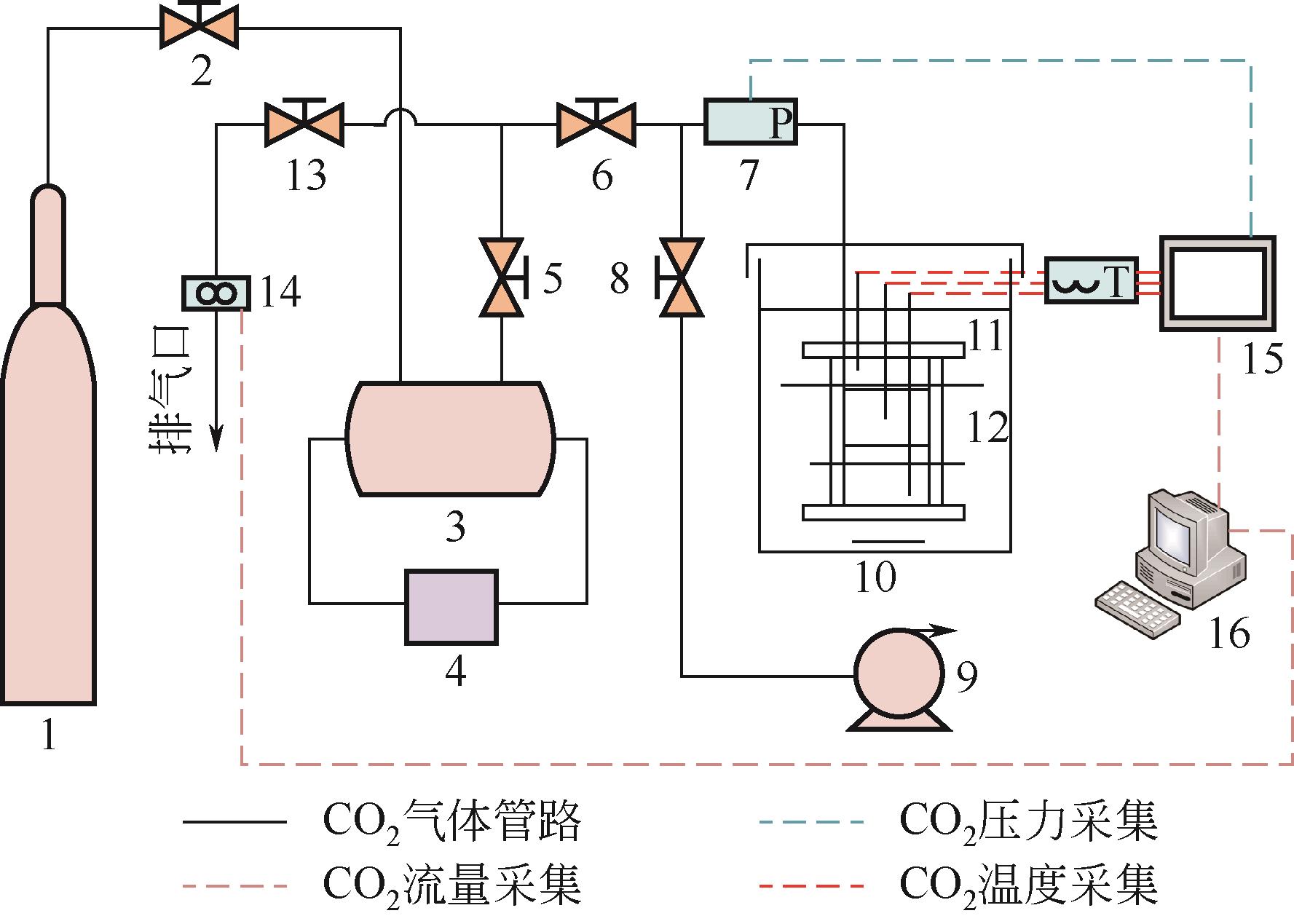
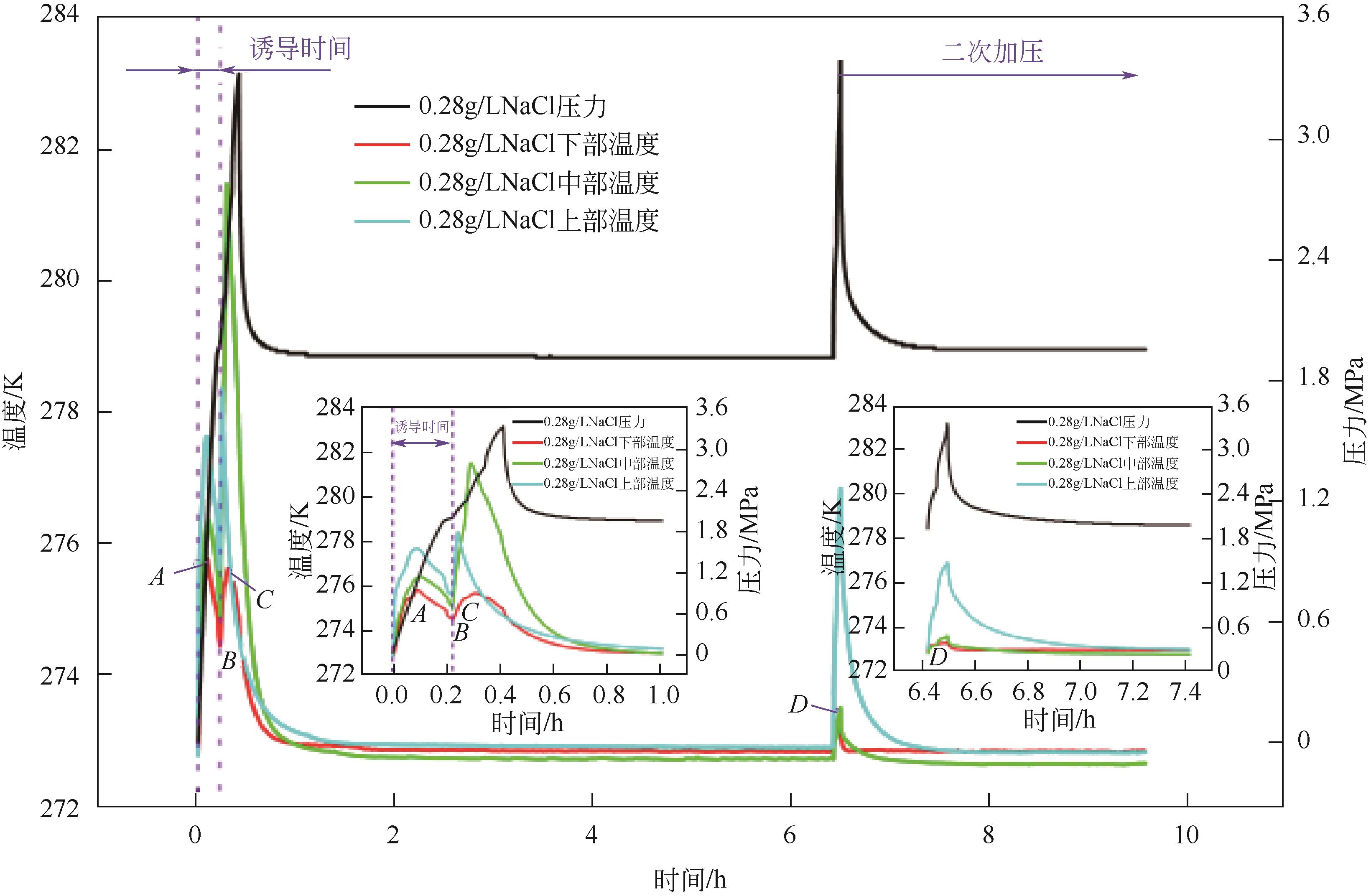
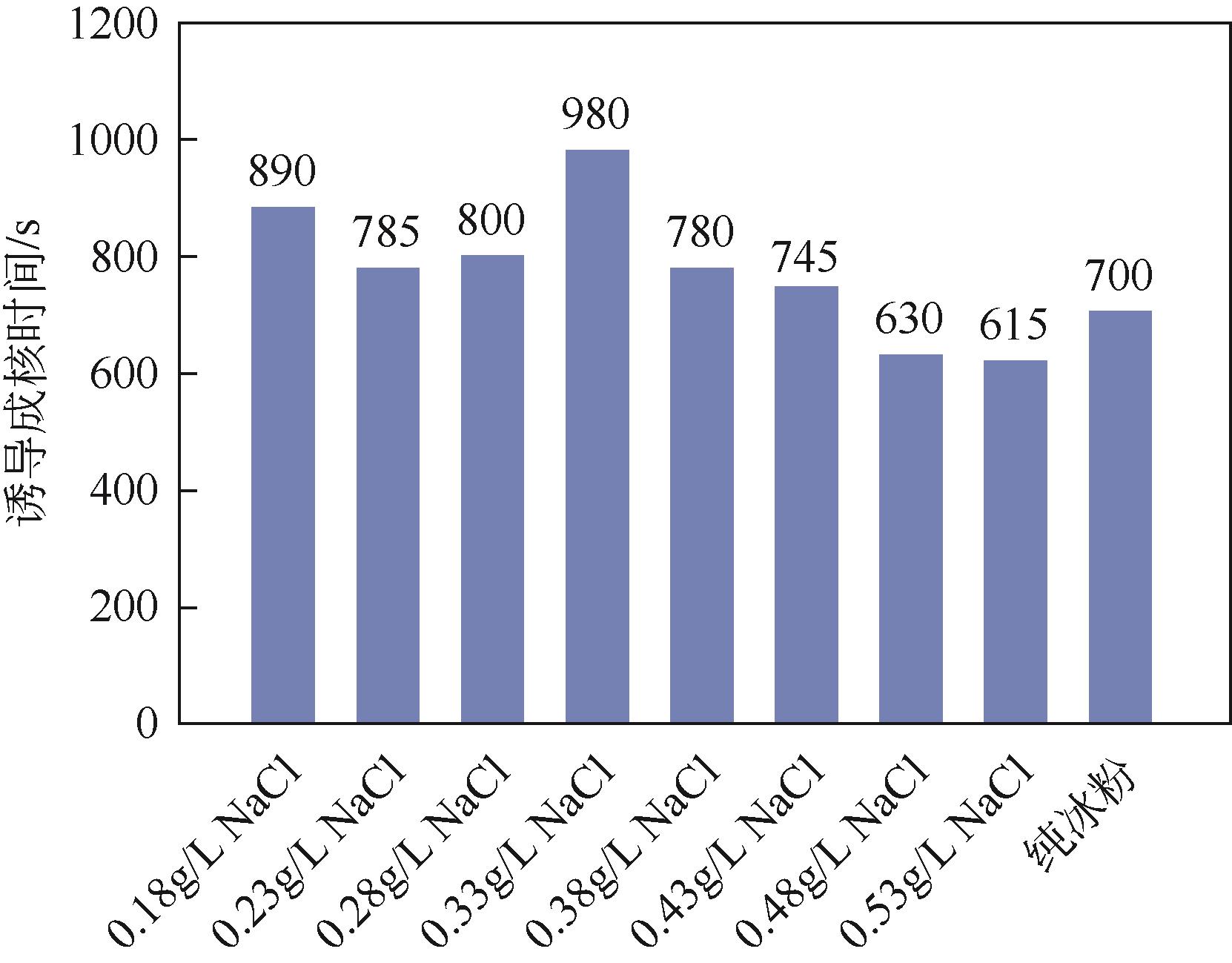
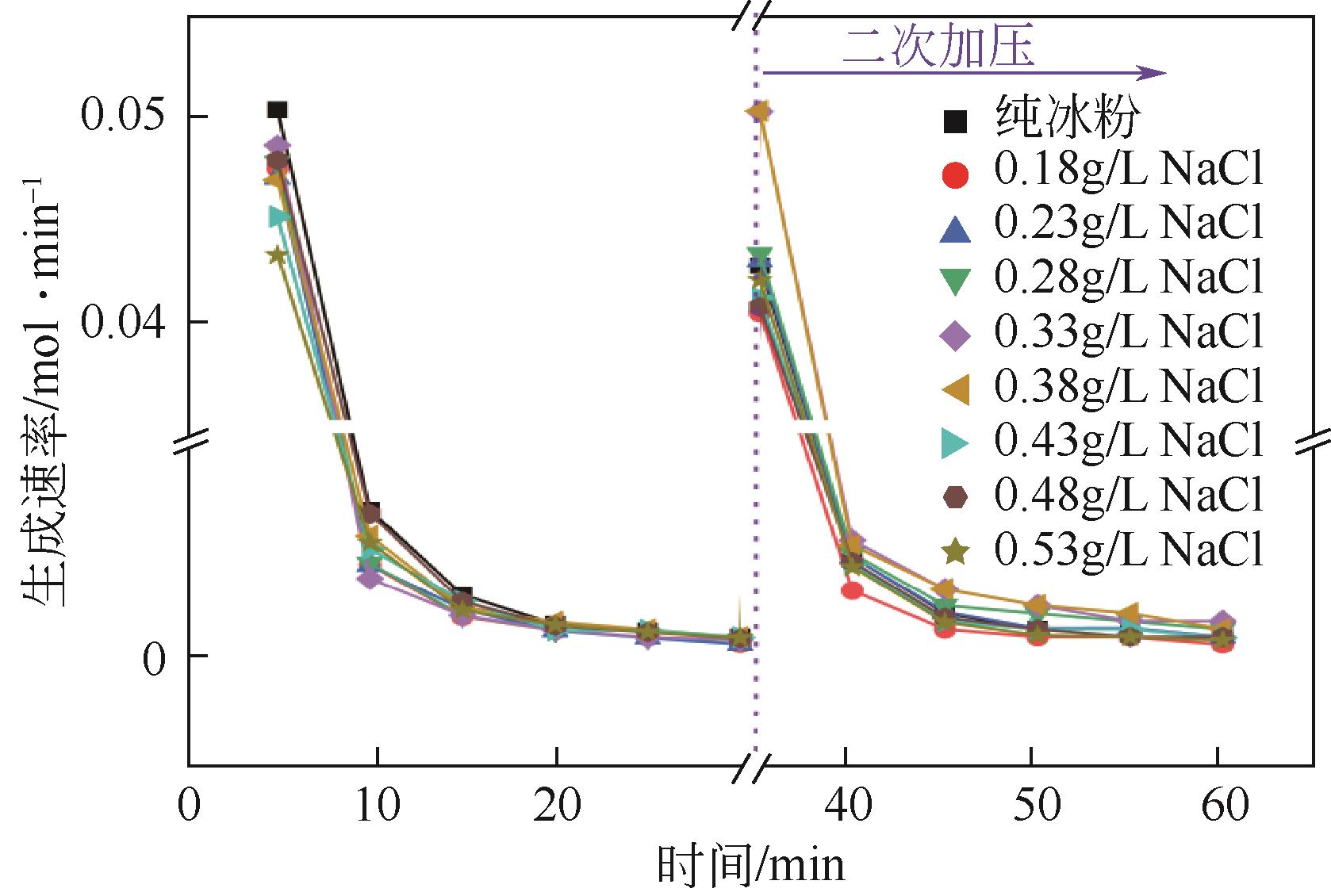
Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (11): 6093-6101.DOI: 10.16085/j.issn.1000-6613.2022-2387
• Resources and environmental engineering • Previous Articles
Effect of NaCl concentration on the formation and stability of CO2 hydrate
WANG Yingmei1,2,3( ), LIU Shenghao1,2,3, TENG Yadong1,2,3, WANG Lijin1,2,3, JIAO Wenze1,2,3
), LIU Shenghao1,2,3, TENG Yadong1,2,3, WANG Lijin1,2,3, JIAO Wenze1,2,3
