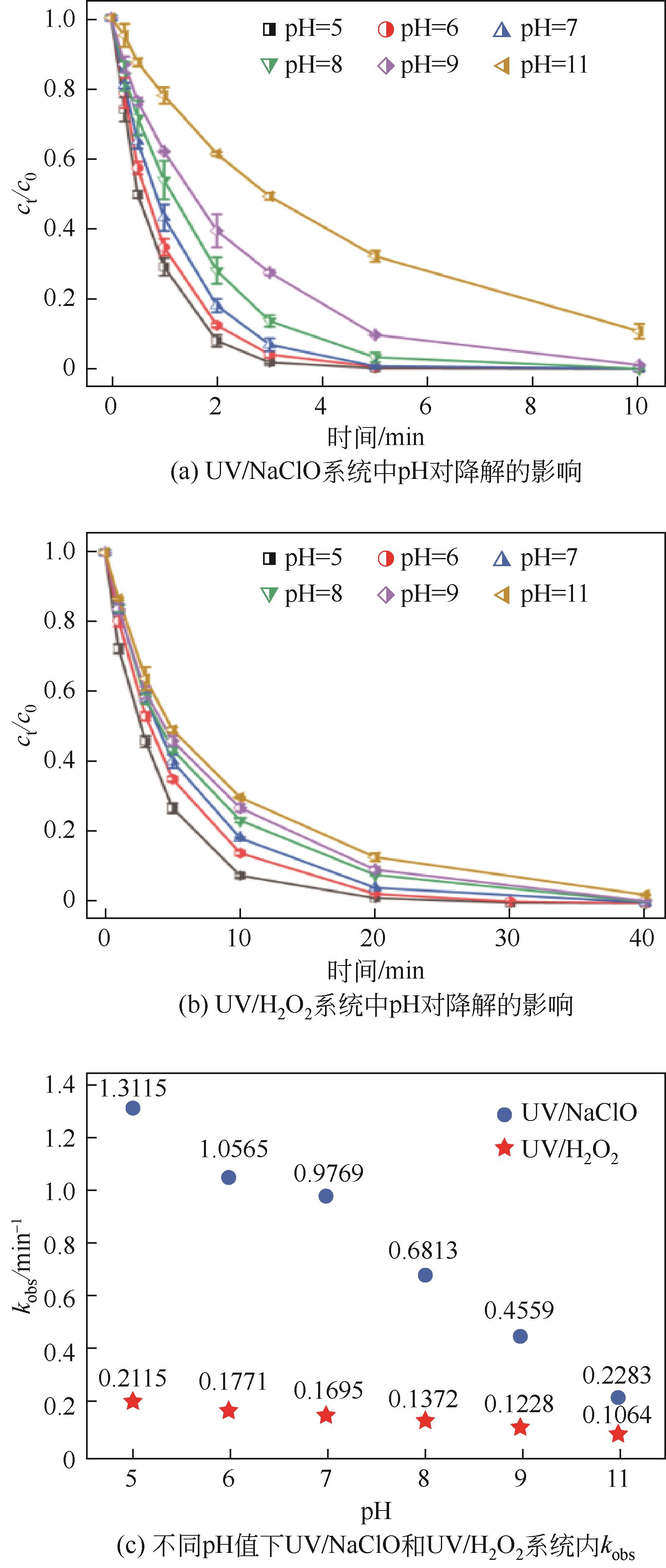
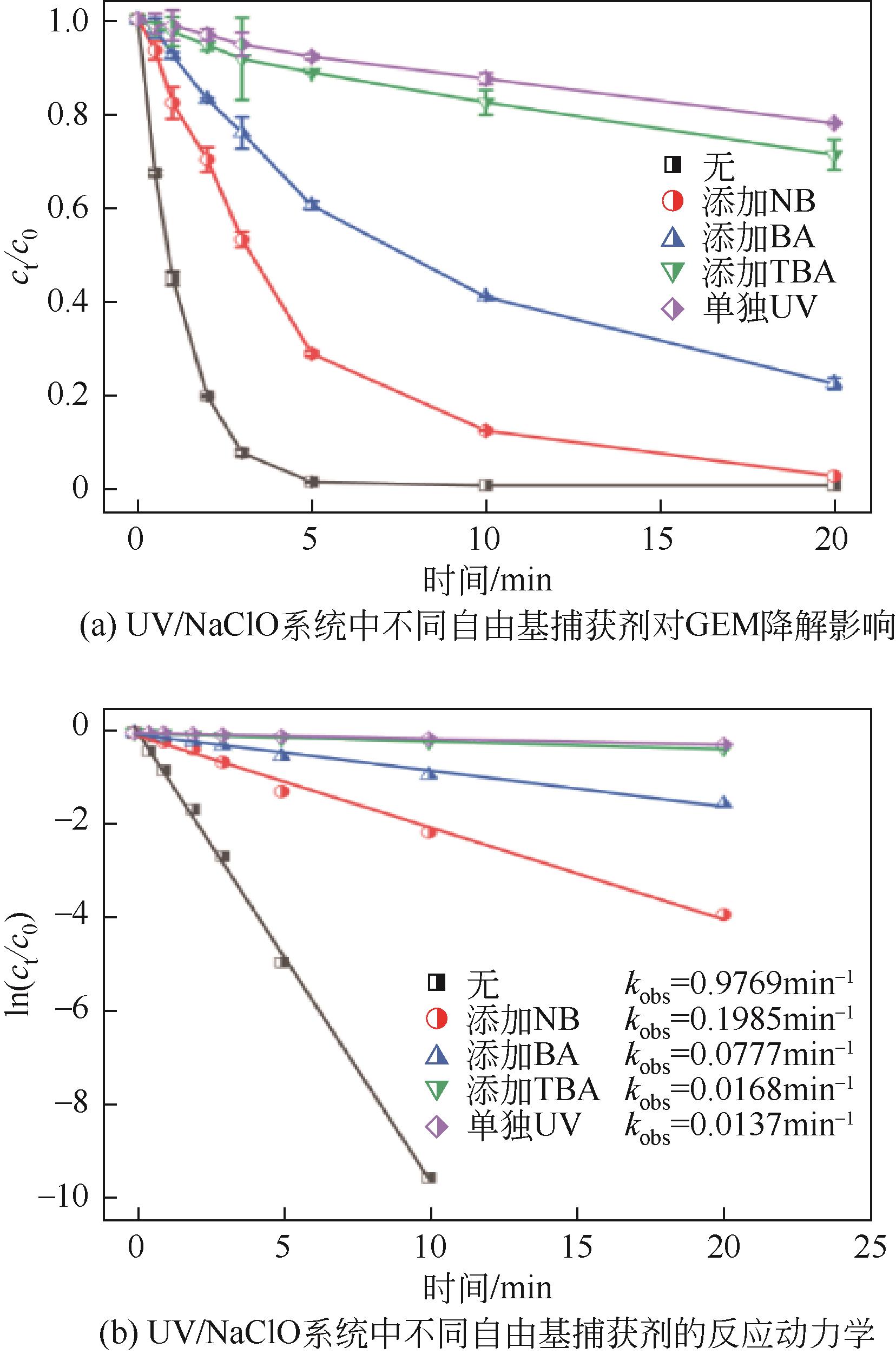
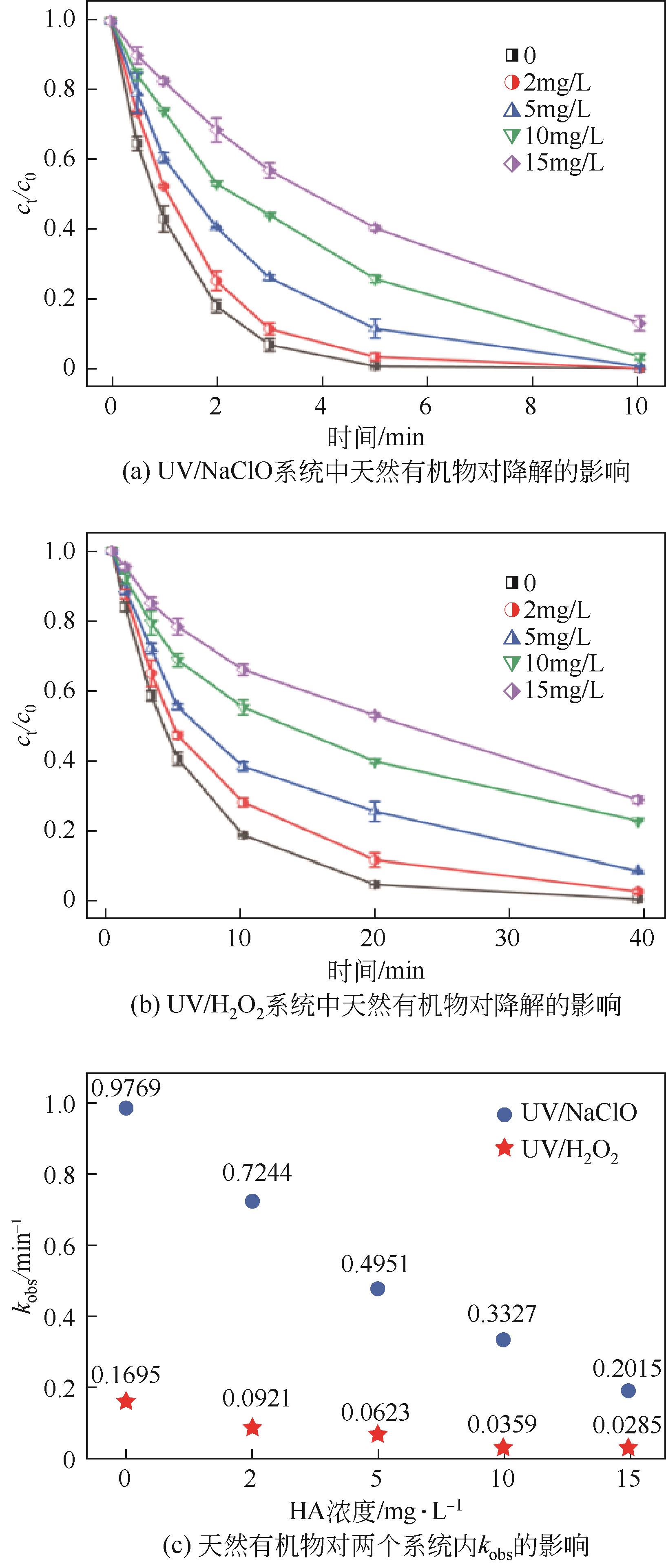
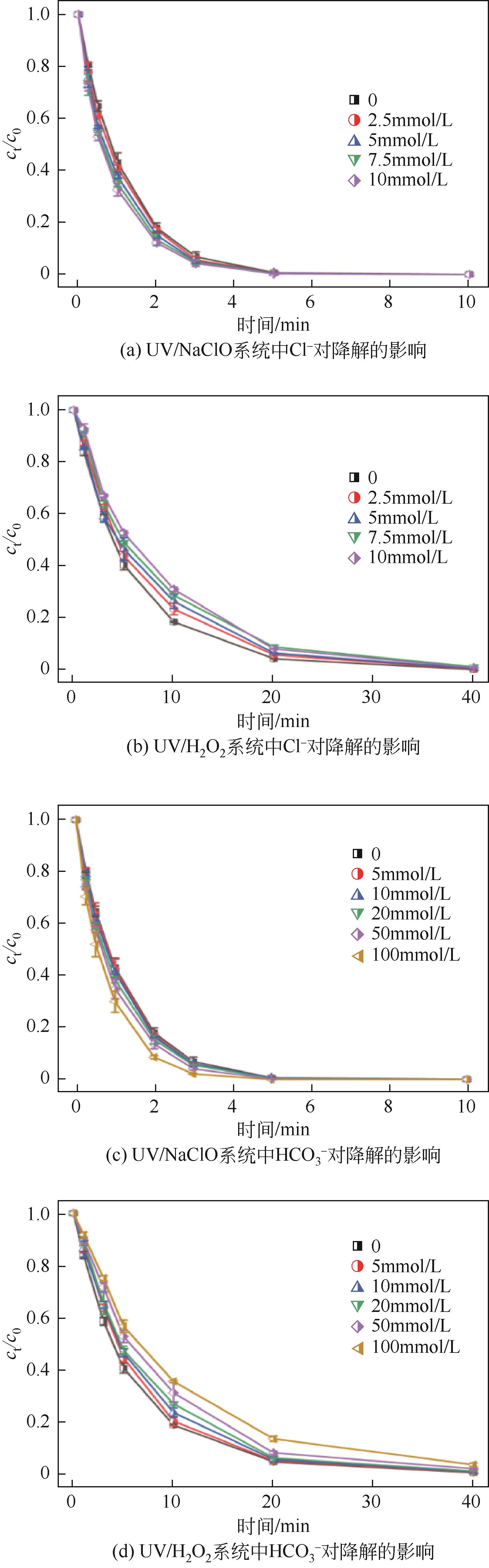
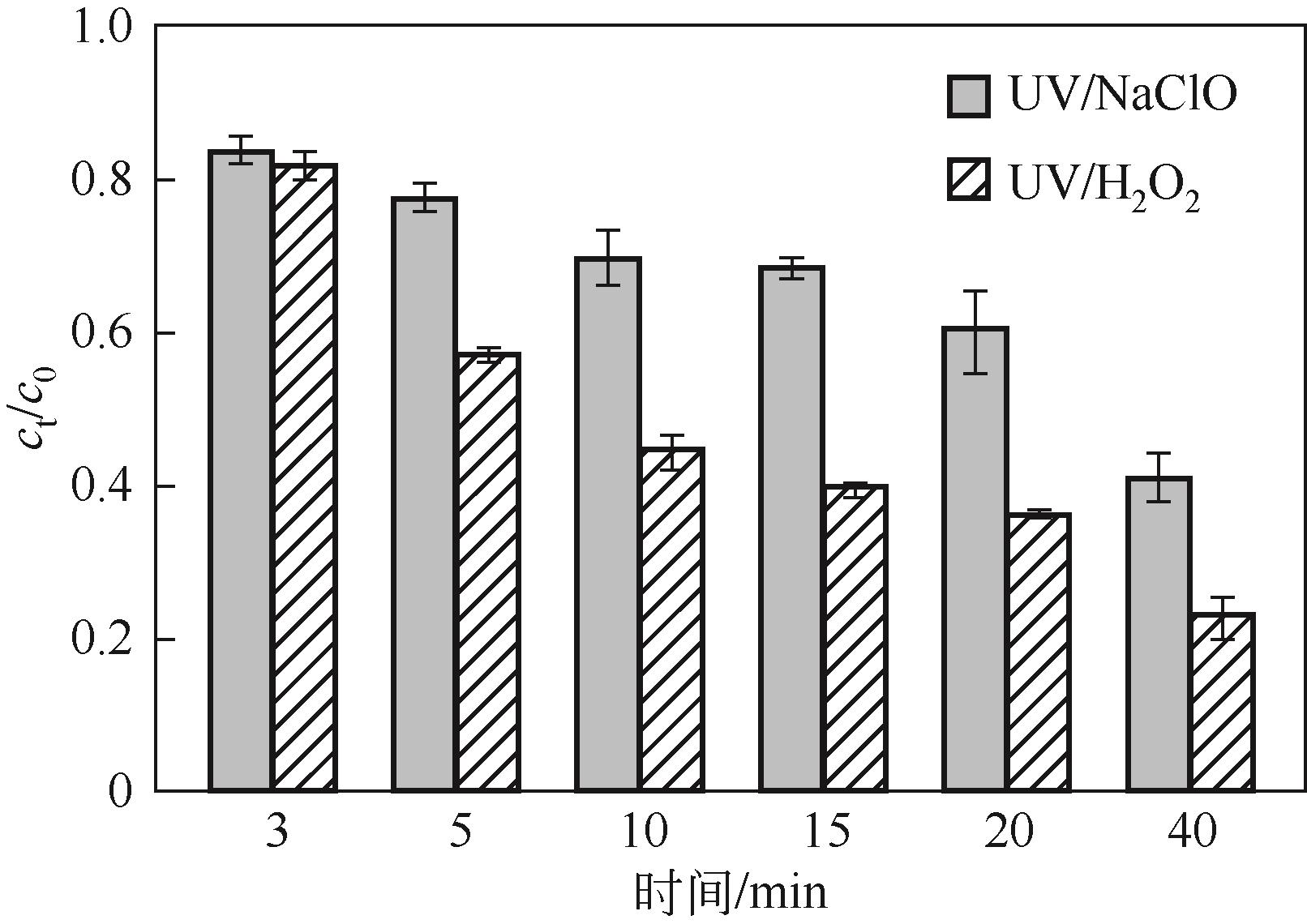
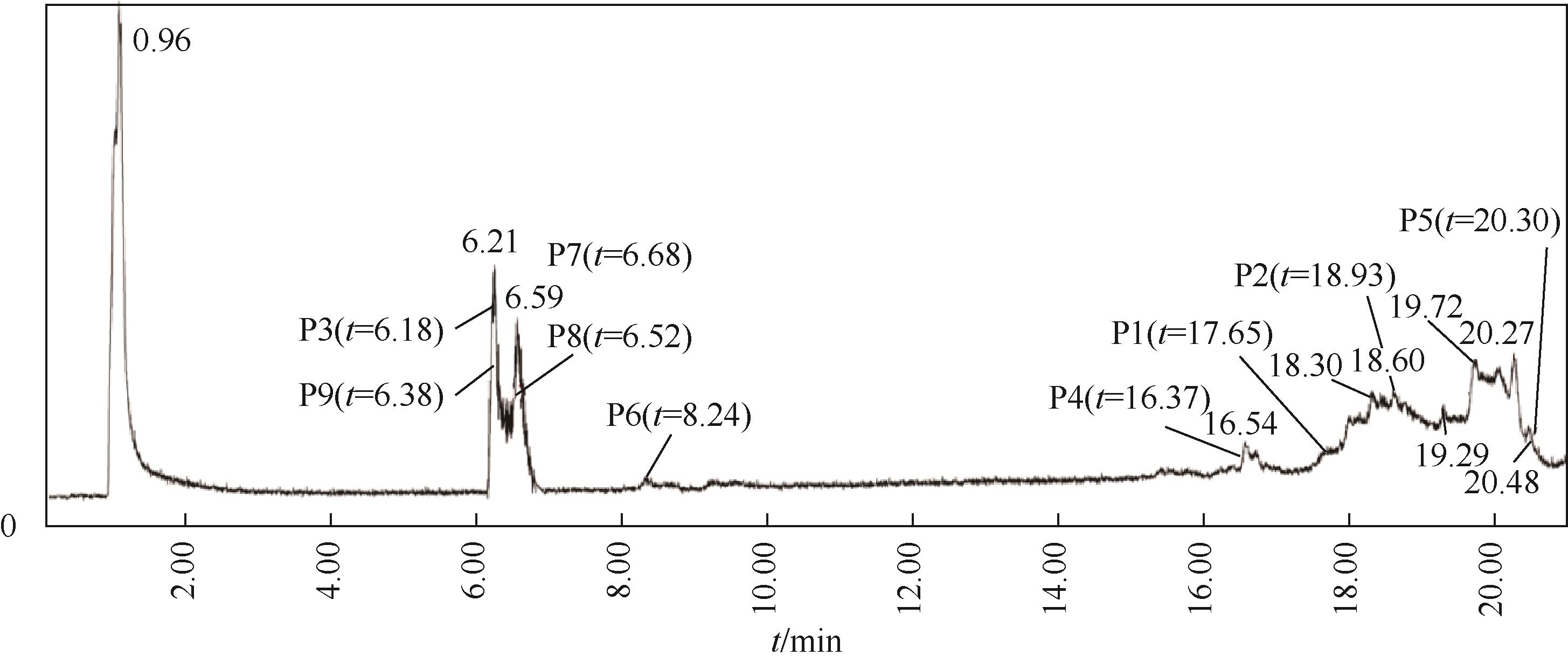
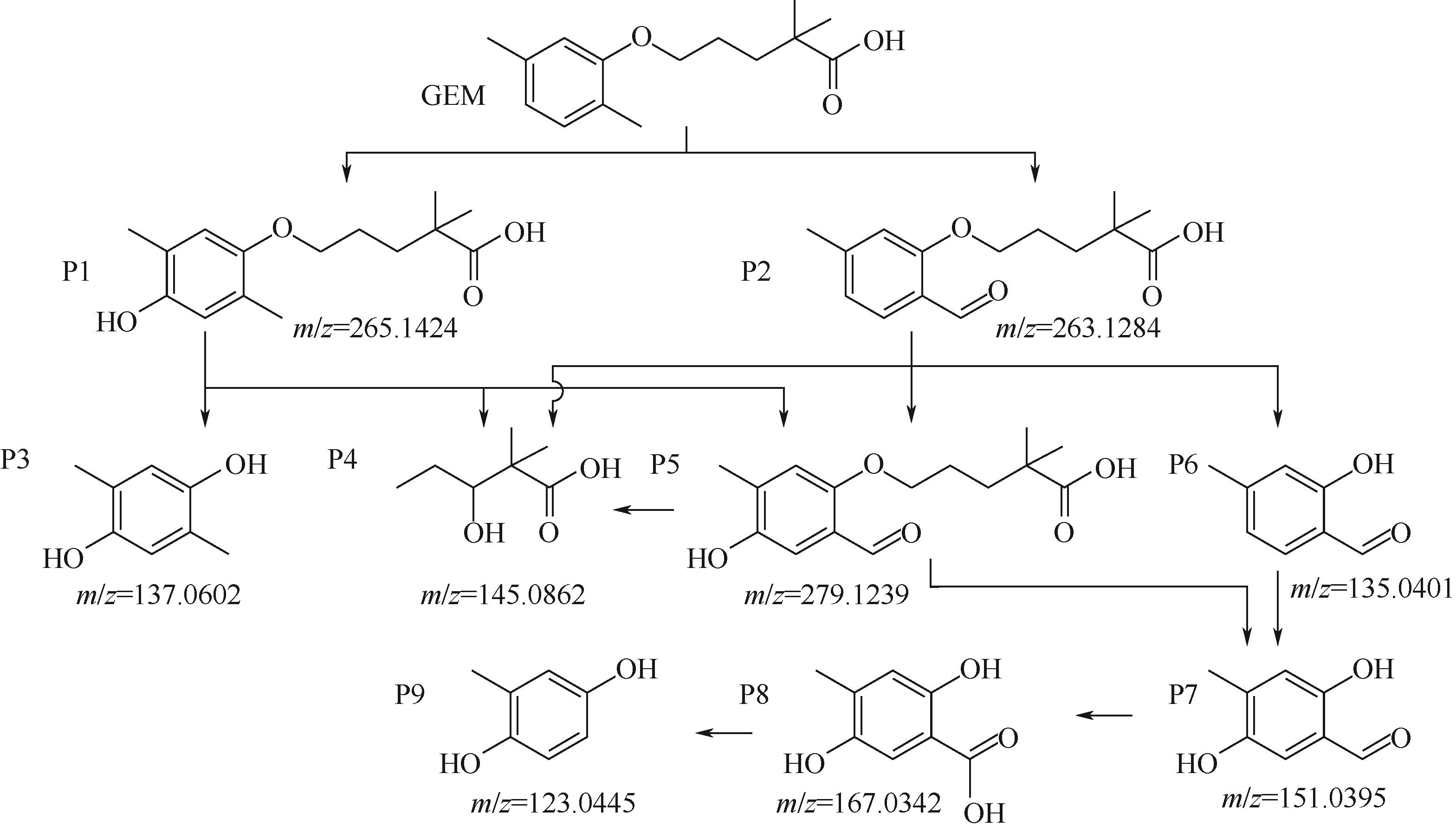
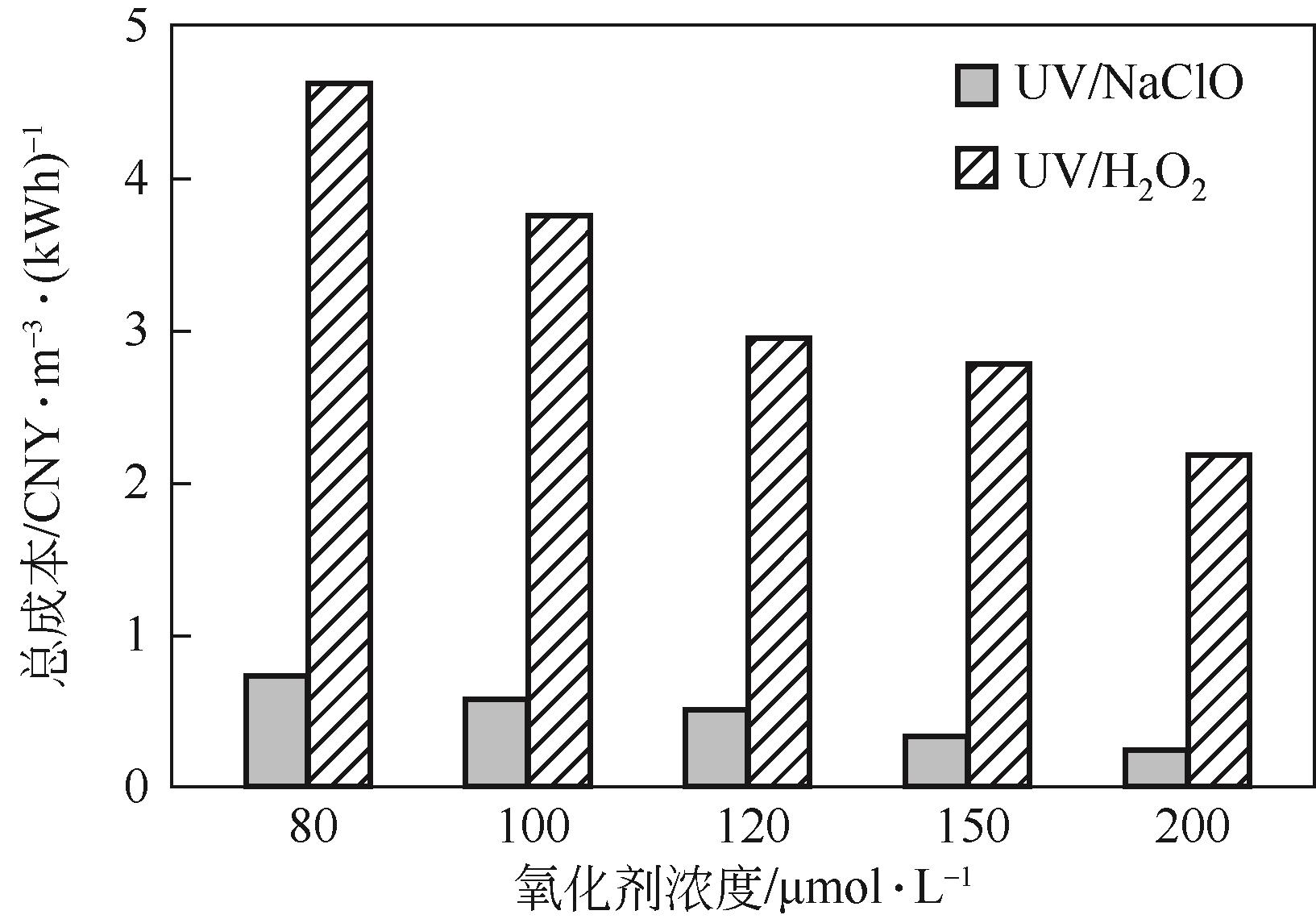
Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (11): 6102-6112.DOI: 10.16085/j.issn.1000-6613.2023-0009
• Resources and environmental engineering • Previous Articles
Comparative investigation of gemfibrozil degradation by UV/H2O2 and UV/NaClO processes
YAN Boyin( ), HAN Chunyu, XIA Jingjing, WANG Songxue, WU Guizhi, XIA Wenxiang, LI Jincheng(
), HAN Chunyu, XIA Jingjing, WANG Songxue, WU Guizhi, XIA Wenxiang, LI Jincheng( )
)
- School of Environmental and Municipal Engineering, Qingdao University of Technology, Qingdao 266033, Shandong, China
-
Received:2023-01-04Revised:2023-02-09Online:2023-12-15Published:2023-11-20 -
Contact:LI Jincheng
UV/H2O2和UV/NaClO工艺降解吉非罗齐的比较
闫博引( ), 韩春宇, 夏晶晶, 王松雪, 武桂芝, 夏文香, 李金成(
), 韩春宇, 夏晶晶, 王松雪, 武桂芝, 夏文香, 李金成( )
)
- 青岛理工大学环境与市政工程学院,山东 青岛 266033
-
通讯作者:李金成 -
作者简介:闫博引(1992—),女,博士,副教授,研究方向为水质净化技术。Email:hit_yanby@126.com。 -
基金资助:山东省自然科学基金(ZR2021QE234);国家自然科学基金(52100010);河海大学浅水湖泊综合治理与资源开发教育部重点实验室开放项目
CLC Number:
Cite this article
YAN Boyin, HAN Chunyu, XIA Jingjing, WANG Songxue, WU Guizhi, XIA Wenxiang, LI Jincheng. Comparative investigation of gemfibrozil degradation by UV/H2O2 and UV/NaClO processes[J]. Chemical Industry and Engineering Progress, 2023, 42(11): 6102-6112.
闫博引, 韩春宇, 夏晶晶, 王松雪, 武桂芝, 夏文香, 李金成. UV/H2O2和UV/NaClO工艺降解吉非罗齐的比较[J]. 化工进展, 2023, 42(11): 6102-6112.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-0009
