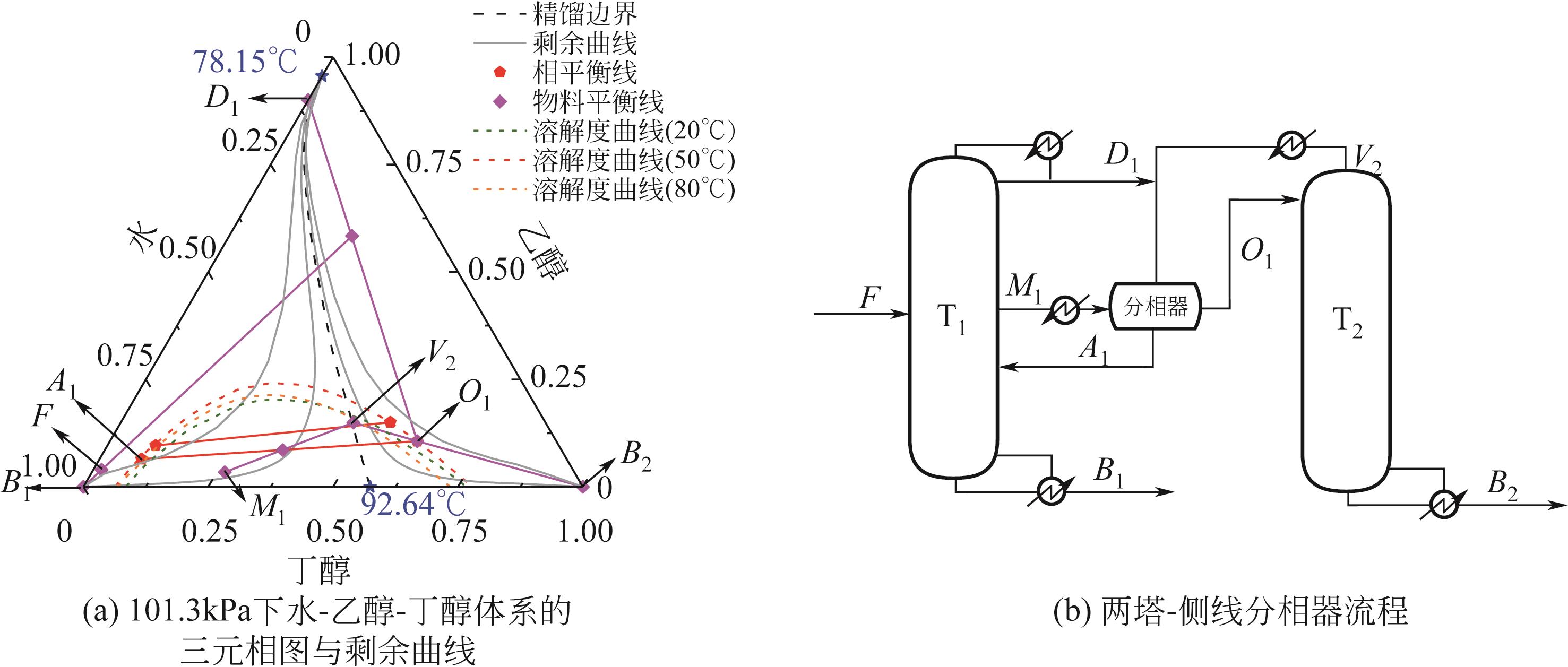
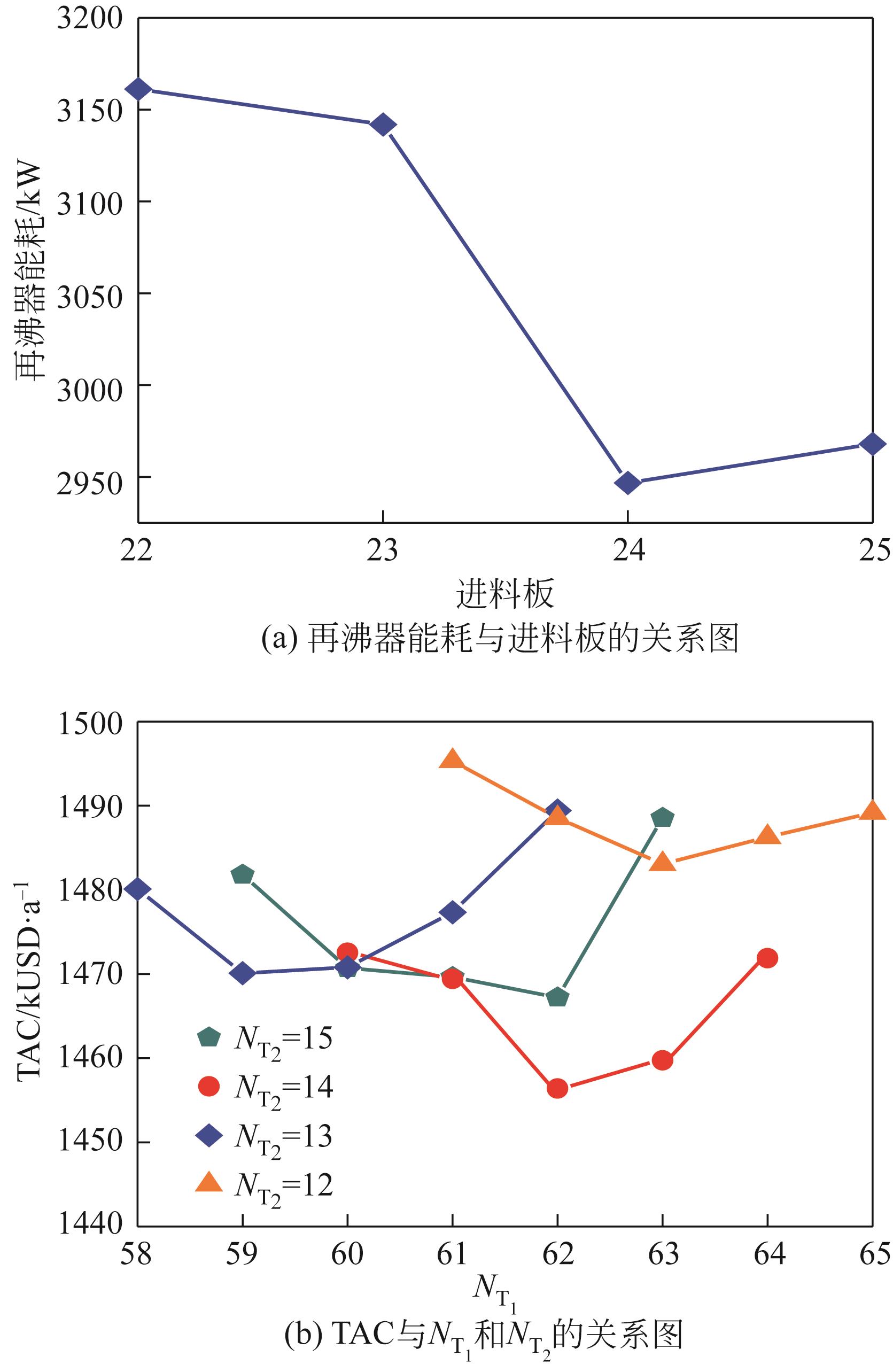
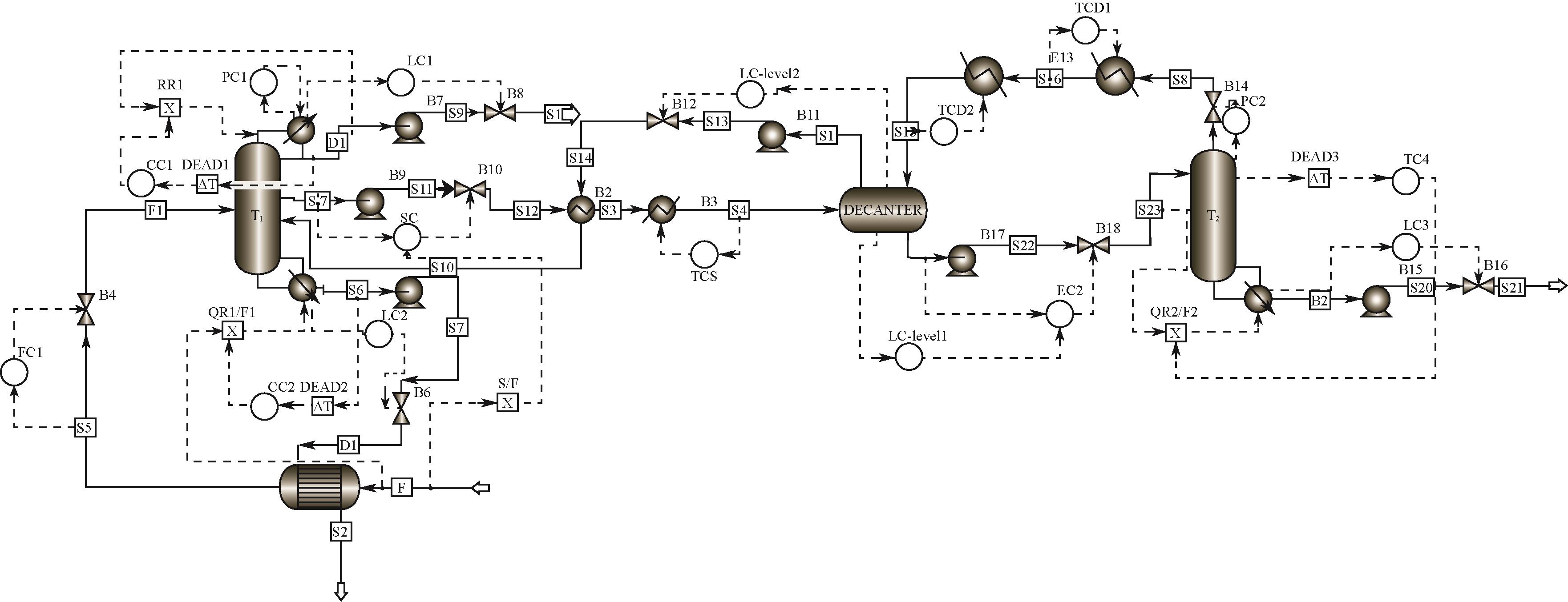
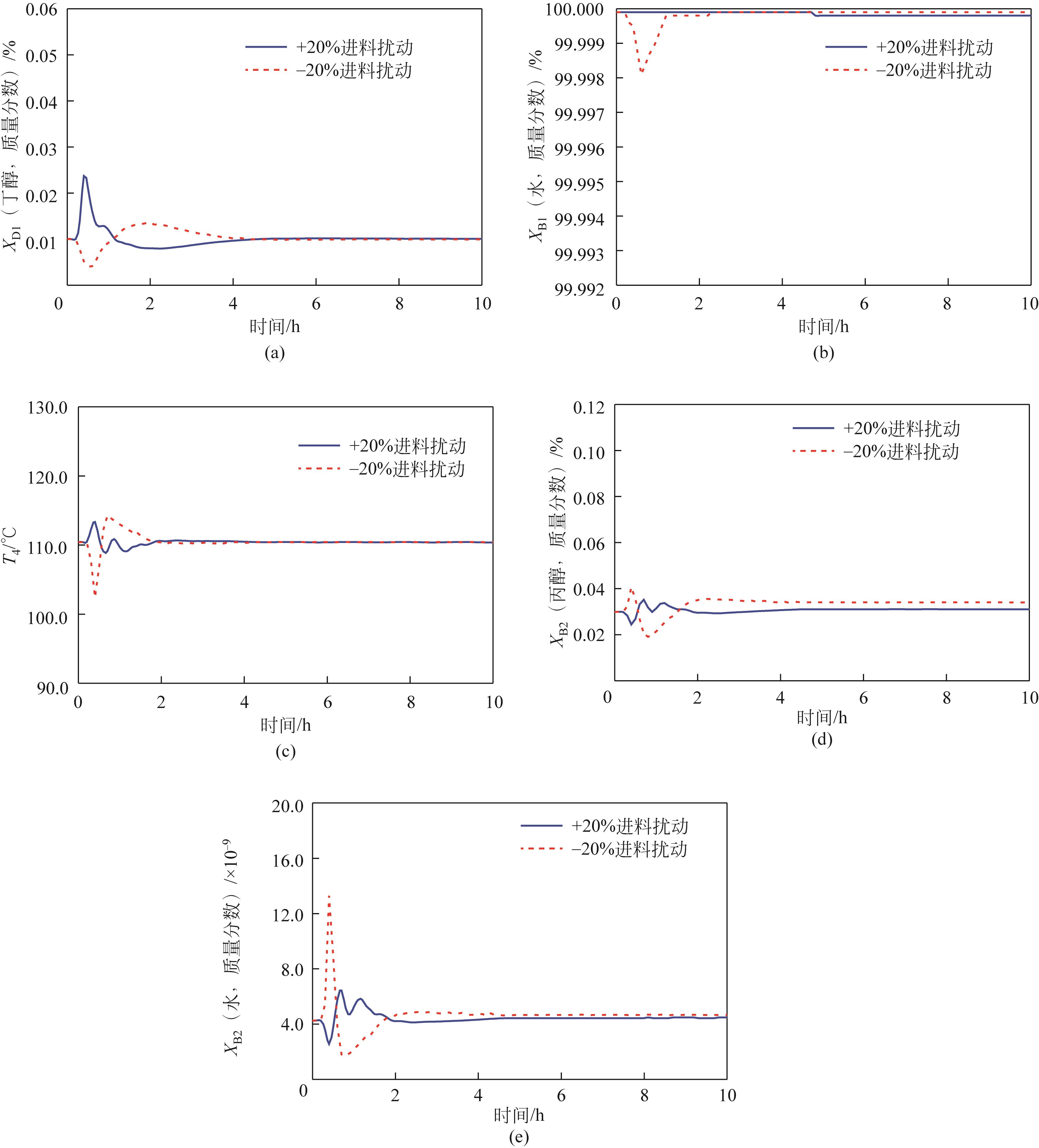
Chemical Industry and Engineering Progress ›› 2022, Vol. 41 ›› Issue (10): 5200-5213.DOI: 10.16085/j.issn.1000-6613.2021-2512
• Chemical processes and equipment • Previous Articles Next Articles
Separation of mixed alcohols from Fischer-Tropsch aqueous by-product: design, optimization and control of fraction cutting
HUANG Yang1( ), ZHANG Jiajun1, LI Jiateng1, XIA Ming2(