- 1.School of Energy and Power Engineering, Lanzhou University of Technology, Lanzhou 730050, Gansu, China
2.Key Lab of Complementary Energy System of Biomass and Solar Energy Gansu Province, Lanzhou 730050, Gansu, China
3.Western China Energy & Environment Research Center, Lanzhou University of Technology, Lanzhou 730050, Gansu, China
-
Received:2022-12-30Revised:2023-01-31Online:2023-12-15Published:2023-11-20 -
Contact:WANG Yingmei
NaCl浓度对CO2水合物形成与稳定性的影响
王英梅1,2,3( ), 刘生浩1,2,3, 滕亚栋1,2,3, 王立瑾1,2,3, 焦雯泽1,2,3
), 刘生浩1,2,3, 滕亚栋1,2,3, 王立瑾1,2,3, 焦雯泽1,2,3
- 1.兰州理工大学能源与动力工程学院,甘肃 兰州 730050
2.甘肃省生物质能与太阳能互补供能系统重点实验室,甘肃 兰州 730050
3.兰州理工大学西部能源与环境研究中心,甘肃 兰州 730050
-
通讯作者:王英梅 -
作者简介:王英梅(1987—),女,副教授,研究方向为气体水合物生成与分解动力学。E-mail:wymch@lzb.ac.cn。 -
基金资助:国家自然科学基金(41661103);国家重点研发计划(2017YFC0307303);中国科学院冻土工程国家重点实验室开放基金(SKLFSE201406)
CLC Number:
Cite this article
WANG Yingmei, LIU Shenghao, TENG Yadong, WANG Lijin, JIAO Wenze. Effect of NaCl concentration on the formation and stability of CO2 hydrate[J]. Chemical Industry and Engineering Progress, 2023, 42(11): 6093-6101.