| 1 | ZHANG Ruochun, MENG Tan, HUANG Chinghua, et al. PPCP degradation by chlorine-UV processes in ammoniacal water: New reaction insights, kinetic modeling, and DBP formation[J]. Environmental Science & Technology, 2018, 52(14): 7833-7841. |
| 2 | KÜMMERER K, DIONYSIOU D D, OLSSON O, et al. A path to clean water[J]. Science, 2018, 361(6399): 222-224. |
| 3 | ZHANG Panwei, ZHOU Huaidong, LI Kun, et al. Occurrence of pharmaceuticals and personal care products, and their associated environmental risks in a large shallow lake in North China[J]. Environmental Geochemistry and Health, 2018, 40(4): 1525-1539. |
| 4 | YIN Haoran, ZHANG Qizhan, SU Yi, et al. A novel UV based advanced oxidation process with electrochemical co-generation of chlorine and H2O2 for carbamazepine abatement: Better performance, lower energy consumption and less DBPs formation[J]. Chemical Engineering Journal, 2021, 425: 131857. |
| 5 | 伊学农, 李京梅, 高玉琼. 紫外-高铁酸盐体系氧化降解水中的萘普生[J]. 化工进展, 2022, 41(8): 4562-4570. |
| YI Xuenong, LI Jingmei, GAO Yuqiong. Oxidative degradation of naproxen in water by UV-Fe(Ⅵ) process[J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4562-4570. | |
| 6 | MIKLOS D B, REMY C, JEKEL M, et al. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review[J]. Water Research, 2018, 139: 118-131. |
| 7 | DAS D, BORDOLOI A, ACHARY M P, et al. Degradation and inactivation of chromosomal and plasmid encoded resistance genes/ARBs and the impact of different matrices on UV and UV/H2O2 based advanced oxidation process[J]. Science of the Total Environment, 2022, 833: 155205. |
