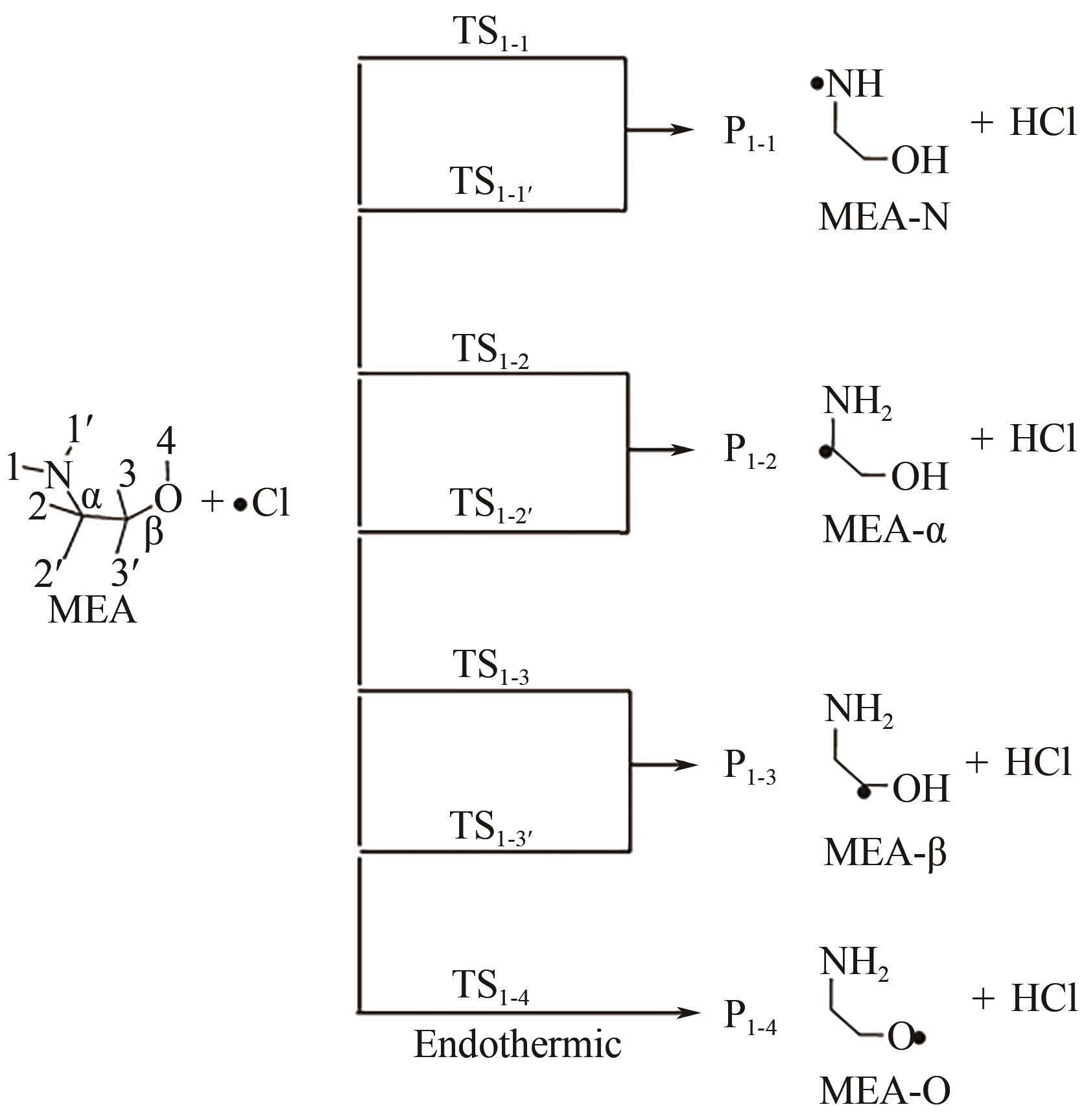
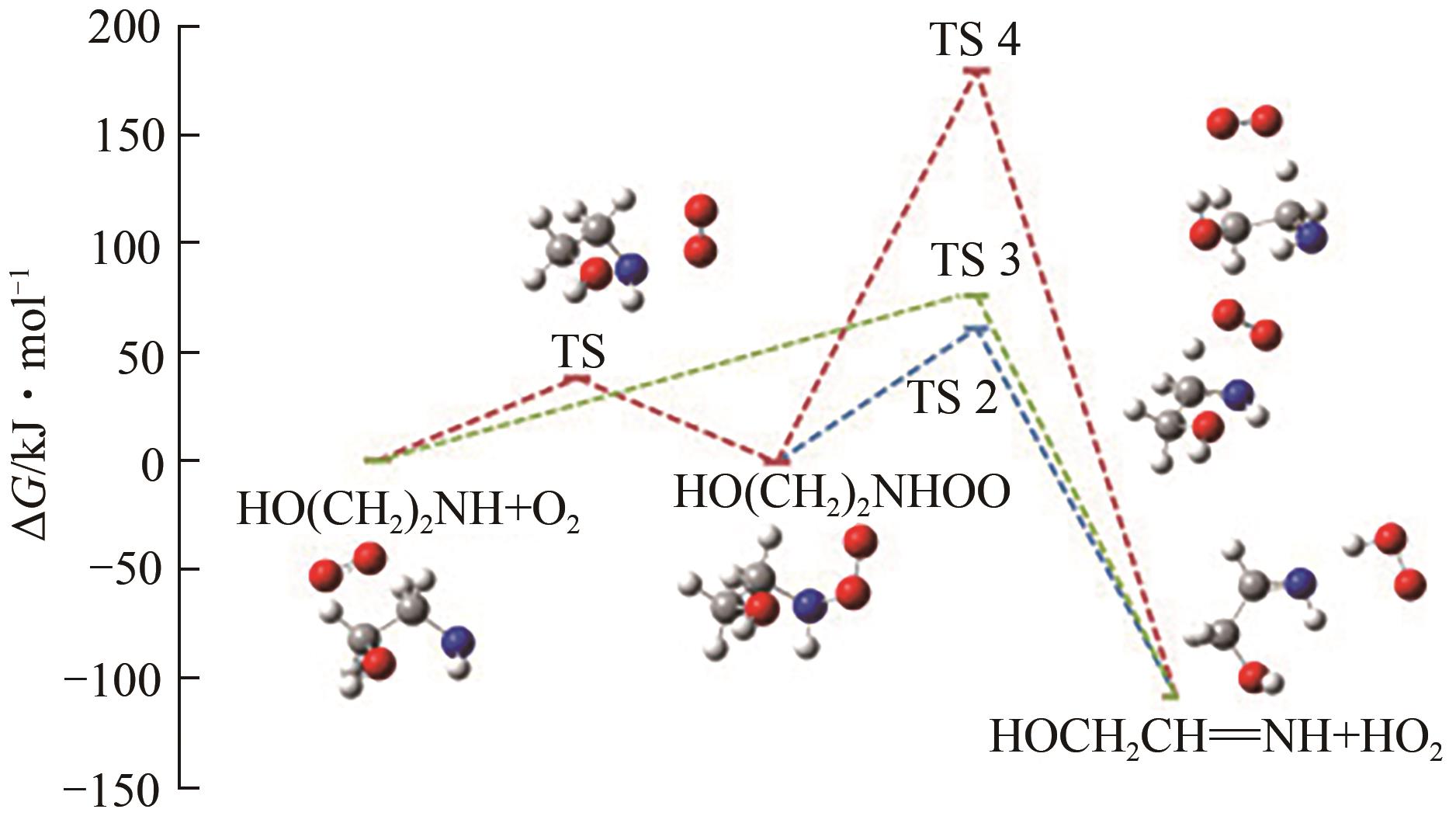
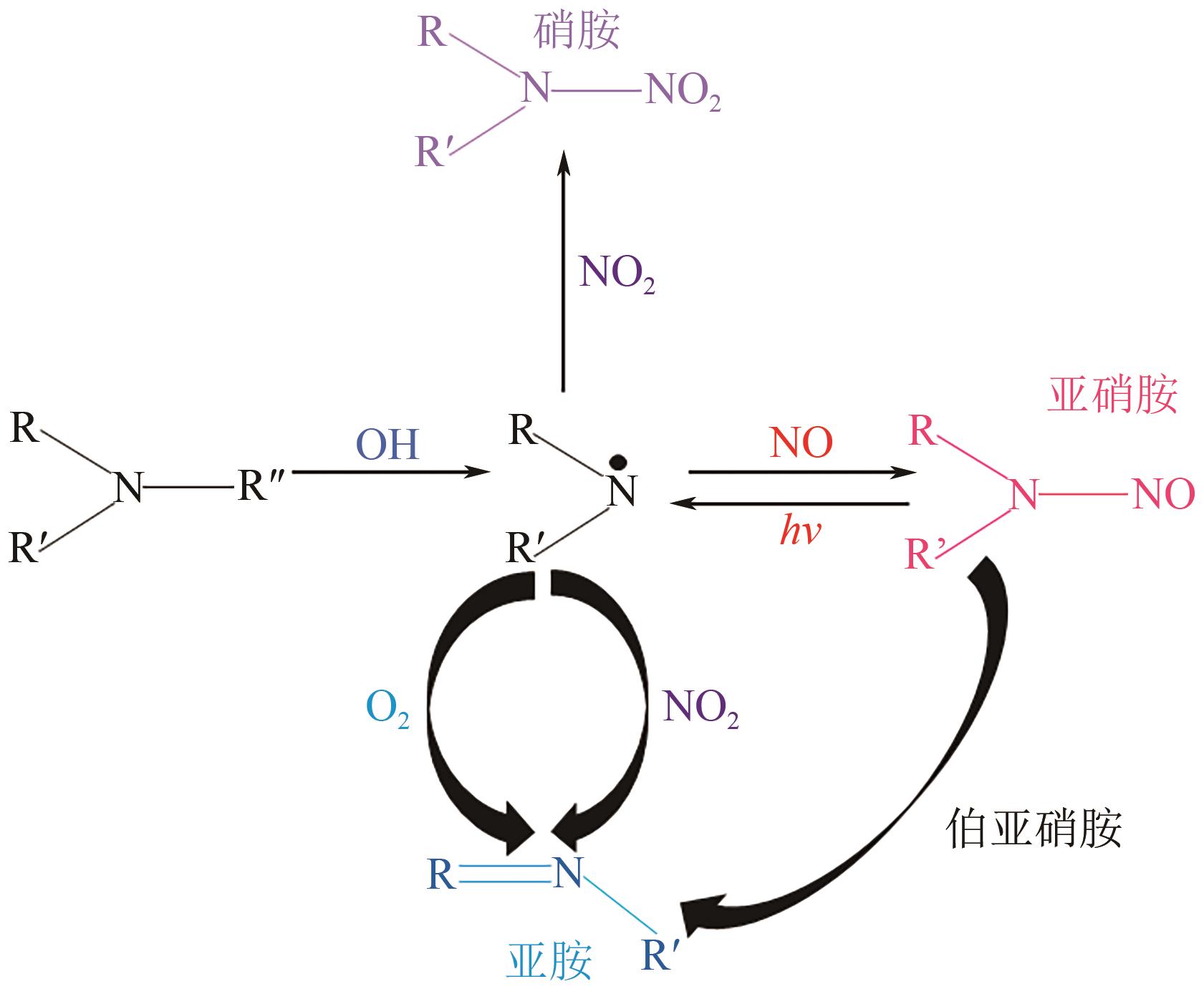
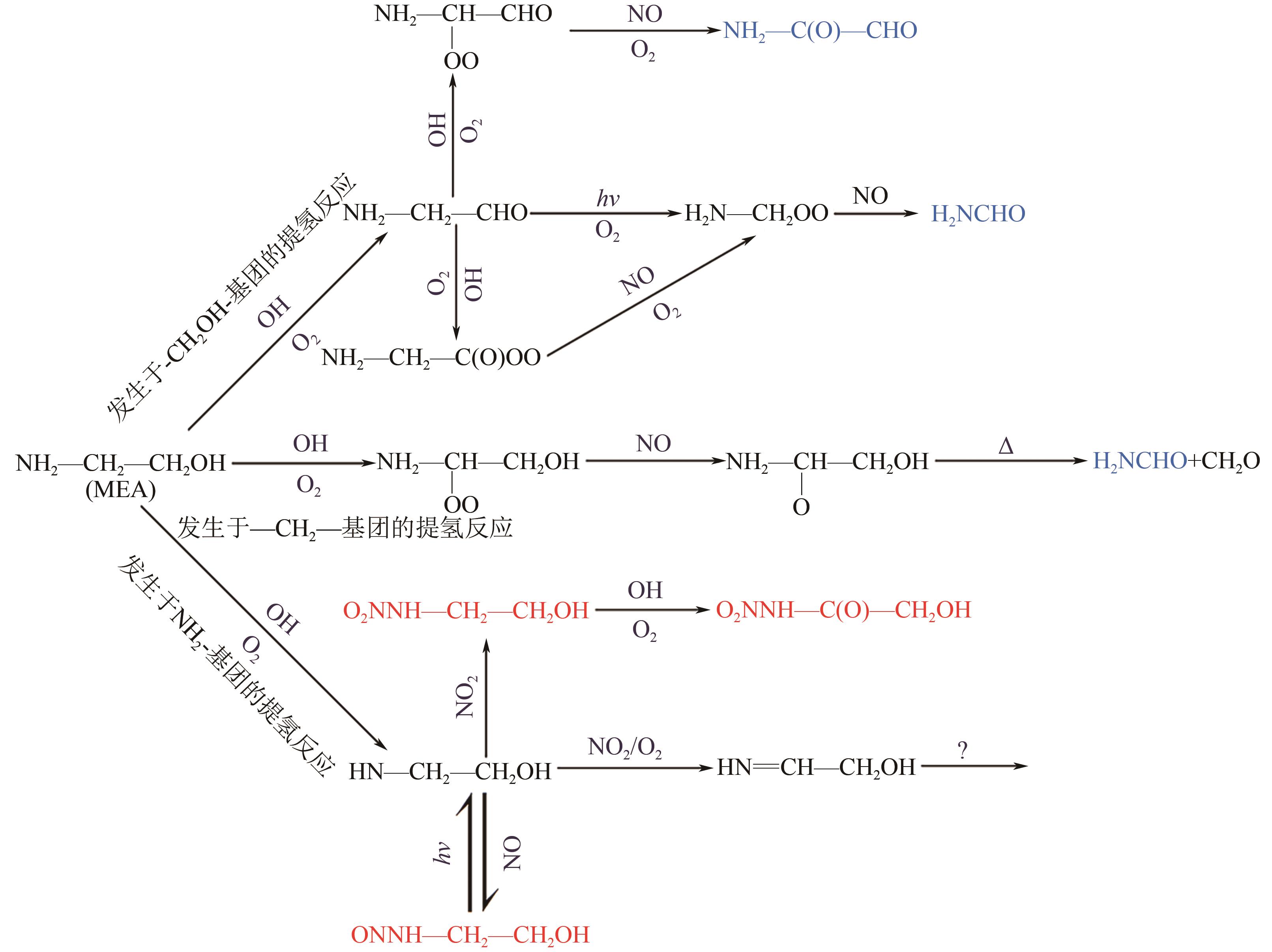
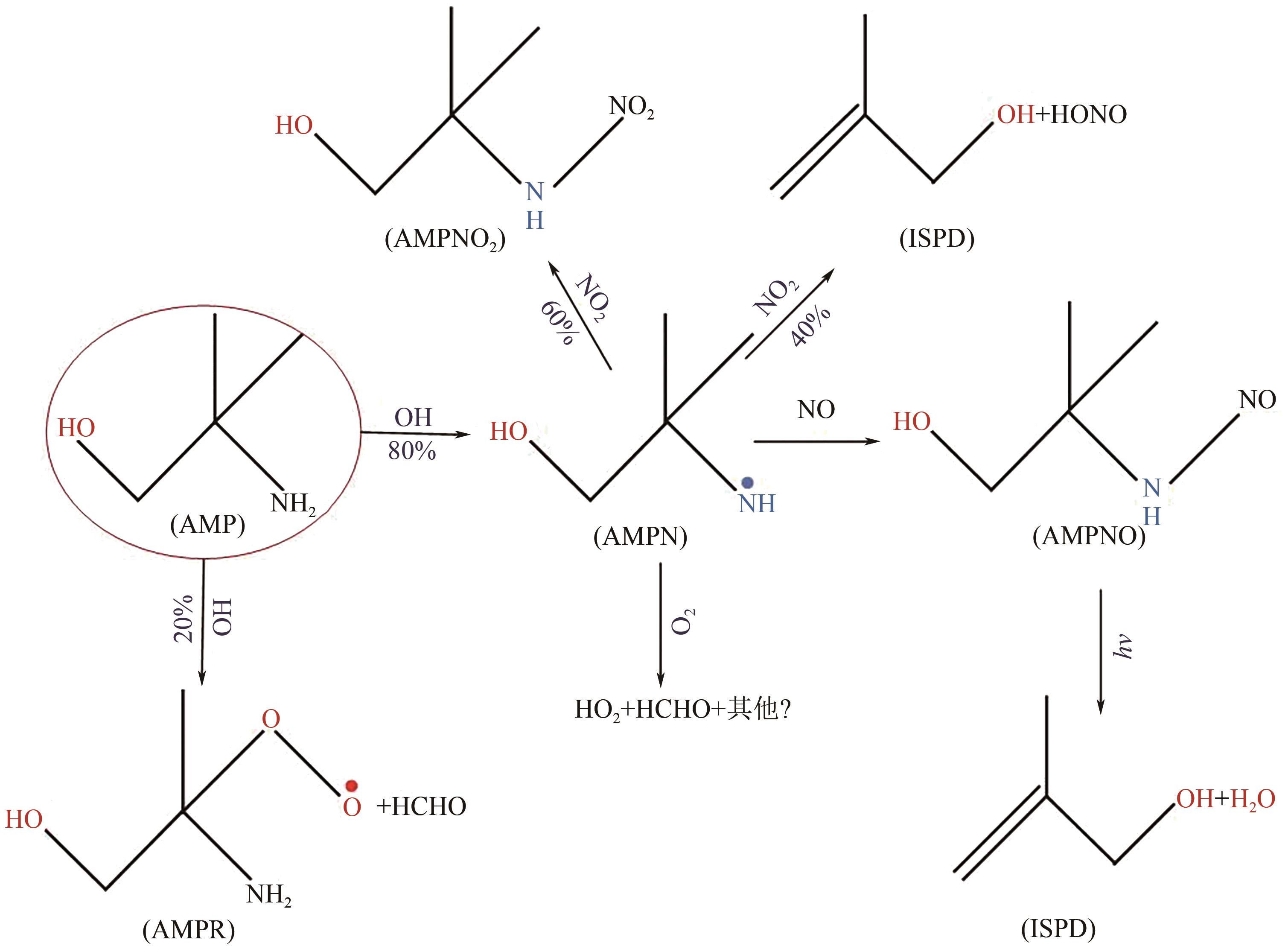
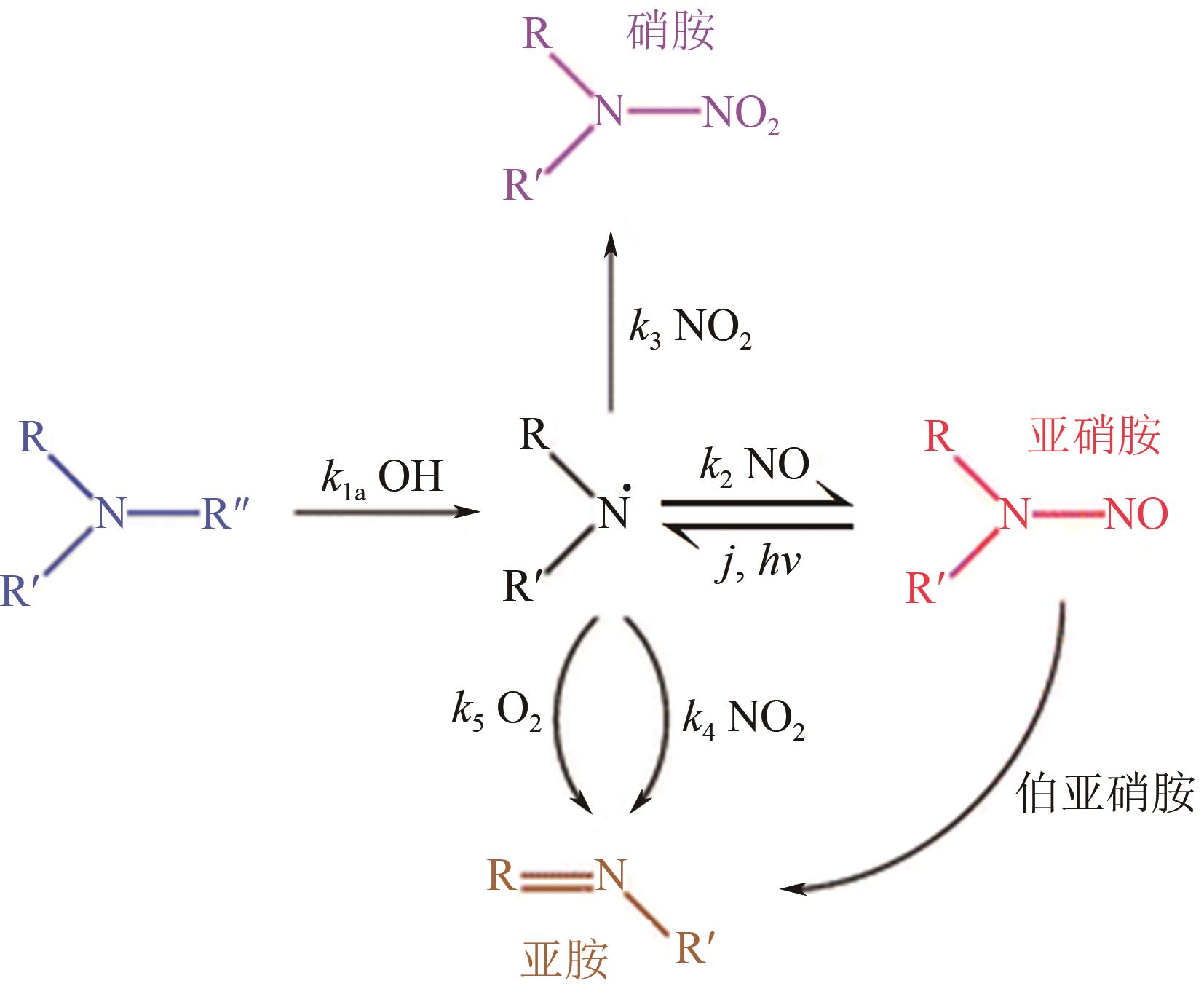
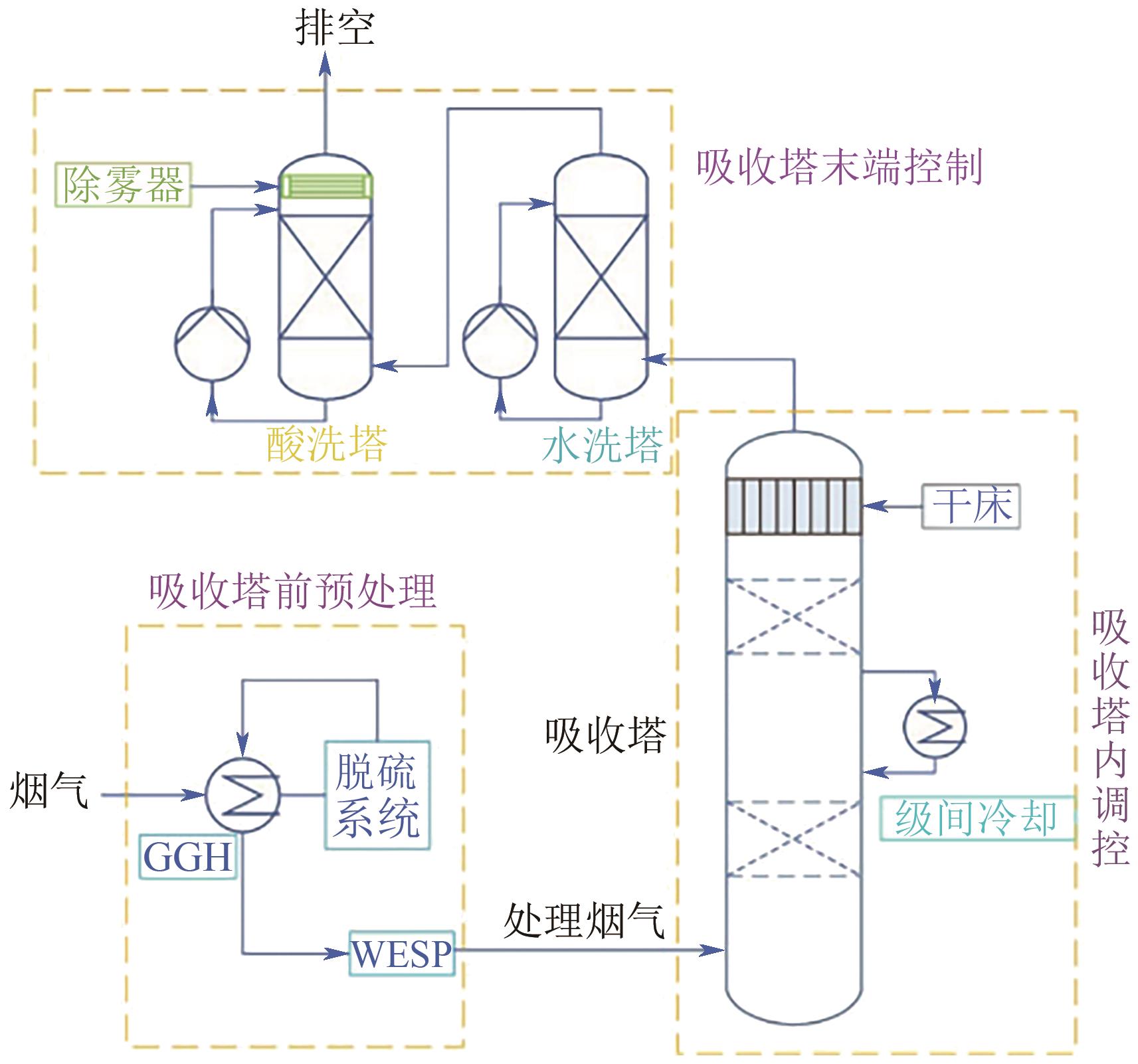
Chemical Industry and Engineering Progress ›› 2024, Vol. 43 ›› Issue (11): 6397-6411.DOI: 10.16085/j.issn.1000-6613.2023-1765
