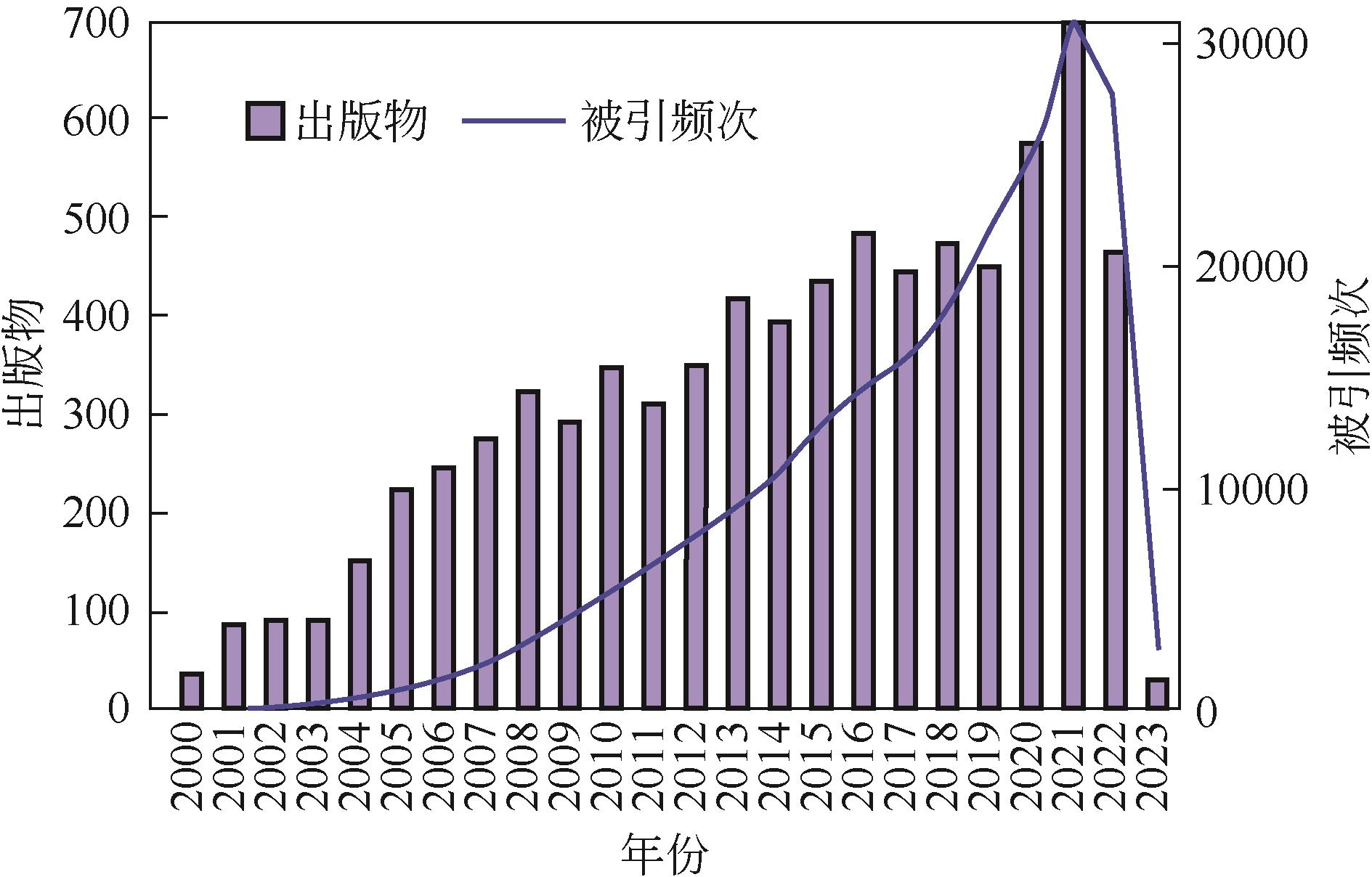
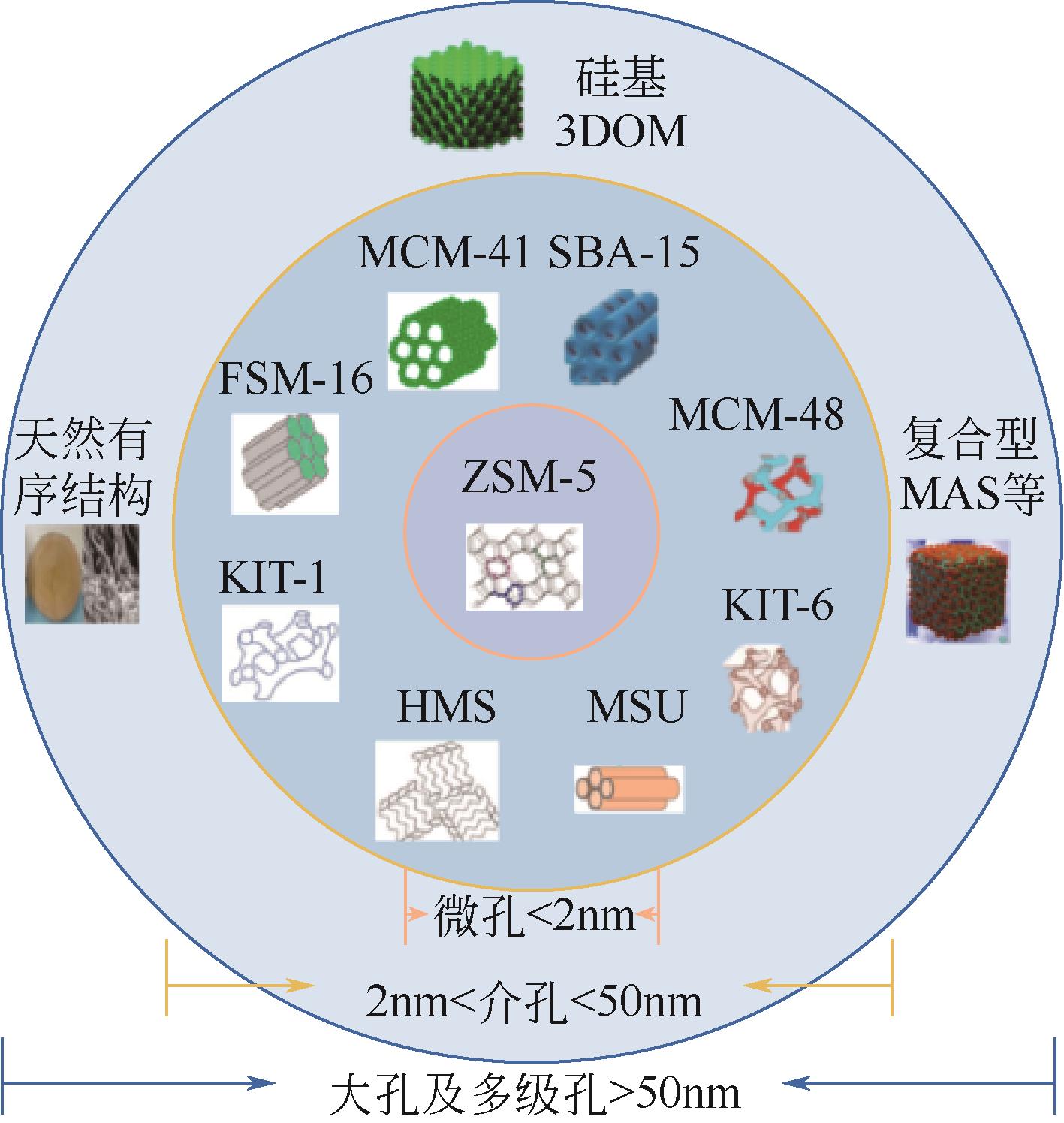
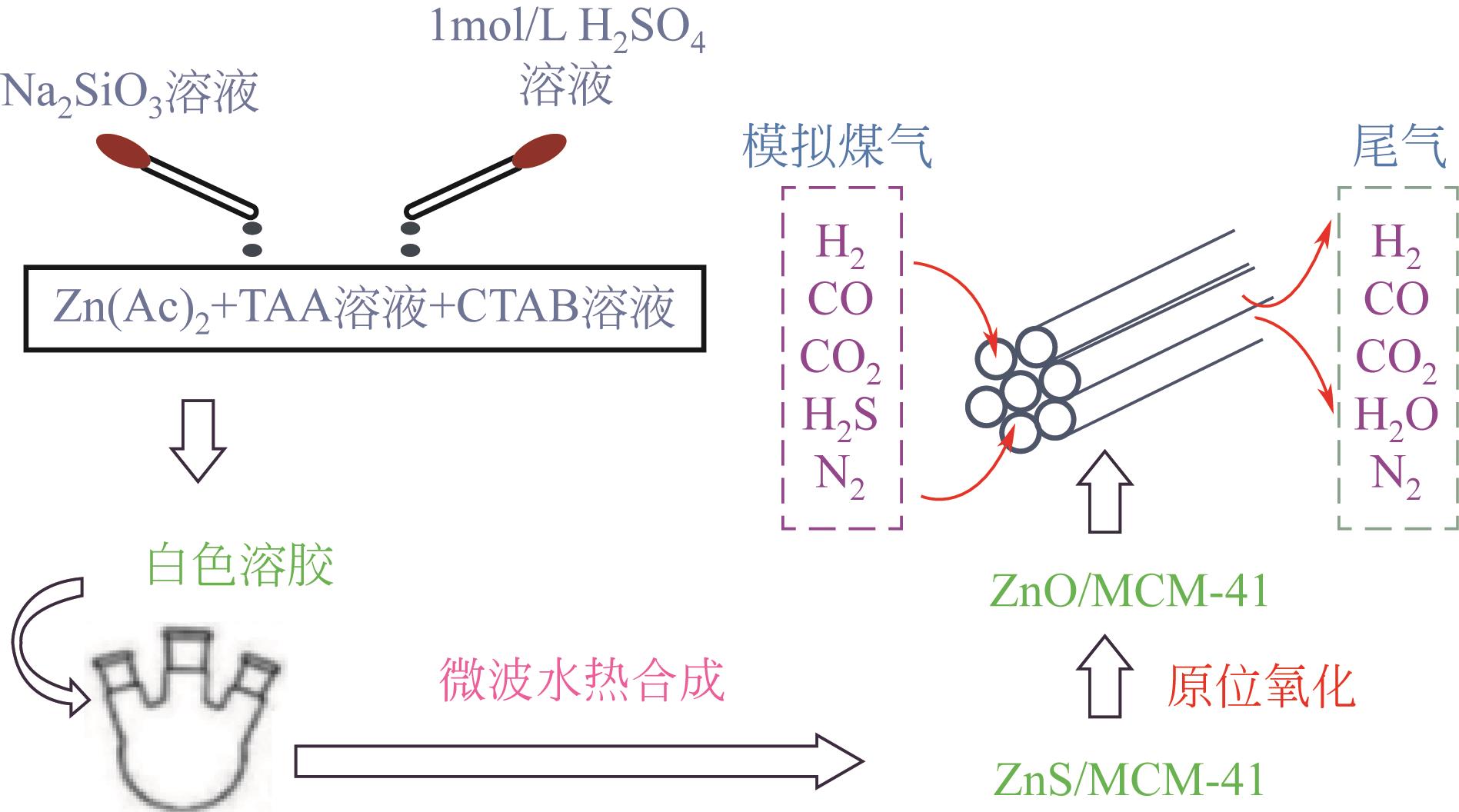
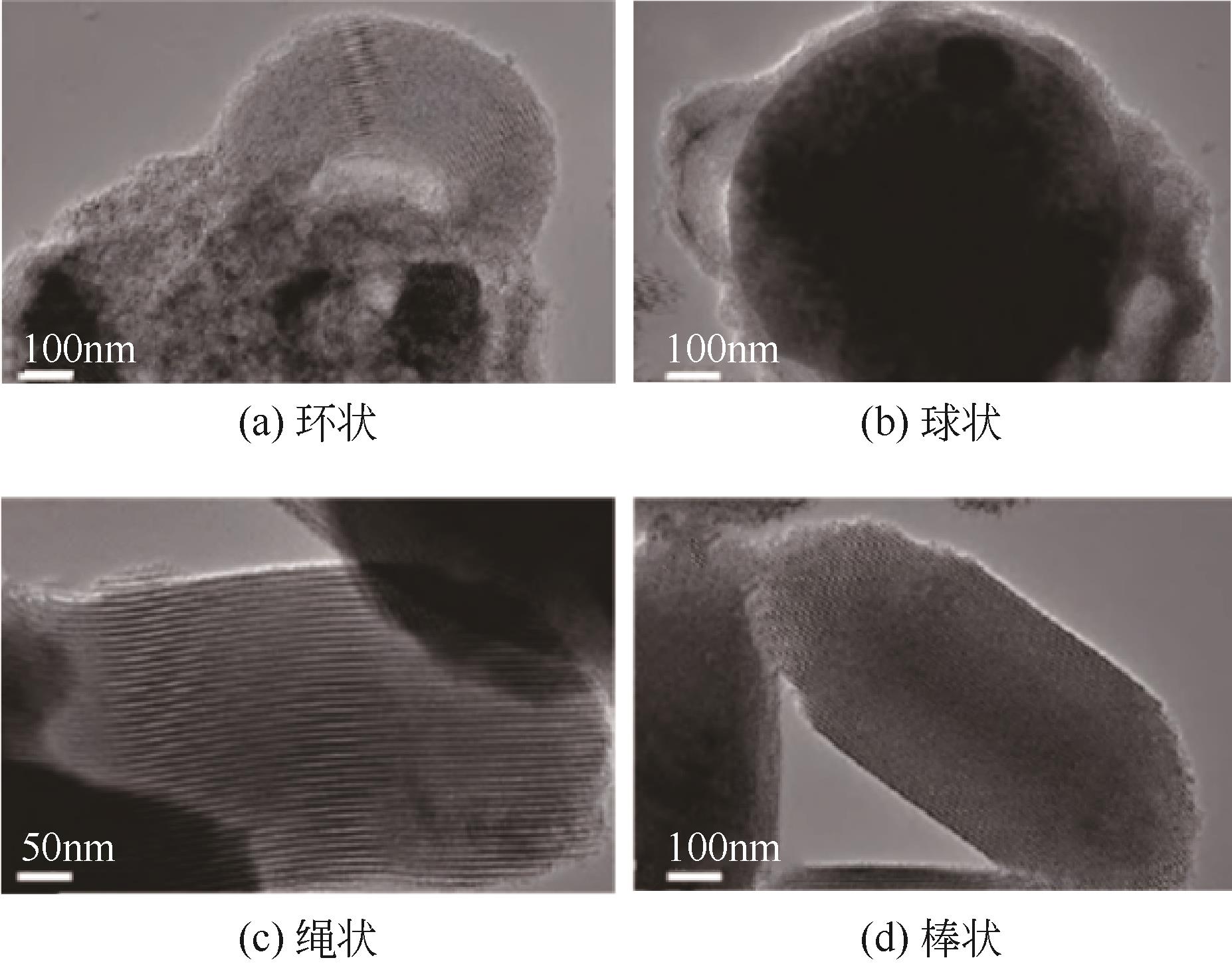
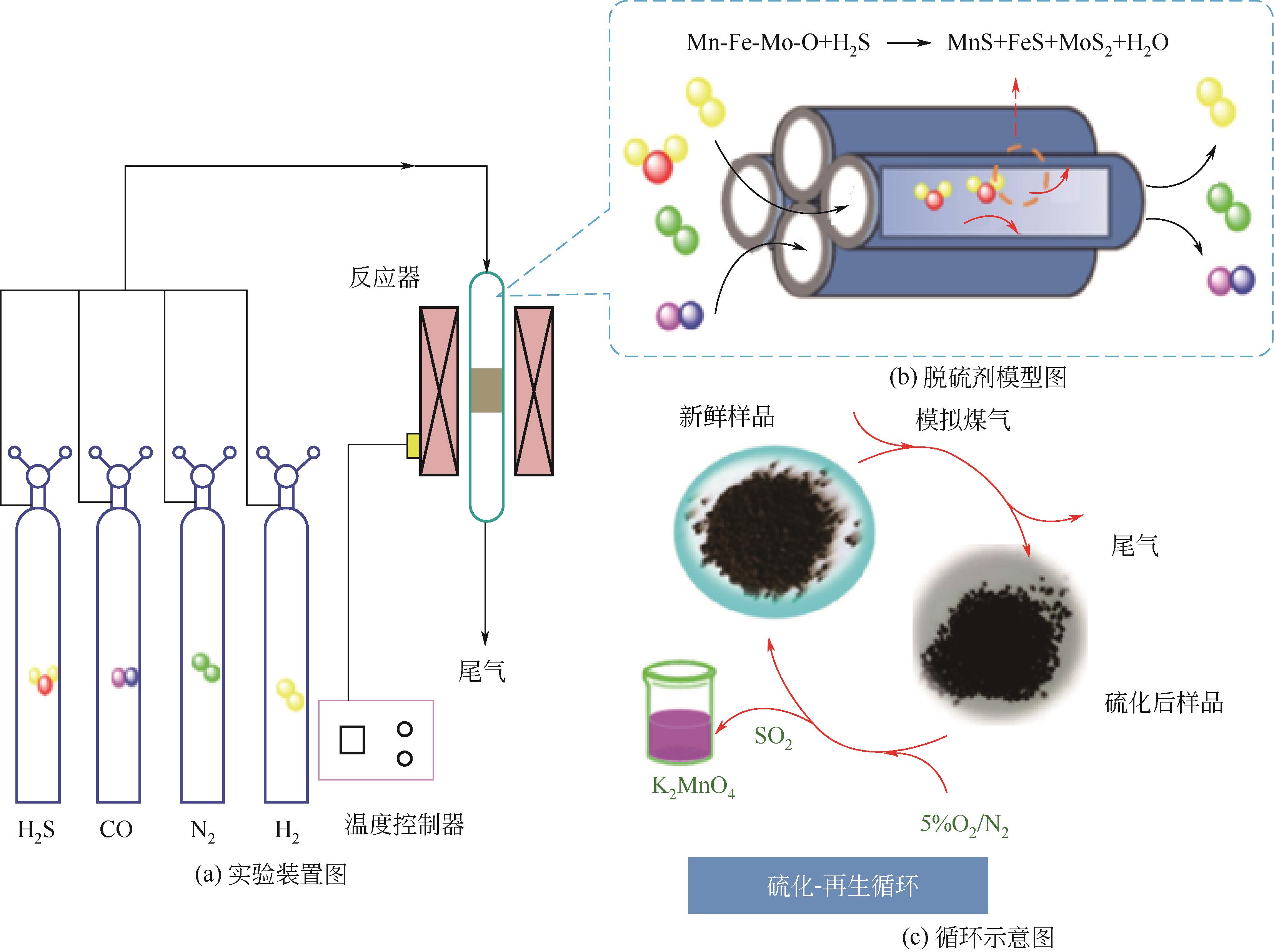
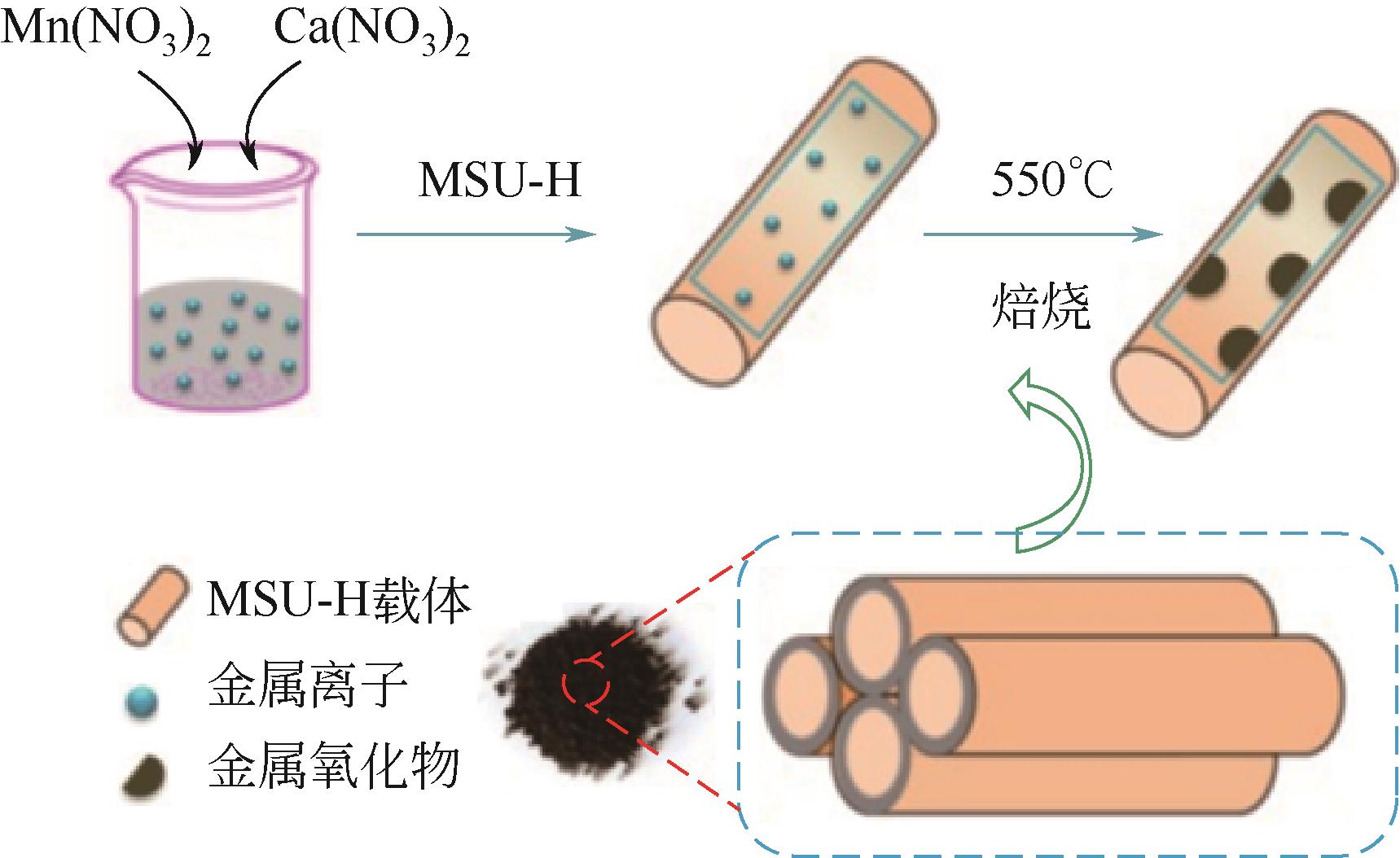
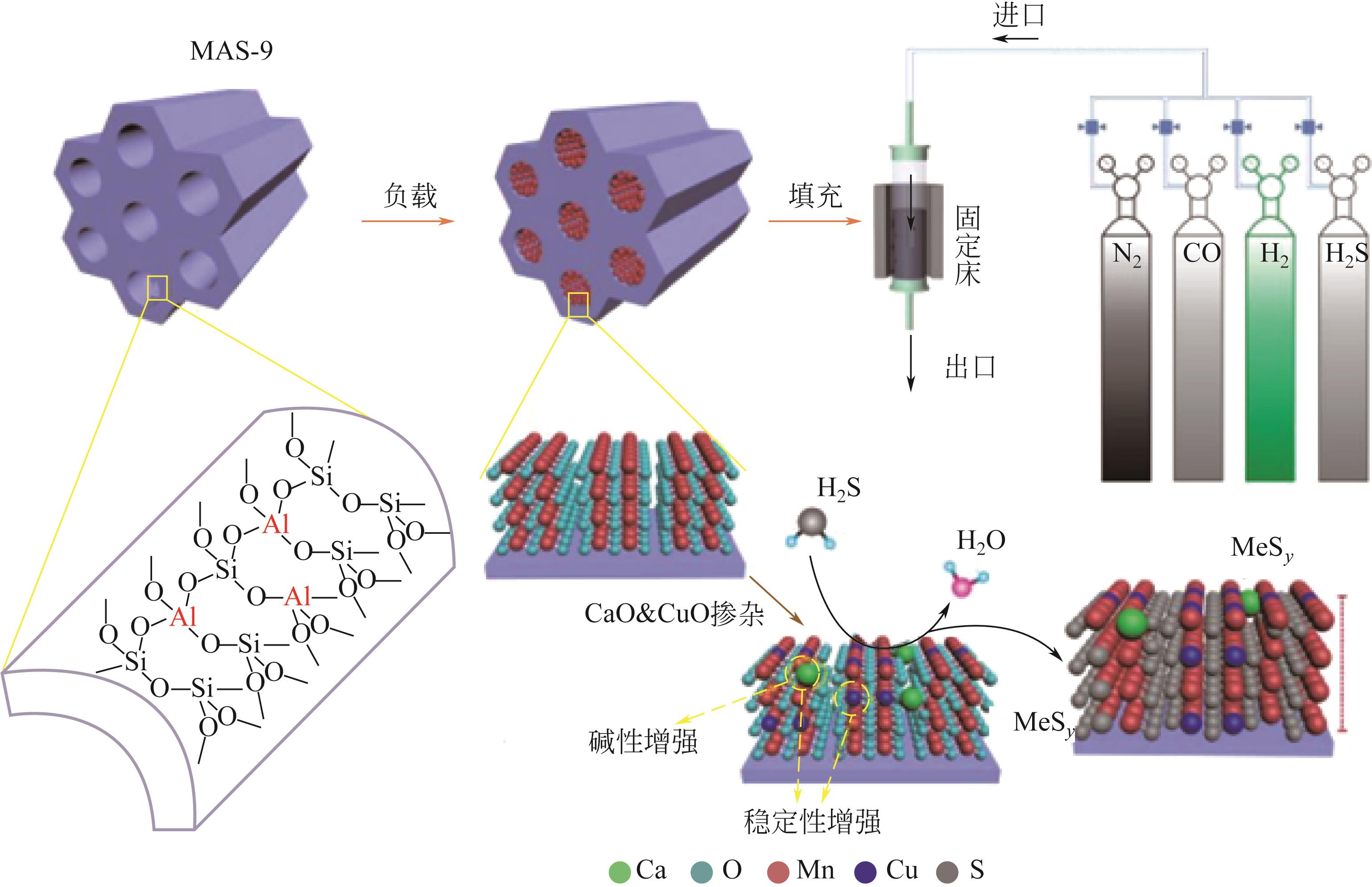
Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (11): 5943-5955.DOI: 10.16085/j.issn.1000-6613.2022-2317
