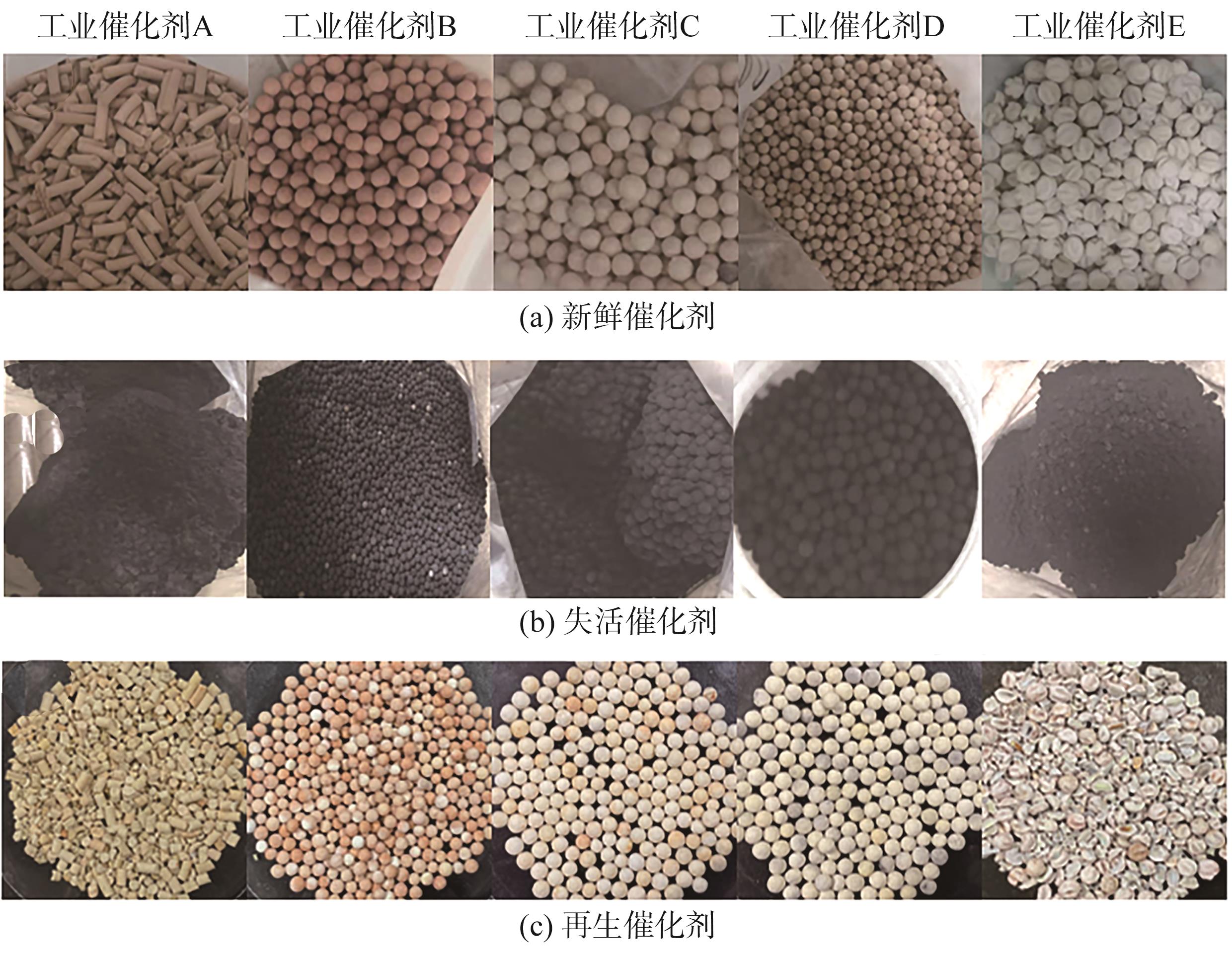
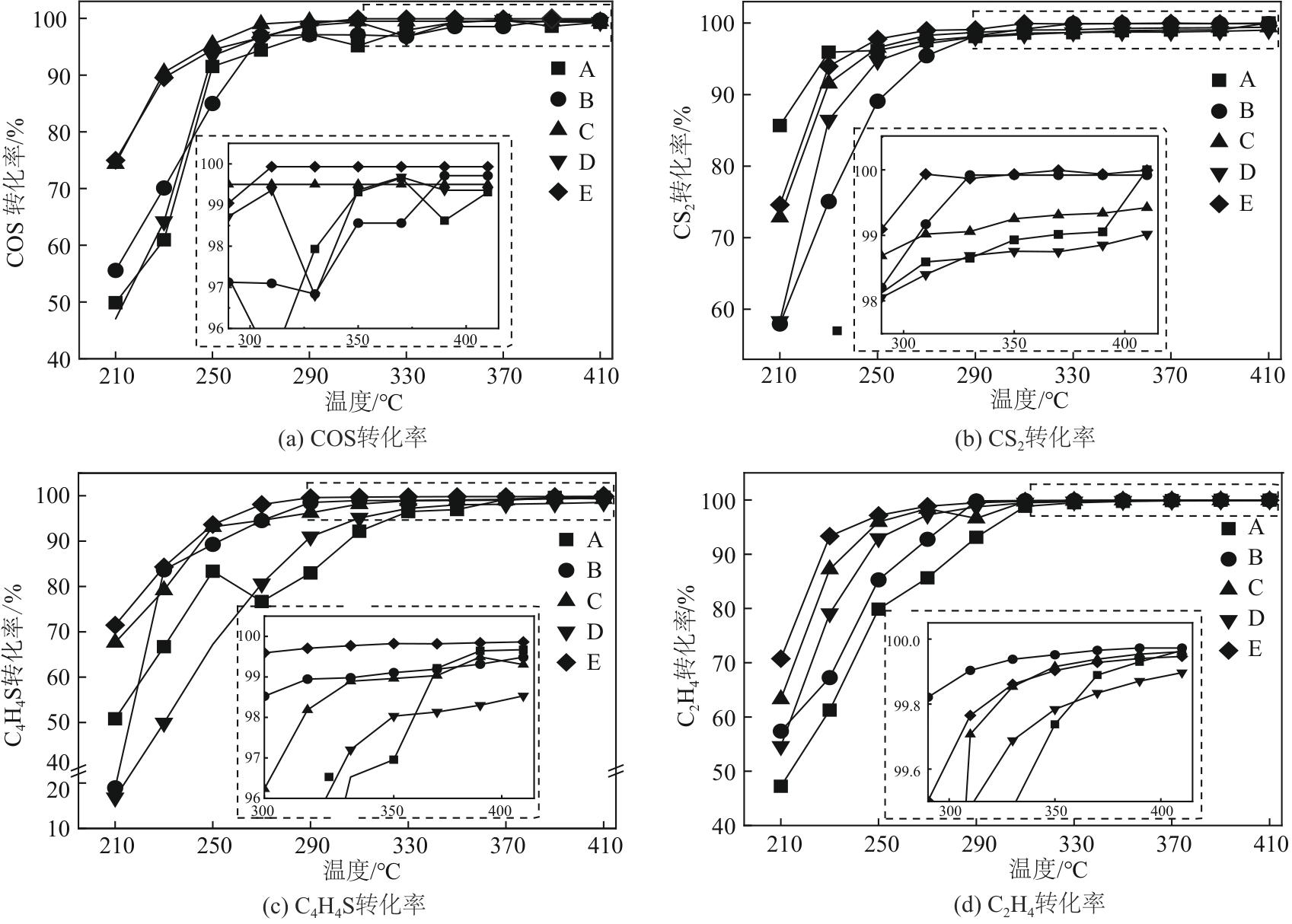
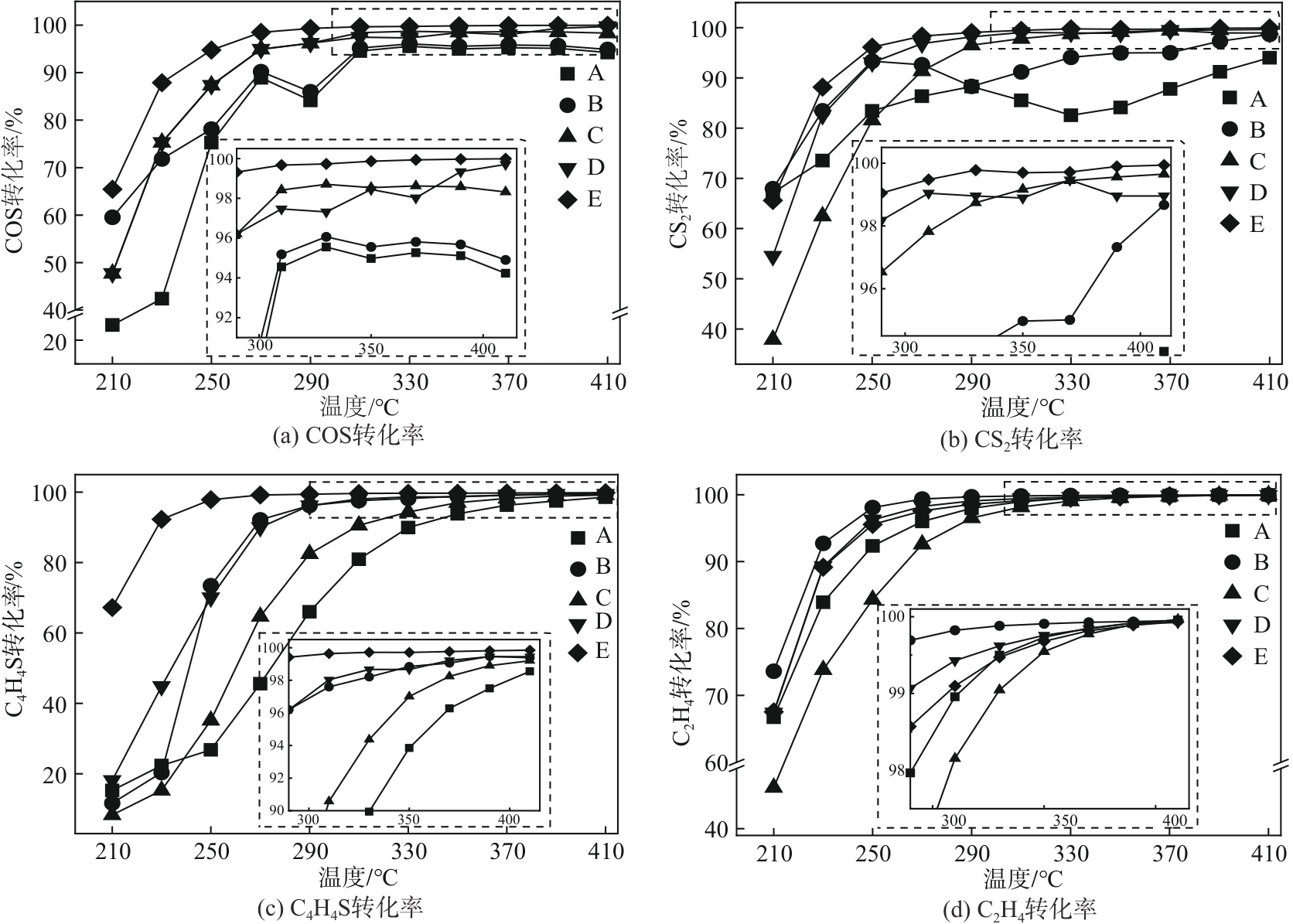
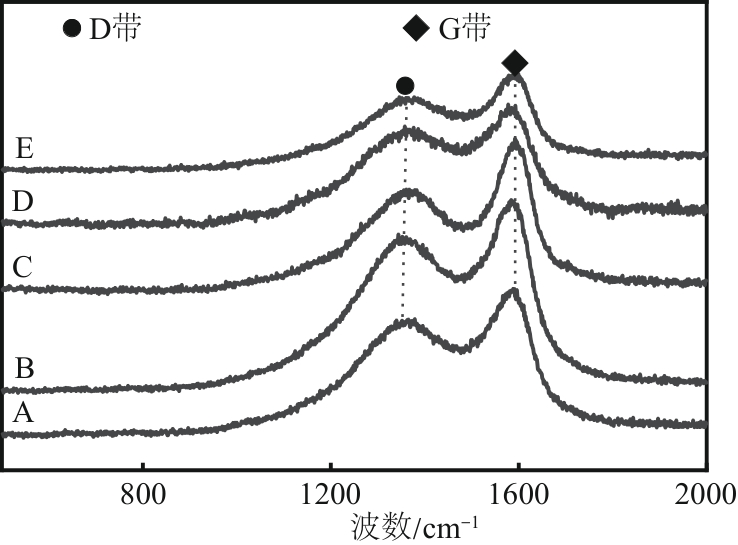
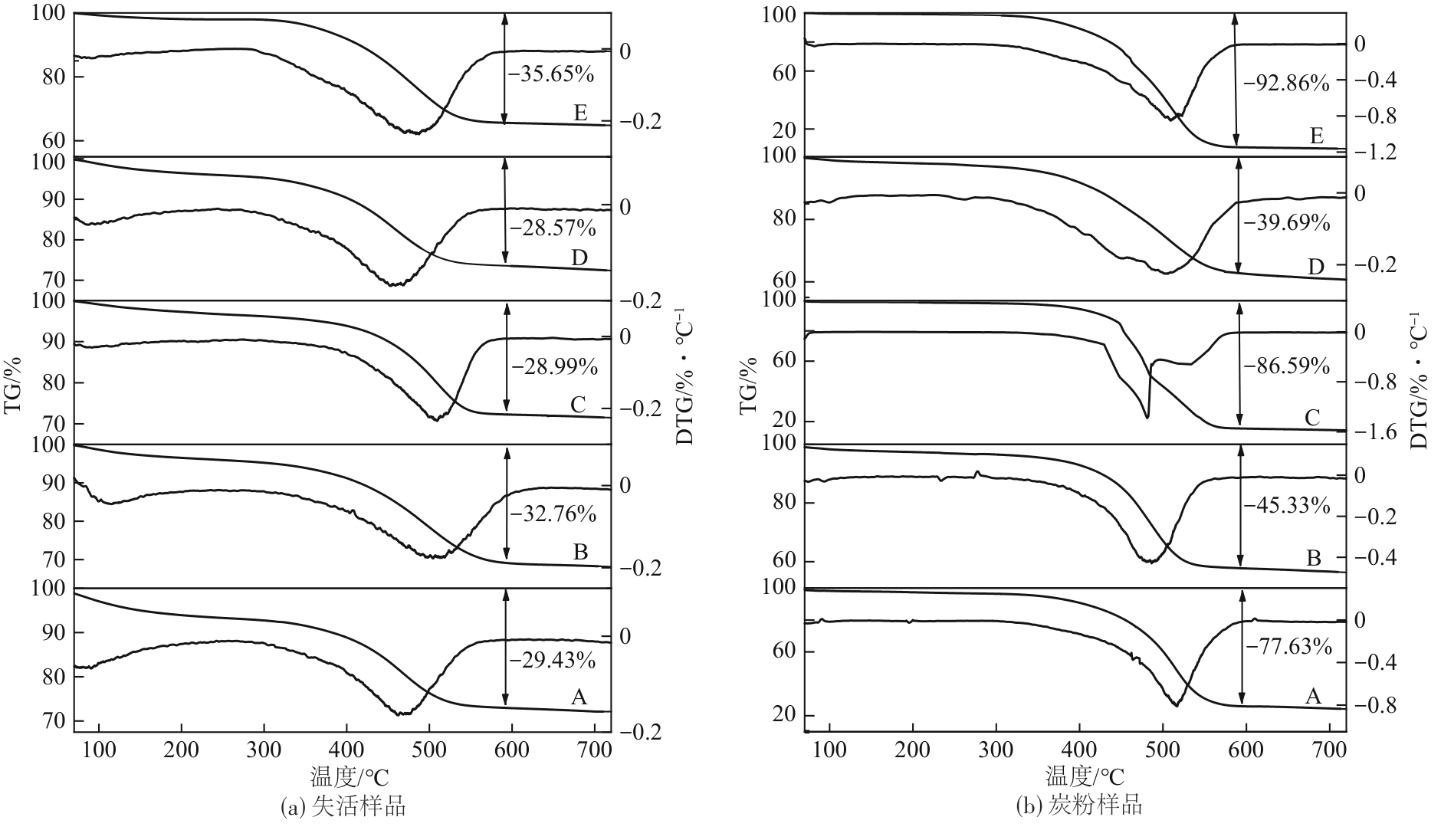
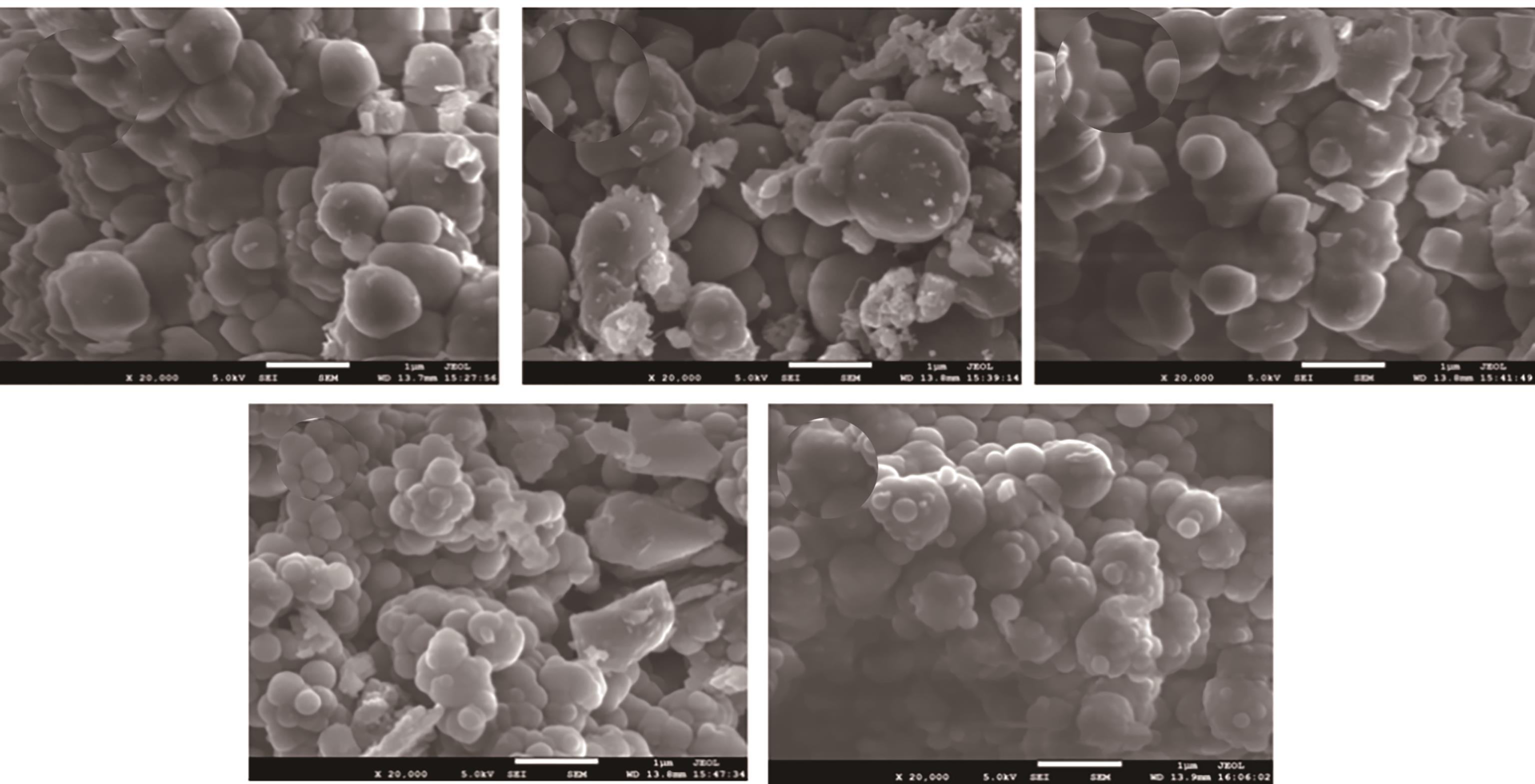
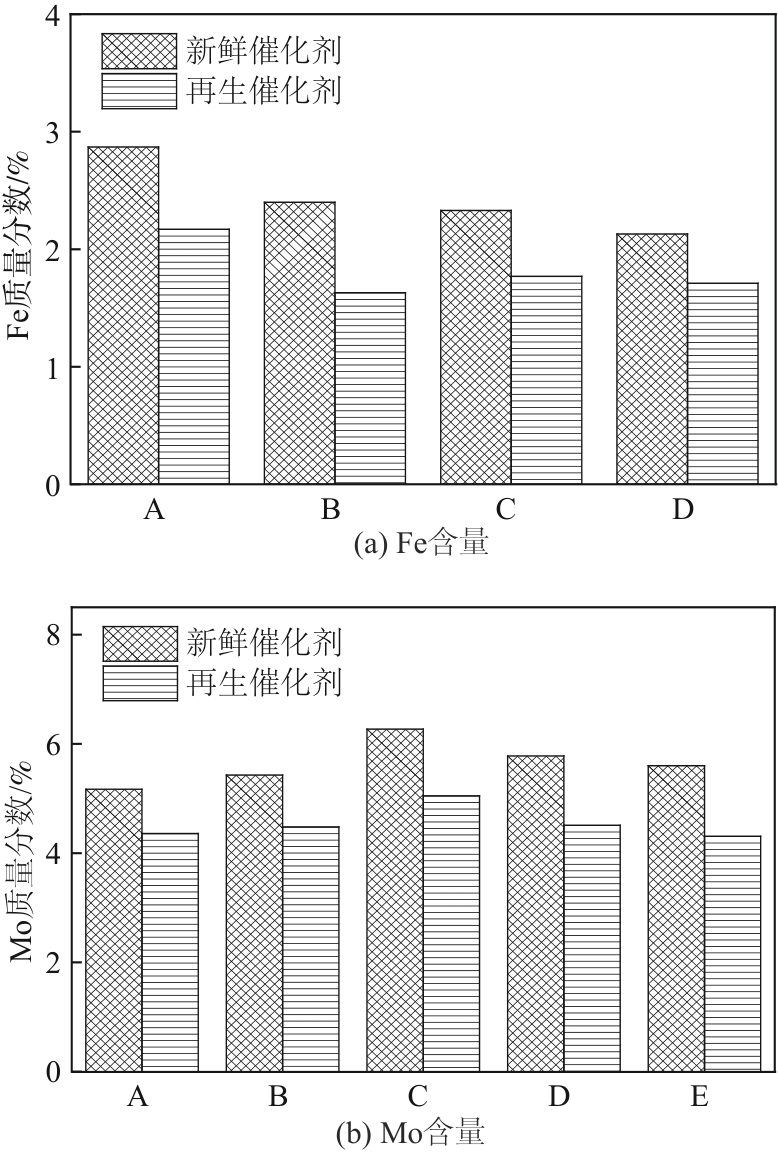
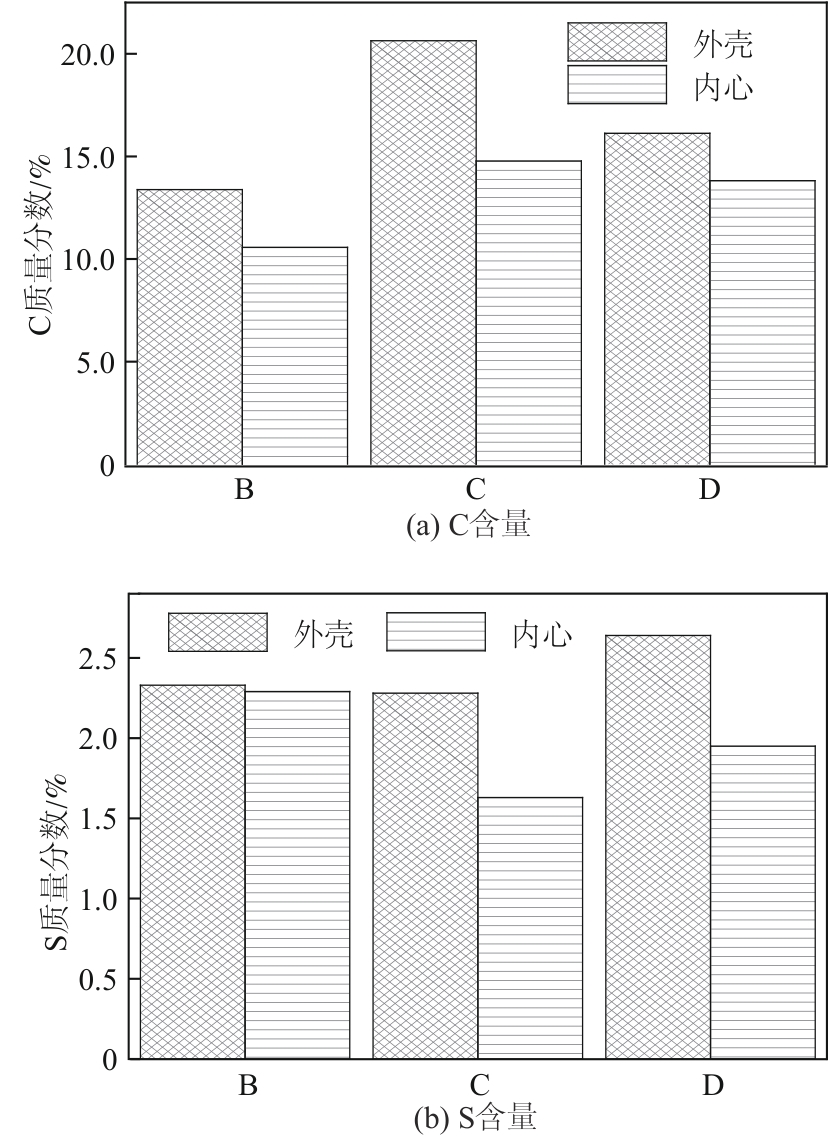
Chemical Industry and Engineering Progress ›› 2024, Vol. 43 ›› Issue (10): 5543-5554.DOI: 10.16085/j.issn.1000-6613.2023-1656
• Industrial catalysis • Previous Articles
Deactivation mechanism of coke oven gas prehydrogenation desulfurization catalyst for industrial application
TAN Tianbing1( ), QIN Zhifeng1,2(
), QIN Zhifeng1,2( ), LI Naizhen1, CHANG Liping1, WU Mengmeng1, YU Feng1, WU Qiongxiao3,4, XIAN Yanli3, JING Jianning3
), LI Naizhen1, CHANG Liping1, WU Mengmeng1, YU Feng1, WU Qiongxiao3,4, XIAN Yanli3, JING Jianning3
- 1.State Key Laboratory of Clean and Efficient Coal Utilization, College of Chemical Engineering and Technology, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
2.Shanxi?Zheda Institute of Advanced Materials and Chemical Engineering, Taiyuan 030002, Shanxi, China
3.Daosheng Xinyu Clean Energy Company Limited, Kelan 036300, Shanxi, China
4.Dongguan Energy Investment Group Co. , Ltd. , Dongguan 523000, Guangdong, China
-
Received:2023-09-19Revised:2023-11-29Online:2024-10-29Published:2024-10-15 -
Contact:QIN Zhifeng
面向工业应用的焦炉煤气预加氢脱硫催化剂失活机理
谭天兵1( ), 秦志峰1,2(
), 秦志峰1,2( ), 李乃珍1, 常丽萍1, 武蒙蒙1, 于峰1, 吴琼笑3,4, 陷艳莉3, 景建宁3
), 李乃珍1, 常丽萍1, 武蒙蒙1, 于峰1, 吴琼笑3,4, 陷艳莉3, 景建宁3
- 1.太原理工大学化学工程与技术学院,省部共建煤基能源清洁高效利用国家重点实验室,山西 太原 030024
2.山西浙大新材料与化工研究院,山西 太原 030002
3.山西道生鑫宇清洁能源有限公司,山西 岢岚 036300
4.东莞市能源投资集团有限公司,广东 东莞 523000
-
通讯作者:秦志峰 -
作者简介:谭天兵(1999—),男,硕士研究生,研究方向为气体净化与工业催化。E-mail:15034596213@163.com。 -
基金资助:2016年度山西省科技重大专项(MJH2016-03);2018年度山西省优秀人才科技创新项目(201805D211037);山西浙大新材料与化工研究院研发项目(2021ST-AT-002);东莞市能源投资集团有限公司攻关项目(RH2000004464)
CLC Number:
Cite this article
TAN Tianbing, QIN Zhifeng, LI Naizhen, CHANG Liping, WU Mengmeng, YU Feng, WU Qiongxiao, XIAN Yanli, JING Jianning. Deactivation mechanism of coke oven gas prehydrogenation desulfurization catalyst for industrial application[J]. Chemical Industry and Engineering Progress, 2024, 43(10): 5543-5554.