• Resources and environmental engineering • Previous Articles
Advances in atmospheric gas-phase reactions initiated by amine absorbent escape
LU Shijian1,2( ), ZHU Wenju1,2(
), ZHU Wenju1,2( ), LIU Ling1,2, KANG Guojun1,2, CHEN Siming1
), LIU Ling1,2, KANG Guojun1,2, CHEN Siming1
- 1.Carbon Neutral Research Institute, China University of Mining and Technology, Xuzhou 221116, Jiangsu, China
2.School of Chemical Engineering, China University of Mining and Technology, Xuzhou 221116, Jiangsu, China
-
Received:2023-10-10Revised:2024-03-04Online:2024-12-07Published:2024-11-15 -
Contact:LU Shijian
胺吸收剂逃逸引发的大气气相反应进展
陆诗建1,2( ), 祝文举1,2(
), 祝文举1,2( ), 刘玲1,2, 康国俊1,2, 陈思铭1
), 刘玲1,2, 康国俊1,2, 陈思铭1
- 1.中国矿业大学碳中和研究院,江苏 徐州 221116
2.中国矿业大学化工学院,江苏 徐州 221116
-
通讯作者:陆诗建 -
作者简介:陆诗建(1984—),男,博士,研究员,研究方向为CCUS与废气治理技术。E-mail:lushijian@cumt.edu.cn
祝文举(2000—),男,硕士研究生,研究方向为CCUS与废气治理技术。E-mail:TS22040155P31@cumt.edu.cn。 -
基金资助:中央高校基本科研业务费专项(XJ2022000501);江苏省科技厅科技项目-碳达峰碳中和科技创新专项(BE2022613)
CLC Number:
Cite this article
LU Shijian, ZHU Wenju, LIU Ling, KANG Guojun, CHEN Siming. Advances in atmospheric gas-phase reactions initiated by amine absorbent escape[J]. Chemical Industry and Engineering Progress, 2024, 43(11): 6397-6411.