| 8 | WANG Pin, BU Lingjun, WU Yangtao, et al. Mechanistic insight into the degradation of ibuprofen in UV/H2O2 process via a combined experimental and DFT study[J]. Chemosphere, 2021, 267: 128883. |
| 9 | 胡晋博, 李梦凯, 蔡恒文, 等. 不同光源UV/H2O2工艺降解四环素动力学[J]. 环境工程学报, 2021, 15(8): 2618-2626. |
| HU Jinbo, LI Mengkai, CAI Hengwen, et al. Degradation kinetics of tetracycline by UV/H2O2 process with various light sources[J]. Chinese Journal of Environmental Engineering, 2021, 15(8): 2618-2626. | |
| 10 | LU Gang, HU Jiangyong. Effect of alpha-hydroxy acids on transformation products formation and degradation mechanisms of carbamazepine by UV/H2O2 process[J]. Science of the Total Environment, 2019, 689: 70-78. |
| 11 | DHAWLE Rebecca, MANTZAVINOS Dionissios, LIANOS Panagiotis. UV/H2O2 degradation of diclofenac in a photocatalytic fuel cell[J]. Applied Catalysis B: Environmental, 2021, 299: 120706. |
| 12 | YAN Boyin, WANG Songxue, LIU Zhiquan, et al. Degradation mechanisms of cyanobacteria neurotoxin β-N-methylamino-l-alanine (BMAA) during UV254/H2O2 process: Kinetics and pathways[J]. Chemosphere, 2022, 302: 134939. |
| 13 | ZHAN Lumeng, LI Wentao, LIU Li, et al. Degradation of micropolluants in flow-through VUV/UV/H2O2 reactors: Effects of H2O2 dosage and reactor internal diameter[J]. Journal of Environmental Sciences, 2021, 110: 28-37. |
| 14 | PARK J A, YANG B, JANG M, et al. Oxidation and molecular properties of microcystin-LR, microcystin-RR and anatoxin-a using UV-light-emitting diodes at 255nm in combination with H2O2 [J]. Chemical Engineering Journal, 2019, 366: 423-432. |
| 15 | HAN Tao, LI Wentao, LI Jin, et al. Degradation of micropollutants in flow-through UV/chlorine reactors: Kinetics, mechanism, energy requirement and toxicity evaluation[J]. Chemosphere, 2022, 307: 135890. |
