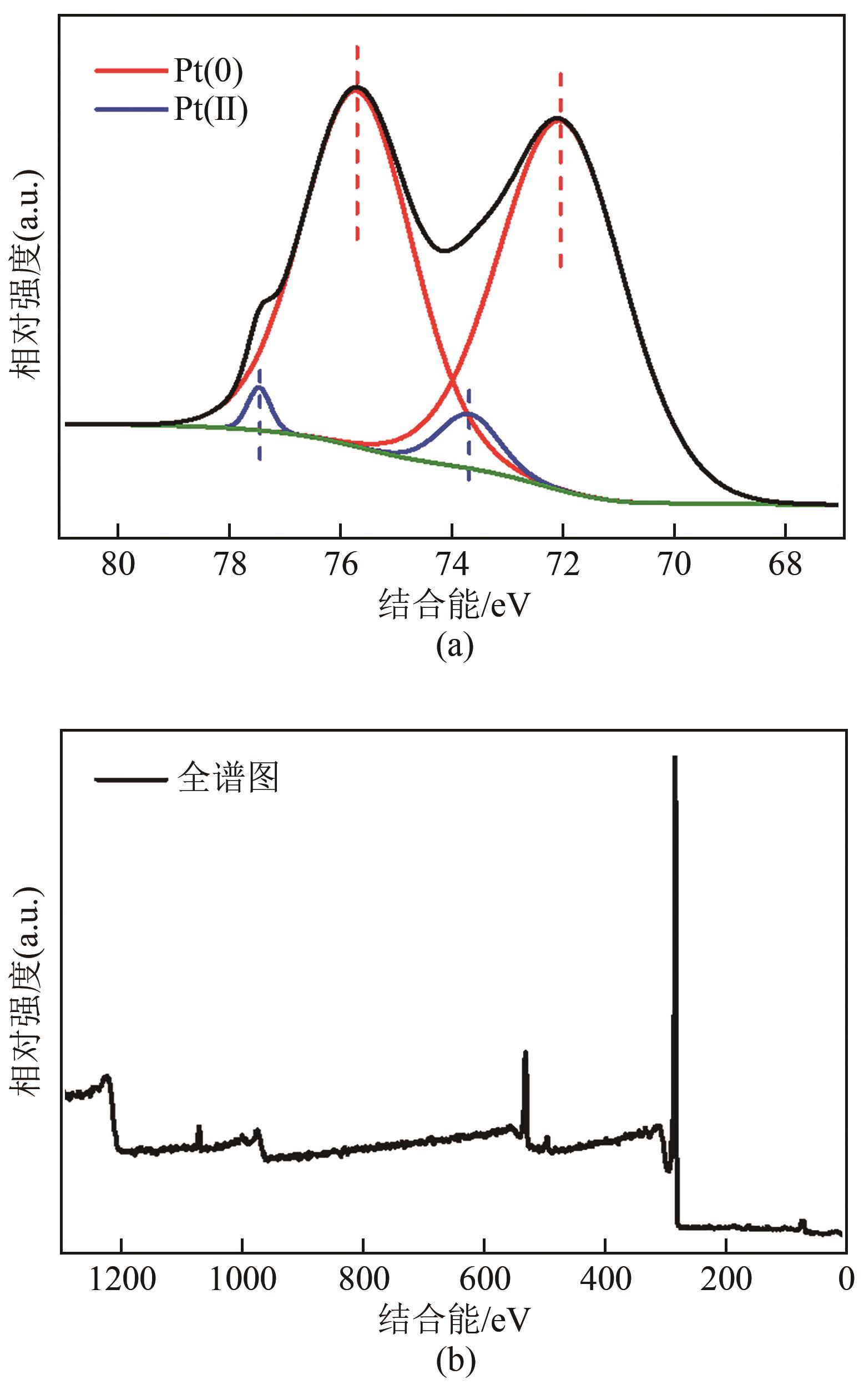
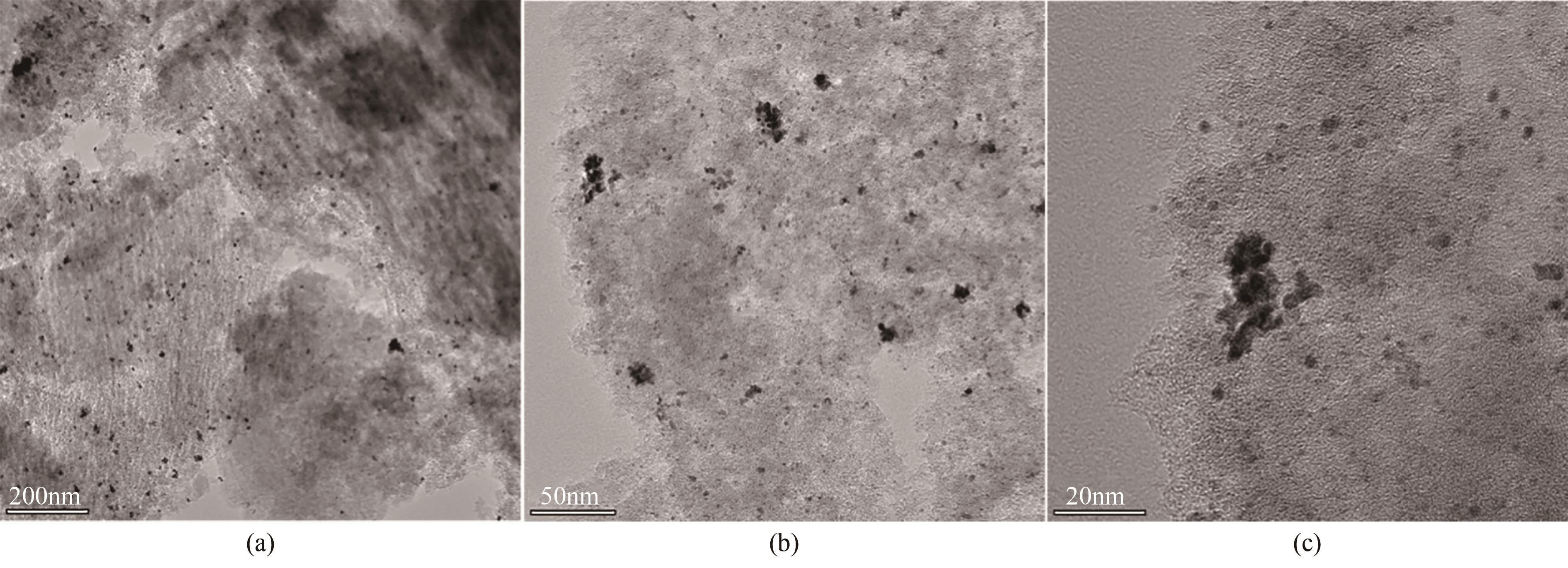
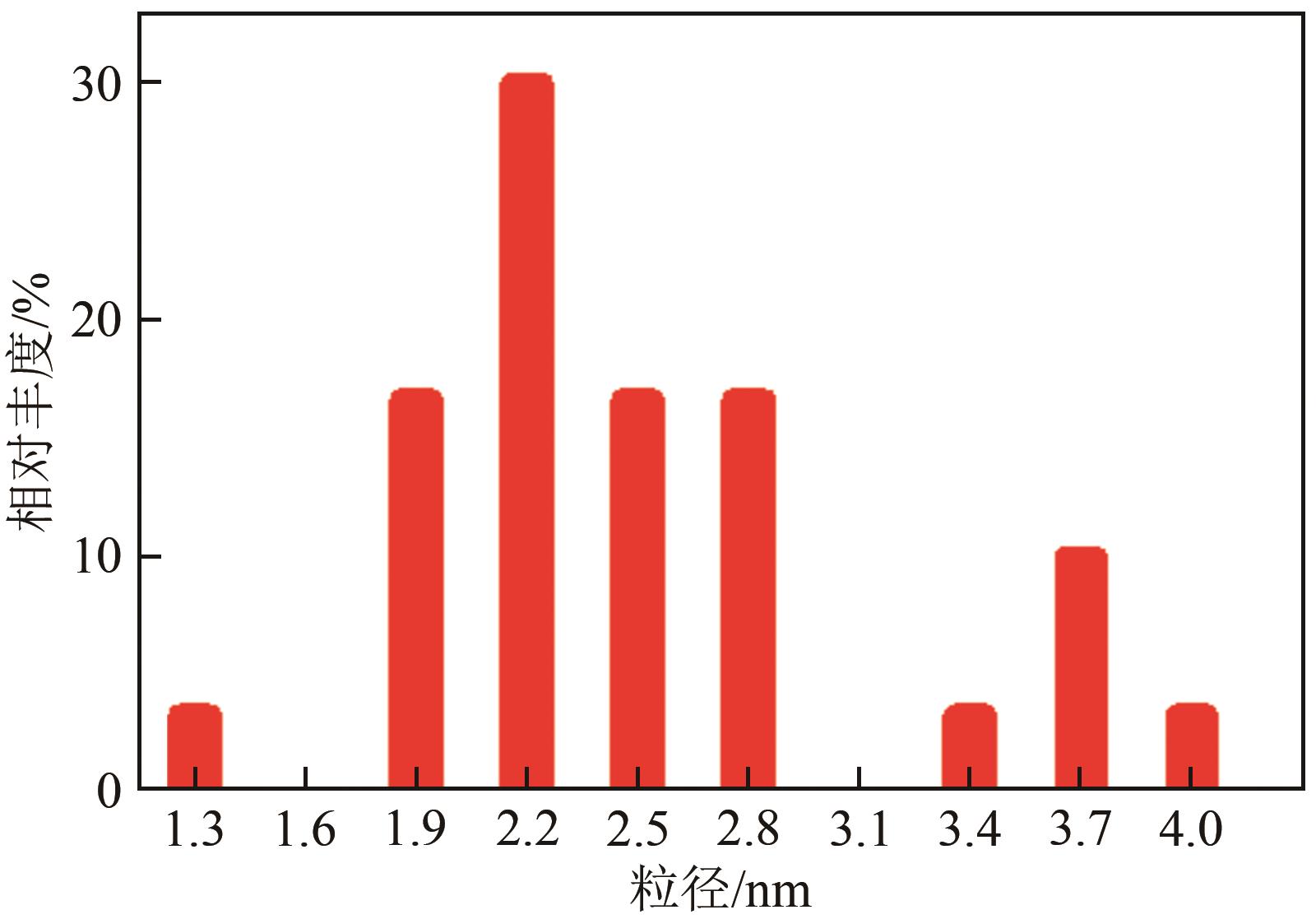
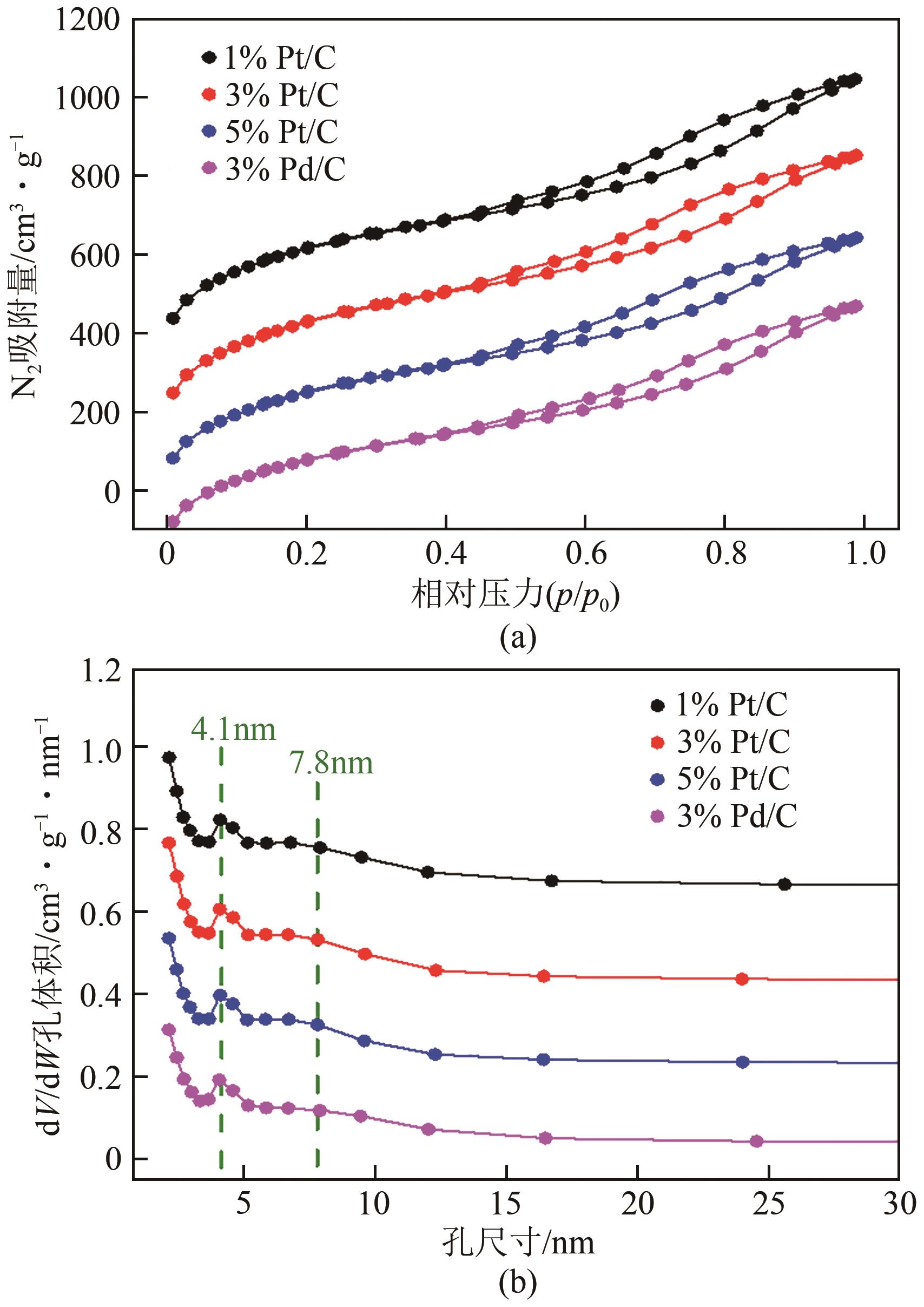
Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (1): 272-281.DOI: 10.16085/j.issn.1000-6613.2022-0505
• Industrial catalysis • Previous Articles Next Articles
Application of carbon-based solid acid in hydrogenation of nitrobenzene to p-aminophenol
HUANG Wei( ), CHU Zheng, REN Lei, LI Shan
), CHU Zheng, REN Lei, LI Shan
- Sinopec Nanjing Research Institute of Chemical Industry Co. , Ltd. , Nanjing 210048, Jiangsu, China
-
Received:2022-03-28Revised:2022-06-11Online:2023-02-20Published:2023-01-25 -
Contact:HUANG Wei
碳基固体酸在硝基苯加氢制备对氨基苯酚中的应用
- 中石化南京化工研究院有限公司,江苏 南京 210048
-
通讯作者:黄伟 -
作者简介:黄伟(1975—),男,硕士,研究方向为精细化学品及催化剂开发。E-mail:huangw.nhgs@sinopec.com。
CLC Number:
Cite this article
HUANG Wei, CHU Zheng, REN Lei, LI Shan. Application of carbon-based solid acid in hydrogenation of nitrobenzene to p-aminophenol[J]. Chemical Industry and Engineering Progress, 2023, 42(1): 272-281.