谭天兵, 秦志峰, 李乃珍, 常丽萍, 武蒙蒙, 于峰, 吴琼笑, 陷艳莉, 景建宁. 面向工业应用的焦炉煤气预加氢脱硫催化剂失活机理[J]. 化工进展, 2024, 43(10): 5543-5554.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-1656
| 名称 | 表示方式 | Fe2O3 | NiO | MoO3 |
|---|---|---|---|---|
| 工业催化剂A | Fe-Mo/Al2O3 | 3.53 | — | 7.76 |
| 工业催化剂B | Fe-Mo/Al2O3 | 2.86 | — | 8.15 |
| 工业催化剂C | Fe-Mo/Al2O3 | 2.76 | — | 9.41 |
| 工业催化剂D | Fe-Mo/Al2O3 | 2.47 | — | 8.67 |
| 工业催化剂E | Ni-Mo/Al2O3 | — | 2.12 | 8.40 |
| 名称 | 表示方式 | Fe2O3 | NiO | MoO3 |
|---|---|---|---|---|
| 工业催化剂A | Fe-Mo/Al2O3 | 3.53 | — | 7.76 |
| 工业催化剂B | Fe-Mo/Al2O3 | 2.86 | — | 8.15 |
| 工业催化剂C | Fe-Mo/Al2O3 | 2.76 | — | 9.41 |
| 工业催化剂D | Fe-Mo/Al2O3 | 2.47 | — | 8.67 |
| 工业催化剂E | Ni-Mo/Al2O3 | — | 2.12 | 8.40 |
| 气体 | 纯度或含量 | 平衡气体 |
|---|---|---|
| 乙烯 | 99.9% | — |
| 预硫化气体 | H2S 3% | H2 |
| 含硫反应气体 | COS 321.43mg/m³、CS2 309.70mg/m³、C4H4S 113.96mg/m³ | H2 |
| 硫化物标气 | H2S 217.54mg/m³、COS 416.25mg/m³、CH3SH 212.89mg/m³、 C2H5SH 272.74mg/m³、CH3SCH3 282.96mg/m³、CS2 532.74mg/m³、 C4H4S 553.38mg/m³、CH3S2CH3 623.46mg/m³、C2H5SCH3 498.03mg/m³、(C2H5)2S 589.59mg/m³ | N2 |
| 气体 | 纯度或含量 | 平衡气体 |
|---|---|---|
| 乙烯 | 99.9% | — |
| 预硫化气体 | H2S 3% | H2 |
| 含硫反应气体 | COS 321.43mg/m³、CS2 309.70mg/m³、C4H4S 113.96mg/m³ | H2 |
| 硫化物标气 | H2S 217.54mg/m³、COS 416.25mg/m³、CH3SH 212.89mg/m³、 C2H5SH 272.74mg/m³、CH3SCH3 282.96mg/m³、CS2 532.74mg/m³、 C4H4S 553.38mg/m³、CH3S2CH3 623.46mg/m³、C2H5SCH3 498.03mg/m³、(C2H5)2S 589.59mg/m³ | N2 |
| 样品 | 比表面积/m2·g-1 | 孔体积/mL·g-1 | 平均孔径/nm |
|---|---|---|---|
| 工业催化剂A | |||
| 新鲜催化剂样品 | 296.3 | 0.26 | 3.5 |
| 失活催化剂样品 | 8.5 | 0.03 | 5.0 |
| 催化剂表面炭粉 | 4.3 | 0.05 | 46.9 |
| 再生催化剂样品 | 230.2 | 0.22 | 3.9 |
| 工业催化剂B | |||
| 新鲜催化剂样品 | 210.4 | 0.38 | 8.7 |
| 失活催化剂样品 | 43.4 | 0.08 | 7.8 |
| 催化剂表面炭粉 | 26.2 | 0.09 | 14.0 |
| 再生催化剂样品 | 173.9 | 0.35 | 6.7 |
| 工业催化剂C | |||
| 新鲜催化剂样品 | 206.5 | 0.41 | 8.0 |
| 失活催化剂样品 | 73.8 | 0.16 | 6.2 |
| 催化剂表面炭粉 | 4.8 | 0.02 | 15.2 |
| 再生催化剂样品 | 156.3 | 0.35 | 8.9 |
| 工业催化剂D | |||
| 新鲜催化剂样品 | 212.9 | 0.39 | 7.4 |
| 失活催化剂样品 | 78.1 | 0.19 | 9.7 |
| 催化剂表面炭粉 | 3.1 | 0.07 | 87.2 |
| 再生催化剂样品 | 170.3 | 0.35 | 8.2 |
| 工业催化剂E | |||
| 新鲜催化剂样品 | 135.7 | 0.48 | 14.6 |
| 失活催化剂样品 | 52.9 | 0.16 | 10.9 |
| 催化剂表面炭粉 | 3.0 | 0.01 | 16.1 |
| 再生催化剂样品 | 125.5 | 0.41 | 12.0 |
| 样品 | 比表面积/m2·g-1 | 孔体积/mL·g-1 | 平均孔径/nm |
|---|---|---|---|
| 工业催化剂A | |||