| 16 | CAI Wenwen, PENG Tao, YANG Bin, et al. Kinetics and mechanism of reactive radical mediated fluconazole degradation by the UV/chlorine process: Experimental and theoretical studies[J]. Chemical Engineering Journal, 2020, 402: 126224. |
| 17 | GUO Kaiheng, WU Zihao, SHANG Chi, et al. Radical chemistry and structural relationships of PPCP degradation by UV/chlorine treatment in simulated drinking water[J]. Environmental Science & Technology, 2017, 51(18): 10431-10439. |
| 18 | NIKRAVESH Behnaz, SHOMALNASAB Asma, NAYYER Aliyehsadat, et al. UV/Chlorine process for dye degradation in aqueous solution: Mechanism, affecting factors and toxicity evaluation for textile wastewater[J]. Journal of Environmental Chemical Engineering, 2020, 8(5): 104244. |
| 19 | GAO Yuqiong, ZHANG Jia, LI Cong, et al. Comparative evaluation of metoprolol degradation by UV/chlorine and UV/H2O2 processes[J]. Chemosphere, 2020, 243: 125325. |
| 20 | DENG Jia, WU Guangxue, YUAN Shoujun, et al. Ciprofloxacin degradation in UV/chlorine advanced oxidation process: Influencing factors, mechanisms and degradation pathways[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2019, 371: 151-158. |
| 21 | YIN Kai, HE Qunying, LIU Chengbin, et al. Prednisolone degradation by UV/chlorine process: Influence factors, transformation products and mechanism[J]. Chemosphere, 2018, 212: 56-66. |
| 22 | GUO Kaiheng, WU Zihao, YAN Shuwen, et al. Comparison of the UV/chlorine and UV/H2O2 processes in the degradation of PPCPs in simulated drinking water and wastewater: Kinetics, radical mechanism and energy requirements[J]. Water Research, 2018, 147: 184-194. |