), ZHANG Jiajun1, LI Jiateng1, XIA Ming2( ), XU Chunjian1(
), XU Chunjian1( )
)
- 1.State Key Laboratory of Chemical Engineering, Chemical Engineering Research Center, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300350, China
2.State Key Laboratory of Coal Conversion, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, Shanxi, China
-
Received:2021-12-09Revised:2021-12-21Online:2022-10-21Published:2022-10-20 -
Contact:XIA Ming, XU Chunjian
费托合成水相副产物混合醇分离: 馏分切割工艺设计及控制
黄洋1( ), 张稼骏1, 李家腾1, 夏铭2(
), 张稼骏1, 李家腾1, 夏铭2( ), 许春建1(
), 许春建1( )
)
- 1.天津大学化工学院,化学工程研究所,化学工程联合国家重点实验室,天津 300350
2.中国科学院山西煤炭 化学研究所煤转化国家重点实验室,山西 太原 030001
-
通讯作者:夏铭,许春建 -
作者简介:黄洋(1997—),男,硕士研究生,研究方向为精馏分离。E-mail:yh_1@tju.edu.cn。
CLC Number:
Cite this article
HUANG Yang, ZHANG Jiajun, LI Jiateng, XIA Ming, XU Chunjian. Separation of mixed alcohols from Fischer-Tropsch aqueous by-product: design, optimization and control of fraction cutting[J]. Chemical Industry and Engineering Progress, 2022, 41(10): 5200-5213.