| 新鲜催化剂样品 | 296.3 | 0.26 | 3.5 |
| 失活催化剂样品 | 8.5 | 0.03 | 5.0 |
| 催化剂表面炭粉 | 4.3 | 0.05 | 46.9 |
| 再生催化剂样品 | 230.2 | 0.22 | 3.9 |
| 工业催化剂B | |||
| 新鲜催化剂样品 | 210.4 | 0.38 | 8.7 |
| 失活催化剂样品 | 43.4 | 0.08 | 7.8 |
| 催化剂表面炭粉 | 26.2 | 0.09 | 14.0 |
| 再生催化剂样品 | 173.9 | 0.35 | 6.7 |
| 工业催化剂C | |||
| 新鲜催化剂样品 | 206.5 | 0.41 | 8.0 |
| 失活催化剂样品 | 73.8 | 0.16 | 6.2 |
| 催化剂表面炭粉 | 4.8 | 0.02 | 15.2 |
| 再生催化剂样品 | 156.3 | 0.35 | 8.9 |
| 工业催化剂D | |||
| 新鲜催化剂样品 | 212.9 | 0.39 | 7.4 |
| 失活催化剂样品 | 78.1 | 0.19 | 9.7 |
| 催化剂表面炭粉 | 3.1 | 0.07 | 87.2 |
| 再生催化剂样品 | 170.3 | 0.35 | 8.2 |
| 工业催化剂E | |||
| 新鲜催化剂样品 | 135.7 | 0.48 | 14.6 |
| 失活催化剂样品 | 52.9 | 0.16 | 10.9 |
| 催化剂表面炭粉 | 3.0 | 0.01 | 16.1 |
| 再生催化剂样品 | 125.5 | 0.41 | 12.0 |
| 样品 | 新鲜催化剂样品/% | 失活催化剂样品/% | 催化剂表面炭粉/% | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O | Al | Fe | Mo | Ni | O | Al | Fe | Mo | S | C | O | Al | Fe | S | C | ||||
| 工业催化剂A | 74.1 | 23.7 | 0.8 | 1.4 | — | 52.1 | 21.8 | 0.7 | 0.6 | 1.4 | 23.4 | 23.8 | 0.2 | 0.3 | 2.1 | 73.6 | |||
| 工业催化剂B | 65.2 | 33.3 | 0.6 | 0.9 | — | 51.1 | 16.1 | 0.5 | 0.4 | 1.5 | 30.4 | 20.7 | 6.1 | 0.4 | 1.7 | 71.1 | |||
| 工业催化剂C | 66.0 | 32.3 | 0.6 | 1.1 | — | 41.9 | 18.6 | 0.6 | 0.4 | 1.3 | 37.2 | 23.2 | 0.2 | 0.3 | 0.1 | 76.2 | |||
| 工业催化剂D | 65.8 | 32.4 | 0.5 | 1.3 | — | 53.5 | 22.5 | 0.5 | 0.8 | 2.0 | 20.7 | 24.5 | 2.0 | 0.4 | 1.5 | 71.6 | |||
| 工业催化剂E | 68.8 | 29.5 | — | 1.1 | 0.6 | 53.8 | 17.7 | — | 0.7 | 0.7 | 27.1 | 25.5 | 0.1 | — | 2.4 | 72.0 | |||
| 样品 | 新鲜催化剂样品/% | 失活催化剂样品/% | 催化剂表面炭粉/% | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O | Al | Fe | Mo | Ni | O | Al | Fe | Mo | S | C | O | Al | Fe | S | C | ||||
| 工业催化剂A | 74.1 | 23.7 | 0.8 | 1.4 | — | 52.1 | 21.8 | 0.7 | 0.6 | 1.4 | 23.4 | 23.8 | 0.2 | 0.3 | 2.1 | 73.6 | |||
| 工业催化剂B | 65.2 | 33.3 | 0.6 | 0.9 | — | 51.1 | 16.1 | 0.5 | 0.4 | 1.5 | 30.4 | 20.7 | 6.1 | 0.4 | 1.7 | 71.1 | |||
| 工业催化剂C | 66.0 | 32.3 | 0.6 | 1.1 | — | 41.9 | 18.6 | 0.6 | 0.4 | 1.3 | 37.2 | 23.2 | 0.2 | 0.3 | 0.1 | 76.2 | |||
| 工业催化剂D | 65.8 | 32.4 | 0.5 | 1.3 | — | 53.5 | 22.5 | 0.5 | 0.8 | 2.0 | 20.7 | 24.5 | 2.0 | 0.4 | 1.5 | 71.6 | |||
| 工业催化剂E | 68.8 | 29.5 | — | 1.1 | 0.6 | 53.8 | 17.7 | — | 0.7 | 0.7 | 27.1 | 25.5 | 0.1 | — | 2.4 | 72.0 | |||