• Resources and environmental engineering • Previous Articles Next Articles
Status of high temperature gas desulfurizer with structural characteristics of molecular sieves
LONG Caimei1,2( ), WU Shuaishan1,2, WANG Jiancheng1,2, MI Jie1,2(
), WU Shuaishan1,2, WANG Jiancheng1,2, MI Jie1,2( ), FENG Yu1,2(
), FENG Yu1,2( )
)
- 1.State Key Laboratory of Clean and Efficient Coal Utilization, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
2.Key Laboratory of Coal Science and Technology, Ministry of Education, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
-
Received:2022-12-15Revised:2023-03-17Online:2023-12-15Published:2023-11-20 -
Contact:MI Jie, FENG Yu
基于分子筛结构特性的高温煤气脱硫剂应用现状
龙彩梅1,2( ), 武帅山1,2, 王建成1,2, 米杰1,2(
), 武帅山1,2, 王建成1,2, 米杰1,2( ), 冯宇1,2(
), 冯宇1,2( )
)
- 1.太原理工大学省部共建煤基能源清洁高效利用国家重点实验室,山西 太原 030024
2.太原理工大学煤科学与技术教育部重点实验室,山西 太原 030024
-
通讯作者:米杰,冯宇 -
作者简介:龙彩梅(1996—),女,硕士研究生,研究方向为气体分离与净化。E-mail:1783139394@qq.com。 -
基金资助:国家自然科学基金(21908156);中国博士后科学基金(2021T140431);山西省自然科学基金(201901D211099)
CLC Number:
Cite this article
LONG Caimei, WU Shuaishan, WANG Jiancheng, MI Jie, FENG Yu. Status of high temperature gas desulfurizer with structural characteristics of molecular sieves[J]. Chemical Industry and Engineering Progress, 2023, 42(11): 5943-5955.