黄洋, 张稼骏, 李家腾, 夏铭, 许春建. 费托合成水相副产物混合醇分离: 馏分切割工艺设计及控制[J]. 化工进展, 2022, 41(10): 5200-5213.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2021-2512
| 组分 | 分子式 | 组成(质量分数)/% | 沸点/℃ | 共沸物中的醇质量分数/% | 共沸物沸点/℃ | 溶解度(水) |
|---|---|---|---|---|---|---|
| 甲醇 | CH4O | 0.96 | 65.40 | — | — | 混溶 |
| 乙醇 | C2H6O | 2.00 | 78.01 | 95.6 | 78.16 | 混溶 |
| 正丙醇 | C3H8O | 1.00 | 97.20 | 69.0 | 87.63 | 混溶 |
| 正丁醇 | C4H10O | 0.83 | 118.66 | 56.5 | 91.78 | 微溶 |
| 正戊醇 | C5H12O | 0.53 | 137.80 | 45.5 | 94.30 | 微溶 |
| 正己醇 | C6H14O | 0.25 | 157.40 | 25.0 | 97.80 | 微溶 |
| 正庚醇 | C7H16O | 0.04 | 176.30 | 17.0 | 98.70 | 微溶 |
| 正辛醇 | C8H18O | 0.01 | 195.20 | 10.0 | 99.40 | 不溶 |
| 水 | H2O | 94.38 | 100.00 | — | — | — |
| 组分 | 分子式 | 组成(质量分数)/% | 沸点/℃ | 共沸物中的醇质量分数/% | 共沸物沸点/℃ | 溶解度(水) |
|---|---|---|---|---|---|---|
| 甲醇 | CH4O | 0.96 | 65.40 | — | — | 混溶 |
| 乙醇 | C2H6O | 2.00 | 78.01 | 95.6 | 78.16 | 混溶 |
| 正丙醇 | C3H8O | 1.00 | 97.20 | 69.0 | 87.63 | 混溶 |
| 正丁醇 | C4H10O | 0.83 | 118.66 | 56.5 | 91.78 | 微溶 |
| 正戊醇 | C5H12O | 0.53 | 137.80 | 45.5 | 94.30 | 微溶 |
| 正己醇 | C6H14O | 0.25 | 157.40 | 25.0 | 97.80 | 微溶 |
| 正庚醇 | C7H16O | 0.04 | 176.30 | 17.0 | 98.70 | 微溶 |
| 正辛醇 | C8H18O | 0.01 | 195.20 | 10.0 | 99.40 | 不溶 |
| 水 | H2O | 94.38 | 100.00 | — | — | — |
| 共沸物 | 模拟值/(kg/kg,℃) | 实验值/(kg/kg,℃) |
|---|---|---|
| 乙醇/水 | 0.9581/0.0419,78.17 | 0.956/0.044,78.16 |
| 丙醇/水 | 0.6914/0.3086,87.73 | 0.690/0.310,87.63 |
| 丁醇/水 | 0.5742/0.4258,92.64 | 0.565/0.435,91.78 |
| 戊醇/水 | 0.4492/0.5508,95.87 | 0.455/0.545,94.30 |
| 己醇/水 | 0.2793/0.7207,98.21 | 0.250/0.750,97.80 |
| 庚醇/水 | 0.1614/0.8386,99.21 | 0.170/0.830,98.70 |
| 辛醇/水 | 0.1159/0.8841,99.52 | 0.100/0.900,99.40 |
| 共沸物 | 模拟值/(kg/kg,℃) | 实验值/(kg/kg,℃) |
|---|---|---|
| 乙醇/水 | 0.9581/0.0419,78.17 | 0.956/0.044,78.16 |
| 丙醇/水 | 0.6914/0.3086,87.73 | 0.690/0.310,87.63 |
| 丁醇/水 | 0.5742/0.4258,92.64 | 0.565/0.435,91.78 |
| 戊醇/水 | 0.4492/0.5508,95.87 | 0.455/0.545,94.30 |
| 己醇/水 | 0.2793/0.7207,98.21 | 0.250/0.750,97.80 |
| 庚醇/水 | 0.1614/0.8386,99.21 | 0.170/0.830,98.70 |
| 辛醇/水 | 0.1159/0.8841,99.52 | 0.100/0.900,99.40 |
| 参数 | 公式 |
|---|---|
| 塔壳费用/USD | (M&S/280)×596.115d1.066H0.802 |
| 塔板费用/USD | (M&S/280)×12.69d1.55H |
| 换热器费用①/USD | (M&S/280)×101.3A0.65(2.29+3.75Fd) |
| 塔高(H)/m | 1.2h (NT-2) |
| 换热面积(A)②/m2 | Q/(UΔT) |
低压蒸汽(500kPa,160℃) /USD·GJ-1 | 13.28 |
| 冷却水(30~35℃)/USD·GJ-1 | 0.354 |
| M&S | 1638.2(2018)[ |
| 参数 | 公式 |
|---|---|
| 塔壳费用/USD | (M&S/280)×596.115d1.066H0.802 |
| 塔板费用/USD | (M&S/280)×12.69d1.55H |
| 换热器费用①/USD | (M&S/280)×101.3A0.65(2.29+3.75Fd) |
| 塔高(H)/m | 1.2h (NT-2) |
| 换热面积(A)②/m2 | Q/(UΔT) |
低压蒸汽(500kPa,160℃) /USD·GJ-1 | 13.28 |
| 冷却水(30~35℃)/USD·GJ-1 | 0.354 |
| M&S | 1638.2(2018)[ |
| 参数 | S1 | S2 |
|---|---|---|
| 进料板 | 19 | 19 |
| 侧线出料位置 | 18 | 49 |
| NT1 | 60 | 60 |
| NT2 | 15 | 15 |
| 塔径/m | ||
| d1 | 1.1 | 1.4 |
| d2 | 0.5 | 0.25 |
| 塔板间距/m | ||
| h1 | 0.6 | 0.8 |
| h2 | 0.4 | 0.2 |
| 再沸器热负荷/kW | ||
| QR1 | 2882.54(差异31.48%①) | 4206.67 |
| QR2 | 278.65 | 99.27 |
| 总再沸器热负荷QR/kW | 3161.19(差异26.59%②) | 4305.94 |
| 冷凝器热负荷QC/kW | -2502.16 | -3828.01 |
| FCI/kUSD·a-1 | 1032.86 | 1275.04 |
| OC/kUSD·a-1 | 1237.21 | 1686.71 |
| TAC/kUSD·a-1 | 1547.07(差异25.23%③) | 2069.22 |
| 参数 | S1 | S2 |
|---|---|---|
| 进料板 | 19 | 19 |