| 1 | 刘丽丽. 焦炉煤气净化工艺研究现状及发展趋势[J]. 山东化工, 2021, 50(8): 63-64. |
| LIU Lili. Research status and development trend of coke oven gas purification process[J]. Shandong Chemical Industry, 2021, 50(8): 63-64. | |
| 2 | 张淑娇, 董丽丽, 王力川, 等. 焦炉煤气净化工艺的研究进展[J]. 山东化工, 2021, 50(23): 79-80, 94. |
| ZHANG Shujiao, DONG Lili, WANG Lichuan, et al. Research progress in coke oven gas purification process[J]. Shandong Chemical Industry, 2021, 50(23): 79-80, 94. | |
| 3 | Portha JEANFRANÇOIS, WILMAR UribeSoto, JEANMARC Commenge, et al. Techno-economic and carbon footprint analyses of a coke oven gas reuse process for methanol production[J]. Processes, 2021, 9(6): 1042. |
| 4 | 李慧敏. 焦炉煤气制天然气的重要意义[J]. 山西化工, 2019, 39(1): 103-104. |
| LI Huimin. The important significance of coke oven gas for natural gas production[J]. Shanxi Chemical Industry, 2019, 39(1): 103-104. | |
| 5 | 杜雄伟. 焦炉煤气制天然气工艺技术探讨[J]. 天然气化工(C1化学与化工), 2014, 39(4): 74-76, 91. |
| DU Xiongwei. A discussion on processes for conversion of coke oven gas to natural gas[J]. Natural Gas Chemical Industry, 2014, 39(4): 74-76, 91. | |
| 6 | 吴越峰, 吴双月. 利用焦炉气生产合成氨工艺路线探讨[C]//全国炼焦行业利用焦炉煤气生产甲醇及应用研讨会. 北京, 2006: 135-150. |
| WU Yuefeng, WU Shuangyue. Discussion on the process route of using coke oven gas to produce synthetic ammonia[C]//National Coking Industry Using Coke Oven Gas to Produce Methanol and Application Seminar. Beijing, 2006: 135-150. | |
| 7 | LIU Xin, YUAN Zengwei. Life cycle environmental performance of by-product coke production in China[J]. Journal of Cleaner Production, 2016, 112: 1292-1301. |
| 8 | VAN ACHT S C J, LAYCOCK C J, CARR S J W, et al. Optimization of VPSA-EHP/C process for high-pressure hydrogen recovery from coke oven gas using CO selective adsorbent[J]. International Journal of Hydrogen Energy, 2021, 46(1): 709-725. |
| 9 | 张华西, 安楚玉, 张礼树, 等. 焦炉煤气加氢脱硫催化剂的制备及应用[J]. 天然气化工(C1化学与化工), 2019, 44(2): 46-49. |
| ZHANG Huaxi, AN Chuyu, ZHANG Lishu, et al. Preparation and application of catalysts for hydrogenation desulfurization of coke-oven gas[J]. Natural Gas Chemical Industry, 2019, 44(2): 46-49. | |
| 10 | DE OLIVEIRA CARNEIRO Lucas, DE VASCONCELOS Suênia Fernandes, DE FARIAS NETO Gilvan Wanderley, et al. Improving H2S removal in the coke oven gas purification process[J]. Separation and Purification Technology, 2021, 257: 117862. |