| 23 | WAN Ying, XIE Pengchao, WANG Zongping, et al. Comparative study on the pretreatment of algae-laden water by UV/persulfate, UV/chlorine, and UV/H2O2: Variation of characteristics and alleviation of ultrafiltration membrane fouling[J]. Water Research, 2019, 158: 213-226. |
| 24 | GHANBARI Farshid, Ali YAGHOOT-NEZHAD, Stanisław WACŁAWEK, et al. Comparative investigation of acetaminophen degradation in aqueous solution by UV/chlorine and UV/H2O2 processes: Kinetics and toxicity assessment, process feasibility and products identification[J]. Chemosphere, 2021, 285: 131455. |
| 25 | 马京帅, 吕文英, 刘国光, 等. 吉非罗齐在热活化过硫酸盐体系中的降解机制研究[J]. 环境科学学报, 2016, 36(10): 3774-3783. |
| MA Jingshuai, Wenying LYU, LIU Guoguang, et al. Degradation mechanism of gemfibrozil in heat-activated persulfate oxidation process[J]. Acta Scientiae Circumstantiae, 2016, 36(10): 3774-3783. | |
| 26 | LUO Congwei, JIANG Jin, MA Jun, et al. Oxidation of the odorous compound 2,4,6-trichloroanisole by UV activated persulfate: Kinetics, products, and pathways[J]. Water Research, 2016, 96: 12-21. |
| 27 | YAN Boyin, XU Dongchuan, LIU Zhiquan, et al. Degradation of neurotoxin β-N-methylamino-L-alanine by UV254 activated persulfate: Kinetic model and reaction pathways[J]. Chemical Engineering Journal, 2021, 404: 127041. |
| 28 | XIANG Yingying, FANG Jingyun, SHANG Chii. Kinetics and pathways of ibuprofen degradation by the UV/chlorine advanced oxidation process[J]. Water Research, 2016, 90: 301-308. |
| 29 | LI Boqiang, MA Xiaoyan, LI Qingsong, et al. Factor affecting the role of radicals contribution at different wavelengths, degradation pathways and toxicity during UV-LED/chlorine process[J]. Chemical Engineering Journal, 2020, 392: 124552. |