| 侧线出料位置 | 18 | 49 |
| NT1 | 60 | 60 |
| NT2 | 15 | 15 |
| 塔径/m | ||
| d1 | 1.1 | 1.4 |
| d2 | 0.5 | 0.25 |
| 塔板间距/m | ||
| h1 | 0.6 | 0.8 |
| h2 | 0.4 | 0.2 |
| 再沸器热负荷/kW | ||
| QR1 | 2882.54(差异31.48%①) | 4206.67 |
| QR2 | 278.65 | 99.27 |
| 总再沸器热负荷QR/kW | 3161.19(差异26.59%②) | 4305.94 |
| 冷凝器热负荷QC/kW | -2502.16 | -3828.01 |
| FCI/kUSD·a-1 | 1032.86 | 1275.04 |
| OC/kUSD·a-1 | 1237.21 | 1686.71 |
| TAC/kUSD·a-1 | 1547.07(差异25.23%③) | 2069.22 |
| 参数 | 两塔-侧线分相器流程 | 常规三塔粗分流程 | ||||
|---|---|---|---|---|---|---|
| 侧线精馏塔 | 重醇塔 | 脱水塔 | 醇分塔 | 重醇塔 | ||
| p/kPa | 101.3 | 121.3 | 101.3 | 121.3 | ||
| NT | 62 | 14 | 47 | 51 | 8 | |
| NF | 25 | 1 | 10 | 29 | 1 | |
| RR | 6.13 | — | 1.61 | 1.69 | — | |
| d/m | 1.1 | 0.5 | 1.0 | 0.7 | 0.25 | |
| h/m | 0.6 | 0.4 | 0.6 | 0.4 | 0.2 | |
| QC/kW | -2224.74 | -272.31 | -2111.65 | -840.84 | -80.20 | |
| QR/kW | 2571.21 | 291.39 | 2446.14 | 858.11 | 101.95 | |
| 合计QR/kW | 2862.60(差异15.96%①) | 3406.20 | ||||
| FCI/kUSD·a-1 | 1016.67 | 1136.08 | ||||
| OC/kUSD·a-1 | 1120.30 | 1333.67 | ||||
| TAC/kUSD·a-1 | 1456.40(差异14.79%②) | 1709.25 | ||||
| 参数 | 两塔-侧线分相器流程 | 常规三塔粗分流程 | ||||
|---|---|---|---|---|---|---|
| 侧线精馏塔 | 重醇塔 | 脱水塔 | 醇分塔 | 重醇塔 | ||
| p/kPa | 101.3 | 121.3 | 101.3 | 121.3 | ||
| NT | 62 | 14 | 47 | 51 | 8 | |
| NF | 25 | 1 | 10 | 29 | 1 | |
| RR | 6.13 | — | 1.61 | 1.69 | — | |
| d/m | 1.1 | 0.5 | 1.0 | 0.7 | 0.25 | |
| h/m | 0.6 | 0.4 | 0.6 | 0.4 | 0.2 | |
| QC/kW | -2224.74 | -272.31 | -2111.65 | -840.84 | -80.20 | |
| QR/kW | 2571.21 | 291.39 | 2446.14 | 858.11 | 101.95 | |
| 合计QR/kW | 2862.60(差异15.96%①) | 3406.20 | ||||
| FCI/kUSD·a-1 | 1016.67 | 1136.08 | ||||
| OC/kUSD·a-1 | 1120.30 | 1333.67 | ||||
| TAC/kUSD·a-1 | 1456.40(差异14.79%②) | 1709.25 | ||||
| 项目 | 控制变量 | 操纵变量 | 增益/Kc | 积分时间τI/min |
|---|---|---|---|---|
| CC1 | xD1(丁醇) | 回流比(侧线精馏塔) | 0.10 | 40.92 |
| CC2 | xB1(水) | 再沸器热负荷(侧线精馏塔) | 0.20 | 77.88 |
| T4 | xB1(丙醇) | 再沸器热负荷(重醇塔) | 0.35 | 10.56 |
| 项目 | 控制变量 | 操纵变量 | 增益/Kc | 积分时间τI/min |
|---|---|---|---|---|
| CC1 | xD1(丁醇) | 回流比(侧线精馏塔) | 0.10 | 40.92 |
| CC2 | xB1(水) | 再沸器热负荷(侧线精馏塔) | 0.20 | 77.88 |
| T4 | xB1(丙醇) | 再沸器热负荷(重醇塔) | 0.35 | 10.56 |
| 1 | LARSON E D, REN T J. Synthetic fuel production by indirect coal liquefaction[J]. Energy for Sustainable Development, 2003, 7(4): 79-102. |
| 2 | LIU Z Y, SHI S D, LI Y W. Coal liquefaction technologies—Development in China and challenges in chemical reaction engineering[J]. Chemical Engineering Science, 2010, 65(1): 12-17. |
| 3 | KHODAKOV A Y, CHU W, FONGARLAND P. Advances in the development of novel cobalt Fischer-Tropsch catalysts for synthesis of long-chain hydrocarbons and clean fuels[J]. Chemical Reviews, 2007, 107(5): 1692-1744. |
| 4 | DRY M E. The fischer-tropsch process: 1950—2000[J]. Catalysis Today, 2002, 71(3/4): 227-241. |
| 5 | DÜRRE P. Biobutanol: an attractive biofuel[J]. Biotechnology Journal: Healthcare Nutrition Technology, 2007, 2(12): 1525-1534. |
| 6 | MOSER W R, CONNOLLY K E. Synthesis and characterization of copper-modified zinc chromites by the high temperature aerosol decomposition process for higher alcohol synthesis[J]. The Chemical Engineering Journal and the Biochemical Engineering Journal, 1996, 64(2). |