黄伟, 储政, 任磊, 李珊. 碳基固体酸在硝基苯加氢制备对氨基苯酚中的应用[J]. 化工进展, 2023, 42(1): 272-281.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-0505
| 催化剂种类 | 催化剂∶固体酸(mg∶mg) | 硝基苯转化率/% | 对氨基苯酚收率/% | 对氨基苯酚选择性/% | 苯胺收率/% | 苯胺选择性/% |
|---|---|---|---|---|---|---|
| 1%Pt/C | 1∶200 | 91.8 | 6.4 | 7.0 | 4.4 | 4.8 |
| 3%Pt/C | 1∶200 | 98.2 | 53 | 53.9 | 29.5 | 30.0 |
| 3%Pt/C | 2∶200 | 100 | 57.8 | 57.8 | 28.3 | 28.3 |
| 3%Pt/C | 3∶200 | 100 | 52.8 | 52.8 | 29.7 | 29.7 |
| 3%Pt/C | 1∶0 | 100 | 0 | 0 | 60.2 | 60.2 |
| 5%Pt/C | 1∶200 | 90.3 | 42.3 | 46.8 | 10.6 | 11.7 |
| 5%Pt/C | 2∶200 | 100 | 42.3 | 42.3 | 34.0 | 34.0 |
| 3%Pd/C | 1∶200 | 91.9 | 20.4 | 22.2 | 15.6 | 17.0 |
| 催化剂种类 | 催化剂∶固体酸(mg∶mg) | 硝基苯转化率/% | 对氨基苯酚收率/% | 对氨基苯酚选择性/% | 苯胺收率/% | 苯胺选择性/% |
|---|---|---|---|---|---|---|
| 1%Pt/C | 1∶200 | 91.8 | 6.4 | 7.0 | 4.4 | 4.8 |
| 3%Pt/C | 1∶200 | 98.2 | 53 | 53.9 | 29.5 | 30.0 |
| 3%Pt/C | 2∶200 | 100 | 57.8 | 57.8 | 28.3 | 28.3 |
| 3%Pt/C | 3∶200 | 100 | 52.8 | 52.8 | 29.7 | 29.7 |
| 3%Pt/C | 1∶0 | 100 | 0 | 0 | 60.2 | 60.2 |
| 5%Pt/C | 1∶200 | 90.3 | 42.3 | 46.8 | 10.6 | 11.7 |
| 5%Pt/C | 2∶200 | 100 | 42.3 | 42.3 | 34.0 | 34.0 |
| 3%Pd/C | 1∶200 | 91.9 | 20.4 | 22.2 | 15.6 | 17.0 |
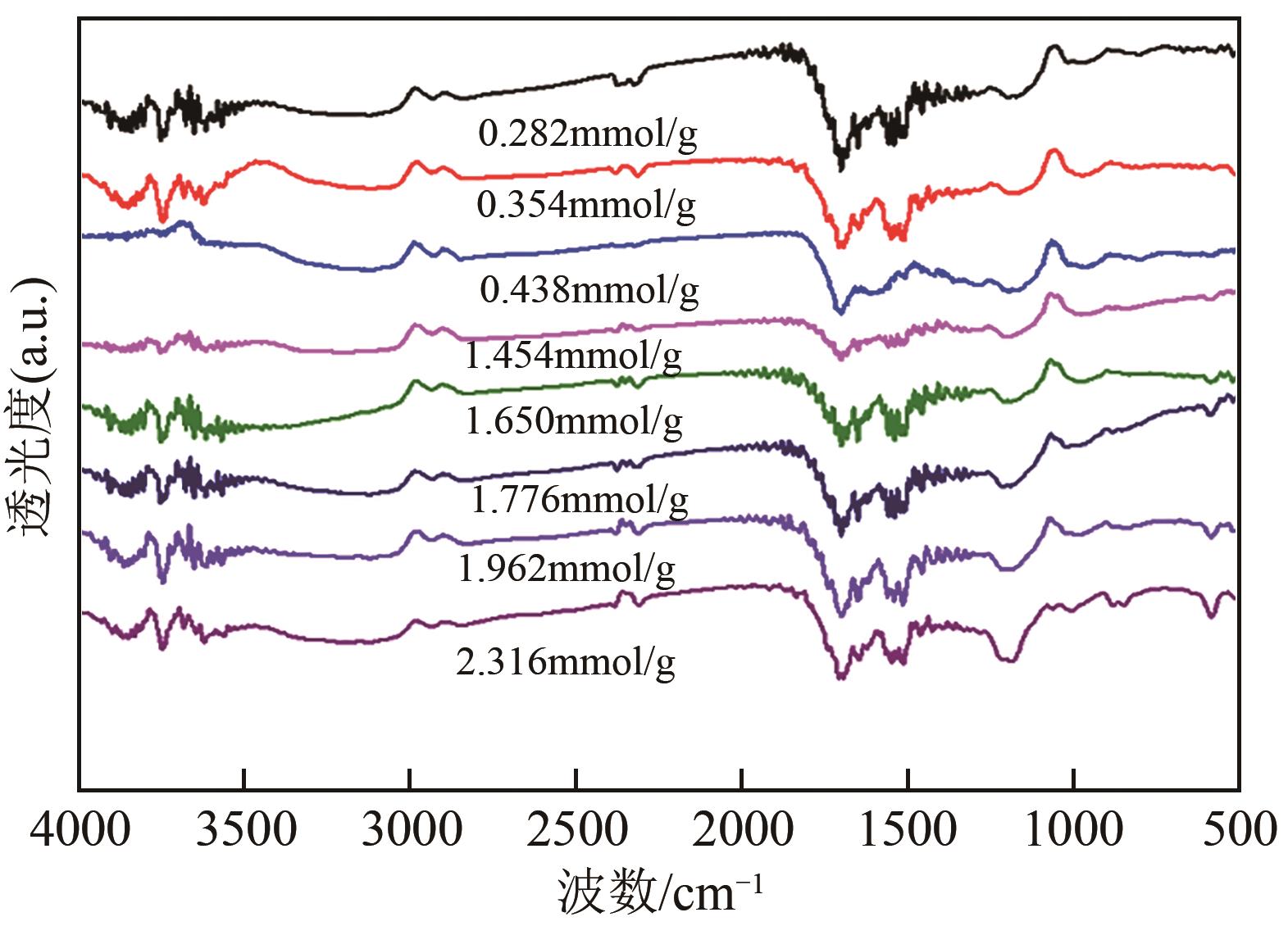
| 固体酸的酸度/mmol·g-1 | 硝基苯转化率/% | 对氨基苯酚收率/% | 对氨基苯酚选择性/% | 苯胺收率/% | 苯胺选择性/% |
|---|---|---|---|---|---|
| 0.282 | 100 | 5.0 | 5.0 | 43.3 | 43.3 |
| 0.354 | 100 | 5.3 | 5.3 | 39.3 | 39.3 |
| 0.438 | 100 | 7.8 | 7.8 | 42.1 | 42.1 |
| 1.454 | 100 | 21.8 | 21.8 | 38.8 | 38.8 |
| 1.650 | 100 | 26.5 | 26.5 | 39.8 | 39.8 |
| 1.776 | 100 | 34.5 | 34.5 | 37.4 | 37.4 |
| 1.962 | 100 | 57.8 | 57.8 | 28.3 | 28.3 |
| 2.316 | 100 | 46.2 | 46.2 | 32.8 | 32.8 |
| 固体酸的酸度/mmol·g-1 | 硝基苯转化率/% | 对氨基苯酚收率/% | 对氨基苯酚选择性/% | 苯胺收率/% | 苯胺选择性/% |
|---|---|---|---|---|---|
| 0.282 | 100 | 5.0 | 5.0 | 43.3 | 43.3 |
| 0.354 | 100 | 5.3 | 5.3 | 39.3 | 39.3 |
| 0.438 | 100 | 7.8 | 7.8 | 42.1 | 42.1 |
| 1.454 | 100 | 21.8 | 21.8 | 38.8 | 38.8 |
| 1.650 | 100 | 26.5 | 26.5 | 39.8 | 39.8 |
| 1.776 | 100 | 34.5 | 34.5 | 37.4 | 37.4 |
| 1.962 | 100 | 57.8 | 57.8 | 28.3 | 28.3 |
| 2.316 | 100 | 46.2 | 46.2 | 32.8 | 32.8 |
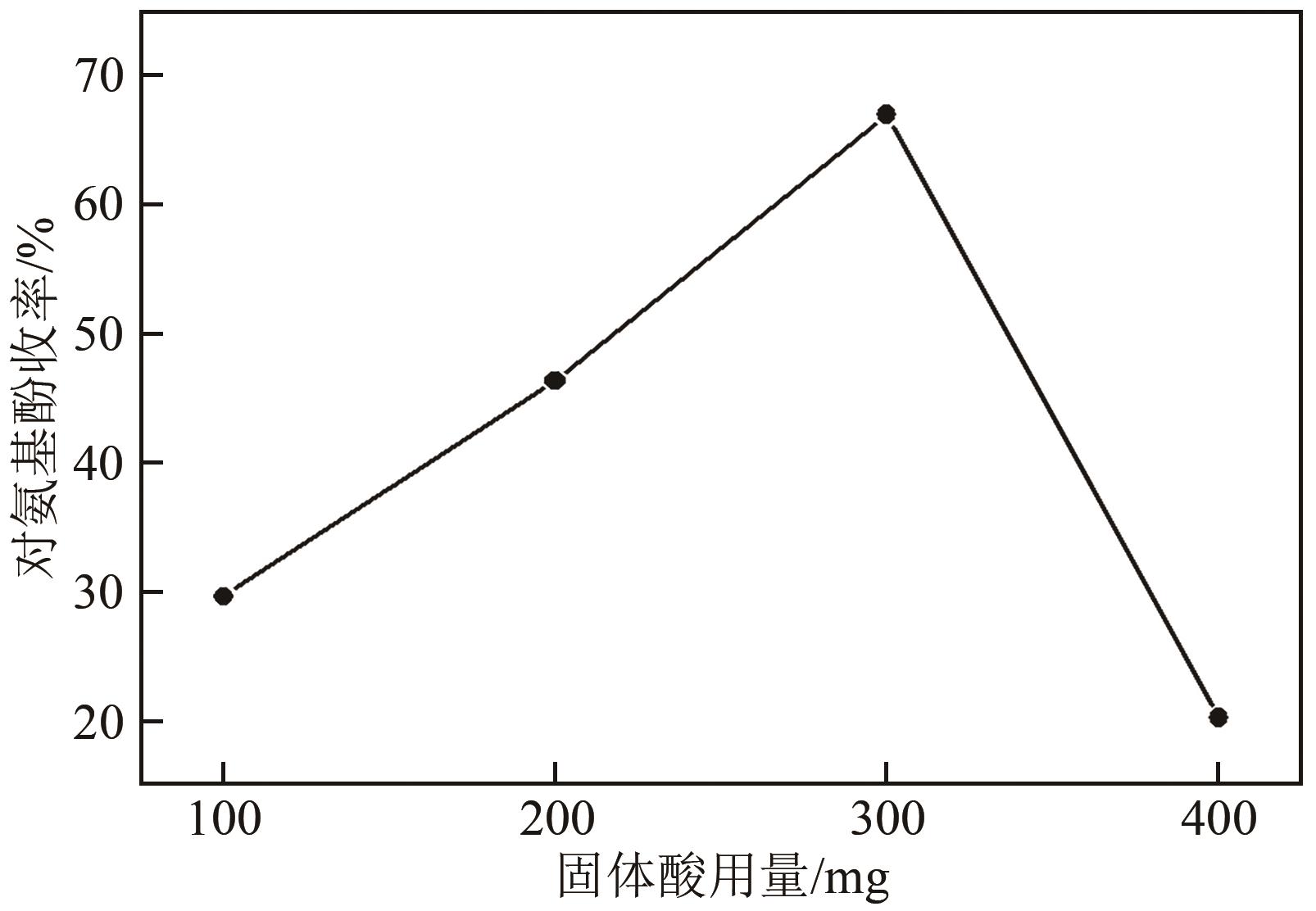
| 固体酸用量/mg | 硝基苯转化率/% | 对氨基苯酚收率/% | 对氨基苯酚选择性/% | 苯胺收率/% | 苯胺选择性/% | 亚硝基苯选择性/% | 苯基羟胺选择性/% |
|---|---|---|---|---|---|---|---|
| 100 | 100 | 29.5 | 29.5 | 41.5 | 41.5 | 0 | 0 |