王英梅, 刘生浩, 滕亚栋, 王立瑾, 焦雯泽. NaCl浓度对CO2水合物形成与稳定性的影响[J]. 化工进展, 2023, 42(11): 6093-6101.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-2387
| 材料 | 参数 | 厂商 |
|---|---|---|
| 二氧化碳气体(CO2) | 分析纯度:99.99% | 兰州中科凯特有限公司 |
| 去离子水(H2O/冰粉) | 电导率:18.25MΩ·cm | 自制 |
| 氯化钠(NaCl) | 分析纯度:99.99% | 国药集团化学试剂有限公司 |
| 硅胶 | 粒径:0.5~1mm,合格率:≥99% | 青岛宸容新材料有限公司 |
| 材料 | 参数 | 厂商 |
|---|---|---|
| 二氧化碳气体(CO2) | 分析纯度:99.99% | 兰州中科凯特有限公司 |
| 去离子水(H2O/冰粉) | 电导率:18.25MΩ·cm | 自制 |
| 氯化钠(NaCl) | 分析纯度:99.99% | 国药集团化学试剂有限公司 |
| 硅胶 | 粒径:0.5~1mm,合格率:≥99% | 青岛宸容新材料有限公司 |
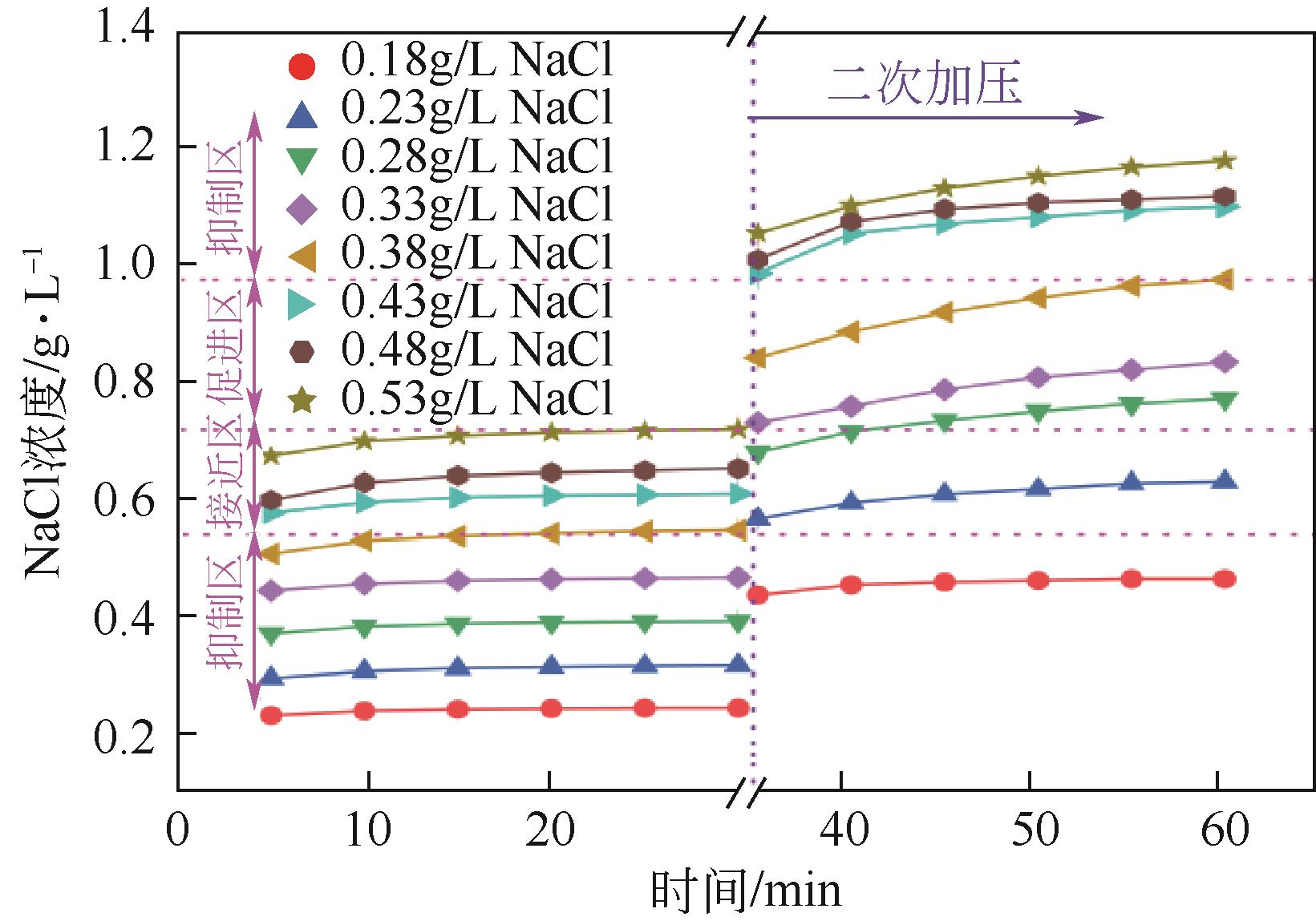
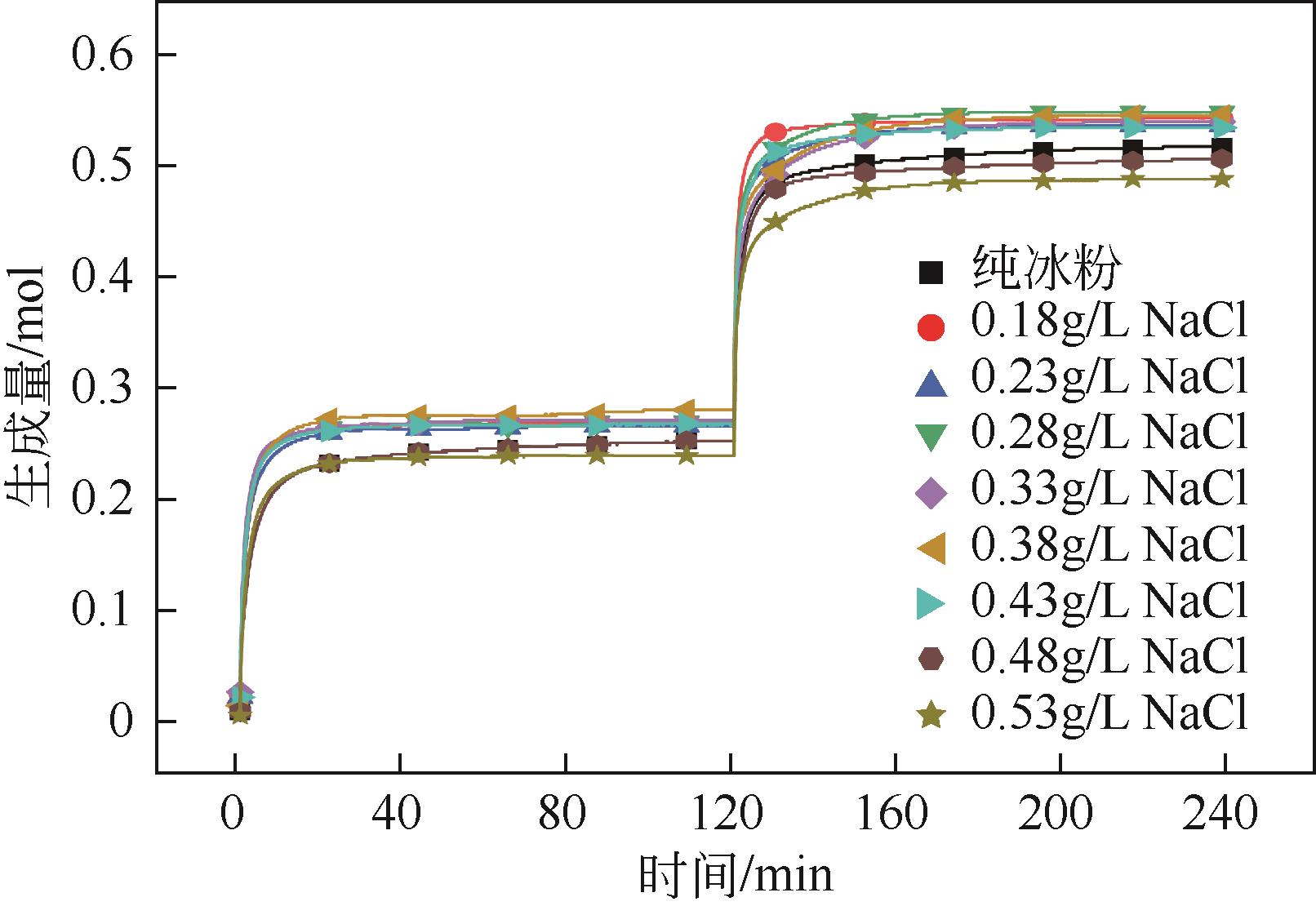
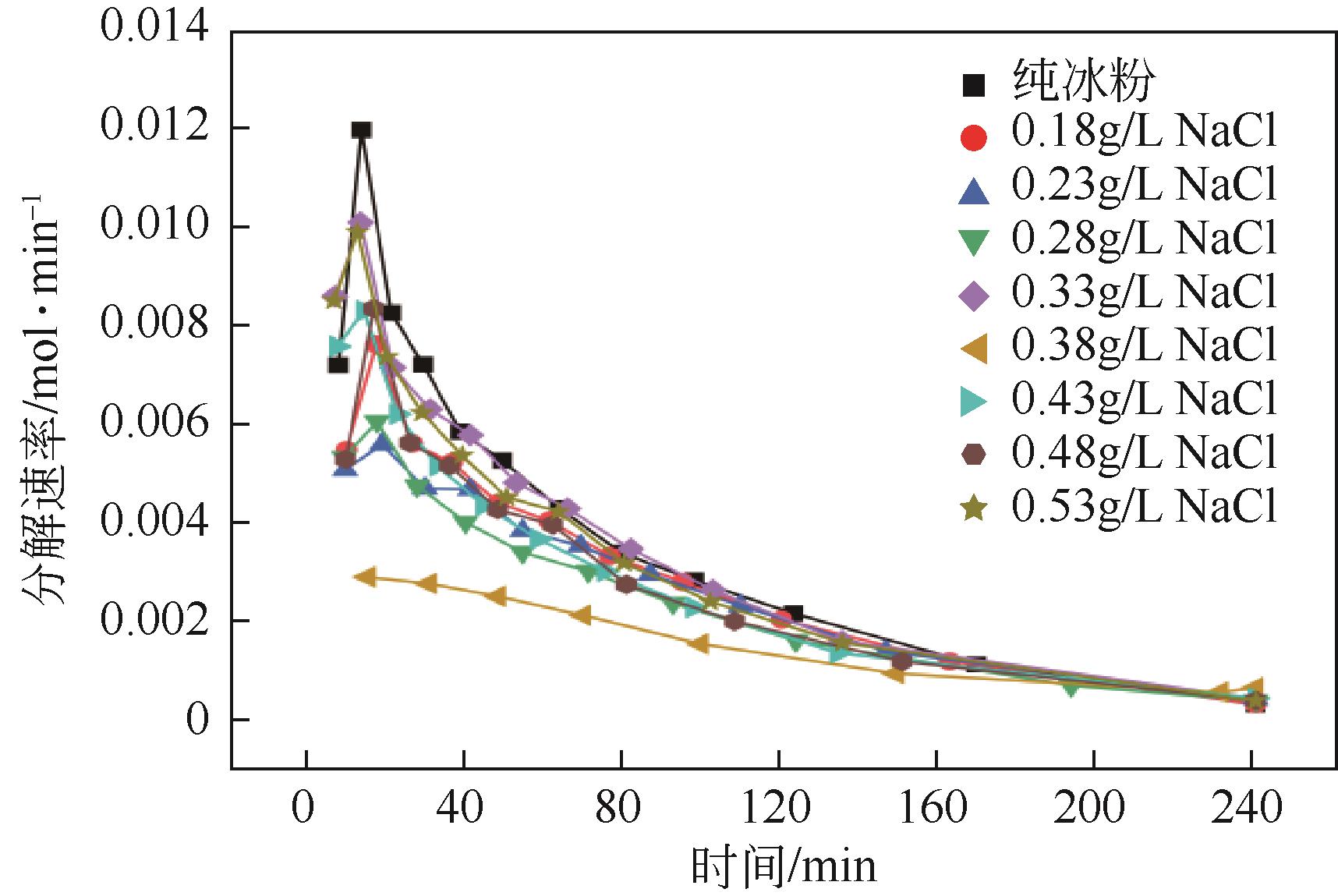
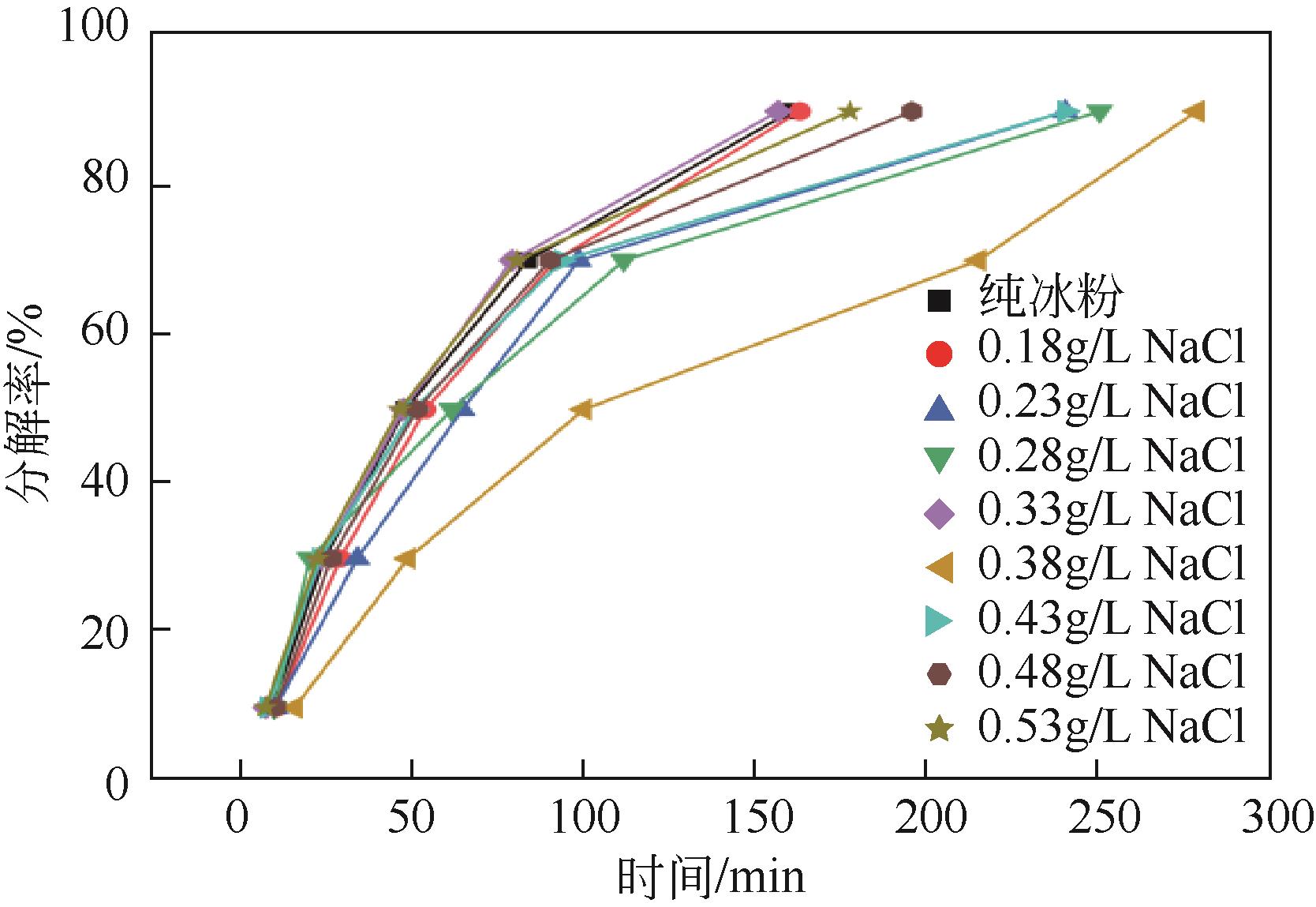
NaCl 浓度/g·L-1 | 一次加压 生成量/mol | 二次加压 生成量/mol | 总生成量/mol | 二次加压/ 一次加压 |
|---|---|---|---|---|
| 0 | 0.2586 | 0.2665 | 0.5251 | 1.0306 |
| 0.18 | 0.2701 | 0.2757 | 0.5458 | 1.0207 |
| 0.23 | 0.2671 | 0.2741 | 0.5412 | 1.0261 |
| 0.28 | 0.2722 | 0.2808 | 0.5530 | 1.0318 |
| 0.33 | 0.2741 | 0.2703 | 0.5444 | 0.9862 |
| 0.38 | 0.2815 | 0.2686 | 0.5501 | 0.9543 |
| 0.43 | 0.2693 | 0.2697 | 0.5390 | 1.0012 |
| 0.48 | 0.2573 | 0.2608 | 0.5181 | 1.0134 |
| 0.53 | 0.2402 | 0.2505 | 0.4907 | 1.0431 |
NaCl 浓度/g·L-1 | 一次加压 生成量/mol | 二次加压 生成量/mol | 总生成量/mol | 二次加压/ 一次加压 |
|---|---|---|---|---|
| 0 | 0.2586 | 0.2665 | 0.5251 | 1.0306 |
| 0.18 | 0.2701 | 0.2757 | 0.5458 | 1.0207 |
| 0.23 | 0.2671 | 0.2741 | 0.5412 | 1.0261 |
| 0.28 | 0.2722 | 0.2808 | 0.5530 | 1.0318 |
| 0.33 | 0.2741 | 0.2703 | 0.5444 | 0.9862 |
| 0.38 | 0.2815 | 0.2686 | 0.5501 | 0.9543 |
| 0.43 | 0.2693 | 0.2697 | 0.5390 | 1.0012 |
| 0.48 | 0.2573 | 0.2608 | 0.5181 | 1.0134 |
| 0.53 | 0.2402 | 0.2505 | 0.4907 | 1.0431 |
| 1 | BOZZANO Giulia, MANENTI Flavio. Efficient methanol synthesis: Perspectives, technologies and optimization strategies[J]. Progress in Energy and Combustion Science, 2016, 56: 71-105. |
| 2 | DUAN Huiming, WANG Siqi, HE Chenglin, et al. Application of a novel grey Bernoulli model to predict the global consumption of renewable energy[J]. Energy Reports, 2021, 7: 7200-7211. |
| 3 | NGUYEN N N, LA V T, HUYNH C D, et al. Technical and economic perspectives of hydrate-based carbon dioxide capture[J]. Applied Energy, 2022, 307: 118237. |
| 4 | 樊栓狮, 尤莎莉, 郎雪梅, 等. 笼型水合物膜分离和捕获二氧化碳研究进展[J]. 化工进展, 2020, 39(4): 1211-1218. |
| FAN Shuanshi, YOU Shali, LANG Xuemei, et al. Separation and capture carbon dioxide by clathrate-hydrate membranes: A review[J]. Chemical Industry and Engineering Progress, 2020, 39(4): 1211-1218. | |
