Al2O3-无定型 | 双齿接枝[ |
| 颗粒 | 修饰机制 |
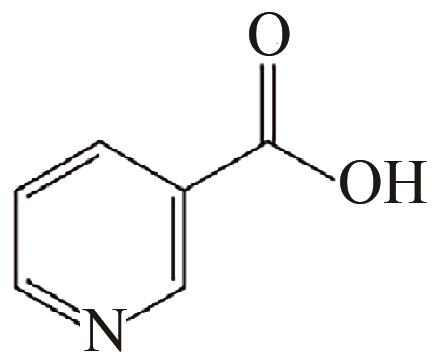
|---|---|
| SiO2 | 氢键[ |
| TiO2 | 双齿接枝[ |
| γ-Al2O3 | 单核单(双)齿接枝[ |
| Al2O3-无定型 | 双齿接枝[ |
| 修饰工艺 | 修饰剂 | 氧化物颗粒 | 主要反应条件 | 研究目标 | 参考文献 |
|---|---|---|---|---|---|
| 高温高压 | (光学活性的)仲醇、正辛醇、环己基甲醇、环己醇等 | SiO2 | 240℃,30atm | 机理研究 | [ |
| 不同碳链长度的正构烷醇 | SiO2 | 235℃,30atm,1h | 调节表面润湿性 | [ | |
| 正丁醇、叔丁醇 | SiO2 | 260℃,30bar,1h | 调节表面润湿性 | [ | |
| 有机溶剂加热回流 | 正辛醇、2-乙基-1己醇; 1-十八醇,甲醇 | SiO2 | 溶剂为二甲苯 加热回流18h | 用于吸附剂、催化剂、传感器等 | [ |
| 8-巯基-1-辛醇 | SiO2 | 溶剂为二甲苯 加热回流18h | 色谱柱、填料 | [ | |
| 干粉加热 | 十六烷醇 | SiO2 | pH=8~9 醇水溶液吸附过滤 气相120℃,12h | 调节表面润湿性 | [ |
三羟甲基氨基甲烷
| SiO2 | 水相混合 喷雾干燥 气相200℃,2h | 氨基功能化 | [ | |
2-氨基-1-丁醇
| SiO2 | 水相混合 喷雾干燥 气相160℃,2h | 氨基功能化 | [ | |
含羟基的联吡啶基分子
| SiO2、TiO2 | 气相160℃,2d | 功能化 | [ |
| 修饰工艺 | 修饰剂 | 氧化物颗粒 | 主要反应条件 | 研究目标 | 参考文献 |
|---|---|---|---|---|---|
| 高温高压 | (光学活性的)仲醇、正辛醇、环己基甲醇、环己醇等 | SiO2 | 240℃,30atm | 机理研究 | [ |
| 不同碳链长度的正构烷醇 | SiO2 | 235℃,30atm,1h | 调节表面润湿性 | [ | |
| 正丁醇、叔丁醇 | SiO2 | 260℃,30bar,1h | 调节表面润湿性 | [ | |
| 有机溶剂加热回流 | 正辛醇、2-乙基-1己醇; 1-十八醇,甲醇 | SiO2 | 溶剂为二甲苯 加热回流18h | 用于吸附剂、催化剂、传感器等 | [ |
| 8-巯基-1-辛醇 | SiO2 | 溶剂为二甲苯 加热回流18h | 色谱柱、填料 | [ | |
| 干粉加热 | 十六烷醇 | SiO2 | pH=8~9 醇水溶液吸附过滤 气相120℃,12h | 调节表面润湿性 | [ |
三羟甲基氨基甲烷
| SiO2 | 水相混合 喷雾干燥 气相200℃,2h | 氨基功能化 | [ | |
2-氨基-1-丁醇
| SiO2 | 水相混合 喷雾干燥 气相160℃,2h | 氨基功能化 | [ | |
含羟基的联吡啶基分子
| SiO2、TiO2 | 气相160℃,2d | 功能化 | [ |
| 溶剂体系 | 修饰剂 | 氧化物颗粒 | 主要反应条件 | 研究目标 | 参考文献 |
|---|---|---|---|---|---|
| 干法 | SiO2 | 球磨8~16h | 提高分散性 | [ | |
| 有机相 | 对氨基苯酚
| SiO2 | 溶剂为乙醇 80℃,8h | 提高分散性 | [ |
| 水相 |
| SiO2 | 溶剂为tris缓冲溶液 pH=8.5,室温,12h | 提高分散性、生物相容性 | [ |
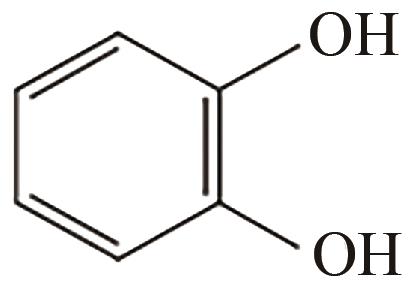
邻苯二酚
| Al2O3 | 溶剂为0.1mol/L KCl溶液 pH=7,室温,6h | 机理研究 | [ |
| 溶剂体系 | 修饰剂 | 氧化物颗粒 | 主要反应条件 | 研究目标 | 参考文献 |
|---|---|---|---|---|---|
| 干法 | SiO2 | 球磨8~16h | 提高分散性 | [ | |
| 有机相 | 对氨基苯酚
| SiO2 | 溶剂为乙醇 80℃,8h | 提高分散性 | [ |
| 水相 |
| SiO2 | 溶剂为tris缓冲溶液 pH=8.5,室温,12h | 提高分散性、生物相容性 | [ |
邻苯二酚
| Al2O3 | 溶剂为0.1mol/L KCl溶液 pH=7,室温,6h | 机理研究 | [ |
| 氧化物颗粒 | 羧酸 | 膦酸 |
|---|---|---|
| SiO2 | 氢键[ | 氢键[ |
| TiO2 | 氢键、单齿[ | 单齿、双齿、 三齿配位[ |
| Al2O3 | 单齿、双齿配位[ | 双齿配位[ |
| Fe2O3, Fe3O4 | 双齿配位[ | 双齿、三齿配位[ |
| 氧化物颗粒 | 羧酸 | 膦酸 |
|---|---|---|
| SiO2 | 氢键[ | 氢键[ |
| TiO2 | 氢键、单齿[ | 单齿、双齿、 三齿配位[ |
| Al2O3 | 单齿、双齿配位[ | 双齿配位[ |
| Fe2O3, Fe3O4 | 双齿配位[ | 双齿、三齿配位[ |
| 溶剂体系 | 修饰剂 | 氧化物颗粒 | 主要反应条件 | 研究目标 | 参考文献 |
|---|---|---|---|---|---|
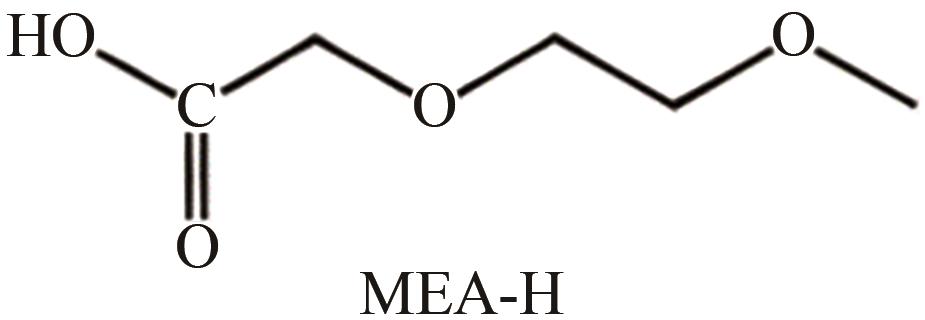
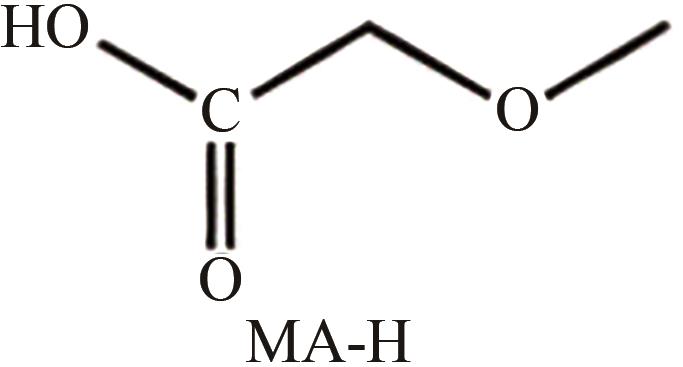
| 水相 | 醋酸、 、 | 拟薄水铝石 AlOOH·nH2O | 水溶液回流72h | 提高分散性 | [ |
 、 、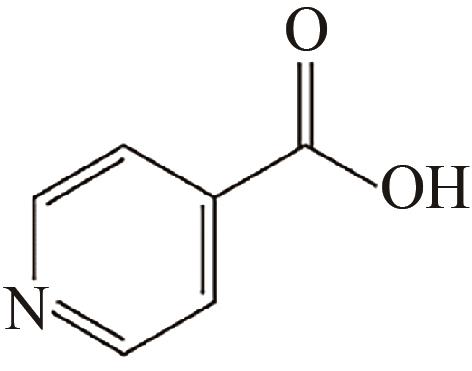 | Al2O3 | pH=7,水溶液回流 | 功能化 重金属离子吸附 | [ | |
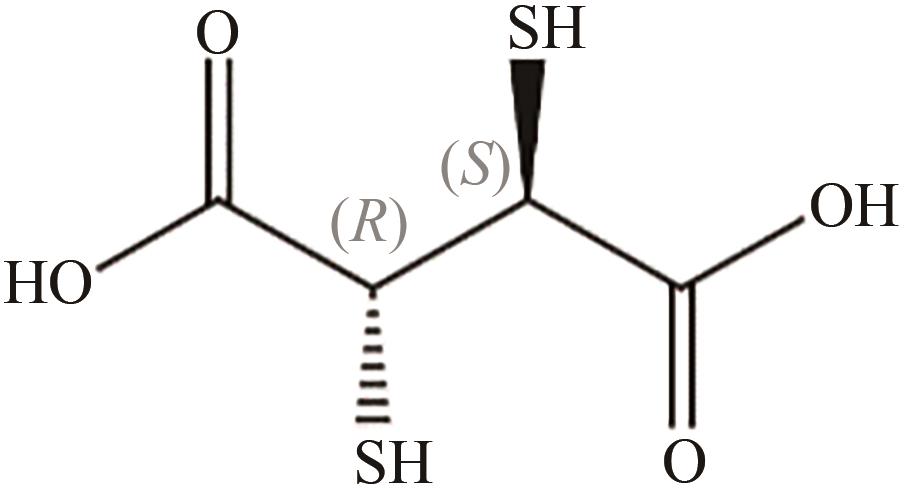
| 甘氨酸、丙二酸 | Fe3O4@SiO2 | 90℃,20min | 功能化 废水处理 | [ | |
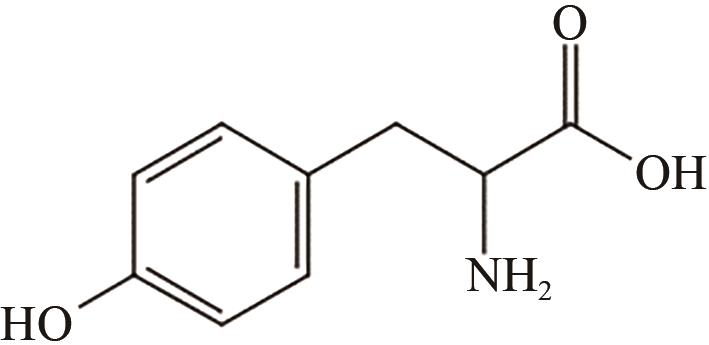 、 、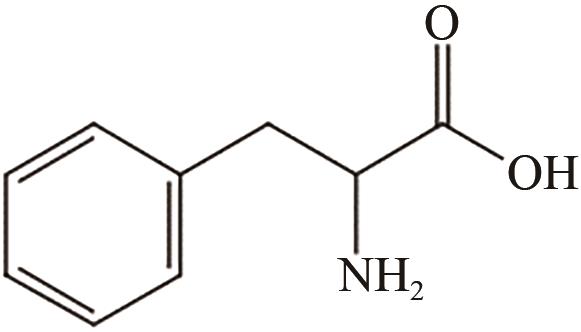 | Fe3O4 | 70℃,1h | 氨基功能化 | [ | |
| 有机相 | 甲酸、乙酸、丙酸、 HOOC(CH2) n COOH n=0, 1, 2, 3, 4, 5 | Al2O3 | 溶剂为四氢呋喃 70℃,24~48h | 功能化 表面电荷调节 | [ |
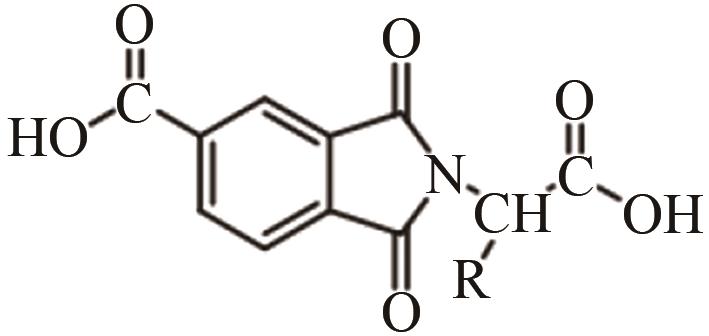 | TiO2 | 溶剂为甲醇 室温,24h 超声30min | 提高分散性 | [ | |
| 硬脂酸 | TiO2 | 溶剂为正己烷 60℃,4h | 提高分散性 | [ | |
| 油酸 | TiO2、Al2O3 | 溶剂为乙醇 75℃,2h | 提高稳定性 | [ | |
| 油酸 | SiO2 | 溶剂为正己烷 60℃,4h | 提高分散性 | [ | |
二巯基丁二酸(DMSA)
| Fe3O4@SiO2 | 溶剂为甲苯、 二甲基亚砜 室温,12h | 提高分散性和生物相容性 | [ |
| 溶剂体系 | 修饰剂 | 氧化物颗粒 | 主要反应条件 | 研究目标 | 参考文献 |
|---|---|---|---|---|---|
| 水相 | 醋酸、 、 | 拟薄水铝石 AlOOH·nH2O | 水溶液回流72h | 提高分散性 | [ |
 、 、 | Al2O3 | pH=7,水溶液回流 | 功能化 重金属离子吸附 | [ | |
| 甘氨酸、丙二酸 | Fe3O4@SiO2 | 90℃,20min | 功能化 废水处理 | [ | |
 、 、 | Fe3O4 | 70℃,1h | 氨基功能化 | [ | |
| 有机相 | 甲酸、乙酸、丙酸、 HOOC(CH2) n COOH n=0, 1, 2, 3, 4, 5 | Al2O3 | 溶剂为四氢呋喃 70℃,24~48h | 功能化 表面电荷调节 | [ |
 | TiO2 | 溶剂为甲醇 室温,24h 超声30min | 提高分散性 | [ | |
| 硬脂酸 | TiO2 | 溶剂为正己烷 60℃,4h | 提高分散性 | [ | |
| 油酸 | TiO2、Al2O3 | 溶剂为乙醇 75℃,2h | 提高稳定性 | [ | |
| 油酸 | SiO2 | 溶剂为正己烷 60℃,4h | 提高分散性 | [ | |
二巯基丁二酸(DMSA)
| Fe3O4@SiO2 | 溶剂为甲苯、 二甲基亚砜 室温,12h | 提高分散性和生物相容性 | [ |
| 溶剂体系 | 修饰剂 | 氧化物颗粒 | 主要反应条件 | 研究目标 | 参考文献 |
|---|---|---|---|---|---|
| 水相 | 正辛基、正戊基、正丙基膦酸 | SiO2@Al2O3 | pH=5,室温,24h | 提高分散性 | [ |
