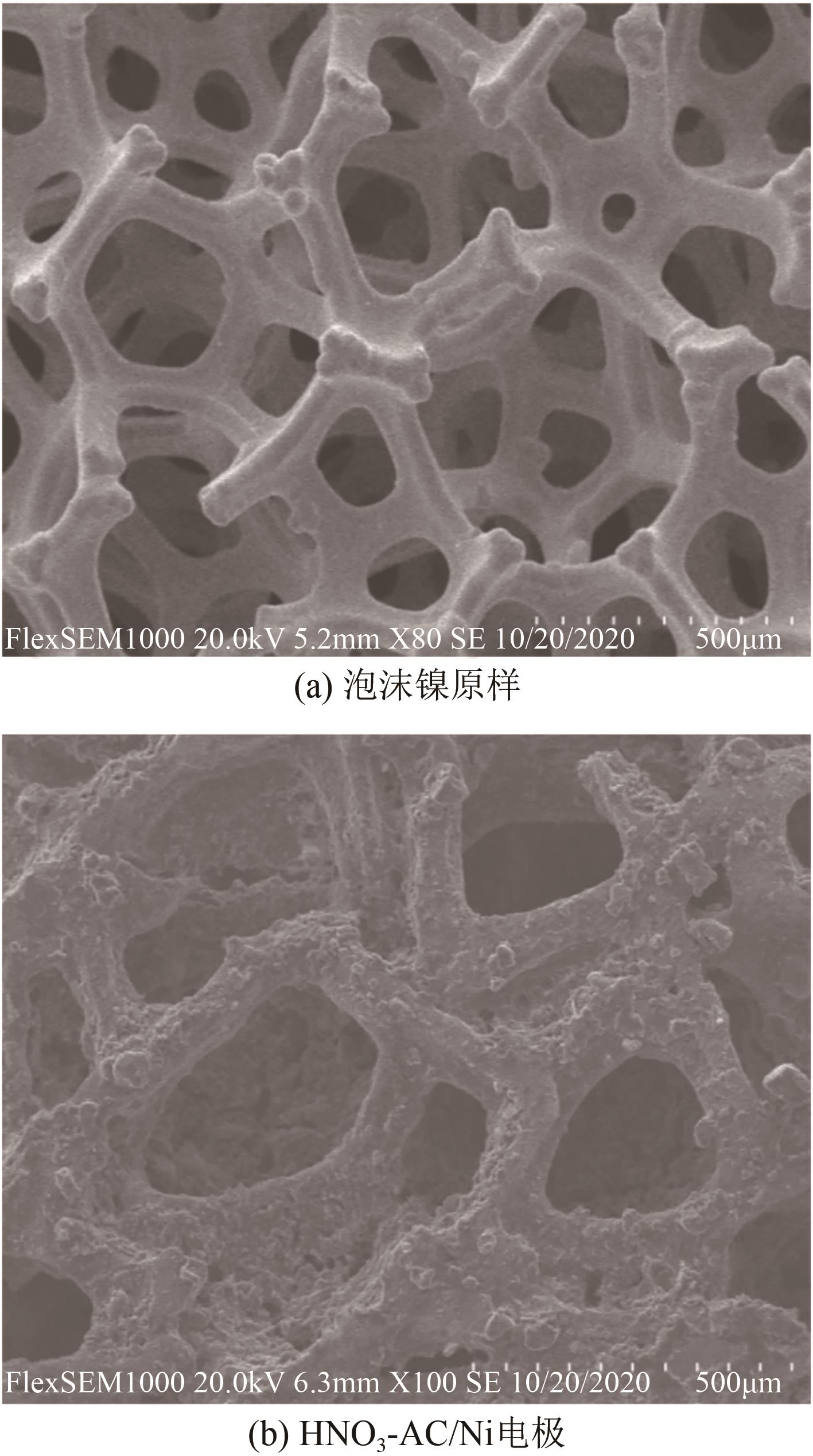
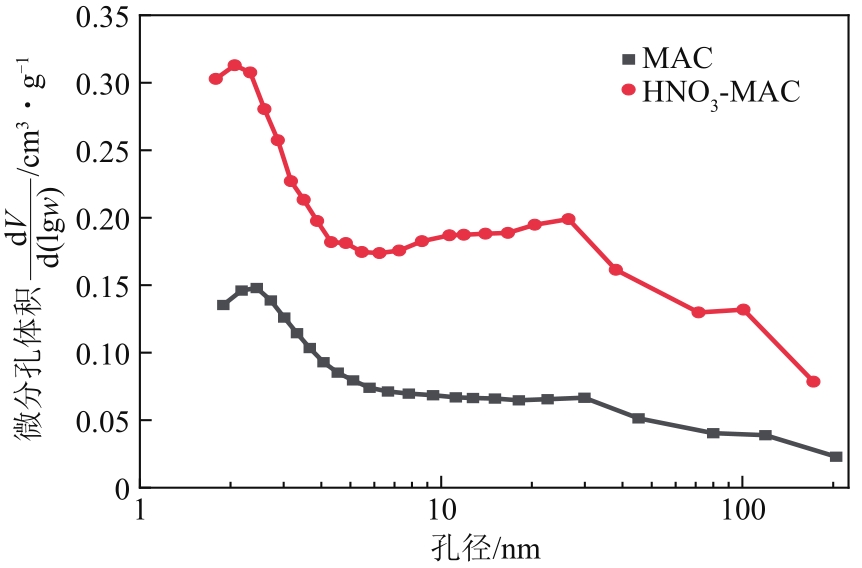
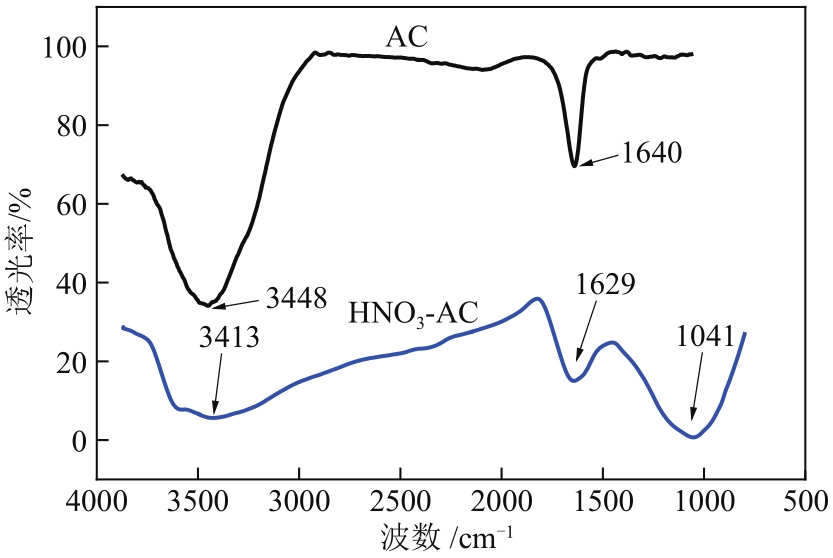
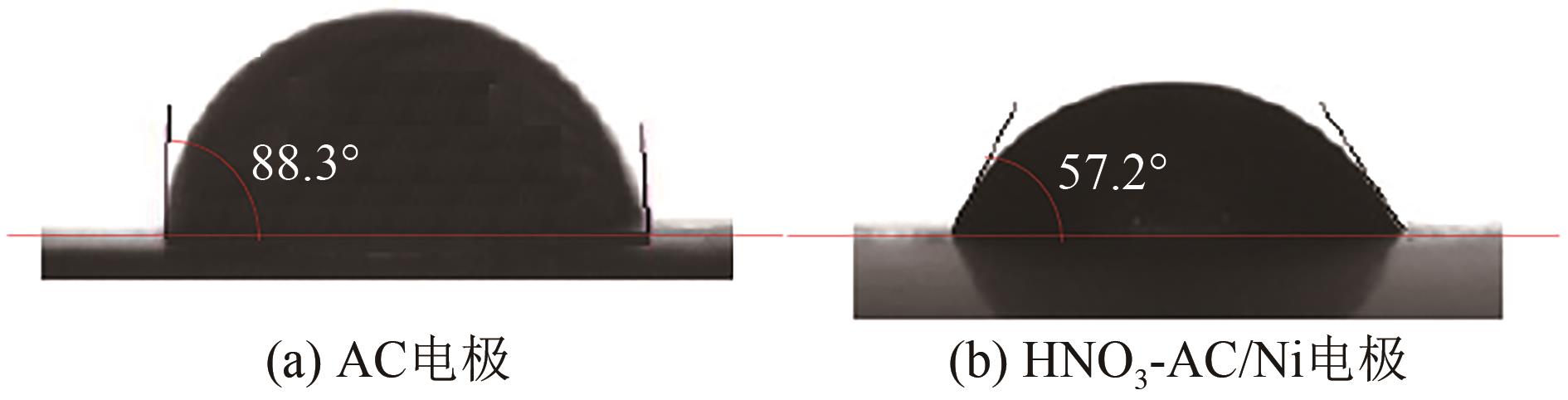
Chemical Industry and Engineering Progress ›› 2022, Vol. 41 ›› Issue (9): 4986-4994.DOI: 10.16085/j.issn.1000-6613.2021-2350
• Materials science and technology • Previous Articles Next Articles
Electric adsorption laws of HNO3-modified activated carbon for different valence ions
YUAN Quan1( ), LI Haihong1(
), LI Haihong1( ), LIU Haojie2
), LIU Haojie2
- 1.College of Environmental and Chemical Engineering, Xi’an Polytechnic University, Xi’an 710048, Shaanxi, China
2.CEC Beijing Survey and Design Institute Co. , Ltd. , Beijing 100024, China
-
Received:2021-11-16Revised:2022-03-21Online:2022-09-27Published:2022-09-25 -
Contact:LI Haihong
HNO3改性活性炭对不同价态离子的电吸附规律
- 1.西安工程大学环境与化学工程学院,陕西 西安 710048
2.中国电建集团北京勘测设计研究院有限公司,北京 100024
-
通讯作者:李海红 -
作者简介:袁权(1998—),男,硕士研究生,研究方向为电化学。E-mail:834878981@qq.com。 -
基金资助:陕西省科技厅社会发展领域项目(2020SF-435);榆林市科技计划(CXY-2020-054)
CLC Number:
Cite this article
YUAN Quan, LI Haihong, LIU Haojie. Electric adsorption laws of HNO3-modified activated carbon for different valence ions[J]. Chemical Industry and Engineering Progress, 2022, 41(9): 4986-4994.