| 7 | MAHDAVI V, PEYROVI M H. Synthesis of C1-C6 alcohols over copper/cobalt catalysts: investigation of the influence of preparative procedures on the activity and selectivity of Cu-Co2O3/ZnO, Al2O3 catalyst[J]. Catalysis Communications, 2006, 7(8): 542-549. |
| 8 | 马爱华, 云志. 费托合成水相副产物中具有共沸组成的低碳混合醇-水体系分离方法的研究进展[J]. 石油学报(石油加工), 2013, 29(4): 738-743. |
| MA A H, YUN Z. Research progress of separation technologies for azeotropic mixture of lower alcohols-water system of the by-product in water of fischer-Tropsch synthesis[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2013, 29(4): 738-743. | |
| 9 | SIE S T. Process development and scale up: Ⅳ. Case history of the development of a Fischer-Tropsch synthesis process[J]. Reviews in Chemical Engineering, 1998, 14(2): 109-157. |
| 10 | 李云华. 费托合成水相副产物馏分切割工艺的技术开发[D]. 天津: 天津大学, 2003. |
| LI Y H. Technology exploitation of cutting the by-product in water of Fischer-Tropsch synthesis[D]. Tianjin: Tianjin University, 2003. | |
| 11 | 汪俊锋, 王红星, 杨金杯, 等. 费托合成水相副产物混合醇分离脱水工艺模拟及优化[J]. 计算机与应用化学, 2015, 32(5): 567-571. |
| WANG J F, WANG H X, YANG J B, et al. F-T of mixed alcohol aqueous by-product separation dehydration technology simulation and optimization[J]. Computers and Applied Chemistry, 2015, 32(5): 567-571. | |
| 12 | 李玲, 柴士阳, 刘来春, 等. 费托合成水相副产物混合醇渗透蒸发分离工艺[J]. 化工进展, 2017, 36(6): 2086-2093. |
| LI L, CHAI S Y, LIU L C, et al. Study on separation of mixed alcohol from water phase by-product in the F-T synthesis by pervaporation technology[J]. Chemical Industry and Engineering Progress, 2017, 36(6): 2086-2093. | |
| 13 | GARCIA M, SANZ M T, BELTRAN S. Separation by pervaporation of ethanol from aqueous solutions and effect of other components present in fermentation broths[J]. Journal of Chemical Technology & Biotechnology, 2009, 84(12): 1873-1882. |
| 14 | ARIFIN S, CHIEN I L. Design and control of an isopropyl alcohol dehydration process via extractive distillation using dimethyl sulfoxide as an entrainer[J]. Industrial & Engineering Chemistry Research, 2008, 47(3): 790-803. |
| 15 | LUYBEN W L. Economic optimum design of the heterogeneous azeotropic dehydration of ethanol[J]. Industrial & Engineering Chemistry Research, 2012, 51(50): 16427-16432. |
| 16 | MILESTONE N B, BIBBY D M. Concentration of alcohols by adsorption on silicalite[J]. Journal of Chemical Technology & Biotechnology, 2010, 31(1): 732-736. |
| 17 | 胡子益, 李洪波, 谭宇鑫, 等. 分子筛膜-精馏耦合用于费托合成水相副产物混合醇回收的工艺流程模拟[J]. 化工进展, 2016, 35(S2): 56-60. |
| HU Z Y, LI H B, TANG Y X, et al. Zeolite membrane dehydration and distillation coupling process simulation of F-T water by-product recovery[J]. Chemical Industry and Engineering Progress, 2016, 35(S2): 56-60. | |
| 18 | CUI C T, ZHANG X D, SUN J S. Design and optimization of energy-efficient liquid-only side-stream distillation configurations using a stochastic algorithm[J]. Chemical Engineering Research and Design, 2019, 145: 48-52. |
| 19 | SMITH R. Chemical process: design and integration[M]. State of New Jersey: John Wiley & Sons, 2005. |
| 20 | ROOKS R E, MALONE M F, DOHERTY M F. A geometric design method for side-stream distillation columns[J]. Industrial & Engineering Chemistry Research, 1996, 35(10): 3653-3664. |