| 苯膦酸、乙烯基膦酸 | SnO2 | 室温,32~35h | 机理研究 | [ | |
| 多种有机膦络合物 | Fe3O4 | pH=2~3,50~60℃,2~6h | 功能化 重金属离子吸附 | [ | |
| 4-氨基苯基膦酸、4-羧基苯基膦酸、磷酰基乙酸、2-羧基乙基膦酸 | Fe3O4 | pH=5~5.5,27℃,超声20min | 表面功能化 | [ | |
| 醇水 | 苯膦酸 | Al2O3 | 溶剂为甲醇-水溶液 pH=4或6,室温,3d | 机理研究 | [ |
| 正十二烷基膦酸 | TiO2、SiO2 | 溶剂为甲醇水溶液 pH=4,室温,15h | 表面功能化 选择性修饰 | [ | |
| 有机相 | 正丁基膦酸 | Al2O3、SiO2 | 溶剂为甲苯 回流2d | 机理研究 | [ |
| 溶剂体系 | 修饰剂 | 氧化物颗粒 | 主要反应条件 | 研究目标 | 参考文献 |
|---|---|---|---|---|---|
| 水相 | 正辛基、正戊基、正丙基膦酸 | SiO2@Al2O3 | pH=5,室温,24h | 提高分散性 | [ |
| 苯膦酸、乙烯基膦酸 | SnO2 | 室温,32~35h | 机理研究 | [ | |
| 多种有机膦络合物 | Fe3O4 | pH=2~3,50~60℃,2~6h | 功能化 重金属离子吸附 | [ | |
| 4-氨基苯基膦酸、4-羧基苯基膦酸、磷酰基乙酸、2-羧基乙基膦酸 | Fe3O4 | pH=5~5.5,27℃,超声20min | 表面功能化 | [ | |
| 醇水 | 苯膦酸 | Al2O3 | 溶剂为甲醇-水溶液 pH=4或6,室温,3d | 机理研究 | [ |
| 正十二烷基膦酸 | TiO2、SiO2 | 溶剂为甲醇水溶液 pH=4,室温,15h | 表面功能化 选择性修饰 | [ | |
| 有机相 | 正丁基膦酸 | Al2O3、SiO2 | 溶剂为甲苯 回流2d | 机理研究 | [ |
| 1 | A Yu OLENIN, LISICHKIN G V. Surface-modified oxide nanoparticles: Synthesis and application[J]. Russian Journal of General Chemistry, 2019, 89(7): 1451-1476. |
| 2 | KIM Taeyoon, KWON Soo-Hyun, KIM Hye Jin, et al. Effect of the surface modification of silica nanoparticles on the viscosity and mechanical properties of silica/epoxy nanocomposites[J]. Composite Interfaces, 2022, 29(13): 1573-1590. |
| 3 | Adriana MARTINEZ-OVIEDO, KSHETRI Yuwaraj K, JOSHI Bhupendra, et al. Surface modification of blue TiO2 with silane coupling agent for NO x abatement[J]. Progress in Natural Science: Materials International, 2021, 31(2): 230-238. |
| 4 | CHINH Nguyen Thuy, Tran Thi MAI, HUNG Dao Phi, et al. Characteristics of organic titanate modified titanium dioxide nanoparticles and its dispersibility in acrylic emulsion coating[J]. Vietnam Journal of Chemistry, 2022, 60(S1): 116-124. |
| 5 | LIU Zhao, YAO Lehan, PAN Xun, et al. A green and facile approach to the efficient surface modification of alumina nanoparticles with fatty acids[J]. Applied Surface Science, 2018, 447: 664-672. |
| 6 | KROPACHEVA T N, ANTONOVA A S, A Yu ZHURAVLEVA. Modification of the surface of magnetic iron oxides with phosphonic complexones[J]. Protection of Metals and Physical Chemistry of Surfaces, 2020, 56(3): 473-479. |
| 7 | HOSAIN Asmaa N A, NEMR Ahmed EL, SIKAILY Amany EL, et al. Surface modifications of nanochitosan coated magnetic nanoparticles and their applications in Pb(Ⅱ), Cu(Ⅱ) and Cd(Ⅱ) removal[J]. Journal of Environmental Chemical Engineering, 2020, 8(5): 104316. |
| 8 | WANG Changyuan, WANG Yang, XIAO Wangchuan, et al. Carboxylated superparamagnetic Fe3O4 nanoparticles modified with 3-amino propanol and their application in magnetic resonance tumor imaging[J]. BMC Cancer, 2023, 23(1): 54. |
| 9 | HOOSHMAND Sara, HAYAT Seyed Mohammad Gheibi, GHORBANI Ahmad, et al. Preparation and applications of superparamagnetic iron oxide nanoparticles in novel drug delivery systems: An overview[J]. Current Medicinal Chemistry, 2021, 28(4): 777-799. |
| 10 | ALTERARY Seham S, ALKHAMEES Anfal. Synthesis, surface modification, and characterization of Fe3O4@SiO2 core@shell nanostructure[J]. Green Processing and Synthesis, 2021, 10(1): 384-391. |
| 11 | AHANGARAN Fatemeh, NAVARCHIAN Amir H. Recent advances in chemical surface modification of metal oxide nanoparticles with silane coupling agents: A review[J]. Advances in Colloid and Interface Science, 2020, 286: 102298. |
| 12 | YILMAZ Betul, OZAY Ozgur. Synthesis of antibiotic-modified silica nanoparticles and their use as a controlled drug release system with antibacterial properties[J]. Phosphorus, Sulfur, and Silicon and the Related Elements, 2022, 197(9): 964-972. |
| 13 | WANG Jingyuan, XING Shiyong, XIE Jiuren, et al. Amination of silica nanoparticles using aminobutanol to increase surface reactivity[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2022, 653: 129958. |
| 14 | VOLOV Alexander, SHKODENKO Liubov, KOSHEL Elena, et al. Bio-inspired surface modification of magnetite nanoparticles with dopamine conjugates[J]. Nanomaterials, 2022, 12(13): 2230. |