袁权, 李海红, 刘浩杰. HNO3改性活性炭对不同价态离子的电吸附规律[J]. 化工进展, 2022, 41(9): 4986-4994.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2021-2350
| 样品 | 比表面积/m2·g-1 | 总孔容/cm3·g-1 | 平均孔径/nm |
|---|---|---|---|
| AC | 148.17 | 0.16 | 4.3 |
| HNO3-AC | 341.38 | 0.42 | 4.9 |
| 样品 | 比表面积/m2·g-1 | 总孔容/cm3·g-1 | 平均孔径/nm |
|---|---|---|---|
| AC | 148.17 | 0.16 | 4.3 |
| HNO3-AC | 341.38 | 0.42 | 4.9 |
| 改性前后AC | C 1s | O 1s | N 1s |
|---|---|---|---|
| AC | 69.22 | 14.49 | 1.05 |
| HNO3-AC | 65.16 | 17.97 | 2.17 |
| 改性前后AC | C 1s | O 1s | N 1s |
|---|---|---|---|
| AC | 69.22 | 14.49 | 1.05 |
| HNO3-AC | 65.16 | 17.97 | 2.17 |
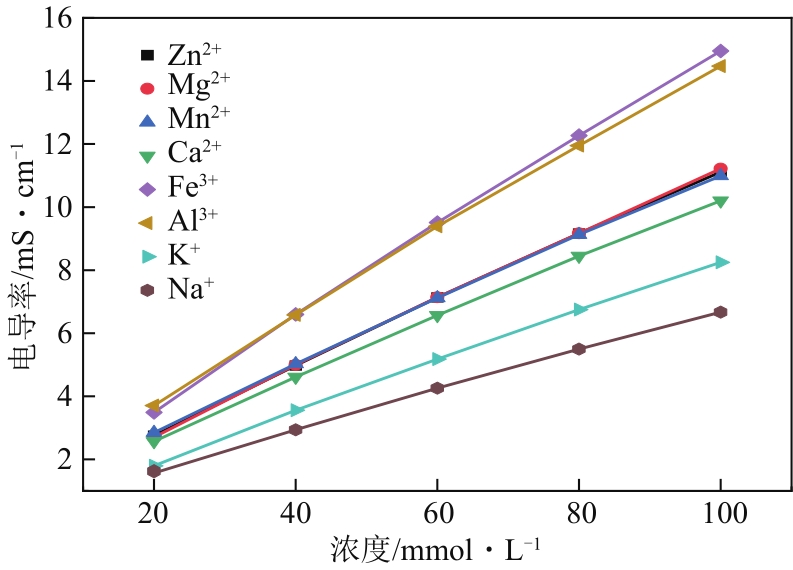
| 离子种类 | σ-c关系方程 | 相关系数R2 |
|---|---|---|
| Zn2+ | σ=0.1046c+0.757 | 0.9993 |
| Mg2+ | σ=0.1061c+0.683 | 0.9993 |
| Mn2+ | σ=0.1019c+0.91 | 0.9991 |
| Ca2+ | σ=0.0955c+0.75 | 0.9991 |
| Fe3+ | σ=0.143c+0.787 | 0.9991 |
| Al3+ | σ=0.1345c+1.153 | 0.9991 |
| K+ | σ=0.0805c+0.281 | 0.9991 |
| Na+ | σ=0.0638c+0.36 | 0.9990 |
| 离子种类 | σ-c关系方程 | 相关系数R2 |
|---|---|---|
| Zn2+ | σ=0.1046c+0.757 | 0.9993 |
| Mg2+ | σ=0.1061c+0.683 | 0.9993 |
| Mn2+ | σ=0.1019c+0.91 | 0.9991 |
| Ca2+ | σ=0.0955c+0.75 | 0.9991 |
| Fe3+ | σ=0.143c+0.787 | 0.9991 |
| Al3+ | σ=0.1345c+1.153 | 0.9991 |
| K+ | σ=0.0805c+0.281 | 0.9991 |
| Na+ | σ=0.0638c+0.36 | 0.9990 |
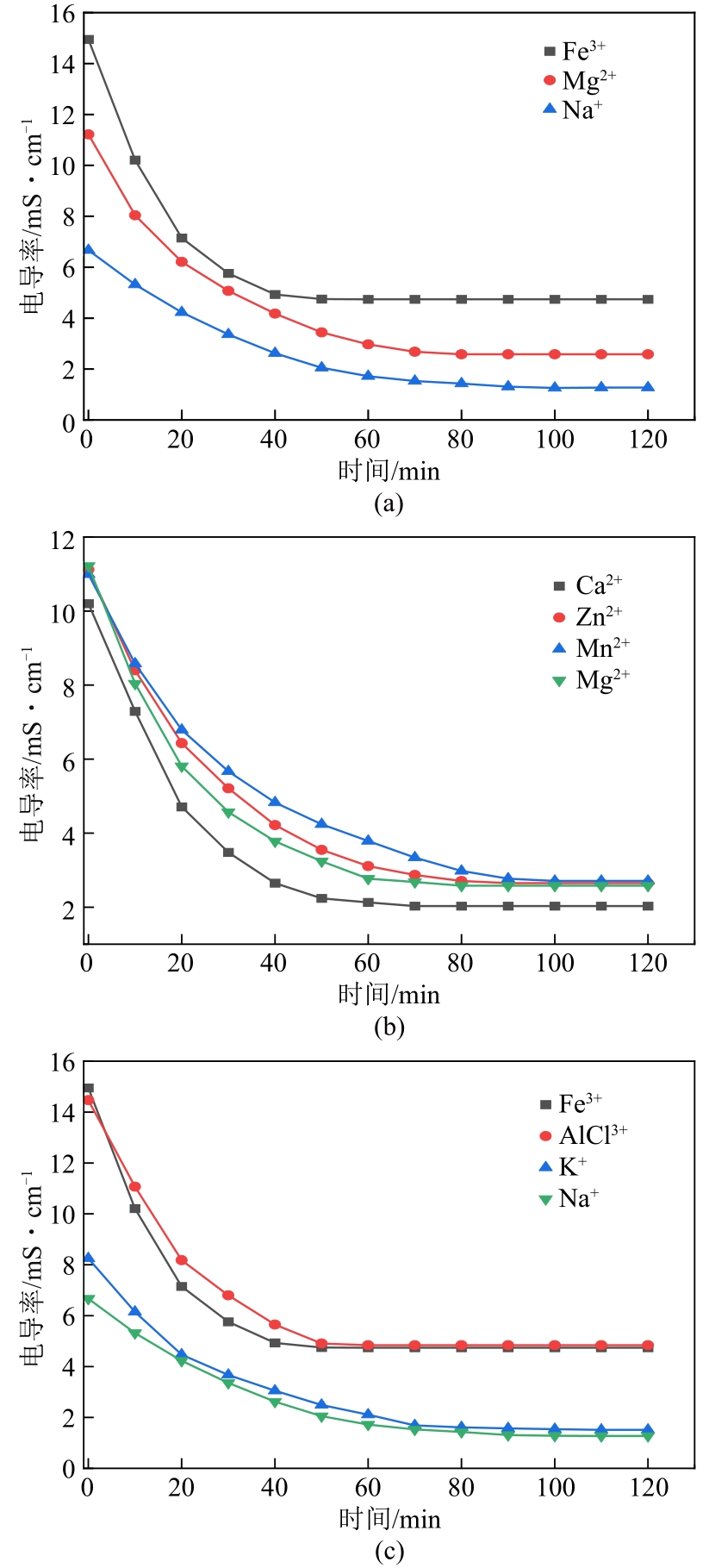