| 200 | 100 | 46.2 | 46.2 | 32.8 | 32.8 | 0.41 | 0 |
| 300 | 100 | 66.8 | 66.8 | 21.2 | 21.2 | 0.45 | 0.09 |
| 400 | 100 | 20.1 | 20.1 | 18.7 | 18.7 | 0.32 | 0.37 |
| 固体酸用量/mg | 硝基苯转化率/% | 对氨基苯酚收率/% | 对氨基苯酚选择性/% | 苯胺收率/% | 苯胺选择性/% | 亚硝基苯选择性/% | 苯基羟胺选择性/% |
|---|---|---|---|---|---|---|---|
| 100 | 100 | 29.5 | 29.5 | 41.5 | 41.5 | 0 | 0 |
| 200 | 100 | 46.2 | 46.2 | 32.8 | 32.8 | 0.41 | 0 |
| 300 | 100 | 66.8 | 66.8 | 21.2 | 21.2 | 0.45 | 0.09 |
| 400 | 100 | 20.1 | 20.1 | 18.7 | 18.7 | 0.32 | 0.37 |
| 反应时间/h | 硝基苯转化率/% | 对氨基苯酚收率/% | 对氨基苯酚选择性/% | 苯胺收率/% | 苯胺选择性/% | 苯基羟胺/mmol·L-1 | 亚硝基苯/mmol·L-1 |
|---|---|---|---|---|---|---|---|
| 1 | 89.6 | 1.2 | 1.3 | 2.1 | 2.4 | 6.94 | 1.63 |
| 3 | 90.6 | 2.8 | 3.1 | 6.0 | 6.6 | 17.70 | 2.41 |
| 5 | 90.3 | 4.3 | 4.8 | 6.9 | 7.7 | 19.09 | 2.46 |
| 1→5(升温)→10 | 100 | 44.9 | 44.9 | 28.4 | 28.4 | 12.34 | 1.86 |
| 10(85℃) | 100 | 46.2 | 46.2 | 32.8 | 32.8 | 0 | 0.48 |
| 反应时间/h | 硝基苯转化率/% | 对氨基苯酚收率/% | 对氨基苯酚选择性/% | 苯胺收率/% | 苯胺选择性/% | 苯基羟胺/mmol·L-1 | 亚硝基苯/mmol·L-1 |
|---|---|---|---|---|---|---|---|
| 1 | 89.6 | 1.2 | 1.3 | 2.1 | 2.4 | 6.94 | 1.63 |
| 3 | 90.6 | 2.8 | 3.1 | 6.0 | 6.6 | 17.70 | 2.41 |
| 5 | 90.3 | 4.3 | 4.8 | 6.9 | 7.7 | 19.09 | 2.46 |
| 1→5(升温)→10 | 100 | 44.9 | 44.9 | 28.4 | 28.4 | 12.34 | 1.86 |
| 10(85℃) | 100 | 46.2 | 46.2 | 32.8 | 32.8 | 0 | 0.48 |
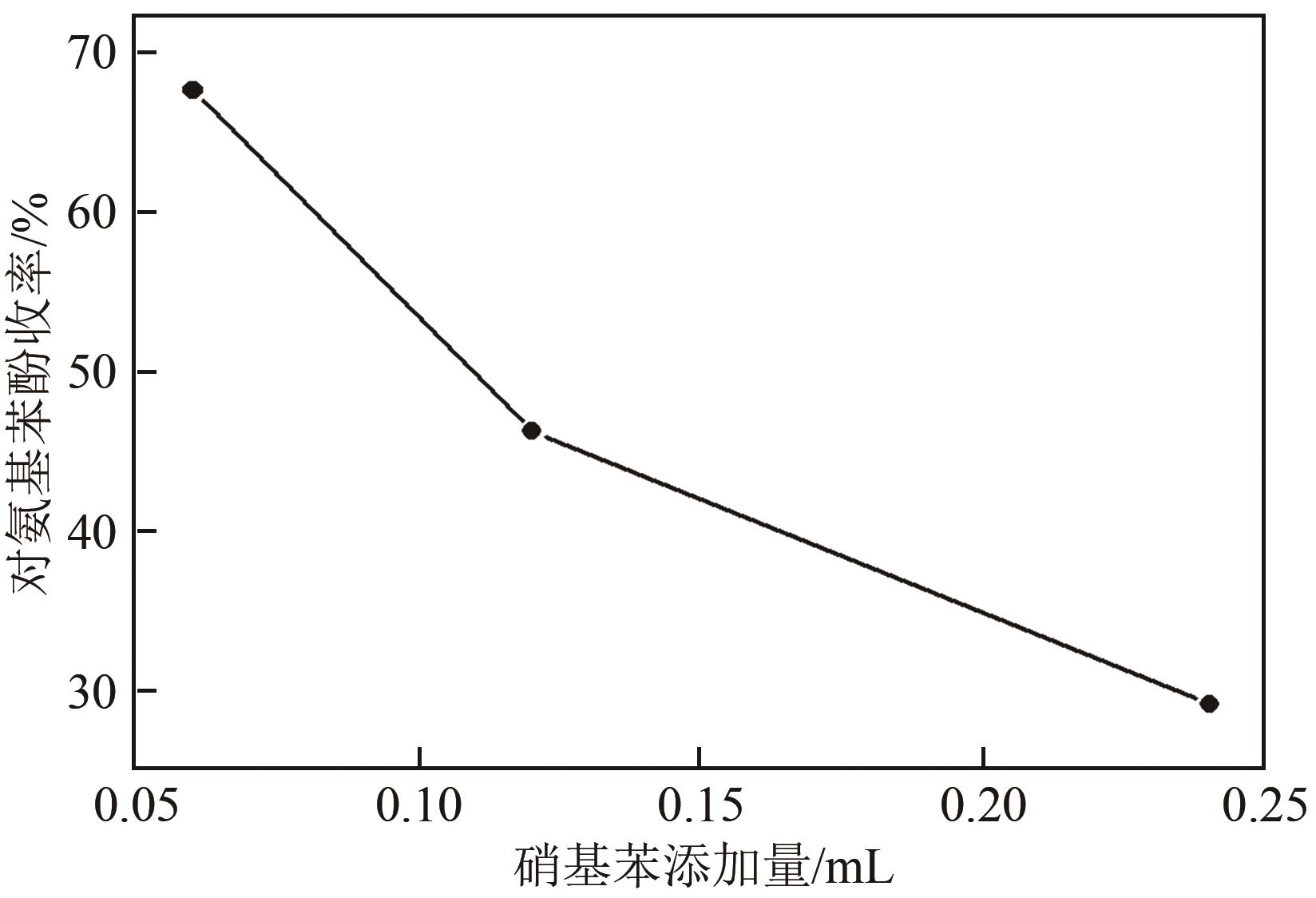
| V硝基苯/mL | 固体酸用量/mg | 硝基苯 转化率/% | 对氨基苯酚收率/% | 对氨基苯酚选择性/% | 苯胺 收率/% | 苯胺 选择性/% |
|---|---|---|---|---|---|---|
| 0.06 | 200 | 100 | 67.6 | 67.6 | 30.1 | 30.1 |
| 0.12 | 200 | 100 | 46.2 | 46.2 | 32.8 | 32.8 |
| 0.24 | 200 | 95.9 | 29.1 | 30.3 | 18.7 | 19.5 |
| V硝基苯/mL | 固体酸用量/mg | 硝基苯 转化率/% | 对氨基苯酚收率/% | 对氨基苯酚选择性/% | 苯胺 收率/% | 苯胺 选择性/% |
|---|---|---|---|---|---|---|
| 0.06 | 200 | 100 | 67.6 | 67.6 | 30.1 | 30.1 |
| 0.12 | 200 | 100 | 46.2 | 46.2 | 32.8 | 32.8 |
| 0.24 | 200 | 95.9 | 29.1 | 30.3 | 18.7 | 19.5 |
| 气体类型 | 固体酸用量 /mg | 硝基苯转化率 /% | 对氨基苯酚收率 /% | 对氨基苯酚选择性 /% | 苯胺收率 /% | 苯胺选择性 /% | 亚硝基苯收率 /% | 氧化偶氮苯收率 /% |
|---|---|---|---|---|---|---|---|---|
| 0.4MPa H2 | 200 | 100 | 36.9 | 36.9 | 36.1 | 36.1 | 0.36 | 0 |
| 0.4MPa H2 | 0 | 100 | 0 | 0 | 78.3 | 78.3 | 0.67 | 0 |
| N2 | 200 | 95.7 | 44.6 | 46.6 | 8.5 | 8.9 | 1.49 | 0.03 |
| 气体类型 | 固体酸用量 /mg | 硝基苯转化率 /% | 对氨基苯酚收率 /% | 对氨基苯酚选择性 /% | 苯胺收率 /% | 苯胺选择性 /% | 亚硝基苯收率 /% | 氧化偶氮苯收率 /% |
|---|---|---|---|---|---|---|---|---|