| 15 | FAHAD Mustafa Raad, ABDULMAJEED Basma Abbas. Surface modification of TiO2-Al2O3 nanoparticles for the enhancement of the rheological properties of base lubricating oil[J]. Journal of Applied Research and Technology, 2022, 20(1): 37-47. |
| 16 | KITAYAMA Yukiya, KATAYAMA Aoi, SHAO Zhicheng, et al. Biocompatible polymer-grafted TiO2 nanoparticle sonosensitizers prepared using phosphonic acid-functionalized RAFT agent[J]. Polymers, 2023, 15(11): 2426. |
| 17 | YANG Lei, QIU Shouji, ZHANG Ya, et al. Preparation of PDMS/SiO2 nanocomposites via ultrasonical modification and miniemulsion polymerization[J]. Journal of Polymer Research, 2012, 20(1): 68. |
| 18 | LIN Jinbin, CHEN Hongling, YAO Licheng. Surface tailoring of SiO2 nanoparticles by mechanochemical method based on simple milling[J]. Applied Surface Science, 2010, 256(20): 5978-5984. |
| 19 | 乔冰. 纳米二氧化硅颗粒高效有机修饰工艺和过程研究[D]. 北京: 清华大学, 2016. |
| QIAO Bing. A novel and effective green process for surface modification of nano-silica particles[D]. Beijing: Tsinghua University, 2016. | |
| 20 | ZHURAVLEV L T. The surface chemistry of amorphous silica. Zhuravlev model[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2000, 173(1/2/3): 1-38. |
| 21 | KHABIBULLIN Amir, BHANGAONKAR Karan, MAHONEY Clare, et al. Grafting PMMA brushes from α-alumina nanoparticles via SI-ATRP[J]. ACS Applied Materials & Interfaces, 2016, 8(8): 5458-5465. |
| 22 | AN Dongmin, WANG Zichen, ZHAO Xu, et al. A new route to synthesis of surface hydrophobic silica with long-chain alcohols in water phase[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2010, 369(1/2/3): 218-222. |
| 23 | YANG Dan, NI Yufeng, KONG Xinxin, et al. Enhanced electromechanical properties of natural rubber using highly efficient and cost-effective mussel-inspired modification of TiO2 nanoparticles[J]. Applied Surface Science, 2019, 495: 143638. |
| 24 | SAHID Mehmood, NISAR Ali, FARMAN Ali, et al. The influence of surface modified silica nanoparticles: Properties of epoxy nanocomposites[J]. Zeitschrift Für Physikalische Chemie, 2020, 235(5): 649-661. |
| 25 | NEOUZE Marie-Alexandra, SCHUBERT Ulrich. Surface modification and functionalization of metal and metal oxide nanoparticles by organic ligands[J]. Monatshefte Für Chemie-Chemical Monthly, 2008, 139(3): 183-195. |
| 26 | KAMIYA Hidehiro, IIJIMA Motoyuki. Surface modification and characterization for dispersion stability of inorganic nanometer-scaled particles in liquid media[J]. Science and Technology of Advanced Materials, 2010, 11(4): 044304. |
| 27 | NGOUANGNA Eugene N, ZAIDI JAAFAR Mohd, NORDDIN MNAM, et al. Surface modification of nanoparticles to improve oil recovery mechanisms: A critical review of the methods, influencing Parameters, advances and prospects[J]. Journal of Molecular Liquids, 2022, 360: 119502. |
| 28 | NASIR Nur Amirah, KAMARUZZAMAN Wan Mohamad Ikhmal Wan Mohamad, BADRUDDIN Malia Athirah, et al. Surface modification effects of CaCO3 and TiO2 nanoparticles in nonpolar solvents[J]. Journal of Dispersion Science and Technology, 2024,45(5): 870-879. |
| 29 | LI Da, SUN Guoli, OUYANG Xueqiong, et al. Surface modification of alumina nanoparticles and its application in tape casting of micro-nano green tape[J]. Applied Surface Science, 2023, 622: 156963. |
| 30 | ZHANG Hao, QING Shan, XU Jiarui, et al. Stability and thermal conductivity of TiO2/water nanofluids: A comparison of the effects of surfactants and surface modification[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2022, 641: 128492. |
| 31 | ZARINWALL Ajmal, WANIEK Tassilo, SAADAT Reza, et al. Comprehensive characterization of APTES surface modifications of hydrous boehmite nanoparticles[J]. Langmuir, 2021, 37(1): 171-179. |
| 32 | ZHANG Wenyu, LAI Edward P C. Chemical functionalities of 3-aminopropyltriethoxy-silane for surface modification of metal oxide nanoparticles[J]. Silicon, 2022, 14(12): 6535-6545. |