| 11 | 裴学国, 亓栋, 丁卉, 等. T202型催化剂在焦炉气二级加氢脱硫工艺中的应用[J]. 化肥设计, 2011, 49(5): 48-51. |
| PEI Xueguo, QI Dong, DING Hui, et al. Application for T202 type catalyst in hydrogenation desulphurization process of coking-oven gas in 2 stages[J]. Chemical Fertilizer Design, 2011, 49(5): 48-51. | |
| 12 | 杨宝刚, 黄成忠, 朱军利. JT-8型加氢催化剂在焦炉煤气制甲醇装置上的工业应用[J]. 工业催化, 2014, 22(8): 628-631. |
| YANG Baogang, HUANG Chengzhong, ZHU Junli. Commercial application of JT-8 hydrogenation catalyst in the plant of methanol synthesized from coke oven gas[J]. Industrial Catalysis, 2014, 22(8): 628-631. | |
| 13 | 朱军利, 张林生, 盛明泽, 等. JT-8型焦炉煤气加氢催化剂失活样品剖析[J]. 工业催化, 2020, 28(9): 45-50. |
| ZHU Junli, ZHANG Linsheng, SHENG Mingze, et al. Analysis of deactivated hydrogenation catalyst JT-8[J]. Industrial Catalysis, 2020, 28(9): 45-50. | |
| 14 | 郭玉峰, 蒋晓娟. 焦炉气净化中的有机硫加氢工艺应用技术[J]. 山西化工, 2017, 37(3): 52-55. |
| GUO Yufeng, JIANG Xiaojuan. Application technology of organic sulfur hydrogenation process in purification of coke oven gas[J]. Shanxi Chemical Industry, 2017, 37(3): 52-55. | |
| 15 | 陈程, 曹晓娜, 徐广通, 等. 渣油加氢失活催化剂的积炭规律[J]. 石油学报(石油加工), 2016, 32(6): 1221-1227. |
| CHEN Cheng, CAO Xiaona, XU Guangtong, et al. Pattern of coke deposition on the spent residue hydrotreating catalysts[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2016, 32(6): 1221-1227. | |
| 16 | 李乃珍, 孙瑞洁, 秦志峰, 等. 焦炉煤气常量含碳气氛对加氢脱硫催化剂活性、选择性和积炭的影响[J]. 化工进展, 2023, 42(2): 783-793. |
| LI Naizhen, SUN Ruijie, QIN Zhifeng, et al. Effects of constant carbon atmosphere on the activity, selectivity and coking of catalysts in hydrodesulfurization of coke oven gas[J]. Chemical Industry and Engineering Progress, 2023, 42(2): 783-793. | |
| 17 | ANCHEYTA J, BETANCOURT G, CENTENO G, et al. Catalyst deactivation during hydroprocessing of maya heavy crude oil. 1. Evaluation at constant operating conditions[J]. Energy & Fuels, 2002, 16(6): 1438-1443. |
| 18 | 韦晋科, 张强, 李永光. 烧结机中温SCR脱硝催化剂失活原因分析[J]. 河北冶金, 2021(12): 75-79. |
| WEI Jinke, ZHANG Qiang, LI Yongguang. Analysis on causation for deactivation of medium temperature denitration catalyst in sintering machine[J]. Hebei Metallurgy, 2021(12): 75-79. | |