龙彩梅, 武帅山, 王建成, 米杰, 冯宇. 基于分子筛结构特性的高温煤气脱硫剂应用现状[J]. 化工进展, 2023, 42(11): 5943-5955.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-2317
| 脱硫剂种类 | 合成方法 | 反应气氛 | 温度/℃ | 硫容 | 参考文献 |
|---|---|---|---|---|---|
| 5Ce5Mn/ZSM-5 | 溶胶-凝胶法 | 0.5%H2S/N2 | 750 | 7653.1µmolS/g | [ |
| 5La5Mn/ZSM-5 | 溶胶-凝胶法 | 0.2%H2S/N2 | 600 | 1020.0µmolS/g | [ |
| CuMn/ZSM-5 | 湿法浸渍 | 0.2%H2S/11%CO2/12.5%CO/13.8%H2/1%CH4/19%H2O/N2 | 871 | NA | [ |
Cu@MCM-41/ Cu-MCM-41 | 浸渍法/水热法 | 1%H2S/10%H2/He | 500 | 23.0gS/100gCuO/ 19.0gS/100gCuO | [ |
| LaFeO3/M41 | 溶胶-凝胶法 | 0.33%H2S/10.5%H2/17.1%CO2/N2 | 500 | 3.2gS/100g脱硫剂 | [ |
| 35%ZnO/MCM-41 | 一步水热法 | 0.2%~0.3%H2S/39%H2/27%CO/12%CO2/10%H2O/N2 | 500 | 11.0gS/100g脱硫剂 | [ |
| 40%ZnO/MCM-41 | 微波水热法 | 2000μL/L H2S/39%H2/27%CO/12%CO2/N2 | 500 | 11.2gS/100g脱硫剂 | [ |
| Al-ZnO/MCM-41 | 微波水热法 | 3000μL/L H2S/39%H2/27%CO/12%CO2/N2 | 500 | 9.1gS/100g脱硫剂 | [ |
ZnO/SBA-15 Fe2O3/SBA-15 | 双溶剂法 | 1.5%H2S/He | 300 | 5.3gS/100g ZnO 40.1gS/100g Fe2O3 | [ |
ZnO/DSBA-15 ZnO/Nb20DSBA-15 Zn5Co/Ti10DSBA-15 | 溶胶-凝胶法 | 2787mg/m3 H2S/10.5%H2/18%CO/5%CO2/N2 | 500 | 5.7gS/100g脱硫剂 6.7gS/100g脱硫剂 7.1gS/100g脱硫剂 | [ |
| Zn20Ni1/SBA-15 | 溶胶-凝胶法 | 2000μL/L H2S/10.5%H2/18%CO/5%CO2/N2 | 500 | 16.0gS/100g脱硫剂 | [ |
| LaFeO3/SBA-15 | 溶胶-凝胶法 | 0.33%H2S/10.5%H2/17.1%±0.3%CO2/72.1%±2%N2 | 500 | 4.9gS/100g脱硫剂 | [ |
| 4Mn1Fe-3%Mo/FSM-16 | 溶胶-凝胶法 | 0.33%H2S/10.5%H2/18%CO/N2 | 600 | 18.2gS/100g脱硫剂 | [ |
| 50% Mn/MCM-48 | 溶胶-凝胶法 | 0.33%H2S/10.5%H2/18%CO/N2 | 550 | 12.4gS/100g脱硫剂 | [ |
| Zn/M48 Zn/M41 | 溶胶-凝胶法 | 0.2%H2S/18%CO/10.5%H2/5%CO2/N2 | 500 | NA | [ |
ZnO/KIT-6 La-ZnO/KIT-6 | 溶胶-凝胶法 | 0.1%H2S/2% CO/5% H2/10%H2O/N2 | 300 | 5.0gS/100g脱硫剂 7.0gS/100g脱硫剂 | [ |
| La3Mn7/KIT-6 | 溶胶-凝胶法 | 0.36%H2S/13.84%H2/19.36%CO/N2 | 800 | 11.6gS/100g脱硫剂 | [ |
| 55%5Sm95Mn/MSU-S | 溶胶-凝胶法 | 0.33%H2S/10.5%H2/18%CO/N2 | 800 | 15.2gS/100g脱硫剂 | [ |
| 90Mn10Ca/MSU-H | 溶胶-凝胶法 | 0.33%H2S/10.5%H2/18%CO/N2 | 750 | 18.7gS/100g脱硫剂 | [ |
| 4Mn1Ce/HMS | 溶胶-凝胶法 | 0.33%H2S/10.6%H2/18%CO/N2 | 600 | 12.1gS/100g脱硫剂 | [ |
| 90Mn10Mo/KIT-1 | 溶胶-凝胶法 | 0.33%H2S/10.6%H2/18%CO/N2 | 700 | 16.9gS/100g脱硫剂 | [ |
| ZnO/SiO2 | 胶晶模板法 | 1mg/m3 H2S/3.0%H2O/N2 | 500 | 17.0gS/100g脱硫剂 | [ |
| ZnCo2/SS | 溶胶-凝胶法 | 0.33%H2S/10.5%H2/18%CO/N2 | 550 | 13.8gS/100g脱硫剂 | [ |
| Ca3Cu10Mn87Oi/MAS-9 | 溶胶-凝胶法 | 0.33%H2S/10.5%H2/18%CO/N2 | 800 | 17.2gS/100g脱硫剂 | [ |
| (8%Ce-Mn)1.5Al0.5O x | 溶胶-凝胶法 | 0.33%H2S/10.5%H2/18%CO/N2 | 700 | 29.0gS/100g脱硫剂 | [ |
| 脱硫剂种类 | 合成方法 | 反应气氛 | 温度/℃ | 硫容 | 参考文献 |
|---|---|---|---|---|---|
| 5Ce5Mn/ZSM-5 | 溶胶-凝胶法 | 0.5%H2S/N2 | 750 | 7653.1µmolS/g | [ |
| 5La5Mn/ZSM-5 | 溶胶-凝胶法 | 0.2%H2S/N2 | 600 | 1020.0µmolS/g | [ |
| CuMn/ZSM-5 | 湿法浸渍 | 0.2%H2S/11%CO2/12.5%CO/13.8%H2/1%CH4/19%H2O/N2 | 871 | NA | [ |
Cu@MCM-41/ Cu-MCM-41 | 浸渍法/水热法 | 1%H2S/10%H2/He | 500 | 23.0gS/100gCuO/ 19.0gS/100gCuO | [ |
| LaFeO3/M41 | 溶胶-凝胶法 | 0.33%H2S/10.5%H2/17.1%CO2/N2 | 500 | 3.2gS/100g脱硫剂 | [ |
| 35%ZnO/MCM-41 | 一步水热法 | 0.2%~0.3%H2S/39%H2/27%CO/12%CO2/10%H2O/N2 | 500 | 11.0gS/100g脱硫剂 | [ |
| 40%ZnO/MCM-41 | 微波水热法 | 2000μL/L H2S/39%H2/27%CO/12%CO2/N2 | 500 | 11.2gS/100g脱硫剂 | [ |
| Al-ZnO/MCM-41 | 微波水热法 | 3000μL/L H2S/39%H2/27%CO/12%CO2/N2 | 500 | 9.1gS/100g脱硫剂 | [ |
ZnO/SBA-15 Fe2O3/SBA-15 | 双溶剂法 | 1.5%H2S/He | 300 | 5.3gS/100g ZnO 40.1gS/100g Fe2O3 | [ |
ZnO/DSBA-15 ZnO/Nb20DSBA-15 Zn5Co/Ti10DSBA-15 | 溶胶-凝胶法 | 2787mg/m3 H2S/10.5%H2/18%CO/5%CO2/N2 | 500 | 5.7gS/100g脱硫剂 6.7gS/100g脱硫剂 7.1gS/100g脱硫剂 | [ |
| Zn20Ni1/SBA-15 | 溶胶-凝胶法 | 2000μL/L H2S/10.5%H2/18%CO/5%CO2/N2 | 500 | 16.0gS/100g脱硫剂 | [ |
| LaFeO3/SBA-15 | 溶胶-凝胶法 | 0.33%H2S/10.5%H2/17.1%±0.3%CO2/72.1%±2%N2 | 500 | 4.9gS/100g脱硫剂 | [ |
| 4Mn1Fe-3%Mo/FSM-16 | 溶胶-凝胶法 | 0.33%H2S/10.5%H2/18%CO/N2 | 600 | 18.2gS/100g脱硫剂 | [ |
| 50% Mn/MCM-48 | 溶胶-凝胶法 | 0.33%H2S/10.5%H2/18%CO/N2 | 550 | 12.4gS/100g脱硫剂 | [ |
| Zn/M48 Zn/M41 | 溶胶-凝胶法 | 0.2%H2S/18%CO/10.5%H2/5%CO2/N2 | 500 | NA | [ |
ZnO/KIT-6 La-ZnO/KIT-6 | 溶胶-凝胶法 | 0.1%H2S/2% CO/5% H2/10%H2O/N2 | 300 | 5.0gS/100g脱硫剂 7.0gS/100g脱硫剂 | [ |
| La3Mn7/KIT-6 | 溶胶-凝胶法 | 0.36%H2S/13.84%H2/19.36%CO/N2 | 800 | 11.6gS/100g脱硫剂 | [ |
| 55%5Sm95Mn/MSU-S | 溶胶-凝胶法 | 0.33%H2S/10.5%H2/18%CO/N2 | 800 | 15.2gS/100g脱硫剂 | [ |
| 90Mn10Ca/MSU-H | 溶胶-凝胶法 | 0.33%H2S/10.5%H2/18%CO/N2 | 750 | 18.7gS/100g脱硫剂 | [ |
| 4Mn1Ce/HMS | 溶胶-凝胶法 | 0.33%H2S/10.6%H2/18%CO/N2 | 600 | 12.1gS/100g脱硫剂 | [ |
| 90Mn10Mo/KIT-1 | 溶胶-凝胶法 | 0.33%H2S/10.6%H2/18%CO/N2 | 700 | 16.9gS/100g脱硫剂 | [ |
| ZnO/SiO2 | 胶晶模板法 | 1mg/m3 H2S/3.0%H2O/N2 | 500 | 17.0gS/100g脱硫剂 | [ |
| ZnCo2/SS | 溶胶-凝胶法 | 0.33%H2S/10.5%H2/18%CO/N2 | 550 | 13.8gS/100g脱硫剂 | [ |
| Ca3Cu10Mn87Oi/MAS-9 | 溶胶-凝胶法 | 0.33%H2S/10.5%H2/18%CO/N2 | 800 | 17.2gS/100g脱硫剂 | [ |
| (8%Ce-Mn)1.5Al0.5O x | 溶胶-凝胶法 | 0.33%H2S/10.5%H2/18%CO/N2 | 700 | 29.0gS/100g脱硫剂 | [ |
| 1 | AZZAM Sara A, ALSHAFEI Faisal H, Tirso LÓPEZ-AUSENS, et al. Effects of morphology and surface properties of copper oxide on the removal of hydrogen sulfide from gaseous streams[J]. Industrial & Engineering Chemistry Research, 2019, 58(40): 18836-18847. |