| 参数 | 一价离子 | 二价离子 | 三价离子 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Na+ | K+ | Mn2+ | Zn2+ | Mg2+ | Ca2+ | Al3+ | Fe3+ | |||
| 去除率/% | 80.86 | 81.7 | 75.34 | 76.17 | 77 | 80.1 | 66.55 | 68.29 | ||
| 吸附平衡时间/min | 100 | 95 | 90 | 85 | 80 | 70 | 55 | 50 | ||
| 水合离子半径/nm | 0.358 | 0.331 | 0.438 | 0.43 | 0.428 | 0.412 | 0.475 | 0.457 | ||
| 参数 | 一价离子 | 二价离子 | 三价离子 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Na+ | K+ | Mn2+ | Zn2+ | Mg2+ | Ca2+ | Al3+ | Fe3+ | |||
| 去除率/% | 80.86 | 81.7 | 75.34 | 76.17 | 77 | 80.1 | 66.55 | 68.29 | ||
| 吸附平衡时间/min | 100 | 95 | 90 | 85 | 80 | 70 | 55 | 50 | ||
| 水合离子半径/nm | 0.358 | 0.331 | 0.438 | 0.43 | 0.428 | 0.412 | 0.475 | 0.457 | ||
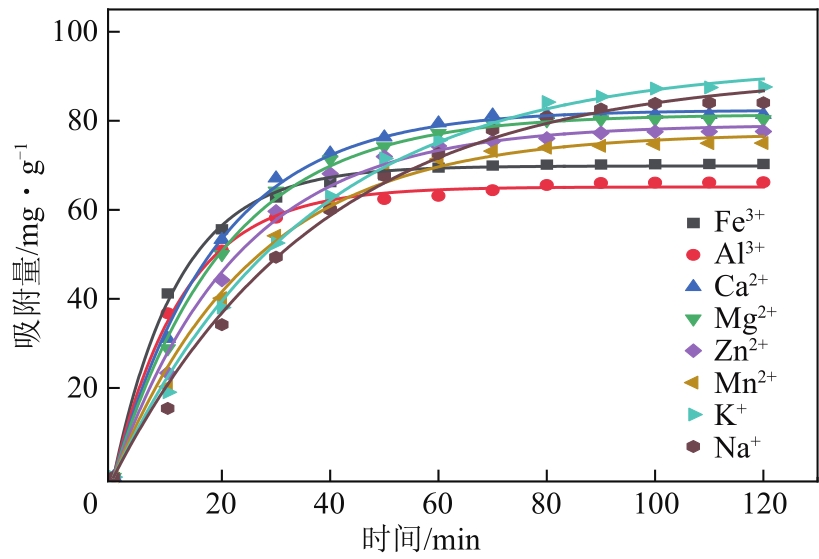
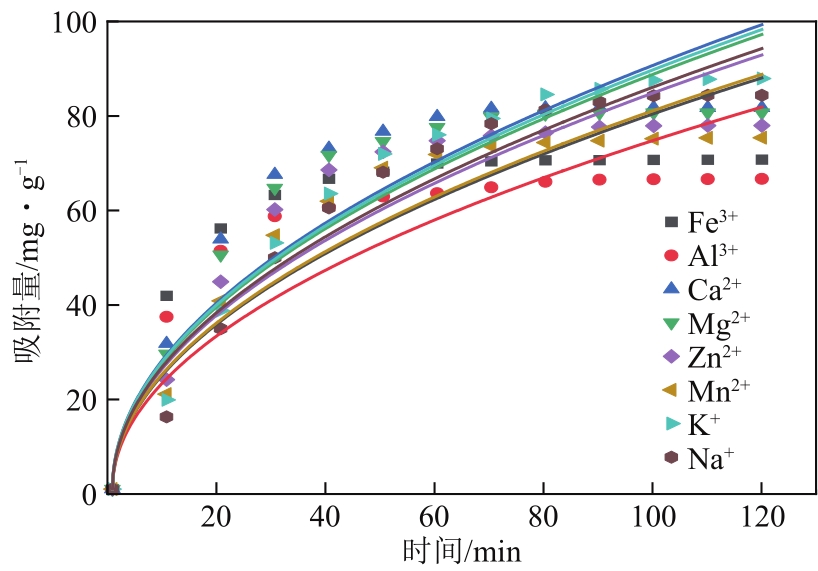
| 离子种类 | qe/mg·g-1 | k1/min-1 | R2 |
|---|---|---|---|
| Fe3+ | 69.84203 | 0.08277 | 0.99792 |
| Al3+ | 65.14445 | 0.07696 | 0.99598 |
| Ca2+ | 82.3935 | 0.05238 | 0.9981 |
| Mg2+ | 81.41524 | 0.04876 | 0.99792 |
| Zn2+ | 79.13729 | 0.04362 | 0.99351 |
| Mn2+ | 77.26577 | 0.03864 | 0.99389 |
| K+ | 92.71654 | 0.02779 | 0.99718 |
| Na+ | 90.71137 | 0.02605 | 0.99354 |
| 离子种类 | qe/mg·g-1 | k1/min-1 | R2 |
|---|---|---|---|
| Fe3+ | 69.84203 | 0.08277 | 0.99792 |
| Al3+ | 65.14445 | 0.07696 | 0.99598 |
| Ca2+ | 82.3935 | 0.05238 | 0.9981 |
| Mg2+ | 81.41524 | 0.04876 | 0.99792 |
| Zn2+ | 79.13729 | 0.04362 | 0.99351 |
| Mn2+ | 77.26577 | 0.03864 | 0.99389 |