| 0.4MPa H2 | 200 | 100 | 36.9 | 36.9 | 36.1 | 36.1 | 0.36 | 0 |
| 0.4MPa H2 | 0 | 100 | 0 | 0 | 78.3 | 78.3 | 0.67 | 0 |
| N2 | 200 | 95.7 | 44.6 | 46.6 | 8.5 | 8.9 | 1.49 | 0.03 |
| 固体酸 | 硝基苯 转化率/% | 对氨基苯酚收率/% | 对氨基苯酚选择性/% | 苯胺 收率/% | 苯胺 选择性/% |
|---|---|---|---|---|---|
| 未循环 | 100 | 34.5 | 34.5 | 37.4 | 37.4 |
| 循环使用3次 | 100 | 0.7 | 0.7 | 43.1 | 43.1 |
| 固体酸 | 硝基苯 转化率/% | 对氨基苯酚收率/% | 对氨基苯酚选择性/% | 苯胺 收率/% | 苯胺 选择性/% |
|---|---|---|---|---|---|
| 未循环 | 100 | 34.5 | 34.5 | 37.4 | 37.4 |
| 循环使用3次 | 100 | 0.7 | 0.7 | 43.1 | 43.1 |
| 催化剂种类 | 比表面积/m2·g-1 | 孔容/cm3·g-1 | 介孔尺寸/nm |
|---|---|---|---|
| 1% Pt/C | 1556.3 | 1.23 | 5.02 |
| 3% Pt/C | 1543.8 | 1.24 | 4.80 |
| 5% Pt/C | 1447.6 | 1.14 | 4.81 |
| 3% Pd/C | 1390.3 | 1.10 | 5.00 |
| 催化剂种类 | 比表面积/m2·g-1 | 孔容/cm3·g-1 | 介孔尺寸/nm |
|---|---|---|---|
| 1% Pt/C | 1556.3 | 1.23 | 5.02 |
| 3% Pt/C | 1543.8 | 1.24 | 4.80 |
| 5% Pt/C | 1447.6 | 1.14 | 4.81 |
| 3% Pd/C | 1390.3 | 1.10 | 5.00 |
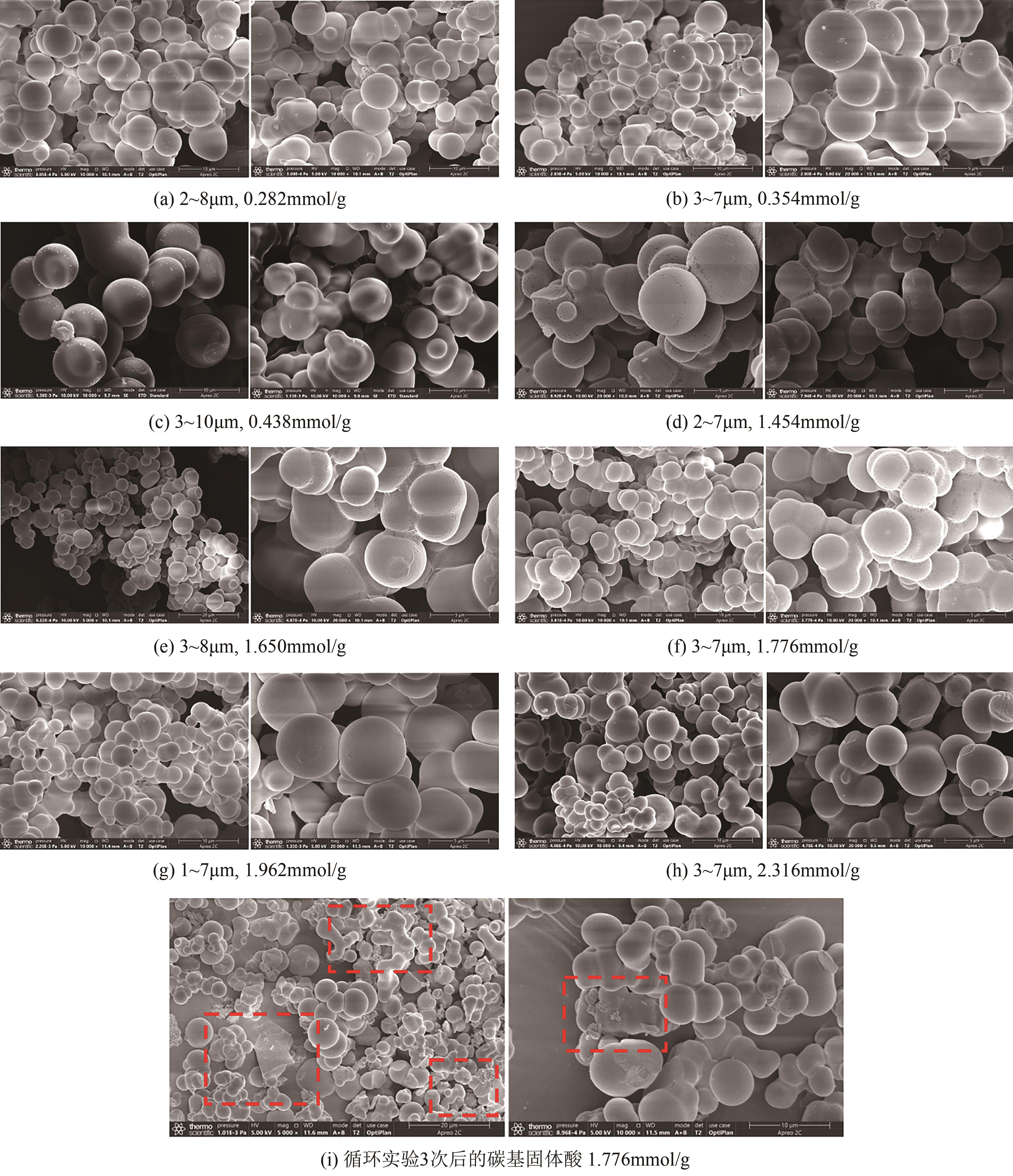
| 固体酸的酸度/mmol·g-1 | 比表面积/m2·g-1 | 孔容/cm3·g-1 | 介孔尺寸/nm |
|---|---|---|---|
| 0.282 | 3.8756 | 0.0250 | 7.3793 |
| 1.962 | 4.5528 | 0.0260 | 7.2610 |
| 1.776 | 5.0573 | 0.0225 | 7.1140 |
| 1.454 | 5.1077 | 0.0244 | 7.2200 |
| 1.650 | 5.3612 | 0.0236 | 7.0779 |
| 0.438 | 5.3905 | 0.0228 | 7.1300 |
| 0.354 | 5.5090 | 0.0254 | 7.3345 |
| 循环3次 | 5.6299 | 0.0282 | 7.1935 |
| 2.316 | 11.2202 | 0.0378 | 6.9448 |
| 固体酸的酸度/mmol·g-1 | 比表面积/m2·g-1 | 孔容/cm3·g-1 | 介孔尺寸/nm |
|---|---|---|---|
| 0.282 | 3.8756 | 0.0250 | 7.3793 |
| 1.962 | 4.5528 | 0.0260 | 7.2610 |
| 1.776 | 5.0573 | 0.0225 | 7.1140 |
| 1.454 | 5.1077 | 0.0244 | 7.2200 |
| 1.650 | 5.3612 | 0.0236 | 7.0779 |
| 0.438 | 5.3905 | 0.0228 | 7.1300 |
| 0.354 | 5.5090 | 0.0254 | 7.3345 |
| 循环3次 | 5.6299 | 0.0282 | 7.1935 |
| 2.316 | 11.2202 | 0.0378 | 6.9448 |
| 1 | 宋龙锋, 袁芝琴, 陈伟兴, 等. 对氨基苯酚的制备[J]. 染料与染色, 2021, 58(4): 33-35. |
| SONG Longfeng, YUAN Zhiqin, CHEN Weixing, et al. Preparation of p-aminophenol[J]. Dyestuffs and Coloration, 2021, 58(4): 33-35. | |
| 2 | 张晓阳, 辛纪衡, 刘晓莲, 等. 常温下合成对乙酰氨基苯酚[J]. 煤炭与化工, 2017, 40(6): 30-32. |
| ZHANG Xiaoyang, XIN Jiheng, LIU Xiaolian, et al. Preparation of acetaminophen at room temperature[J]. Coal and Chemical Industry, 2017, 40(6): 30-32. | |
| 3 | 王凯还, 孙哲, 高秋荣, 等. 扑炎痛的合成路线及其主要影响因素[J]. 中国科技博览, 2015(23): 276-276. |