陆诗建, 祝文举, 刘玲, 康国俊, 陈思铭. 胺吸收剂逃逸引发的大气气相反应进展[J]. 化工进展, 2024, 43(11): 6397-6411.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-1765
| 排放类型 | 形态 | 产生原因 | 吸收塔后胺排放数量级/μL·L-1 |
|---|---|---|---|
| 物理夹带 | 液滴D≥100μm | 气液接触时部分吸收剂被气流携带 | <1 |
| 气体 | 气态 | 吸收剂及其降解产物挥发 | 0~102 |
| 气溶胶 | 气溶胶颗粒100μm>D>1nm | 吸收塔内非均相成核、均相成核、气泡破裂等 | 0~103 |
| 排放类型 | 形态 | 产生原因 | 吸收塔后胺排放数量级/μL·L-1 |
|---|---|---|---|
| 物理夹带 | 液滴D≥100μm | 气液接触时部分吸收剂被气流携带 | <1 |
| 气体 | 气态 | 吸收剂及其降解产物挥发 | 0~102 |
| 气溶胶 | 气溶胶颗粒100μm>D>1nm | 吸收塔内非均相成核、均相成核、气泡破裂等 | 0~103 |
| 来源 | 报道的排放浓度/mg·m-3 | 组分 |
|---|---|---|
| IEAGHG技术报告试验厂[ | 0.5~3 | MEA |
| Niederaussem中试工厂[ | 0.02~0.03 | MEA |
| CESAR中试工厂[ | <0.3 | MEA |
| ENEL中试工厂[ | 1.2~1.5 | MEA(WESP运行与否) |
| Maasvlakte火电站CO2捕集中试厂,1500m3/h[ | 0.97~4 | MEA(除雾器后) |
| 206 | MEA(水洗塔后) | |
| 336~460 | MEA(吸收塔后) | |
| Loy Yang中试工厂[ | 2.4 | MEA |
| KIT中试火电厂CO2捕集系统,180m3/h[ | 3000 | MEA |
| TNO微型移动捕集试验厂,4m3/h[ | 100~200 | MEA(进口烟气含有烟尘) |
| 600~1100 | MEA(进口烟气含有SO3) | |
| TNO微型移动捕集试验厂,4m3/h[ | 1000~1900 | MEA(贫液温度40~80℃)) |
| 100~2940 | AMP(贫液pH=9.4~11.0,硫酸气溶胶浓度变化) | |
| 0~416 | PZ(CO2体积分数0.7%~13%,硫酸气溶胶浓度变化) | |
| TNO微型移动捕集试验厂,4m3/h[ | 383 1051 | MEA(进口烟气无SO3) MEA(进口烟气SO3浓度5.25μL/L) |
| 来源 | 报道的排放浓度/mg·m-3 | 组分 |
|---|---|---|
| IEAGHG技术报告试验厂[ | 0.5~3 | MEA |
| Niederaussem中试工厂[ | 0.02~0.03 | MEA |
| CESAR中试工厂[ | <0.3 | MEA |
| ENEL中试工厂[ | 1.2~1.5 | MEA(WESP运行与否) |
| Maasvlakte火电站CO2捕集中试厂,1500m3/h[ | 0.97~4 | MEA(除雾器后) |
| 206 | MEA(水洗塔后) | |
| 336~460 | MEA(吸收塔后) | |
| Loy Yang中试工厂[ | 2.4 | MEA |
| KIT中试火电厂CO2捕集系统,180m3/h[ | 3000 | MEA |
| TNO微型移动捕集试验厂,4m3/h[ | 100~200 | MEA(进口烟气含有烟尘) |
| 600~1100 | MEA(进口烟气含有SO3) | |
| TNO微型移动捕集试验厂,4m3/h[ | 1000~1900 | MEA(贫液温度40~80℃)) |
| 100~2940 | AMP(贫液pH=9.4~11.0,硫酸气溶胶浓度变化) | |
| 0~416 | PZ(CO2体积分数0.7%~13%,硫酸气溶胶浓度变化) | |
| TNO微型移动捕集试验厂,4m3/h[ | 383 1051 | MEA(进口烟气无SO3) MEA(进口烟气SO3浓度5.25μL/L) |
| 胺吸收剂种类 | 结构 | kOH/cm3·mol-1·s-1 |
|---|---|---|
| MEA | 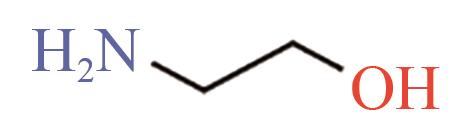 | (7.61±0.76)×10-11[ (9.2±1.1)×10-11[ (7.02±0.46)×10-11[ |
| DMEA | 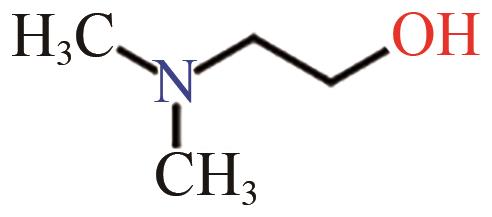 | (9.0±2.0)×10-11[ (4.7±1.2)×10-11[ (7.29±0.72)×10-11[ |
| AMP | 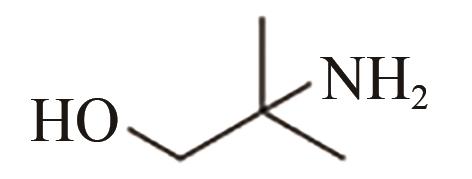 | (2.8±0.5)×10-11[ |
| PZ | 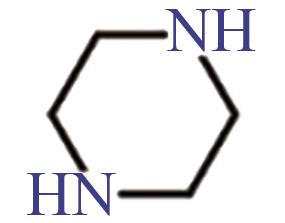 | (2.8±0.6)×10-10[ |
| MMEA | 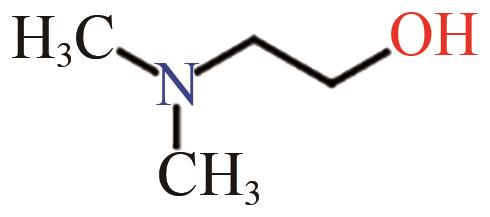 | (8.26±0.82)×10-11[ |
| 胺吸收剂种类 | 结构 | kOH/cm3·mol-1·s-1 |
|---|---|---|
| MEA |  | (7.61±0.76)×10-11[ (9.2±1.1)×10-11[ (7.02±0.46)×10-11[ |
| DMEA |  | (9.0±2.0)×10-11[ (4.7±1.2)×10-11[ (7.29±0.72)×10-11[ |
| AMP |  | (2.8±0.5)×10-11[ |
| PZ |  | (2.8±0.6)×10-10[ |
| MMEA |  | (8.26±0.82)×10-11[ |
| 胺吸收剂种类 | 结构 | k |
|---|---|---|
| MEA | 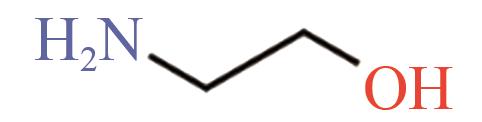 | (1.09±0.05)×10-18[ |
| AMP | 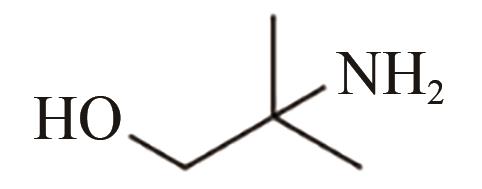 | 1.9×10-19[ |
| DMAE | 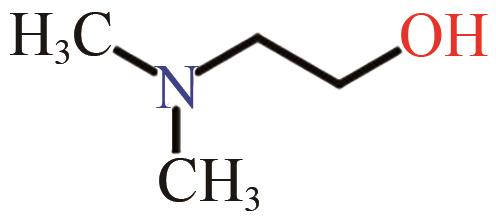 | (6.76±0.83)×10-18[ |
| 胺吸收剂种类 | 结构 | k |
|---|---|---|
| MEA |  | (1.09±0.05)×10-18[ |
| AMP |  | 1.9×10-19[ |
| DMAE |  | (6.76±0.83)×10-18[ |
| 化学名称/种类 | 缩写名称 | 是否鉴定 |
|---|---|---|
| 2-氨基-2-甲基-1-丙醇(碱胺) | AMP | 是 |
| AMP的氨基 | AMPN | 否(未观察到自由基) |
| AMP的过氧基团 | AMPR | 否(未观察到自由基) |
| AMP硝酸气溶胶 | AMPNTR | 通过SMPS测量间接观察到 |
| AMP亚硝胺 | AMPNO | 否(亚硝胺很难观察到) |
| AMP硝胺 | AMPNO2 | 通过SIFT-MS观察到, 但很难量化 |
| 甲烯丙醇等产品 | ISPD | 否 |
| 化学名称/种类 | 缩写名称 | 是否鉴定 |
|---|---|---|
| 2-氨基-2-甲基-1-丙醇(碱胺) | AMP | 是 |
| AMP的氨基 | AMPN | 否(未观察到自由基) |
| AMP的过氧基团 | AMPR | 否(未观察到自由基) |
| AMP硝酸气溶胶 | AMPNTR | 通过SMPS测量间接观察到 |
| AMP亚硝胺 | AMPNO | 否(亚硝胺很难观察到) |
| AMP硝胺 | AMPNO2 | 通过SIFT-MS观察到, 但很难量化 |
| 甲烯丙醇等产品 | ISPD | 否 |
| 污染物 | 最高允许排放浓度/mg·m-3 | 最高允许排放速率/kg·h-1 | 无组织排放监控浓度限值 | |||
|---|---|---|---|---|---|---|
| 排放气筒高度/m | 二级 | 三级 | 监控点 | 浓度/mg·m-3 | ||
| 苯胺类 | 20 | 15 | 0.52 | 0.78 | 周界外最高浓度点 | 0.40 |
| 20 | 0.87 | 7.3 | ||||
| 30 | 2.9 | 4.4 | ||||
| 40 | 5.0 | 7.6 | ||||
| 50 | 7.7 | 12 | ||||
| 60 | 11 | 17 | ||||
| 氮氧化物 | 240(硝酸使用和其他) | 15 | 0.77 | 1.2 | 周界外最高浓度点 | 0.12 |
| 20 | 1.3 | 2.0 | ||||
| 30 | 4.4 | 6.6 | ||||
| 40 | 7.5 | 11 | ||||
| 50 | 12 | 18 | ||||
| 60 | 16 | 25 | ||||
| 70 | 23 | 35 | ||||
| 80 | 31 | 47 | ||||
| 90 | 40 | 61 | ||||
| 100 | 52 | 78 | ||||
| 甲醛 | 25 | 15 | 0.26 | 0.39 | 周界外最高浓度点 | 0.20 |
| 20 | 0.43 | 0.65 | ||||
| 30 | 1.4 | 2.2 | ||||
| 40 | 2.6 | 3.8 | ||||
| 50 | 3.8 | 5.9 | ||||
| 60 | 5.4 | 8.3 | ||||
| 污染物 | 最高允许排放浓度/mg·m-3 | 最高允许排放速率/kg·h-1 | 无组织排放监控浓度限值 | |||
|---|---|---|---|---|---|---|
| 排放气筒高度/m | 二级 | 三级 | 监控点 | 浓度/mg·m-3 | ||
| 苯胺类 | 20 | 15 | 0.52 | 0.78 | 周界外最高浓度点 | 0.40 |
| 20 | 0.87 | 7.3 | ||||
| 30 | 2.9 | 4.4 | ||||
| 40 | 5.0 | 7.6 | ||||
| 50 | 7.7 | 12 | ||||
| 60 | 11 | 17 | ||||
| 氮氧化物 | 240(硝酸使用和其他) | 15 | 0.77 | 1.2 | 周界外最高浓度点 | 0.12 |
| 20 | 1.3 | 2.0 | ||||
| 30 | 4.4 | 6.6 | ||||
| 40 | 7.5 | 11 | ||||
| 50 | 12 | 18 | ||||
| 60 | 16 | 25 | ||||
| 70 | 23 | 35 | ||||
| 80 | 31 | 47 | ||||
| 90 | 40 | 61 | ||||
| 100 | 52 | 78 | ||||
| 甲醛 | 25 | 15 | 0.26 | 0.39 | 周界外最高浓度点 | 0.20 |
| 20 | 0.43 | 0.65 | ||||
| 30 | 1.4 | 2.2 | ||||
| 40 | 2.6 | 3.8 | ||||
| 50 | 3.8 | 5.9 | ||||
| 60 | 5.4 | 8.3 | ||||
| 1 | NABAT Mohammad Hossein, ZEYNALIAN Mirhadi, RAZMI Amir Reza, et al. Energy, exergy, and economic analyses of an innovative energy storage system; liquid air energy storage (LAES) combined with high-temperature thermal energy storage (HTES)[J]. Energy Conversion and Management, 2020, 226: 113486. |