| 5 | 王磊, 方桂英, 阳庆元. 金属-有机骨架材料CO2捕获性能的大规模计算筛选[J]. 化工学报, 2019, 70(3): 1135-1143. |
| WANG Lei, FANG Guiying, YANG Qingyuan. Performance of metal-organic frameworks for CO2 capture from large-scale computational screening[J]. CIESC Journal, 2019, 70(3): 1135-1143. | |
| 6 | SUN Q B, KANG Y T. Review on CO2 hydrate formation/dissociation and its cold energy application[J]. Renewable and Sustainable Energy Reviews, 2016, 62: 478-494. |
| 7 | DASHTI H, ZHEHAO Y L, LOU X. Recent advances in gas hydrate-based CO2 capture[J]. Journal of Natural Gas Science and Engineering, 2015, 23: 195-207. |
| 8 | ZHANG Lunxiang, YANG Lei, WANG Jiaqi, et al. Enhanced CH4 recovery and CO2 storage via thermal stimulation in the CH4/CO2 replacement of methane hydrate[J]. Chemical Engineering Journal, 2017, 308: 40-49. |
| 9 | XU Chungang, YU Yisong, XIE Wenjun, et al. Study on developing a novel continuous separation device and carbon dioxide separation by process of hydrate combined with chemical absorption[J]. Applied Energy, 2019, 255: 113791. |
| 10 | CHUNG W, ROH K, LEE J H. Design and evaluation of CO2 capture plants for the steelmaking industry by means of amine scrubbing and membrane separation[J]. International Journal of Greenhouse Gas Control, 2018, 74: 259-270. |
| 11 | WANG Tian, ZHANG Lunxiang, SUN Lingjie, et al. Methane recovery and carbon dioxide storage from gas hydrates in fine marine sediments by using CH4/CO2 replacement[J]. Chemical Engineering Journal, 2021, 425: 131562. |
| 12 | WANG Yanhong, LANG Xuemei, FAN Shuanshi. Hydrate capture CO2 from shifted synthesis gas, flue gas and sour natural gas or biogas[J]. Journal of Energy Chemistry, 2013, 22(1): 39-47. |
| 13 | SUN Ronghui, YANG Mingjun, SONG Yongchen. Effect of NaCl concentration on depressurization-induced methane hydrate dissociation near ice-freezing point: Associated with metastable phases[J]. Journal of Natural Gas Science and Engineering, 2021, 96: 104304. |
| 14 | Junghoon MOK, CHOI Wonjung, KIM Sungwoo, et al. NaCl-induced enhancement of thermodynamic and kinetic CO2 selectivity in CO2+N2 hydrate formation and its significance for CO2 sequestration[J]. Chemical Engineering Journal, 2023, 451: 138633. |
| 15 | SMIT Berend. Molecular simulations of fluid phase equilibria[J]. Fluid Phase Equilibria, 1996, 116(1/2): 249-256. |