| 33 | HE Wentao, WU Danhua, LI Juan, et al. Surface modification of colloidal silica nanoparticles: Controlling the size and grafting process[J]. Bulletin of the Korean Chemical Society, 2013, 34(9): 2747-2752. |
| 34 | HAN Chunli, DENG Jian, LUO Guangsheng. Hydrolysis and condensation kinetic studies of mercaptopropyl trimethoxysilane using in-situ Raman spectroscopy[J]. Chemical Engineering Journal, 2023, 469: 143887. |
| 35 | Ramzi BEL-HASSEN, BOUFI Sami, SALON Marie-Christine Brochier, et al. Adsorption of silane onto cellulose fibers. Ⅱ. The effect of pH on silane hydrolysis, condensation, and adsorption behavior[J]. Journal of Applied Polymer Science, 2008, 108(3): 1958-1968. |
| 36 | KHURANA Nikhita, ARORA Pinklesh, PENTE Avinash S, et al. Surface modification of zinc oxide nanoparticles by vinyltriethoxy silane (VTES)[J]. Inorganic Chemistry Communications, 2021, 124: 108347. |
| 37 | ZHAO Hongran, ZHANG Tong, QI Rongrong, et al. Humidity sensor based on solution processible microporous silica nanoparticles[J]. Sensors and Actuators B: Chemical, 2018, 266: 131-138. |
| 38 | Hubert MUTIN P, GUERRERO Gilles, VIOUX André. Organic-inorganic hybrid materials based on organophosphorus coupling molecules: From metal phosphonates to surface modification of oxides[J]. Comptes Rendus Chimie, 2003, 6(8/9/10): 1153-1164. |
| 39 | YAN Yongli, CAI Yuxiu, LIU Xiaochun, et al. Hydrophobic modification on the surface of SiO2 nanoparticle: Wettability control[J]. Langmuir, 2020, 36(49): 14924-14932. |
| 40 | MARCINKO Stephen, FADEEV Alexander Y. Hydrolytic stability of organic monolayers supported on TiO2 and ZrO2 [J]. Langmuir, 2004, 20(6): 2270-2273. |
| 41 | SOSNOV Evgeni A, MALKOV A A, MALYGIN A A. Hydrolytic stability of the Si—O—Ti bonds in the chemical assembly of titania nanostructures on silica surfaces[J]. Russian Chemical Reviews, 2010, 79(10): 907-920. |
| 42 | NAVIROJ Somsak, KOENIG Jack L, ISHIDA Hatsuo. Diffuse reflectance Fourier transform infrared spectroscopic study of chemical bonding and hydrothermal stability of an aminosilane on metal oxide surfaces[J]. The Journal of Adhesion, 1985, 18(2): 93-110. |
| 43 | SCHINDLER F, SCHMIDBAUR H. Siloxane compounds of the transition metals[J]. Angewandte Chemie International Edition in English, 1967, 6(8): 683-694. |
| 44 | ROZYYEV Vepa, MURPHY Julia G, BARRY Edward, et al. Vapor-phase grafting of a model aminosilane compound to Al2O3, ZnO, and TiO2 surfaces prepared by atomic layer deposition[J]. Applied Surface Science, 2021, 562: 149996. |
| 45 | MAHTABANI Amirhossein, LA ZARA Damiano, ANYSZKA Rafał, et al. Gas phase modification of silica nanoparticles in a fluidized bed: Tailored deposition of aminopropylsiloxane[J]. Langmuir, 2021, 37(15): 4481-4492. |
| 46 | HWANG Ha Soo, Jae Hyun BAE, KIM Hyun Gyu, et al. Synthesis of silica-polystyrene core-shell nanoparticles via surface thiol-lactam initiated radical polymerization[J]. European Polymer Journal, 2010, 46(8): 1654-1659. |
| 47 | KIM Young-Jae, KIM Jong-Heon, Shin-Woo HA, et al. Polyimide nanocomposites with functionalized SiO2 nanoparticles: Enhanced processability, thermal and mechanical properties[J]. RSC Advances, 2014, 4(82): 43371-43377. |
| 48 | NI Weishuang, WU Songping, REN Qun. Preparation and characterization of silanized TiO2 nanoparticles and their application in toner[J]. Industrial & Engineering Chemistry Research, 2012, 51(40): 13157-13163. |
| 49 | Phi Hung DAO, NGUYEN Thuy Chinh, PHUNG Thi Lan, et al. Assessment of some characteristics and properties of zirconium dioxide nanoparticles modified with 3-(trimethoxysilyl) propyl methacrylate silane coupling agent[J]. Journal of Chemistry, 2021, 2021: 9925355. |
| 50 | SHIN Youngchan, LEE Deokkyu, LEE Kangtaek, et al. Surface properties of silica nanoparticles modified with polymers for polymer nanocomposite applications[J]. Journal of Industrial and Engineering Chemistry, 2008, 14(4): 515-519. |