| 2 | AGENCY International Energy. Energy technology perspectives 2020 - special report on carbon capture utilisation and storage: CCUS in clean energy transitions[M]. OECD, 2020. |
| 3 | GCCSI. Global status of CCS 2022[R]. Melbourne: Global CCS Institute, 2022. |
| 4 | AFKHAMIPOUR Morteza, MOFARAHI Masoud. A modeling-optimization framework for assessment of CO2 absorption capacity by novel amine solutions: 1DMA2P, 1DEA2P, DEEA, and DEAB[J]. Journal of Cleaner Production, 2018, 171: 234-249. |
| 5 | REYNOLDS Alicia J, Vincent VERHEYEN T, ADELOJU Samuel B, et al. Towards commercial scale postcombustion capture of CO2 with monoethanolamine solvent: Key considerations for solvent management and environmental impacts[J]. Environmental Science & Technology, 2012, 46(7): 3643-3654. |
| 6 | LEPAUMIER Hélène, SILVA Eirik F DA, EINBU Aslak, et al. Comparison of MEA degradation in pilot-scale with lab-scale experiments[J]. Energy Procedia, 2011, 4: 1652-1659. |
| 7 | SHAO R, STANGELAND A. Amines used in CO2 capture-health and environmental impacts[J]. Bellona report, 2009, 49: 1-49. |
| 8 | NIELSEN C, D’ANNA B, KARL M, et al. Atmospheric degradation of amines (ADA) summary report: Photo-oxidation of methylamine, dimethylamine and trimethylamine CLIMIT project no. 201604[M]. NILU, 2011. |
| 9 | KHAKHARIA Purvil, BRACHERT Leonie, MERTENS Jan, et al. Understanding aerosol based emissions in a post combustion CO2 capture process: Parameter testing and mechanisms[J]. International Journal of Greenhouse Gas Control, 2015, 34: 63-74. |
| 10 | SILVA Eirik F DA, KOLDERUP Herman, GOETHEER Earl, et al. Emission studies from a CO2 capture pilot plant[J]. Energy Procedia, 2013, 37: 778-783. |
| 11 | NGUYEN Thu, HILLIARD Marcus, ROCHELLE Gary. Volatility of aqueous amines in CO2 capture[J]. Energy Procedia, 2011, 4: 1624-1630. |
| 12 | NGUYEN Thu, HILLIARD Marcus, ROCHELLE Gary T. Amine volatility in CO2 capture[J]. International Journal of Greenhouse Gas Control, 2010, 4(5): 707-715. |
| 13 | IEA GHG. Environmental impact of solvent scrubbing of CO2 [R/OL]. (2006-10)[2023-07-18]. . |
| 14 | MOSER Peter, SCHMIDT Sandra, STAHL Knut. Investigation of trace elements in the inlet and outlet streams of a MEA-based post-combustion capture process results from the test programme at the Niederaussem pilot plant[J]. Energy Procedia, 2011, 4: 473-479. |
| 15 | Project CESAR EU. SINTEF materials and chemistry. emission measurements at Dong’s pilot plant for CO2 capture in esbjerg[EB/OL]. [2023-7-18]. . |
| 16 | OCTAVIUS Evénements. Workshop emissions[C/OL]// Projets IFPEN. OCTAVIUS FP 7. Allemagne: EnBW Heilbronn, 2014: 2.13-2.14. . |
| 17 | HALLIBURTON B, DAY S J, LAVRENCIC S, et al. Project A-Establishing sampling and analytical procedures for potentially harmful components from post-combustion amine based CO2 capture[C]. 2010. |
| 18 | MERTENS Jan, BRACHERT L, DESAGHER D, et al. ELPI+ measurements of aerosol growth in an amine absorption column[J]. International Journal of Greenhouse Gas Control, 2014, 23: 44-50. |
| 19 | KHAKHARIA Purvil, BRACHERT Leonie, MERTENS Jan, et al. Investigation of aerosol based emission of MEA due to sulphuric acid aerosol and soot in a Post Combustion CO2 Capture process[J]. International Journal of Greenhouse Gas Control, 2013, 19: 138-144. |
| 20 | HARSHA Shreyas, KHAKHARIA Purvil, HUIZINGA Arjen, et al. In-situ experimental investigation on the growth of aerosols along the absorption column in post combustion carbon capture[J]. International Journal of Greenhouse Gas Control, 2019, 85: 86-99. |
| 21 | NIELSEN Claus J, HERRMANN Hartmut, WELLER Christian. Atmospheric chemistry and environmental impact of the use of amines in carbon capture and storage (CCS)[J]. Chemical Society Reviews, 2012, 41(19): 6684-6704. |
| 22 | ATKINSON Roger. Kinetics and mechanisms of the gas-phase reactions of the hydroxyl radical with organic compounds under atmospheric conditions[J]. Chemical Reviews, 1986, 86(1): 69-201. |
| 23 | ATKINSON Roger, CARTER William P L. Kinetics and mechanisms of the gas-phase reactions of ozone with organic compounds under atmospheric conditions[J]. Chemical Reviews, 1984, 84(5): 437-470. |
| 24 | ONEL L, BLITZ M A, SEAKINS P W. Direct determination of the rate coefficient for the reaction of OH radicals with monoethanol amine (MEA) from 296 to 510 K[J]. The Journal of Physical Chemistry Letters, 2012, 3(7): 853-856. |
| 25 | KARL M, DYE C, SCHMIDBAUER N, et al. Study of OH-initiated degradation of 2-aminoethanol[J]. Atmospheric Chemistry & Physics, 2012, 12(4): 1881-1901. |
| 26 | BORDUAS Nadine, ABBATT Jonathan P D, MURPHY Jennifer G. Gas phase oxidation of monoethanolamine (MEA) with OH radical and ozone: Kinetics, products, and particles[J]. Environmental Science & Technology, 2013, 47(12): 6377-6383. |
| 27 | ANDERSON Larry G, STEPHENS Robert D. Kinetics of the reaction of hydroxyl radicals with 2-(dimethylamino)ethanol from 234–364 K[J]. International Journal of Chemical Kinetics, 1988, 20(2): 103-110. |
| 28 | HARRIS Geoffrey W, PITTS James N. Notes. Rates of reaction of hydroxyl radicals with 2-(dimethylamino)ethanol and 2-amino-2-methyl-1-propanol in the gas phase at 300±2K[J]. Environmental Science & Technology, 1983, 17(1): 50-51. |
| 29 | ONEL L, BLITZ M A, BREEN J, et al. Branching ratios for the reactions of OH with ethanol amines used in carbon capture and the potential impact on carcinogen formation in the emission plume from a carbon capture plant[J]. Physical Chemistry Chemical Physics, 2015, 17(38): 25342-25353. |