| 21 | TEDDER D W, RUDD D F. Parametric studies in industrial distillation: Part Ⅰ. Design comparisons[J]. AIChE Journal, 1978, 24(2): 303-315. |
| 22 | GLINOS K N, MALONE M F. Design of sidestream distillation columns[J]. Industrial & Engineering Chemistry Process Design and Development, 1985, 24(3): 822-828. |
| 23 | FERREIRA M C, MEIRELLES A J A, BATISTA E A C. Study of the fusel oil distillation process[J]. Industrial & Engineering Chemistry Research, 2013, 52(6): 2336-2351. |
| 24 | PUENTES C, JOULIA X, ATHÈS V, et al. Review and thermodynamic modeling with NRTL model of vapor-liquid equilibria (VLE) of aroma compounds highly diluted in ethanol-water mixtures at 101.3kPa[J]. Industrial & Engineering Chemistry Research, 2018, 57(10): 3443-3470. |
| 25 | IWAKABE K, KOSUGE H. Isobaric vapor-liquid-liquid equilibria with a newly developed still[J]. Fluid Phase Equilibria, 2001, 192(1/2): 171-186. |
| 26 | 任冉冉. 费托合成水中醇分离过程的热力学模型评价[D]. 北京: 北京石油化工学院, 2016. |
| REN R R. Evaluation of thermodynamic model for alcohol separation process of Fischer-Tropsch synthesis water[D]. Beijing: Beijing Institute of Petrochemical Technology, 2016. | |
| 27 | ORCHILLÉS A V, VERCHER E, MARTÍNEZ-ANDREU A, et al. Isobaric vapor-liquid equilibria for 1-propanol + water + 1-ethyl-3-methylimidazolium trifluoromethanesulfonate at 100kPa[J]. Journal of Chemical & Engineering Data, 2008, 53(10): 2426-2431. |
| 28 | KAMIHAMA N, MATSUDA H, KURIHARA K, et al. Isobaric vapor-liquid equilibria for ethanol + water + ethylene glycol and its constituent three binary systems[J]. Journal of Chemical & Engineering Data, 2012, 57(2): 339-344. |
| 29 | IWAKABE K, KOSUGE H. Isobaric vapor-liquid-liquid equilibria with a newly developed still[J]. Fluid Phase Equilibria, 2001, 192(1/2): 171-186. |
| 30 | 程能林. 溶剂手册[M]. 4版. 北京: 化学工业出版社, 2007: 405-439. |
| CHENG N L. Solvents Handbook[M]. 4th ed. Beijing: Chemical Industry Press, 2007: 405-439. | |
| 31 | STEPHENSON R, STUART J, TABAK M. Mutual solubility of water and aliphatic alcohols[J]. Journal of Chemical and Engineering Data, 1984, 29(3): 287-290. |
| 32 | GMEHLING J, MENKE J, KRAFCZYK J, et al. Azeotropic data[M]. Weinheim: Wiley-VCH, 2004. |
| 33 | DOUGLAS J M. Conceptual design of chemical processes[M]. New York: McGraw-Hill, 1988. |
| 34 | WEI F, DIAO B T, GAO J, et al. Process design, evaluation and control for separation of 2, 2, 3, 3‐tetrafluoro‐1‐propanol and water by extractive distillation using ionic liquid 1‐ethyl‐3‐methylimidazolium acetate[J]. Journal of Chemical Technology & Biotechnology, 2021, 96(11): 3175-3184. |
| 35 | FIEN G J A F, LIU Y A. Heuristic synthesis and shortcut design of separation processes using residue curve maps: a review[J]. Industrial & Engineering Chemistry Research, 1994, 33(11): 2505-2522. |
| 36 | BARBOSA D, DOHERTY M F. The simple distillation of homogeneous reactive mixtures[J]. Chemical Engineering Science, 1988, 43(3): 541-550. |
| 37 | WALPOT H E. Theoretical modeling of residue curve maps for a reactive distillation concept for the production of n-propyl propionate[D]. Vancouver: the University of British Columbia, 2011. |
| 38 | LUYBEN W L. Distillation design and control using Aspen simulation[M]. New York: John Wiley & Sons Inc., 2013. |