| 2 | LIU Dongjing, WANG Qian, WU Jiang, et al. A review of sorbents for high-temperature hydrogen sulfide removal from hot coal gas[J]. Environmental Chemistry Letters, 2019, 17(1): 259-276. |
| 3 | KHABAZIPOUR Maryam, ANBIA Mansoor. Removal of hydrogen sulfide from gas streams using porous materials: A review[J]. Industrial & Engineering Chemistry Research, 2019, 58(49): 22133-22164. |
| 4 | SUN Jian, MODI Shruti, LIU Ke, et al. Kinetics of zinc oxide sulfidation for packed-bed desulfurizer modeling[J]. Energy & Fuels, 2007, 21(4): 1863-1871. |
| 5 | JIANG Bolong, ZHANG Jiaojing, CHEN Yanguang, et al. Ultrasonic-assisted preparation of highly active Co3O4/MCM-41 adsorbent and its desulfurization performance for low H2S concentration gas[J]. RSC Advances, 2020, 10(50): 30214-30222. |
| 6 | WANG Xiaowen, ZHANG Ran, LI Qiaochun, et al. Insights into H2S-absorption and oxidation-regeneration behavior of Ni-doped ZnO-based sorbents supported on SBA-15 for desulfurization of hot coal gas[J]. Fuel, 2023, 332: 126052. |
| 7 | 陈宪宏. 天然沸石改性及其去除氮磷性能研究[D]. 广州: 广州大学, 2022. |
| CHEN Xianhong. Study on modification of natural zeolite and its nitrogen and phosphorus removal performance[D]. Guangzhou: Guangzhou University, 2022. | |
| 8 | KOOHSARYAN Esmat, ANBIA Mansoor. Nanosized and hierarchical zeolites: A short review[J]. Chinese Journal of Catalysis, 2016, 37(4): 447-467. |
| 9 | WILSON Stephen T, Brent M LOK, MESSINA Celeste A, et al. Aluminophosphate molecular sieves: A new class of microporous crystalline inorganic solids[J]. Journal of the American Chemical Society, 1982, 104(4): 1146-1147. |
| 10 | ZHANG Jie, TAN Yan, SONG Wenjun. Zeolitic imidazolate frameworks for use in electrochemical and optical chemical sensing and biosensing: A review[J]. Microchimica Acta, 2020, 187(4): 234. |
| 11 | ZACHARIOU Andrea, HAWKINS Alexander, HOWE Russell, et al. Counting the acid sites in a commercial ZSM-5 zeolite catalyst[J]. ACS Physical Chemistry Au, 2023, 3(1): 74-83. |
| 12 | WATANABE Shingo. Chemistry of H2S over the surface of common solid sorbents in industrial natural gas desulfurization[J]. Catalysis Today, 2021, 371: 204-220. |
| 13 | 邱广敏, 黄宝丽, 王新民, 等. HZSM-5沸石分子筛吸附H2S的理论研究[J]. 石油与天然气化工, 2006, 35(2): 107-109. |
| QIU Guangmin, HUANG Baoli, WANG Xinmin, et al. The theoretical study of H2S adsorption on HZSM-5 zeolite[J]. Chemical Engineering of Oil & Gas, 2006, 35(2): 107-109. | |
| 14 | LIU Dongjing, ZHOU Weiguo, WU Jiang. CeO2-MnO x /ZSM-5 sorbents for H2S removal at high temperature[J]. Chemical Engineering Journal, 2016, 284: 862-871. |
| 15 | LIU Dongjing, ZHOU Weiguo, WU Jiang. Perovskite LaMnO3/ZSM-5 composites for H2S reactive adsorption at high temperature[J]. Adsorption, 2016, 22(3): 327-334. |
| 16 | ATIMTAY Aysel T, GASPER-GALVIN Lee D, POSTON James A. Novel supported sorbent for hot gas desulfurization[J]. Environmental Science & Technology, 1993, 27(7): 1295-1303. |
| 17 | CHENG Chifeng, HE Heyong, ZHOU Wuzong, et al. Crystal morphology supports the liquid crystal formation mechanism for the mesoporous molecular sieve MCM-41[J]. Chemical Physics Letters, 1995, 244(1/2): 117-120. |
| 18 | FENG Yu, LU Jianjun, WANG Jiancheng, et al. Desulfurization sorbents for green and clean coal utilization and downstream toxics reduction: A review and perspectives[J]. Journal of Cleaner Production, 2020, 273: 123080. |
| 19 | CHENG Chifeng, ZHOU Wuzong, PARK Dong Ho, et al. Controlling the channel diameter of the mesoporous molecularsieve MCM-41[J]. Journal of the Chemical Society, Faraday Transactions, 1997, 93(2): 359-363. |
| 20 | OZAYDIN Zeynep, YASYERLI Sena, DOGU Gulsen. Synthesis and activity comparison of copper-incorporated MCM-41-type sorbents prepared by one-pot and impregnation procedures for H2S removal[J]. Industrial & Engineering Chemistry Research, 2008, 47(4): 1035-1042. |
| 21 | LIU Bingsi, WAN Zhengyong, ZHAN Yueping, et al. Desulfurization of hot coal gas over high-surface-area LaMeO x /MCM-41 sorbents[J]. Fuel, 2012, 98: 95-102. |
| 22 | 贾磊. MCM-41负载纳米ZnO中高温煤气脱硫剂的制备及其硫化性能研究[D]. 太原: 太原理工大学, 2017. |
| JIA Lei. Preparation and desulfurization performance of zinc oxide load in MCM-41 sorbent for hot coal gas[D]. Taiyuan: Taiyuan University of Technology, 2017. | |
| 23 | WU Mengmeng, SHI Lei, Teik Thye LIM, et al. Ordered mesoporous Zn-based supported sorbent synthesized by a new method for high-efficiency desulfurization of hot coal gas[J]. Chemical Engineering Journal, 2018, 353: 273-287. |
| 24 | 史磊. 微波原位制备ZnO/MCM-41中高温煤气脱硫剂及其性能的研究[D]. 太原: 太原理工大学, 2018. |
| SHI Lei. The study on desulfurization performance of ZnO/MCM-41 sorbent prepared by microwave assisted in-situ method for hot coal gas[D]. Taiyuan: Taiyuan University of Technology, 2018. | |
| 25 | 李阳. 锌基有序介孔高温煤气脱硫剂性能稳定性及动力学研究[D]. 太原: 太原理工大学, 2020. |
| LI Yang. Performance stability and kinetics of zinc-based ordered mesoporous sorbent for high temperature coal gas[D]. Taiyuan: Taiyuan University of Technology, 2020. | |