| 30 | CAO Y, YAO J, KNUDSEN T Š, et al. Radical chemistry, degradation mechanism and toxicity evolution of BPA in the UV/chlorine and UV/H2O2 [J]. Chemosphere, 2023, 312: 137169. |
| 31 | LIU Huaying, HOU Zhichao, LI Yingjie, et al. Modeling degradation kinetics of gemfibrozil and naproxen in the UV/chlorine system: Roles of reactive species and effects of water matrix[J]. Water Research, 2021, 202: 117445. |
| 32 | REMUCAL C K, MANLEY D. Emerging investigators series: The efficacy of chlorine photolysis as an advanced oxidation process for drinking water treatment[J]. Environmental Science: Water Research & Technology, 2016, 2(4): 565-579. |
| 33 | WU Zihao, GUO Kaiheng, FANG Jingyun, et al. Factors affecting the roles of reactive species in the degradation of micropollutants by the UV/chlorine process[J]. Water Research, 2017, 126: 351-360. |
| 34 | CHEN Chunyan, WU Zihao, HOU Shaodong, et al. Transformation of gemfibrozil by the interaction of chloride with sulfate radicals: Radical chemistry, transient intermediates and pathways[J]. Water Research, 2022, 209: 117944. |
| 35 | FANG Jingyun, FU Yun, SHANG Chii. The roles of reactive species in micropollutant degradation in the UV/free chlorine system[J]. Environmental Science & Technology, 2014, 48(3): 1859-1868. |
| 36 | LI Tong, JIANG Yan, AN Xiaoqiang, et al. Transformation of humic acid and halogenated byproduct formation in UV-chlorine processes[J]. Water Research, 2016, 102: 421-427. |
| [1] | BAI Zhihua, ZHANG Jun. Oxidative removal of NO in DTPMPA/Fenton system [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4967-4973. |