| 16 | NASEH Moeinoddin, FALAMAKI Cavus, MOHEBBI Vahid. Equilibrium conditions of CO2+C3H8 hydrates in pure and saline water solutions of NaCl[J]. Journal of Natural Gas Science and Engineering, 2022, 106: 104734. |
| 17 | WANG Jianlong, SUN Jinsheng, WANG Ren, et al. Mechanisms of synergistic inhibition of NaCl and glycine mixtures on methane hydrate formation: Experimental and molecular dynamic simulation[J]. Gas Science and Engineering, 2023: 204880. |
| 18 | HOLZAMMER Christine, FINCKENSTEIN Agnes, WILL Stefan, et al. How sodium chloride salt inhibits the formation of CO2 gas hydrates[J]. The Joural of Physical Chemistry B, 2022, 120(9): 2452-2459. |
| 19 | CHOI Wonjung, LEE Yohan, Junghoon MOK, et al. Thermodynamic and kinetic influences of NaCl on HFC-125a hydrates and their significance in gas hydrate-based desalination[J]. Chemical Engineering Journal, 2019, 358: 598-605. |
| 20 | SUN Xian, LIU Dejun, CHANG Dongchao, et al. Analysis of natural gas hydrate formation in sodium dodecyl sulfate and quartz sand complex system under saline environment[J]. Petroleum Science and Technology, 2018, 36(14): 1073-1079. |
| 21 | XU Jiafang, DU Shuai, HAO Yongchao, et al. Molecular simulation study of methane hydrate formation mechanism in NaCl solutions with different concentrations[J]. Chemical Physics, 2021, 551: 111323. |
| 22 | 张金锋. 甲烷水合物在不同体系中的生成动力学研究[D]. 杭州: 浙江工业大学, 2003. |
| ZHANG Jinfeng. Research on the kinetics of methane hydrate formation in various systems[D]. Hangzhou: Zhejiang University of Technology, 2003. | |
| 23 | ZHANG Baoyong, WU Qiang, GAO Xia, et al. Memory effect on hydrate formation and influential factors of its sustainability in new hydrate-based coal mine methane separation method[J]. International Journal of Environment and Pollution, 2013, 53(3/4): 201. |
| 24 | SMITH J M, VAN NESS H C, ABBOTT M M, et al. Introduction to chemical engineering thermodynamics[M]. Eighth edition. New York, NY: McGraw-Hill Education, 2018. |
| 25 | SUN Duo, ENGLEZOS Peter. Storage of CO2 in a partially water saturated porous medium at gas hydrate formation conditions[J]. International Journal of Greenhouse Gas Control, 2014, 25: 1-8. |
| 26 | NATARAJAN V, BISHNOI P R, KALOGERAKIS N. Induction phenomena in gas hydrate nucleation[J]. Chemical Engineering Science, 1994, 49(13): 2075-2087. |
| 27 | DENDY S E. Clathrate hydrates of natural gases[M]. Boca Raton, FL: CRC Press, 2008. |
| 28 | 张强, 吴强, 张保勇, 等. NaCl-SDS复合溶液中多组分瓦斯水合物成核动力学机理[J]. 煤炭学报, 2015, 40(10): 2430-2436. |