| WANG Kaihuan, SUN Zhe, GAO Qiurong, et al. Synthetic route of paracetamol and its main influencing factors[J]. China Science and Technology Review, 2015(23): 276-276. | |
| 4 | 王公应. 对氨基苯酚研究进展[J]. 医药化工, 2007(10): 26-30. |
| WANG Gongying. Research progress of p-aminophenol[J]. Pharmaceutical Chemicals, 2007(10): 26-30. | |
| 5 | 梁克民, 王凤娟, 潘世伟. 常压合成防老剂4020的工业生产工艺[J]. 合成橡胶工业, 2003, 26(6): 339-341. |
| LIANG Kemin, WANG Fengjuan, PAN Shiwei. Industrial production process for synthesizing antioxidant 4020 under normal pressure[J]. Synthtrc Rubber Industry, 2003, 26(6): 339-341. | |
| 6 | 陈天生. 对氨基苯酚的生产、应用及发展[J]. 精细化工原料及中间体, 2007(5): 29-32. |
| CHEN Tiansheng. Produce, development and uses of para-aminophenol[J]. Fine Chemical Industrial Raw Materials & Intermediates, 2007(5): 29-32. | |
| 7 | 李静, 侯端杰. 对氨基苯酚生产新工艺[J]. 广州化学, 1995, 20(1): 63-67. |
| LI Jing, HOU Duanjie. The new producing technique of p-aminophenol[J]. Guangzhou Chemistry, 1995, 20(1): 63-67. | |
| 8 | 姜元国, 陈日志, 邢卫红. 对硝基苯酚催化加氢研究进展[J]. 化工进展, 2011, 30(2): 309-313. |
| JIANG Yuanguo, CHEN Rizhi, XING Weihong. Research progress of p-nitrophenol catalytic hydrogenation[J]. Chemical Industry and Engineering Progress, 2011, 30(2): 309-313. | |
| 9 | 高全昌,陈拴虎. 对硝基苯酚的电解还原[J]. 化学世界, 1994, 35(8): 432-434. |
| GAO Jinchang, CHEN Shuanghu. Electrolytic reduction of p-nitrophenol[J]. Chemical World, 1994, 35(8): 432-434. | |
| 10 | 赵利军, 程海洋, 王承学, 等. 硝基苯催化加氢合成对氨基苯酚的绿色清洁工艺[J]. 应用化学, 2015, 32(9): 977-986. |
| ZHAO Lijun, CHENG Haiyang, WANG Chengxue, et al. Green and clean technology for preparation of p-aminophenol by catalytic hydrogenation of nitrobenzene[J]. Chinese Journal of Applied Chemistry, 2015, 32(9): 977-986. | |
| 11 | 韦少平, 覃国红, 韦志明, 翁德洪. 硝基苯电解还原制对氨基苯酚[J]. 化工技术与开发, 2003, 32(4): 1-3. |
| WEI Shaoping, QIN Guohong, WEI Zhiming, et al. Preparation of p-aminophenol by electrochemical reduction of nitrobenzene[J]. Guangxi & Development of Chemical Industry, 2003, 32(4): 1-3. | |
| 12 | 李远军, 陈丽, 刚丽霞. 铝粉还原硝基苯制对氨基苯酚的研究[J]. 西昌学院学报(自然科学版), 2014, 28(2): 41-43. |
| LI Yuanjun, CHEN Li, GANG Lixia. Study on p-amionphenol yield by using aluminum reduction of nitrobenzene[J]. Journal of Xichang College (Natural Science Edition), 2014, 28(2): 41-43. | |
| 13 | 黎耀忠, 程溥明, 陈华, 等. 硝基苯锌法合成对氨基苯酚的研究[J]. 石油化工, 1998, 27(8): 7-10. |
| LI Yaozhong, CHENG Puming, CHEN Hua, et al. Preparation of p-aminophenol by the reduction of nitrobenzene with zinc powder[J]. Petrochemical Technology, 1998, 27(8): 7-10. | |