| K+ | 92.71654 | 0.02779 | 0.99718 |
| Na+ | 90.71137 | 0.02605 | 0.99354 |
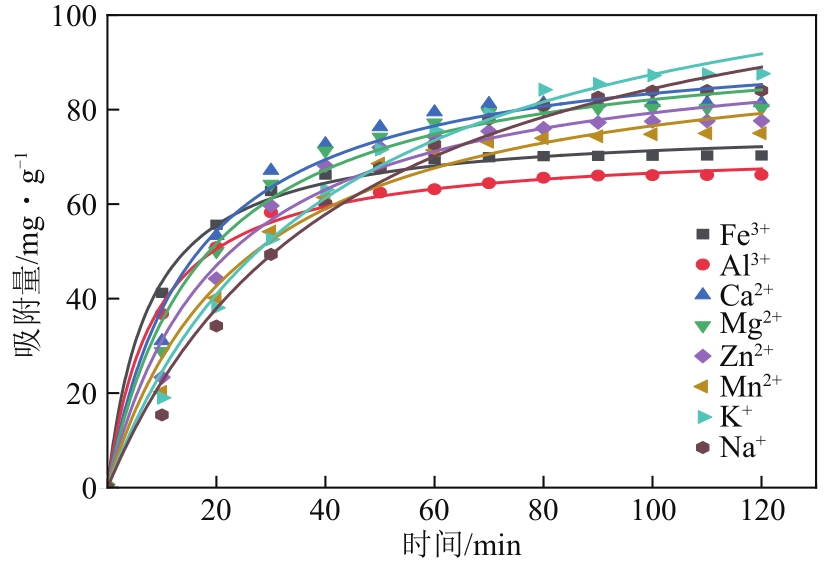
| 离子种类 | qe/mg·g-1 | k2/min-1 | R2 |
|---|---|---|---|
| Fe3+ | 76.74263 | 0.00171 | 0.99503 |
| Al3+ | 72.30318 | 0.00160 | 0.99679 |
| Ca2+ | 96.3138 | 0.00007 | 0.98231 |
| Mg2+ | 96.30625 | 0.00060 | 0.98293 |
| Zn2+ | 95.72711 | 0.00050 | 0.97558 |
| Mn2+ | 95.52012 | 0.00042 | 0.97795 |
| K+ | 122.19321 | 0.00021 | 0.98011 |
| Na+ | 121.75304 | 0.00019 | 0.98467 |
| 离子种类 | qe/mg·g-1 | k2/min-1 | R2 |
|---|---|---|---|
| Fe3+ | 76.74263 | 0.00171 | 0.99503 |
| Al3+ | 72.30318 | 0.00160 | 0.99679 |
| Ca2+ | 96.3138 | 0.00007 | 0.98231 |
| Mg2+ | 96.30625 | 0.00060 | 0.98293 |
| Zn2+ | 95.72711 | 0.00050 | 0.97558 |
| Mn2+ | 95.52012 | 0.00042 | 0.97795 |
| K+ | 122.19321 | 0.00021 | 0.98011 |
| Na+ | 121.75304 | 0.00019 | 0.98467 |
| 离子种类 | kn /min-1 | R2 |
|---|---|---|
| Fe3+ | 8.01149 | 0.46896 |
| Al3+ | 7.44364 | 0.40818 |
| Ca2+ | 9.04892 | 0.41207 |
| Mg2+ | 8.8568 | 0.37921 |
| Zn2+ | 8.45852 | 0.34302 |
| Mn2+ | 8.07978 | 0.29454 |
| K+ | 8.95356 | 0.21725 |
| Na+ | 8.58248 | 0.22528 |
| 离子种类 | kn /min-1 | R2 |
|---|---|---|
| Fe3+ | 8.01149 | 0.46896 |
| Al3+ | 7.44364 | 0.40818 |
| Ca2+ | 9.04892 | 0.41207 |
| Mg2+ | 8.8568 | 0.37921 |
| Zn2+ | 8.45852 | 0.34302 |
| Mn2+ | 8.07978 | 0.29454 |
| K+ | 8.95356 | 0.21725 |