| 30 | TAN Wen, ZHU Liang, MIKOVINY Tomas, et al. Experimental and theoretical study of the OH-initiated degradation of piperazine under simulated atmospheric conditions[J]. The Journal of Physical Chemistry A, 2021, 125(1): 411-422. |
| 31 | XIE Hongbin, LI Chao, HE Ning, et al. Atmospheric chemical reactions of monoethanolamine initiated by OH radical: Mechanistic and kinetic study[J]. Environmental Science & Technology, 2014, 48(3): 1700-1706. |
| 32 | TAN Wen, ZHU Liang, MIKOVINY Tomáš, et al. Atmospheric chemistry of 2-amino-2-methyl-1-propanol: A theoretical and experimental study of the OH-initiated degradation under simulated atmospheric conditions[J]. The Journal of Physical Chemistry A, 2021, 125(34): 7502-7519. |
| 33 | XIE Hongbin, MA Fangfang, WANG Yuanfang, et al. Quantum chemical study on ·Cl-initiated atmospheric degradation of monoethanolamine[J]. Environmental Science & Technology, 2015, 49(22): 13246-13255. |
| 34 | MA Fangfang, DING Zhezheng, Jonas ELM, et al. Atmospheric oxidation of piperazine initiated by ·Cl: Unexpected high nitrosamine yield[J]. Environmental Science & Technology, 2018, 52(17): 9801-9809. |
| 35 | MANZOOR Saba, SIMPERLER Alexandra, KORRE Anna. A theoretical study of the reaction kinetics of amines released into the atmosphere from CO2 capture[J]. International Journal of Greenhouse Gas Control, 2015, 41: 219-228. |
| 36 | LI Kangwei, WHITE Stephen, ZHAO Bin, et al. Evaluation of a new chemical mechanism for 2-amino-2-methyl-1-propanol in a reactive environment from CSIRO smog chamber experiments[J]. Environmental Science & Technology, 2020, 54(16): 9844-9853. |
| 37 | TUAZON E C, ATKINSON R, ASCHMANN S M, et al. Kinetics and products of the gas-phase reactions of O3 with amines and related compounds[J]. Research on Chemical Intermediates, 1994, 20(3): 303-320. |
| 38 | NIELSEN Claus Jørgen, Barbara D’ANNA, Christian DYE, et al. Atmospheric chemistry of 2-aminoethanol (MEA)[J]. Energy Procedia, 2011, 4: 2245-2252. |
| 39 | DAG T. Update and improvement of dispersion calculations for emissions to air from TCM’s amine plant. Part II-Likely case nitrosamines, nitramines and formaldehyde[R/OL]. (2011)[2023-8-14]. . |
| 40 | 国家环境保护局. 大气污染物综合排放标准: [S]. 北京: 中国标准出版社, 1997. |
| State Bureau of Environmental Protection of the People’s Republic of China. Comprehensive emission standard of air pollutants: [S]. Beijing: Standards Press of China, 1997. | |
| 41 | HSE. EH40/2005 Workplace exposure limits [S/OL]. 2007. [2023-8-15]. . |
| 42 | LAG M P, LINDEMAN B, INSTANES C, et al. Health effects of amines and derivatives associated with CO2 capture[C].2011. |
| 43 | Review of Amine Emissions from Carbon Capture Systems[C].2015. |
| 44 | BERGLEN T, TØNNESEN D, DYE C, et al. CO2 Technology Centre Mongstad-updated air dispersion calculations[C]. 2010. |
| 45 | 中华人民共和国环境保护部. 环境空气和废气 酰胺类化合物的测定 液相色谱法: [S]. 北京: 中国环境科学出版社, 2016. |
| Ministry of Environmental Protection of the People’s Republic of China. Ambient air and waste gas-Determination of amide compounds-Liquid chromatography: [S]. Beijing: China Environmental Science Press, 2016. | |
| 46 | 环境空气. 挥发性有机物的测定 吸附管采样-热脱附/气相色谱-质谱法: [S]. |
| Determination of volatile organic compounds in ambient air Adsorbent tube sampling-thermal desorption/gas chromatography-mass spectrometry: [S]. | |
| 47 | ALAWODE A O. Oxidative degradation of piperazine in the absorption of carbon dioxide[D]. The University of Texas at Austin, 2005. |
| 48 | EINBU Aslak, DASILVA Eirik, HAUGEN Geir, et al. A new test rig for studies of degradation of CO2 absorption solvents at process conditions; comparison of test rig results and pilot plant data for degradation of MEA[J]. Energy Procedia, 2013, 37: 717-726. |
| 49 | FINE Nathan A, ROCHELLE Gary T. Thermal decomposition of N-nitrosopiperazine[J]. Energy Procedia, 2013, 37: 1678-1686. |
| 50 | FINE Nathan A, GOLDMAN Mark J, NIELSEN Paul T, et al. Managing N-nitrosopiperazine and dinitrosopiperazine[J]. Energy Procedia, 2013, 37: 273-284. |
| 51 | FINE Nathan A, NIELSEN Paul T, ROCHELLE Gary T. Decomposition of nitrosamines in CO2 capture by aqueous piperazine or monoethanolamine[J]. Environmental Science and Technology, 2014, 48(10): 5996-6002. |
| 52 | GOLDMAN Mark J, FINE Nathan A, ROCHELLE Gary T. Kinetics of N-nitrosopiperazine formation from nitrite and piperazine in CO2 capture[J]. Environmental Science & Technology, 2013, 47(7): 3528-3534. |
| 53 | CHEN Yifei, LIN Qinhao, LI Guiying, et al. A new method of simultaneous determination of atmospheric amines in gaseous and particulate phases by gas chromatography-mass spectrometry[J]. Journal of Environmental Sciences, 2022, 114: 401-411. |
| 54 | Berit FOSTÅS, GANGSTAD Audun, NENSETER Bjarne, et al. Effects of NO x in the flue gas degradation of MEA[J]. Energy Procedia, 2011, 4: 1566-1573. |
| 55 | 杨正大, 贤振楠, 邵凌宇, 等. 胺法碳捕集过程胺气溶胶形成与排放研究现状[J]. 能源环境保护, 2023, 37(3): 195-203. |
| YANG Zhengda, XIAN Zhennan, SHAO Lingyu, et al. Research status of amine aerosol formation and emission in amine carbon capture process[J]. Energy Environmental Protection, 2023, 37(3): 195-203. | |
| 56 | 方梦祥, 狄闻韬, 易宁彤, 等. CO2化学吸收系统污染物排放与控制研究进展[J]. 洁净煤技术, 2021, 27(2): 8-16. |