| 19 | MAHIPALREDDY B. Dispersion and activity of molybdena-alumina catalysts prepared by impregnation and solid/solid wetting methods[J]. Journal of Catalysis, 1992, 136(1): 50-58. |
| 20 | 余长春, 李然家, 王伟, 等. CO2/CH4干重整转化催化剂的积碳控制研究[J]. 石油化工, 2020, 49(10): 925-930. |
| YU Changchun, LI Ranjia, WANG Wei, et al. Study of carbon deposition controlling over CO2/CH4 dry reforming catalyst[J]. Petrochemical Technology, 2020, 49(10): 925-930. | |
| 21 | 徐军科, 羌宁. 反应器材质对Ni-Co双金属催化剂上沼气重整制氢性能与积炭的影响[J]. 天然气化工(C1化学与化工), 2021, 46(4): 52-57, 69. |
| XU Junke, QIANG Ning. Effect of reactor material on performance and carbon deposition of biogas reforming for hydrogen production over Ni-Co bimetallic catalyst[J]. Natural Gas Chemical Industry, 2021, 46(4): 52-57, 69. | |
| 22 | SHAMSI Abolghasem, BALTRUS John P, SPIVEY James J. Characterization of coke deposited on Pt/alumina catalyst during reforming of liquid hydrocarbons[J]. Applied Catalysis A: General, 2005, 293: 145-152. |
| 23 | LI Qing, SUI Zhijun, ZHOU Xinggui, et al. Coke formation on Pt-Sn/Al2O3 catalyst in propane dehydrogenation: Coke characterization and kinetic study[J]. Topics in Catalysis, 2011, 54(13): 888. |
| 24 | Pedro CASTAÑO, ELORDI Gorka, OLAZAR Martin, et al. Insights into the coke deposited on HZSM-5, Hβ and HY zeolites during the cracking of polyethylene[J]. Applied Catalysis B: Environmental, 2011, 104(1/2): 91-100. |
| 25 | 王雪, 孙昱东, 张强, 等. 催化剂上积炭结构和组成的分析研究方法[J]. 分析测试技术与仪器, 2013, 19(1): 6-11. |
| WANG Xue, SUN Yudong, ZHANG Qiang, et al. Analytical methods for structure and composition of coke deposited on catalysts[J]. Analysis and Testing Technology and Instruments, 2013, 19(1): 6-11. | |
| 26 | BAUER F, GEIDEL E, GEYER W, et al. TG-FTIR and isotopic studies on coke formation during the MTG process[J]. Microporous and Mesoporous Materials, 1999, 29(1): 109-115. |
| 27 | RODRIGUEZ Andrea C, María E SAD, CRUCHADE Hugo, et al. Study of catalyst deactivation during 1,3-butanediol dehydration to produce butadiene[J]. Microporous and Mesoporous Materials, 2021, 320: 111066. |
| 28 | 张萍, 辛靖, 范文轩, 等. 柴油加氢精制剂失活研究[J]. 工业催化, 2021, 29(1): 53-58. |
| ZHANG Ping, XIN Jing, FAN Wenxuan, et al. Study on the deactivation of hydrorefining catalyst[J]. Industrial Catalysis, 2021, 29(1): 53-58. | |