| 26 | 姜晓庆, 郭宇, 吴红梅. 2-吡啶甲醛功能化SBA-15介孔材料的制备及其对Cr(Ⅲ)离子的吸附[J]. 化工进展, 2022, 41(7): 3915-3924. |
| JIANG Xiaoqing, GUO Yu, WU Hongmei. Synthesis of 2-pyridinecarboxaldehyde functionalized SBA-15 mesoporous material for the adsorption of Cr(Ⅲ) ions from aqueous solution[J]. Chemical Industry and Engineering Progress, 2022, 41(7): 3915-3924. | |
| 27 | MUREDDU Mauro, FERINO Italo, MUSINU Anna, et al. MeO x /SBA-15 (Me=Zn, Fe): Highly efficient nanosorbents for mid-temperature H2S removal[J]. Journal of Materials Chemistry A, 2014, 2(45): 19396-19406. |
| 28 | 张旭阳, 武蒙蒙, 李俏春, 等. 载体形貌对ZnO/SBA-15煤气脱硫剂结构及性能的影响[J]. 天然气化工——C1化学与化工, 2022, 47(3): 33-40. |
| ZHANG Xuyang, WU Mengmeng, LI Qiaochun, et al. Effect of carrier morphology on structure and properties of ZnO/SBA-15 coal gas desulfurizer[J]. Natural Gas Chemical Industry—C1 Chemistry and Chemical Industry, 2022, 47(3): 33-40. | |
| 29 | 李俏春. SBA-15负载锌基氧化物的煤气脱硫与再生行为研究[D]. 太原: 太原理工大学, 2021. |
| LI Qiaochun. Study on desulfurization and regeneration behavior of coal gas with SBA-15 loaded zinc-based oxides[D]. Taiyuan: Taiyuan University of Technology, 2021. | |
| 30 | LIU Bingsi, WEI Xiaona, ZHAN Yueping, et al. Preparation and desulfurization performance of LaMeO x /SBA-15 for hot coal gas[J]. Applied Catalysis B: Environmental, 2011, 102(1/2): 27-36. |
| 31 | SAMARI Mahya, ZINADINI Sirus, ZINATIZADEH Ali Akbar, et al. A new fouling resistance polyethersulfone ultrafiltration membrane embedded by metformin-modified FSM-16: Fabrication, characterization and performance evaluation in emulsified oil-water separation[J]. Journal of Environmental Chemical Engineering, 2021, 9(4): 105386. |
| 32 | XIA Hong, LIU Bingsi, LI Qian, et al. High capacity Mn-Fe-Mo/FSM-16 sorbents in hot coal gas desulfurization and mechanism of elemental sulfur formation[J]. Applied Catalysis B: Environmental, 2017, 200: 552-565. |
| 33 | DUAN Yongzheng, ZHAI Decui, ZHANG Xin, et al. Synthesis of CuO/Ti-MCM-48 photocatalyst for the degradation of organic pollutions under solar-simulated irradiation[J]. Catalysis Letters, 2018, 148(1): 51-61. |
| 34 | 黄兆彪. 高温煤气脱硫剂的制备及反应条件对脱硫性能的影响[D]. 天津: 天津大学, 2016. |
| HUANG Zhaobiao. Preparation of sorbents for hot coal gas desulfurization and performance of sorbents under different reaction conditions[D]. Tianjin: Tianjin University, 2016. | |
| 35 | WU Mengmeng, LI Qiaochun, WANG Xiaowen, et al. Structure characteristics and hot-coal-gas desulfurization properties of Zn-based sorbents supported on mesoporous silica with different pore-arrangement patterns: A comparison study[J]. Energy & Fuels, 2021, 35(3): 2456-2467. |
| 36 | 周一思, 马守涛, 汪颖军, 等. KIT-6介孔分子筛的研究进展[J]. 化学与粘合, 2022, 44(2): 158-161. |
| ZHOU Yisi, MA Shoutao, WANG Yingjun, et al. Progress in research on the mesoporous molecular sieves KIT-6[J]. Chemistry and Adhesion, 2022, 44(2): 158-161. | |
| 37 | LI Lu, ZHOU Pin, ZHANG Hongbo, et al. Mid-temperature deep removal of hydrogen sulfide on rare earth (RE = Ce, La, Sm, Gd) doped ZnO supported on KIT-6: Effect of RE dopants and interaction between active phase and support matrix[J]. Applied Surface Science, 2017, 407: 197-208. |
| 38 | 张凤梅. 介孔高温煤气脱硫剂的研制与性能研究[D]. 天津: 天津大学, 2012. |
| ZHANG Fengmei. Preparation and performance of mesoporous sorbents for hot coal gas desulfurization[D]. Tianjin: Tianjin University, 2012. | |
| 39 | CHANG Xiaoqian, LIU Bingsi, XIA Hong, et al. High catalytic activity and stability of Ni/Ce x Zr1- x O2/MSU-H for CH4/CO2 reforming reaction[J]. Applied Surface Science, 2018, 442: 342-351. |
| 40 | 王芳. 钐-锰/MSU-S吸附剂的高温煤气脱硫研究[D]. 天津: 天津大学, 2015. |
| WANG Fang. High-temperature desulfurization of coal gas with Sm adopted Mn-based support MSU-S sorbents[D]. Tianjin: Tianjin University, 2015. | |
| 41 | XIA Hong, LIU Bingsi. High H2O-resistance CaO-MnO x /MSU-H sorbents for hot coal gas desulfurization[J]. Journal of Hazardous Materials, 2017, 324: 281-290. |
| 42 | 熊鹏辉. Ti改性的PtSn/Ti-HMS催化剂的丙烷直接脱氢制丙烯的研究[D]. 北京: 中国石油大学(北京), 2019. |
| XIONG Penghui. Study on the titanium-modified PtSn/Ti-HMS catalysts for the dehydrogenation of propane to propylene[D]. Beijing: China University of Petroleum (Beijing), 2019. | |
| 43 | 张照飞. 锰基-蠕虫状介孔高温煤气脱硫剂的制备及性能研究[D]. 天津: 天津大学, 2014. |
| ZHANG Zhaofei. Prepared and performance of Mn-based mesoporous sorbents with wormlike-hole for hot coal gas desulfurization[D]. Tianjin: Tianjin University, 2014. | |
| 44 | 宋佳音. 纳米Al-KIT-1介孔分子筛的合成、表征和催化性质[D]. 大连: 大连理工大学, 2012. |
| SONG Jiayin. Synthesis, characterization and catalytic performances of nanosized Al-KIT-1 mesoprous molecular sieve[D]. Dalian: Dalian University of Technology, 2012. | |
| 45 | WANG Longjiang, FAN Huiling, SHANGGUAN Ju, et al. Design of a sorbent to enhance reactive adsorption of hydrogen sulfide[J]. ACS Applied Materials & Interfaces, 2014, 6(23): 21167-21177. |