| 51 | IDRIS Alamin, MAN Zakaria, MAULUD Abdulhalim S, et al. Investigation on particle properties and extent of functionalization of silica nanoparticles[J]. Applied Surface Science, 2020, 506: 144978. |
| 52 | KARNATI Sidharth Reddy, OLDHAM Daniel, FINI Elham H, et al. Application of surface-modified silica nanoparticles with dual silane coupling agents in bitumen for performance enhancement[J]. Construction and Building Materials, 2020, 244: 118324. |
| 53 | HODOROABA Vasile-Dan, RADES Steffi, BORGHETTI Patrizia, et al. Organic surface modification and analysis of titania nanoparticles for self-assembly in multiple layers[J]. Surface and Interface Analysis, 2020, 52(12): 829-834. |
| 54 | ZHAO Jie, MILANOVA Maria, WARMOESKERKEN Marijn M C G, et al. Surface modification of TiO2 nanoparticles with silane coupling agents[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2012, 413: 273-279. |
| 55 | MERTENS G, FRIPIAT J J. The methanol-silica gel system. Ⅲ. Kinetics of the methoxylation process[J]. Journal of Colloid and Interface Science, 1973, 42(1): 169-180. |
| 56 | LUO Ting, ZHANG Ruidan, ZENG Weiwang, et al. Alkoxylation reaction of alcohol on silica surfaces studied by sum frequency vibrational spectroscopy[J]. The Journal of Physical Chemistry C, 2021, 125(16): 8638-8646. |
| 57 | LUO Ting, ZENG Weiwang, ZHANG Ruidan, et al. Hydrophobic modification of silica surfaces via grafting alkoxy groups[J]. ACS Applied Electronic Materials, 2021, 3(4): 1691-1698. |
| 58 | UTSUGI Hiroshi, HORIKOSHI Hideo, MATSUZAWA Toshiharu. Mechanism of esterification of alcohols with surface silanols and hydrolysis of surface esters on silica gels[J]. Journal of Colloid and Interface Science, 1975, 50(1): 154-161. |
| 59 | PAOPRASERT Peerasak, KANDALA Srikanth, SWEAT Daniel P, et al. Versatile grafting chemistry for creation of stable molecular layers on oxides[J]. Journal of Materials Chemistry, 2012, 22(3): 1046-1053. |
| 60 | DILEEP P, NARAYANANKUTTY Sunil K. A novel method for preparation of nanosilica from bamboo leaves and its green modification as a multi-functional additive in styrene butadiene rubber[J]. Materials Today Communications, 2020, 24: 100957. |
| 61 | ABADIKHAH Hamidreza, KALALI Ehsan Naderi, BEHZADI Shabnam, et al. Amino functionalized silica nanoparticles incorporated thin film nanocomposite membrane with suppressed aggregation and high desalination performance[J]. Polymer, 2018, 154: 200-209. |
| 62 | TIAN Jianwen, ZHANG Haoxuan, LIU Meiying, et al. A bioinspired strategy for surface modification of silica nanoparticles[J]. Applied Surface Science, 2015, 357: 1996-2003. |
| 63 | LE-MASURIER Solomon Pradhan, DUONG Hien Thi Thu, BOYER Cyrille, et al. Surface modification of polydopamine coated particles via glycopolymer brush synthesis for protein binding and FLIM testing[J]. Polymer Chemistry, 2015, 6(13): 2504-2511. |
| 64 | MO Min, DU Shuo, GAO Yujie, et al. Bioinspired Janus particles for hydrophobic modification of hydrogels with photothermal antibacterial capability[J]. Journal of Colloid and Interface Science, 2022, 616: 93-100. |
| 65 | ANDERSON Travers H, YU Jing, ESTRADA Abril, et al. The contribution of DOPA to substrate-peptide adhesion and internal cohesion of mussel-inspired synthetic peptide films[J]. Advanced Functional Materials, 2010, 20(23): 4196-4205. |
| 66 | DALSIN Jeffrey L, LIN Lijun, TOSATTI Samuele, et al. Protein resistance of titanium oxide surfaces modified by biologically inspired mPEG-DOPA[J]. Langmuir, 2005, 21(2): 640-646. |
| 67 | MARTIN Scot T, KESSELMAN Janet M, PARK David S, et al. Surface structures of 4-chlorocatechol adsorbed on titanium dioxide[J]. Environmental Science & Technology, 1996, 30(8): 2535-2542. |
| 68 | MCBRIDE Murray B, WESSELINK Lambert G. Chemisorption of catechol on gibbsite, boehmite, and noncrystalline alumina surfaces[J]. Environmental Science & Technology, 1988, 22(6): 703-708. |