| [2] | WANG Junjie, PAN Yanqiu, NIU Yabin, YU Lu. Molecular level catalytic reforming model construction and application [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3404-3412. |
| [3] | XU Peiyao, CHEN Biaoqi, KANKALA Ranjith Kumar, WANG Shibin, CHEN Aizheng. Research progress of nanomaterials for synergistic ferroptosis anticancer therapy [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3684-3694. |
| [4] | GE Weitong, LIAO Yalong, LI Mingyuan, JI Guangxiong, XI Jiajun. Preparation and dechlorination kinetics of Pd-Fe/MWCNTs bimetallic catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 1885-1894. |
| [5] | SUN Qianqian, LIU Zhen, LI Rui, ZHANG Xi, YANG Mingde, WU Yulong. Low temperature hydrothermal coupling of ferrous ion activated persulfate to improve the dewatering performance of waste activated sludge [J]. Chemical Industry and Engineering Progress, 2023, 42(2): 595-602. |
| [6] | WANG Qinghong, JIANG Chenxu, WANG Xin, YU Meiqi, ZHU Shuai, LI Yiming, CHEN Chunmao. An overview of natural mineral catalytic oxidation of refractory organic contaminants in wastewater [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 417-434. |
| [7] | DUAN Yi, ZOU Ye, ZHOU Shukui, YANG Liu. Progress in the degradation of organic pollutants by H2O2/PMS/PDS activated by transition metal single-atom catalysts [J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4147-4158. |
| [8] | PAN Jie, WANG Mingxin, GAO Shengwang, XIA Xunfeng, HAN Xue. Nitrogen-sulfur doped biochar/permonosulfate for degradation of sulfisoxazole in water [J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4204-4212. |
| [9] | JI Xuanyu, LIN Weijian, ZHOU Xiong, BAI Jisong, YANG Yu, KONG Jie, LIAO Chongyang. Research status and progress of waste tire pyrolysis technology [J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4498-4512. |