| Na+ | 8.58248 | 0.22528 |
| 1 | 马双忱, 刘畅, 马岚, 等. 电吸附用于微污染水处理: 技术选择、工艺原理、未来发展[J]. 化工进展, 2020, 39(7): 2841-2849. |
| MA Shuangchen, LIU Chang, MA Lan, et al. Electro-adsorption for micro-polluted water treatment: technology selection, process principle, future development[J]. Chemical Industry and Engineering Progress, 2020, 39(7): 2841-2849. | |
| 2 | MAHER M, HASSAN S, SHOUEIR K, et al. Activated carbon electrode with promising specific capacitance based on potassium bromide redox additive electrolyte for supercapacitor application[J]. Journal of Materials Research and Technology, 2021, 11: 1232-1244. |
| 3 | FOLARANMI G, BECHELANY M, SISTAT P, et al. Comparative investigation of activated carbon electrode and a novel activated carbon/graphene oxide composite electrode for an enhanced capacitive deionization[J]. Materials, 2020, 13(22): 5185. |
| 4 | ALENCHERRY T, A R N, GHOSH S, et al. Effect of increasing electrical conductivity and hydrophilicity on the electrosorption capacity of activated carbon electrodes for capacitive deionization[J]. Desalination, 2017, 415: 14-19. |
| 5 | CHEN Zhaolin, SONG Cunyi, SUN Xiaowei, et al. Kinetic and isotherm studies on the electrosorption of NaCl from aqueous solutions by activated carbon electrodes[J]. Desalination, 2011, 267(2/3): 239-243. |
| 6 | 骆青虎, 武福平, 李锡锋, 等. 碱改性活性炭纤维电吸附处理RO浓水效果及除盐动力学特性[J]. 环境工程学报, 2019, 13(11): 2545-2552. |