| FANG Mengxiang, DI Wentao, YI Ningtong, et al. Research progress on pollutant emission and control from CO2 chemical absorption system[J]. Clean Coal Technology, 2021, 27(2): 8-16. | |
| 57 | MERTENS Jan, ANDERLOHR C, ROGIERS P, et al. A wet electrostatic precipitator (WESP) as countermeasure to mist formation in amine based carbon capture[J]. International Journal of Greenhouse Gas Control, 2014, 31: 175-181. |
| 58 | FUJITA Koshito, MURAOKA Daigo, OGAWA Takashi, et al. Evaluation of amine emissions from the post-combustion CO2 capture pilot plant[J]. Energy Procedia, 2013, 37: 727-734. |
| 59 | MERTENS Jan, KHAKHARIA P, ROGIERS Pieter, et al. Prevention of mist formation in amine based carbon capture: Field testing using a wet ElectroStatic precipitator (WESP) and a gas-gas heater (GGH)[J]. Energy Procedia, 2017, 114: 987-999. |
| 60 | HUANG Jiayu, ZHANG Fan, SHI Yingjie, et al. Investigation of a pilot-scale wet electrostatic precipitator for the control of sulfuric acid mist from a simulated WFGD system[J]. Journal of Aerosol Science, 2016, 100: 38-52. |
| 61 | KHAKHARIA Purvil, HUIZINGA Arjen, TRAP Henk, et al. Lab scale investigation on the formation of aerosol nuclei by a wet electrostatic precipitator in the presence of SO2 in a gas stream[J]. International Journal of Greenhouse Gas Control, 2019, 86: 22-33. |
| 62 | LI Chao, YI Ningtong, FANG Mengxiang, et al. Solvent emissions control in large scale chemical absorption CO2 capture plant[J]. International Journal of Greenhouse Gas Control, 2021, 111: 103444. |
| 63 | MOSER Peter, SCHMIDT Sandra, STAHL Knut, et al. Demonstrating emission reduction-results from the post-combustion capture pilot plant at niederaussem[J]. Energy Procedia, 2014, 63: 902-910. |
| 64 | LU Shijian, YANG Fei, ZHANG Juanjuan, et al. Research and design experience of a 150kt/a CO2 capture and purification project in the Shaanxi Guohua Jinjie power plant[J]. Separation and Purification Technology, 2023, 320: 124089. |
| [1] | YU Mengjie, WU Yutong, LUO Faxiang, DOU Yibo. Research progress on structural design of photocatalysts for diluted carbon dioxide reduction [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 335-350. |
| [2] | ZHANG Xi, LI Haoxin, ZHANG Tianyang, LI Zifu, SUN Wenjun, AO Xiuwei. Degradation of per- and polyfluoroalkyl substances in water by UV-based advanced oxidation or advanced reduction processes [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4587-4600. |
| [3] | LU Shijian, ZHANG Juanjuan, YANG Fei, LIU Ling, CHEN Siming, KANG Guojun, FANG Qinqin. Research progress of amine escape control technology by chemical absorption method [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4562-4570. |
| [4] | LUO Congjia, DOU Yibo, WEI Min. Research progress on structural regulation of layered double hydroxides for photocatalytic CO2 reduction [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 3891-3909. |
| [5] | FANG Yao, LIU Lei, GAO Zhihua, HUANG Wei, ZUO Zhijun. Advances in anode catalysts for photo-assisted direct methanol fuel cells [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2611-2628. |
| [6] | JIAO Jing, LIU Linlin, DU Jian. Optimization of carbon capture and power plant integrated scheduling based on proxy model [J]. Chemical Industry and Engineering Progress, 2024, 43(11): 6059-6067. |
| [7] | HUANG Zhixin, WANG Junyao, YUAN Xiangzhou, DENG Shuai, ZHAO Jie, ZHANG Xinyi. Research advances on upcycling organic solid waste into CO2 adsorbents: A cross-research review [J]. Chemical Industry and Engineering Progress, 2024, 43(10): 5748-5764. |
| [8] | YU Xiaoxiao, CHAO Yanhong, LIU Haiyan, ZHU Wenshuai, LIU Zhichang. Enhanced photoelectric properties and photocatalytic CO2 conversion by D-A conjugated polymerization [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 292-301. |
| [9] | LYU Chengyuan, ZHANG Han, YANG Mingwang, DU Jianjun, FAN Jiangli. Recent advances of dioxetane-based afterglow system for bio-imaging [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4108-4122. |
| [10] | XU Wei, LI Kaijun, SONG Linye, ZHANG Xinghui, YAO Shunhua. Research progress of photocatalysis and co-electrochemical degradation of VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3520-3531. |
| [11] | FAN Sihan, YU Guoxi, LAI Chaochao, HE Huan, HUANG Bin, PAN Xuejun. Effect of abiotic modification on photochemical activity of anaerobic microbial products [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2180-2189. |
| [12] | SANG Wei, TANG Jianfeng, HUA Yihuai, CHEN Jie, SUN Peiyuan, XU Yifei. Effects of physical solvent and amine properties on the performance of biphasic solvent [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2151-2159. |
| [13] | FU Le, YANG Yang, XU Wenqing, GENG Zanbu, ZHU Tingyu, HAO Runlong. Research progress in CO2 capture technology using novel biphasic organic amine absorbent [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2068-2080. |
| [14] | CHEN Shuhui, WU Yue, ZHANG Wenxiang, WANG Shanshan, MA Heping. Preparation of ionic organic porous polymer and its coupled desulfurization and decarbonization properties in flue gas [J]. Chemical Industry and Engineering Progress, 2023, 42(2): 1028-1038. |
| [15] | LIU Yanhui, ZHOU Mingfang, MA Ming, WANG Kai, TAN Tianwei. Recent advances on the bio-fixation of CO2 driven by renewable energy [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 1-15. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||