| [10] | YI Xuenong, LI Jingmei, GAO Yuqiong. Oxidative degradation of naproxen in water by UV-Fe(Ⅵ) process [J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4562-4570. |
| [11] | ZHUANG Yuting, WANG Jianhua, XIANG Zhiyan, ZHAO Juan, XU Qiong, LIU Xianxiang, YIN Dulin. Research progress in preparation and kinetics of γ-valerolactone synthesis from hemicellulose and its derivatives [J]. Chemical Industry and Engineering Progress, 2022, 41(7): 3519-3533. |
| [12] | LI Guixian, ZHANG Junqiang, YANG Yong, FAN Xueying, WANG Dongliang. A novel PX production shortcut through PX selectivity intensification in toluene and methanol methylation [J]. Chemical Industry and Engineering Progress, 2022, 41(6): 2939-2947. |
| [13] | LIAO Bing, XU Wen, YE Qiuyue. A review of activated percarbonate and peroxymonocarbonate in the field of water treatment [J]. Chemical Industry and Engineering Progress, 2022, 41(6): 3235-3248. |
| [14] | XIONG Zhe, DENG Wei, LIU Jia, WANG Xuepeng, XU Jun, JIANG Long, SU Sheng, WANG Yi, HU Song, XIANG Jun. Research progress in coke formation characteristics of bio-oil during its non-catalytic thermal conversion process [J]. Chemical Industry and Engineering Progress, 2022, 41(4): 1802-1813. |
| [15] | ZHEN Jianzheng, NIE Shisong, PAN Shiyuan, LYU Weiyang, YAO Yuyuan. Research progress on advanced activation of peroxymonosulfate by multidimensional carbon-supported metal catalyst for degradation of organic pollutants in water [J]. Chemical Industry and Engineering Progress, 2022, 41(4): 1858-1872. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||