| 69 | OSSENKAMP Gabriel C, KEMMITT Tim, JOHNSTON Jim H. New approaches to surface-alkoxylated silica with increased hydrolytic stability[J]. Chemistry of Materials, 2001, 13(11): 3975-3980. |
| 70 | OSSENKAMP Gabriel C, KEMMITT Tim, JOHNSTON Jim H. Toward functionalized surfaces through surface esterification of silica[J]. Langmuir, 2002, 18(15): 5749-5754. |
| 71 | FUJI M, UENO S, TAKEI T, et al. Surface structural analysis of fine silica powder modified with butyl alcohol[J]. Colloid and Polymer Science, 2000, 278(1): 30-36. |
| 72 | FUJI Masayoshi, MACHIDA Kotoe, TAKEI Takashi, et al. Surface geometric structure of chemically modified silica studied by direct atomic force microscopy (AFM) imaging and adsorption method[J]. Langmuir, 2000, 16(7): 3281-3287. |
| 73 | FUJI Masayoshi, TAKEI Takashi, WATANABE Tohoru, et al. Wettability of fine silica powder surfaces modified with several normal alcohols[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 1999, 154(1/2): 13-24. |
| 74 | KAWAMURA Aki, UENO Shoichi, TAKAI Chika, et al. Effect of steric hindrance on surface wettability of fine silica powder modified by n- or t-butyl alcohol[J]. Advanced Powder Technology, 2017, 28(10): 2488-2495. |
| 75 | WANG Jingyuan, YANG Ling, XIE Jiuren, et al. Surface amination of silica nanoparticles using tris(hydroxymethyl)aminomethane[J]. Industrial & Engineering Chemistry Research, 2020, 59(49): 21383-21392. |
| 76 | PUJARI Sidharam P, SCHERES Luc, MARCELIS Antonius T M, et al. Covalent surface modification of oxide surfaces[J]. Angewandte Chemie International Edition, 2014, 53(25): 6322-6356. |
| 77 | HOTCHKISS Peter J, JONES Simon C, PANIAGUA Sergio A, et al. The modification of indium tin oxide with phosphonic acids: Mechanism of binding, tuning of surface properties, and potential for use in organic electronic applications[J]. Accounts of Chemical Research, 2012, 45(3): 337-346. |
| 78 | Florence BRODARD-SEVERAC, GUERRERO Gilles, MAQUET Jocelyne, et al. High-field 17O MAS NMR investigation of phosphonic acid monolayers on titania[J]. Chemistry of Materials, 2008, 20(16): 5191-5196. |
| 79 | DING Xuefeng, ZHAO Jingzhe, LIU Yanhua, et al. Silica nanoparticles encapsulated by polystyrene via surface grafting and in situ emulsion polymerization[J]. Materials Letters, 2004, 58(25): 3126-3130. |
| 80 | LI Zongwei, ZHU Yongfa. Surface-modification of SiO2 nanoparticles with oleic acid[J]. Applied Surface Science, 2003, 211(1/2/3/4): 315-320. |
| 81 | Hubert MUTIN P, LAFOND Vincent, POPA Aurelian F, et al. Selective surface modification of SiO2-TiO2 supports with phosphonic acids[J]. Chemistry of Materials, 2004, 16(26): 5670-5675. |
| 82 | MALLAKPOUR Shadpour, NIKKHOO Elham. Surface modification of nano-TiO2 with trimellitylimido-amino acid-based diacids for preventing aggregation of nanoparticles[J]. Advanced Powder Technology, 2014, 25(1): 348-353. |
| 83 | BAEK Naerin, KIM Young T, MARCY Joe E, et al. Physical properties of nanocomposite polylactic acid films prepared with oleic acid modified titanium dioxide[J]. Food Packaging and Shelf Life, 2018, 17: 30-38. |
| 84 | MOHAPATRA Sasmita, PRAMANIK Panchanan. Synthesis and stability of functionalized iron oxide nanoparticles using organophosphorus coupling agents[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2009, 339(1/2/3): 35-42. |
| 85 | BERTAZZO Sergio, REZWAN Kurosch. Control of α-alumina surface charge with carboxylic acids[J]. Langmuir, 2010, 26(5): 3364-3371. |
| 86 | GUERRERO Gilles, Hubert MUTIN P, VIOUX André. Organically modified aluminas by grafting and sol-gel processes involving phosphonate derivatives[J]. Journal of Materials Chemistry, 2001, 11(12): 3161-3165. |
| 87 | PAULY Céline Schmitt, GENIX Anne-Caroline, ALAUZUN Johan G, et al. Surface modification of alumina-coated silica nanoparticles in aqueous sols with phosphonic acids and impact on nanoparticle interactions[J]. Physical Chemistry Chemical Physics, 2015, 17(29): 19173-19182. |