| ZHANG Qiang, WU Qiang, ZHANG Baoyong, et al. Nucleation kinetics mechanism of multi-component mine gas hydrate in NaCl-SDS mixed solutions[J]. Journal of China Coal Society, 2015, 40(10): 2430-2436. | |
| 29 | BAI Dongsheng, WU Ziyan, LIN Cijie, et al. The effect of aqueous NaCl solution on methane hydrate nucleation and growth[J]. Fluid Phase Equilibria, 2019, 487: 76-82. |
| 30 | WANG Xiaohui, WANG Yunfei, XIE Yan, et al. Study on the decomposition conditions of gas hydrate in quartz sand-brine mixture systems[J]. The Journal of Chemical Thermodynamics, 2019, 131: 247-253. |
| 31 | 曹学文, 杨凯然, 夏闻竹, 等. CO2水合物分解实验及分解速率模型[J]. 天然气工业, 2021, 41(7): 152-159. |
| CAO Xuewen, YANG Kairan, XIA Wenzhu, et al. Dissociation experiment and dissociation rate model of CO2 hydrate[J]. Natural Gas Industry, 2021, 41(7): 152-159. | |
| 32 | 陈雪萍, 张鹏, 吴青柏. “自保护”态CO2水合物分解动力及影响因素[J]. 天然气地球科学, 2020, 31(2): 184-193. |
| CHEN Xueping, ZHANG Peng, WU Qingbai. Decomposition dynamics and influencing factors of CO2 hydrate in self-preservation state[J]. Natural Gas Geoscience, 2020, 31(2): 184-193. | |
| 33 | FALENTY A, KUHS W F, GLOCKZIN M, et al. “self-preservation” of CH4 hydrates for gas transport technology: Pressure-temperature dependence and ice microstructures[J]. Energy & Fuels, 2014, 28(10): 6275-6283. |
| 34 | YAGASAKI Takuma, MATSUMOTO Masakazu, TANAKA Hideki. Effects of thermodynamic inhibitors on the dissociation of methane hydrate: A molecular dynamics study[J]. Physical Chemistry Chemical Physics: PCCP, 2015, 17(48): 32347-32357. |
| 35 | QURESHI M F, KHANDELWAL H, USADI A, et al. CO2 hydrate stability in oceanic sediments under brine conditions[J]. Energy, 2022, 256: 124625. |
| [1] | WANG Yinmei, ZHANG Zhaohui, LIU Shenghao, JIAO Wenze, WANG Lijin, TENG Yadong, LIU Jie. Atmospheric pressure decomposition of carbon dioxide hydrate in accelerator system [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 141-149. |
| [2] | HUANG Ye, YAN Xing, WU Qiaowei, CHAI Xiaotao, PAN Gongying, ZHANG Jinfeng, LI Xiangqian. Study of silica gel regeneration applied on cyclosporine A column chromatography [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 461-468. |
| [3] | WANG Zepeng, YUAN Zhongxian, WANG Jie, WEN Xin, LIU Yimo. Effect of particulate diameter of silica gel on performance of solar adsorption refrigeration system [J]. Chemical Industry and Engineering Progress, 2022, 41(7): 3545-3552. |