| 14 | 刘迎新, 刘晓爽, 曾茂, 等. 硝基苯催化加氢合成对氨基苯酚的研究进展[J]. 石油化工, 2018, 47(1): 79-85. |
| LIU Yingxin, LIU Xiaoshuang, ZENG Mao, et al. Progress in synthesis of p-aminophenol by catalytic hydrogenation of nitrobenzene[J]. Petrochemical Technology, 2018, 47(1): 79-85. | |
| 15 | 尹春凯, 王幸宜, 卢冠忠. Pt/C催化剂催化硝基苯加氢制对氨基苯酚工艺过程剖析[J]. 工业催化, 2000, 8(5): 13-17. |
| YIN Chunkai, WANG Xingyi, LU Guanzhong. Technological analysis on the process for preparation of p-aminophenol over Pt/C[J]. Industrial Catalysis, 2000, 8(5): 13-17. | |
| 16 | TERNAY A L. Contemporary organic chemistry[M]. Philadelphia:W.B.Saunders Compang, 1976:611. |
| 17 | JUANG T M, HWANG J C, HO H O, et al. Selectivity in the phase transfer catalytic hydrogenation of nitrobenzene by transition Ⅷ metals[J]. Journal of the Chinese Chemical Society, 1988, 35(2): 135-140. |
| 18 | YAMABE S, ZENG G X, GUAN W, et al. An aniline dication-like transition state in the Bamberger rearrangement[J]. Beilstein Journal of Organic Chemistry, 2013, 9: 1073-1082. |
| 19 | 马原辉. 金属/固体酸催化剂上合成对氨基苯酚反应研究[D]. 天津: 河北工业大学, 2007. |
| MA Yuanhui. Study on the synthesis of p-aminophenol over metal/solid acid catalyst[D]. Tianjin: Hebei University of Technology, 2007. | |
| 20 | 崔咏梅. 铂-酸性离子液体双功能催化剂及催化合成对氨基苯酚研究[D]. 天津: 河北工业大学, 2009. |
| CUI Yongmei. Study on the preparation of platinum-acidic ionic liquid bifunctional catalyst and the catalytic synthesis of p-aminophenol[D]. Tianjin: Hebei University of Technology, 2009. |
| [1] | ZHANG Mingyan, LIU Yan, ZHANG Xueting, LIU Yake, LI Congju, ZHANG Xiuling. Research progress of non-noble metal bifunctional catalysts in zinc-air batteries [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 276-286. |
| [2] | SHI Yongxing, LIN Gang, SUN Xiaohang, JIANG Weigeng, QIAO Dawei, YAN Binhang. Research progress on active sites in Cu-based catalysts for CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 287-298. |
| [3] | XIE Luyao, CHEN Songzhe, WANG Laijun, ZHANG Ping. Platinum-based catalysts for SO2 depolarized electrolysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 299-309. |
| [4] | YANG Xiazhen, PENG Yifan, LIU Huazhang, HUO Chao. Regulation of active phase of fused iron catalyst and its catalytic performance of Fischer-Tropsch synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 310-318. |