| 88 | PAVÓN-HERNÁNDEZ Arturo I, Eustolia RODRÍGUEZ-VELÁZQUEZ, Manuel ALATORRE-MEDA, et al. Magnetic nanocomposite with fluorescence enhancement effect based on amino acid coated-Fe3O4 functionalized with quantum dots[J]. Materials Chemistry and Physics, 2020, 251: 123082. |
| 89 | HOSSAIN Khohinur, FLOREAN Luca, DEL TEDESCO Anna, et al. Modification of amorphous mesoporous zirconia nanoparticles with bisphosphonic acids: A straightforward approach for tailoring the surface properties of the nanoparticles[J]. Chemistry-A European Journal, 2021, 27(71): 17941-17951. |
| 90 | MINGALYOV P G, BUCHNEV M V, LISICHKIN G V. Chemical modification of alumina and silica with alkylphosphonic acids and their esters[J]. Russian Chemical Bulletin, 2001, 50(9): 1693-1695. |
| 91 | YE Lu, ZHOU Lin, LU Yangcheng. Direct continuous synthesis of oleic acid-modified Fe3O4 nanoparticles in a microflow system[J]. Industrial & Engineering Chemistry Research, 2022, 61(12): 4320-4328. |
| 92 | AGUSTIN Anggi Regiana, TAMURA Kazuhiro. Surface modification of TiO2 nanoparticles with terephthalic acid in supercritical carbon dioxide[J]. The Journal of Supercritical Fluids, 2021, 174: 105245. |
| 93 | CALLENDER Rhonda L, Jeff HARLAN C, SHAPIRO Noah M, et al. Aqueous synthesis of water-soluble alumoxanes: Environmentally benign precursors to alumina and aluminum-based ceramics[J]. Chemistry of Materials, 1997, 9(11): 2418-2433. |
| 94 | GOWENLOCK Cathren E, MCGETTRICK James D, MCNAUGHTER Paul D, et al. Copper-complexed isonicotinic acid functionalized aluminum oxide nanoparticles[J]. Main Group Chemistry, 2015, 15(1): 1-15. |
| 95 | GUO Xueyi, MAO Fangfang, WANG Weijia, et al. Sulfhydryl-modified Fe3O4@SiO2 core/shell nanocomposite: Synthesis and toxicity assessment in vitro[J]. ACS Applied Materials & Interfaces, 2015, 7(27): 14983-14991. |
| 96 | GHEONEA Ramona, Carmen MAK, CRASMAREANU Eleonora Cornelia, et al. Surface modification of SnO2 with phosphonic acids[J]. Journal of Chemistry, 2017, 2017: 2105938. |
| 97 | 乔梁, 付丽, 郑精武, 等. 一种氧化铝粉末的表面疏水改性方法: CN108410215A[P]. 2018-08-17. |
| QIAO Liang, FU Li, ZHENG Jingwu, et al. Surface hydrophobic modification method of alumina powder: CN108410215A[P]. 2018-08-17. | |
| 98 | 马志领, 郝学辉, 何佳音, 等. 羟基硅油原位改性制备疏水性沉淀二氧化硅[J]. 无机盐工业, 2011, 43(3): 36-38. |
| MA Zhiling, HAO Xuehui, HE Jiayin, et al. Preparation of hydrophobic precipitated SiO2 by insitu modification of hydroxyl silicone oil[J]. Inorganic Chemicals Industry, 2011, 43(3): 36-38. | |
| 99 | 李亚, 陈洪龄. 用含氢硅油机械化学法改性SiO2粉体[J]. 机械工程材料, 2008, 32(1): 76-78, 83. |
| LI Ya, CHEN Hongling. Modification of silica by mechanochemical treatment with polymethylhydrogensiloxane[J]. Materials for Mechanical Engineering, 2008, 32(1): 76-78, 83. | |
| 100 | 卜军, 陈洪龄, 沈斌, 等. 机械力化学法疏水改性高岭土[J]. 化学研究与应用, 2009, 21(5): 760-763. |
| BU Jun, CHEN Hongling, SHEN Bin, et al. Hydrophobic modification of Kaolin by mechanochemical method[J]. Chemical Research and Application, 2009, 21(5): 760-763. | |
| 101 | 施利毅, 孙小英, 沈骁遥, 等. 一种改性纳米二氧化钛及其制备方法: CN110835119A[P]. 2020-02-25. |
| SHI Liyi, SUN Xiaoying, SHEN Xiaoyao, et al. Modified nanometermeter titanium dioxide and preparation method thereof: CN110835119A[P]. 2020-02-25. | |
| 102 | MEKURIA Tadele Daniel, ZHANG Chunhong, LIU Yingnan, et al. Surface modification of nano-silica by diisocyanates and their application in polyimide matrix for enhanced mechanical, thermal and water proof properties[J]. Materials Chemistry and Physics, 2019, 225: 358-364. |
| 103 | Baoli OU, LI Duxin. Preparation of polystyrene/silica nanocomposites by radical copolymerization of styrene with silica macromonomer[J]. Science in China Series B: Chemistry, 2007, 50(3): 385-391. |
| [1] | WAN Zhen, WANG Shaoqing, LI Zhihe, ZHAO Tiansheng. Advances in HZSM-5 catalyzed pyrolysis of lignin to aromatic hydrocarbons [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 517-532. |