| 46 | WANG Jialu, GUO Xiaolin, SHI Yijun, et al. Synergistic effect of Pt nanoparticles and micro-mesoporous ZSM-5 in VOCs low-temperature removal[J]. Journal of Environmental Sciences, 2021, 107: 87-97. |
| 47 | LI Xin, REZAEI Fateme, LUDLOW Douglas K, et al. Synthesis of SAPO-34@ZSM-5 and SAPO-34@Silicalite-1 core-shell zeolite composites for ethanol dehydration[J]. Industrial & Engineering Chemistry Research, 2018, 57(5): 1446-1453. |
| 48 | LIU Qiang, LIU Bingsi, LIU Qinze, et al. Lattice substitution and desulfurization kinetic analysis of Zn-based spinel sorbents loading onto porous silicoaluminophosphate zeolites[J]. Journal of Hazardous Materials, 2020, 383: 121151. |
| 49 | LIU Qiang, LIU Bingsi, LIU Qinze, et al. Probing mesoporous character, desulfurization capability and kinetic mechanism of synergistic stabilizing sorbent Ca x CuyMn z O i /MAS-9 in hot coal gas[J]. Journal of Colloid and Interface Science, 2021, 587: 743-754. |
| 50 | LIU Qiang, LIU Bingsi, LIU Qinze, et al. Walnut wood-derived hierarchically 3D self-assembly of (a%Ce-Mn) y Al2-yO x and rapid diffusion character of straight micron channel during hot coal gas desulfurization[J]. Chemical Engineering Journal, 2020, 393: 124761. |
| [1] | SHENG Weiwu, CHENG Yongpan, CHEN Qiang, LI Xiaoting, WEI Jia, LI Linge, CHEN Xianfeng. Operating condition analysis of the microbubble and microdroplet dual-enhanced desulfurization reactor [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 142-147. |
| [2] | ZHU Jie, JIN Jing, DING Zhenghao, YANG Huipan, HOU Fengxiao. Modification of CaSO4 oxygen carrier by Zhundong coal ash in chemical looping gasification and its mechanism [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4628-4635. |
| [3] | GAO Yanjing. Analysis of international research trend of single-atom catalysis technology [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4667-4676. |
| [4] | ZHANG Lihong, JIN Yaoru, CHENG Fangqin. Resource utilization of coal gasification slag [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4447-4457. |
| [5] | WANG Yungang, JIAO Jian, DENG Shifeng, ZHAO Qinxin, SHAO Huaishuang. Experimental analysis of condensation heat transfer and synergistic desulfurization [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4230-4237. |
| [6] | WANG Xiaohan, ZHOU Yasong, YU Zhiqing, WEI Qiang, SUN Jinxiao, JIANG Peng. Synthesis and hydrocracking performance of Y molecular sieves with different crystal sizes [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4283-4295. |
| [7] | SUN Xudong, ZHAO Yuying, LI Shirui, WANG Qi, LI Xiaojian, ZHANG Bo. Textual quantitative analysis on China’s local hydrogen energy development policies [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3478-3488. |
| [8] | YU Zhiqing, HUANG Wenbin, WANG Xiaohan, DENG Kaixin, WEI Qiang, ZHOU Yasong, JIANG Peng. B-doped Al2O3@C support for CoMo hydrodesulfurization catalyst and their hydrodesulfurization performance [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3550-3560. |
| [9] | GONG Pengcheng, YAN Qun, CHEN Jinfu, WEN Junyu, SU Xiaojie. Properties and mechanism of eriochrome black T degradation by carbon nanotube-cobalt ferrite composites activated persulfate [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3572-3581. |
| [10] | WANG Darui, SUN Hongmin, XUE Mingwei, WANG Yiyan, LIU Wei, YANG Weimin. Efficient synthesis of fully crystalline ZSM-5 zeolite catalyst by microwave method and its catalytic performance [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3582-3588. |
| [11] | ZHANG Wei, QIN Chuan, XIE Kang, ZHOU Yunhe, DONG Mengyao, LI Jie, TANG Yunhao, MA Ying, SONG Jian. Application and performance enhancement challenges of H2-SCR modified platinum-based catalysts for low-temperature denitrification [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2954-2962. |
| [12] | CHEN Yixin, ZHEN Yaoyao, CHEN Ruihao, WU Jiwei, PAN Limei, YAO Chong, LUO Jie, LU Chunshan, FENG Feng, WANG Qingtao, ZHANG Qunfeng, LI Xiaonian. Preparation of platinum based nanocatalysts and their recent progress in hydrogenation [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2904-2915. |
| [13] | WANG Baowen, LIU Tongqing, ZHANG Gang, LI Weiguang, LIN Deshun, WANG Mengjia, MA Jingjing. Reaction characteristics of CuFe2O4 modified desulfurization slag oxygen carrier with lignite [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2884-2894. |
| [14] | ZHANG Ning, WU Haibin, LI Yu, LI Jianfeng, CHENG Fangqin. Recent advances in preparation and application of floating photocatalysts in water treatment [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2475-2485. |
| [15] | REN Zhongyuan, HE Jinlong, YUAN Qing. Research progress on intercrystalline defects control and remediation technologies for zeolite membranes [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2454-2463. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||