| 29 | 孙锦宜. 工业催化剂的失活与再生[M]. 北京: 化学工业出版社, 2006. |
| SUN Jinyi. Deactivation and regeneration of industrial catalysts[M]. Beijing: Chemical Industry Press, 2006. | |
| 30 | 汪佩华, 秦志峰, 吴琼笑, 等. 磷添加方式对NiMo/Al2O3催化剂加氢脱硫性能的影响[J]. 化工进展, 2021, 40(2): 890-900. |
| WANG Peihua, QIN Zhifeng, WU Qiongxiao, et al. Effect of phosphorus adding manners on the performance of NiMo/Al2O3 catalyst in hydrodesulfurization[J]. Chemical Industry and Engineering Progress, 2021, 40(2): 890-900. | |
| 31 | KALUŽA L, PALCHEVA R, JIRÁTOVÁ K, et al. Characterization and HDS activity of Mo and NiMo sulfided catalyst prepared by thioglycolic acid assisted hydrothermal deposition method[J]. Journal of Alloys and Compounds, 2022, 903: 163925. |
| [1] | WANG Jia, LI Wencui, WU Fan, GAO Xinqian, LU Anhui. Regulation active components distribution of NiMo/Al2O3 catalysts for hydrodesulfurization [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4393-4402. |
| [2] | DUAN Xiang, TIAN Ye, DONG Wenwei, SONG Song, LI Xingang. Research progress on reaction networks and catalytic reaction mechanisms of phthalic anhydride synthesis [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2587-2599. |
| [3] | LI Jingying, MA Longfei, PAN Yibo, LU Shan, ZHANG Hongjuan, XU Long, MA Xiaoxun. Life cycle environmental analysis of coke oven gas to liquefied natural gas based on decarburization and methanation processes [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2872-2879. |
| [4] | DI Zichen, LEI Feixia, CHANG Chenggong, CHEN Wenhui, CHENG Fangqin. Evaluation of hydrocarbon resource utilization potential and low-carbon path in the coking industry [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2862-2871. |
| [5] | GU Xingpeng, MA Hongqin, LIU Jiahao. Modification of Rainey nickel with phosphorus quantum dots and its catalytic hydrodesulfurization performances [J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1293-1301. |
| [6] | CHEN Xiaozhen, LIU Li, YANG Chengmin, ZHENG Bumei, YIN Xiaoying, SUN Jin, YAO Yunhai, DUAN Weiyu. Research progress of alumina-supported hydrodesulfurization catalyst [J]. Chemical Industry and Engineering Progress, 2024, 43(2): 948-961. |