| [2] | MAO Ningxuan, WAN Xiaowei, JU Jie, HU Yanjie, JIANG Hao. Numerical simulation and structural optimization of flow field in industrial gas-solid fluidized beds based on CFD-PBM [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 13-20. |
| [3] | XIE Yulin, RAU Jui-yeh, HUANG Jian, HAO Jiayi, WANG Youyi, HUANG Qi. Preparation of continuous ZIF-8 membrane and its progress in hydrogen separation [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 403-418. |
| [4] | FU Wei, NING Shuying, CAI Chen, CHEN Jiayin, ZHOU Hao, SU Yaxin. SCR-C3H6 denitrification performance of Cu-modified MIL-100(Fe) catalysts [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 4951-4960. |
| [5] | SUN Shiwan, LI Xin, ZHOU Han. Review of radiative cooling paint and its applications in the fields of energy and environment [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 4961-4969. |
| [6] | LI Ying, WANG Qizhao, BAI Bo, ZHANG Qian, SUN Wenjing, ZHANG Linxuan. Unidirectional water transportation and fog collection performance of Janus surface-patterned structures [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5133-5141. |
| [7] | OU Hongxiang, MIN Zheng, XUE Honglai, CAO Haizhen, BI Haipu, WANG Junqi. Effect of hydrophobic modified magnesium oxide nanoparticles on the properties of short fluorocarbon chain foam [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5177-5184. |
| [8] | WU Yuqi, LI Jiangtao, DING Jianzhi, SONG Xiulan, SU Bingqin. Calcined Mg/Al hydrotalcites for CO2 removal in anaerobic digestion biogas: Performances and mechanisms [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5250-5261. |
| [9] | SUN Yan, XIE Xiaoyang, FENG Qianying, ZHENG Lu, HE Jiaojie, YANG Liwei, BAI Bo. Preparation of forward osmosis membrane modified by tannic acid-iron (Ⅲ) and its antifouling performance [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5309-5319. |
| [10] | LIANG Guowei, JIN Jing, DONG Bo, HOU Fengxiao. Effect of in-situ modification of coal ash on carbon deposition of Ca-based oxygen carrier in chemical looping combustion [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4253-4261. |
| [11] | SONG Jiakai, KONG Lingzhen, CHEN Jiaqing, SUN Huan, LI Qi, LI Changhe, WANG Sicheng, KONG Biao. Liquid film flow and separation characteristics in the swirl separation section of a tubular deliquidiser [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4297-4306. |
| [12] | WANG Jia, LI Wencui, WU Fan, GAO Xinqian, LU Anhui. Regulation active components distribution of NiMo/Al2O3 catalysts for hydrodesulfurization [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4393-4402. |
| [13] | FU Tao, LI Li, GAO Lining, ZHU Fuwei, CAO Weiye, CHEN Huaxin. Cement-based boron-doped graphite phase carbon nitride material degrades NO [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4403-4410. |
| [14] | LONG Tao, ZHOU Feng, ZHANG Wei, WU Hong, WANG Jian, CHEN Lin. Synthesis and modification of deuterated methanol catalyst used in CO-CO2 system [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4411-4420. |
| [15] | LI li, CAI Xinyu, CHEN Yinjie, ZHANG Wenqi, LI Guanghui, RAO Pinhua. Preparation and properties of superhydrophobic-highly oleophobic SiC membrane [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4516-4522. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
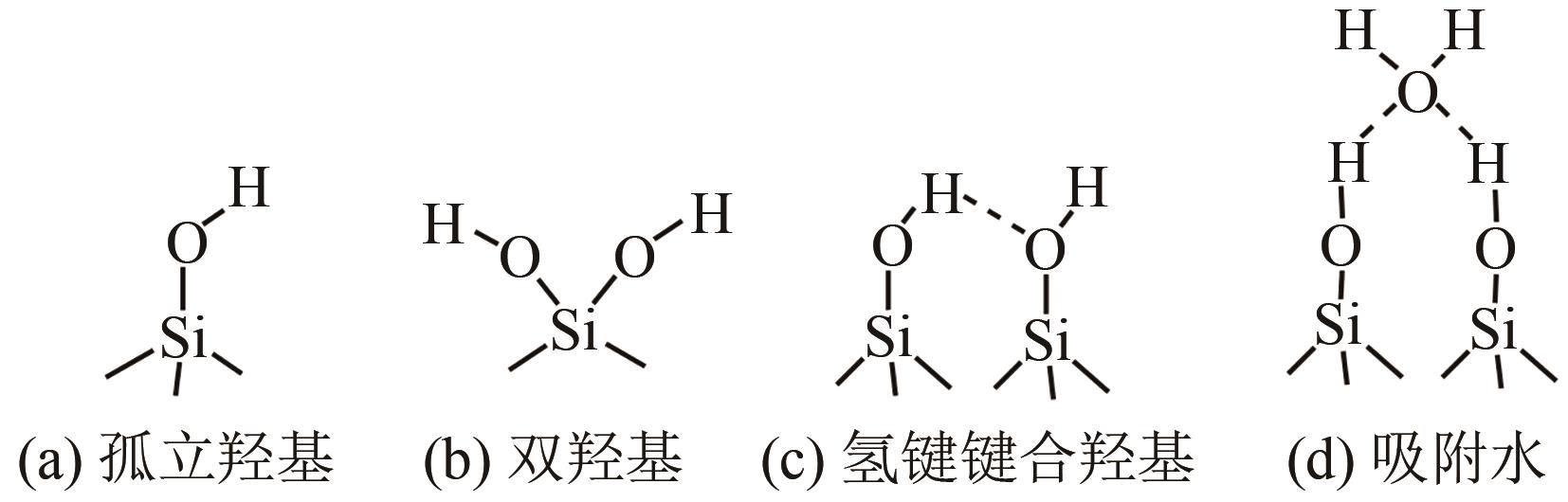
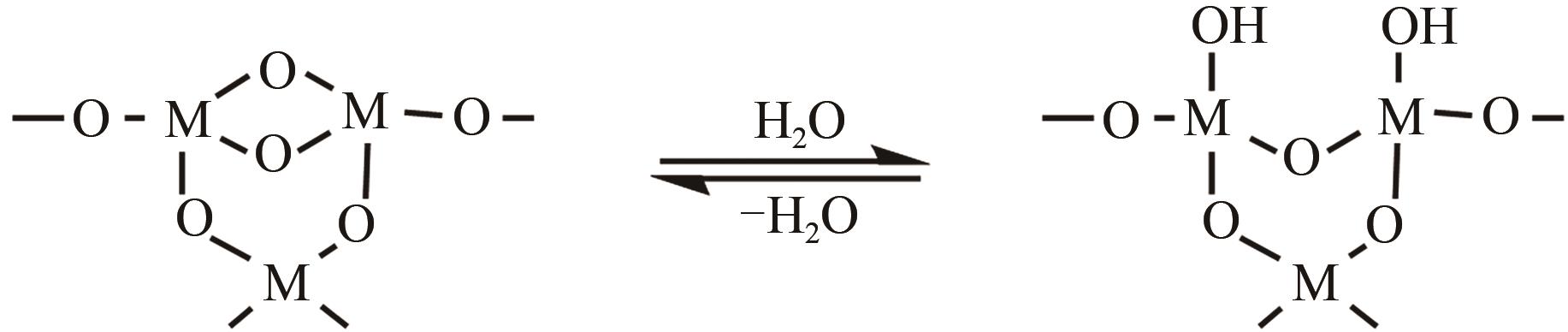


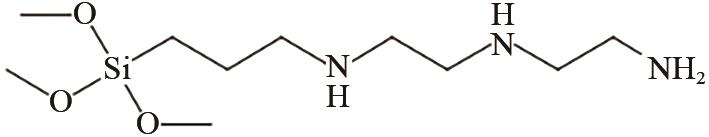

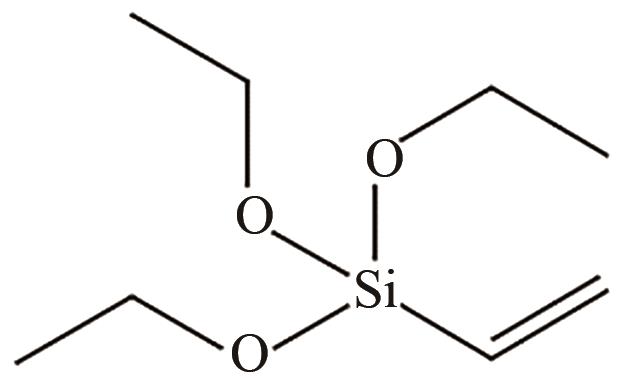
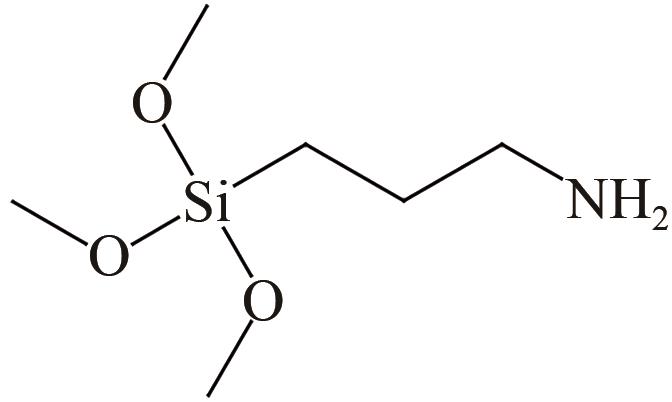
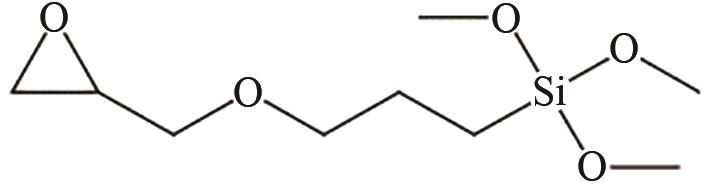

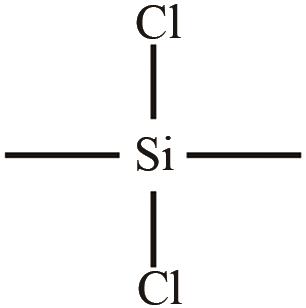

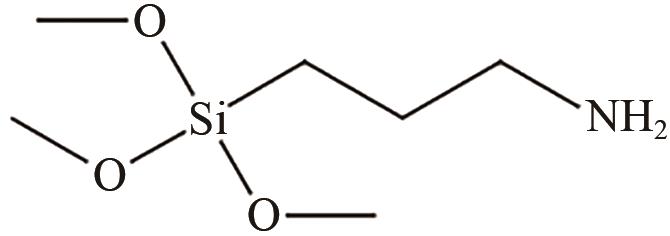
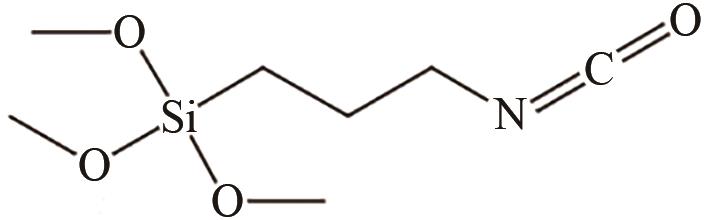
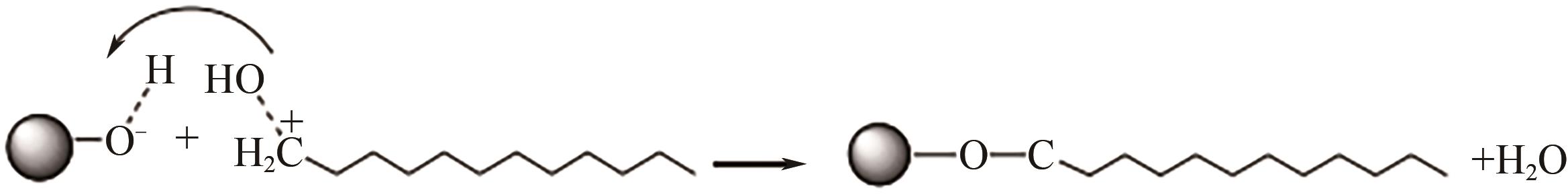
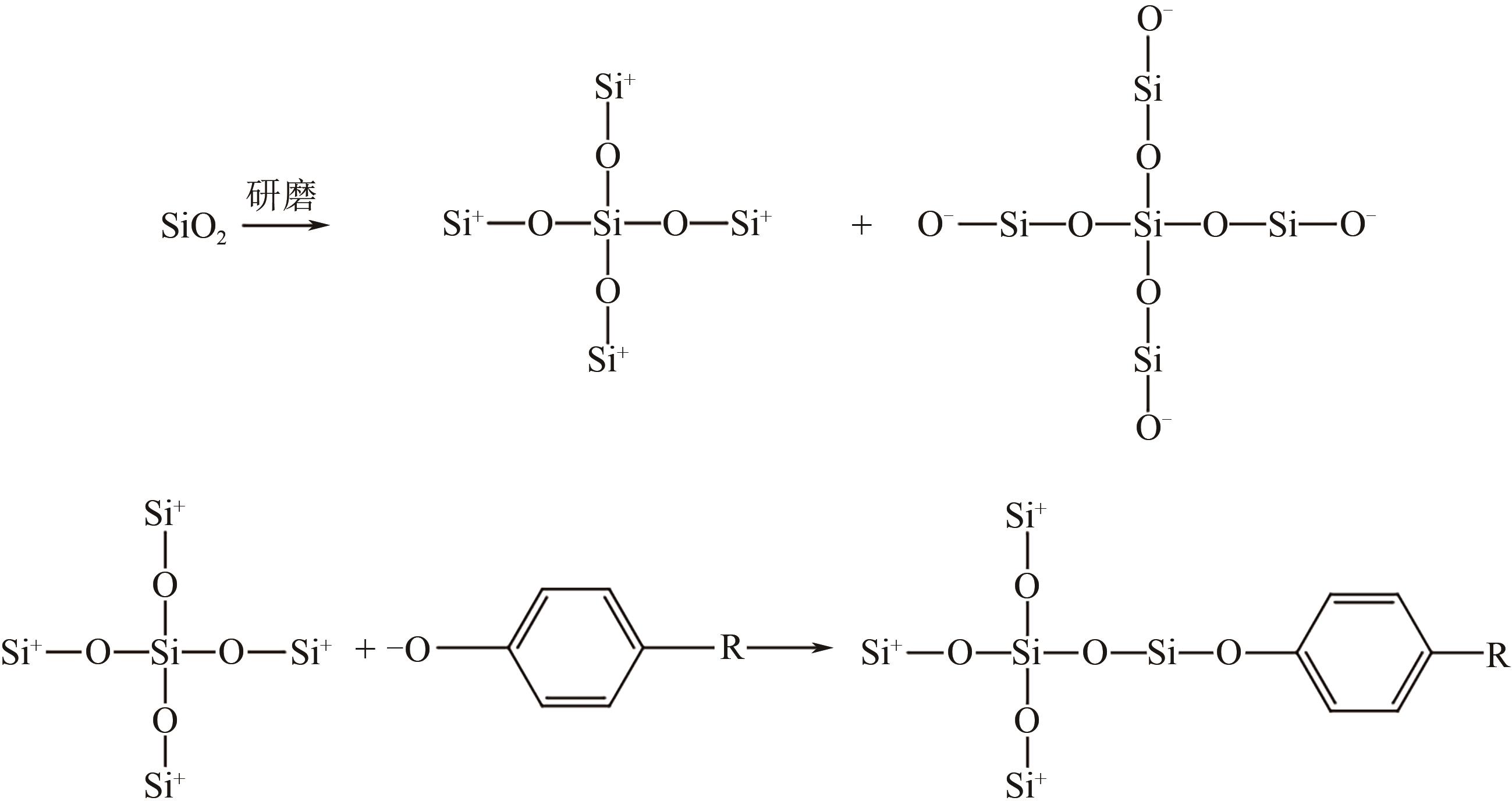
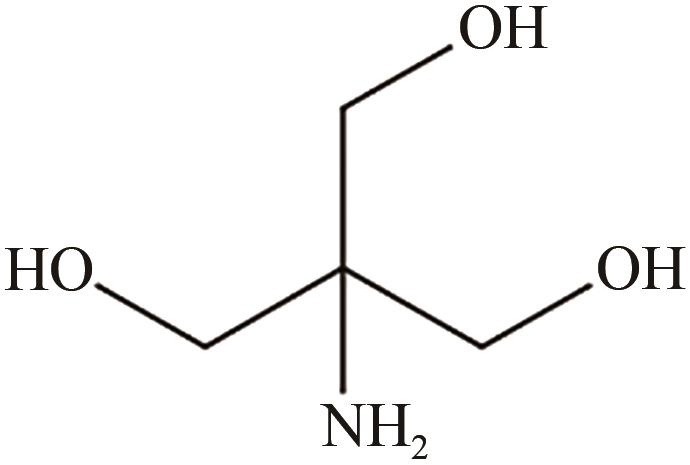

 、
、