| [5] | ZHAO Wei, ZHAO Deyin, LI Shihan, LIU Hongda, SUN Jin, GUO Yanqiu. Synthesis and application of triazine drag reducing agent for nature gas pipeline [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 391-399. |
| [6] | WANG Zhengkun, LI Sifang. Green synthesis of gemini surfactant decyne diol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 400-410. |
| [7] | WANG Lele, YANG Wanrong, YAO Yan, LIU Tao, HE Chuan, LIU Xiao, SU Sheng, KONG Fanhai, ZHU Canghai, XIANG Jun. Influence of spent SCR catalyst blending on the characteristics and deNO x performance for new SCR catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 489-497. |
| [8] | DENG Liping, SHI Haoyu, LIU Xiaolong, CHEN Yaoji, YAN Jingying. Non-noble metal modified vanadium titanium-based catalyst for NH3-SCR denitrification simultaneous control VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 542-548. |
| [9] | CHENG Tao, CUI Ruili, SONG Junnan, ZHANG Tianqi, ZHANG Yunhe, LIANG Shijie, PU Shi. Analysis of impurity deposition and pressure drop increase mechanisms in residue hydrotreating unit [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4616-4627. |
| [10] | WANG Peng, SHI Huibing, ZHAO Deming, FENG Baolin, CHEN Qian, YANG Da. Recent advances on transition metal catalyzed carbonylation of chlorinated compounds [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4649-4666. |
| [11] | ZHANG Qi, ZHAO Hong, RONG Junfeng. Research progress of anti-toxicity electrocatalysts for oxygen reduction reaction in PEMFC [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4677-4691. |
| [12] | GE Quanqian, XU Mai, LIANG Xian, WANG Fengwu. Research progress on the application of MOFs in photoelectrocatalysis [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4692-4705. |
| [13] | WANG Weitao, BAO Tingyu, JIANG Xulu, HE Zhenhong, WANG Kuan, YANG Yang, LIU Zhaotie. Oxidation of benzene to phenol over aldehyde-ketone resin based metal-free catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4706-4715. |
| [14] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| [15] | WU Haibo, WANG Xilun, FANG Yanxiong, JI Hongbing. Progress of the development and application of 3D printing catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 3956-3964. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||