| [4] | MA Xingxing, FENG Yakai. Improvement of thermal oxygen aging resistance of silicon gel by adding modified cerium oxide with coupling agent [J]. Chemical Industry and Engineering Progress, 2022, 41(2): 874-880. |
| [5] | WANG Yingmei, NIU Aili, ZHANG Zhaohui, ZHAN Jing, ZHANG Xuemin. Review of rapid generation methods of carbon dioxide hydrate [J]. Chemical Industry and Engineering Progress, 2021, 40(S2): 117-125. |
| [6] | Huaming MAO,Bin ZHANG,Shuai CUI,Xiaoning TANG,Su’e YANG,Xianfa JIANG. Preparation and antibacterial method of Cu2+/Tb2O3 antibacterial silica gel [J]. Chemical Industry and Engineering Progress, 2019, 38(11): 5024-5032. |
| [7] | ZHOU Shidong, CHEN Xiaokang, BIAN Hui, HE Chengyuan, WANG Shuli, LÜ Xiaofang. CO2 hydrate formation in pipeline and its plugging characteristics [J]. Chemical Industry and Engineering Progress, 2018, 37(11): 4250-4256. |
| [8] | XIAO Haiping, QI Chao, SUN Baomin. Reaction kinetics calculations of gaseous NaCl sulfation pathway when burning high alkali coal [J]. Chemical Industry and Engineering Progress, 2018, 37(05): 1760-1766. |
| [9] | YU Dongmei, CHEN Shuo, WANG Shuli, RAO Yongchao, LÜ Xiaofang. Experiment on attapulgite for CO2 hydrate formation kinetics [J]. Chemical Industry and Engineering Progress, 2018, 37(02): 546-553. |
| [10] | DAI Mimi, ZOU Tonghua, YAN Lei, JIA Gu. Experimental investigation on the performances of different desiccant wheels [J]. Chemical Industry and Engineering Progree, 2015, 34(07): 1841-1845. |
| [11] | XIN Feng, YUAN Zhongxian, WANG Wenchao. Adsorption and desorption characteristics of allochroic silica gel and ZSM-5 zeolite to water [J]. Chemical Industry and Engineering Progree, 2015, 34(06): 1730-1736. |
| [12] | LEI Ming,WANG Yan,ZHAO Hao,PENG Qijun . Study on the reaction of type C silica gel and KH-560 coupling agent [J]. Chemical Industry and Engineering Progree, 2012, 31(06): 1263-1268. |
| [13] | HU Wenna,SU Baogen,SU Yun,YANG Yiwen,REN Qilong. Purification of cholesterol from lanolin by column chromatography [J]. Chemical Industry and Engineering Progree, 2011, 30(7): 1426-. |
| [14] | ZHANG Cuiling,ZHANG Peng,LIU Wenxia,LI Li,LIU Qiang. Preparation process of a high purity macroporous silica gel [J]. Chemical Industry and Engineering Progree, 2011, 30(6): 1313-. |
| [15] | ZHANG Cuiling,LIU Wenxia,ZHANG Peng,LIU Qiang,LI Li. Preparation of novel chromium-based catalyst and study on its carrier performance [J]. Chemical Industry and Engineering Progree, 2011, 30(6): 1253-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||