| [1] | CUI Shoucheng, XU Hongbo, PENG Nan. Simulation analysis of two MOFs materials for O2/He adsorption separation [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 382-390. |
| [2] | WANG Zhengkun, LI Sifang. Green synthesis of gemini surfactant decyne diol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 400-410. |
| [3] | LI Shilin, HU Jingze, WANG Yilin, WANG Qingji, SHAO Lei. Research progress in separation and extraction of high value components by electrodialysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 420-429. |
| [4] | XU Ruosi, TAN Wei. Flow field simulation and fluid-structure coupling analysis of C-tube pool boiling two-phase flow model [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 47-55. |
| [5] | ZHANG Fengqi, CUI Chengdong, BAO Xuewei, ZHU Weixuan, DONG Hongguang. Design and evaluation of sweetening process with amine solution absorption and multiple desorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 518-528. |
| [6] | GUO Qiang, ZHAO Wenkai, XIAO Yonghou. Numerical simulation of enhancing fluid perturbation to improve separation of dimethyl sulfide/nitrogen via pressure swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 64-72. |
| [7] | SHAO Boshi, TAN Hongbo. Simulation on the enhancement of cryogenic removal of volatile organic compounds by sawtooth plate [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 84-93. |
| [8] | LI Mengyuan, GUO Fan, LI Qunsheng. Simulation and optimization of the third and fourth distillation columns in the recovery section of polyvinyl alcohol production [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 113-123. |
| [9] | ZHANG Ruijie, LIU Zhilin, WANG Junwen, ZHANG Wei, HAN Deqiu, LI Ting, ZOU Xiong. On-line dynamic simulation and optimization of water-cooled cascade refrigeration system [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 124-132. |
| [10] | WANG Tai, SU Shuo, LI Shengrui, MA Xiaolong, LIU Chuntao. Dynamic behavior of single bubble attached to the solid wall in the AC electric field [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 133-141. |
| [11] | SUN Jipeng, HAN Jing, TANG Yangchao, YAN Bowen, ZHANG Jieyao, XIAO Ping, WU Feng. Numerical simulation and optimization of operating parameters of sulfur wet molding process [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 189-196. |
| [12] | WANG Fu'an. Consumption and emission reduction of the reactor of 300kt/a propylene oxide process [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 213-218. |
| [13] | CHEN Kuangyin, LI Ruilan, TONG Yang, SHEN Jianhua. Structure design of gas diffusion layer in proton exchange membrane fuel cell [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 246-259. |
| [14] | HE Meijin. Application and development trend of molecular management in separation technology in petrochemical field [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 260-266. |
| [15] | YANG Yudi, LI Wentao, QIAN Yongkang, HUI Junhong. Analysis of influencing factors of natural gas turbulent diffusion flame length in industrial combustion chamber [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 267-275. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||