| [7] | SUN Jin, CHEN Xiaozhen, LIU Mingrui, LIU Li, NIU Shikun, GUO Rong. Deactivation mechanism of sodium poisoning hydrodesulfurization catalyst [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 407-413. |
| [8] | YU Zhiqing, HUANG Wenbin, WANG Xiaohan, DENG Kaixin, WEI Qiang, ZHOU Yasong, JIANG Peng. B-doped Al2O3@C support for CoMo hydrodesulfurization catalyst and their hydrodesulfurization performance [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3550-3560. |
| [9] | LI Naizhen, SUN Ruijie, QIN Zhifeng, MIAO Maoqian, WU Qiongxiao, CHANG Liping, SUN Pengcheng, ZENG Jian, LIU Yi. Effects of constant carbon atmosphere on the activity, selectivity and coking of catalysts in hydrodesulfurization of coke oven gas [J]. Chemical Industry and Engineering Progress, 2023, 42(2): 783-793. |
| [10] | CHEN Jian, JI Cunmin, BU Lingbing. Research and application of hydrogen production technology from industrial by-product gas under the background of carbon neutrality [J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1479-1486. |
| [11] | Peihua WANG, Zhifeng QIN, Qiongxiao WU, Congming LI, Maoqian MIAO, Liping CHANG, Pengcheng SUN, Jian ZENG, Lihua WANG, Kechang XIE. Effect of phosphorus adding manners on the performance of NiMo/Al2O3 catalyst in hydrodesulfurization [J]. Chemical Industry and Engineering Progress, 2021, 40(2): 890-900. |
| [12] | Hui WANG, Rui ZHANG, Haiyan LIU, Xianghai MENG. Advances in the desulfurization of fluid catalytic cracking naphtha [J]. Chemical Industry and Engineering Progress, 2020, 39(6): 2354-2362. |
| [13] | Yonghong ZHU,Pengju HUO,Tianhua YANG,Wei LI,Lei HAN,Cheng YANG. Lumped kinetic models for VGO hydrodesulfurization [J]. Chemical Industry and Engineering Progress, 2020, 39(4): 1339-1347. |
| [14] | Qi WANG, Ming KE, Pei YU, Yang LIU, Lei ZHANG, Chengjie XIA, Wen LIU. Effect of impregnation way of Ce on the performance of CoMo/CeAl2O3 catalysys in hydrodesulfurization [J]. Chemical Industry and Engineering Progress, 2019, 38(07): 3163-3169. |
| [15] | Shuo LI, Yibin LIU, Xiang FENG, Chaohe YANG. Research progress in active phase structure and reaction mechanism of MoS2-based catalysts for hydrodesulfurization [J]. Chemical Industry and Engineering Progress, 2019, 38(02): 867-875. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||