| LUO Qinghu, WU Fuping, LI Xifeng, et al. Electrosorption effect and desalination kinetic characteristics of reverse osmosis concentrated water with alkaline modified activated carbon fiber electrode[J]. Chinese Journal of Environmental Engineering, 2019, 13(11): 2545-2552. | |
| 7 | MORENO-CASTILLA C, CARRASCO-MARÍN F, MALDONADO-HÓDAR F J, et al. Effects of non-oxidant and oxidant acid treatments on the surface properties of an activated carbon with very low ash content[J]. Carbon, 1998, 36(1/2): 145-151. |
| 8 | 宾齐, 李海红, 张田田. 活化剂改性秸秆基活性炭的制备及其表征[J]. 纺织高校基础科学学报, 2020, 33(4): 111-117. |
| Qi BIN, LI Haihong, ZHANG Tiantian. Preparation and characterization of cotton stalk-based activated carbon modified by activator[J]. Basic Sciences Journal of Textile Universities, 2020, 33(4): 111-117. | |
| 9 | 李海红, 李红艳, 夏禹周. 活性炭涂层电极的制备及其电化学性能[J]. 材料科学与工程学报, 2014, 32(1): 101-106. |
| LI Haihong, LI Hongyan, XIA Yuzhou. Preparation and electrochemical performance of activated carbon coated electrodes[J]. Journal of Materials Science and Engineering, 2014, 32(1): 101-106. | |
| 10 | 李海红, 张超, 董军旗, 等. 活性炭负载TiO2改性处理及其性能表征[J]. 粉末冶金材料科学与工程, 2015, 20(3): 438-443. |
| LI Haihong, ZHANG Chao, DONG Junqi, et al. Preparation and characterization of active carbon material modified by TiO2 [J]. Materials Science and Engineering of Powder Metallurgy, 2015, 20(3): 438-443. | |
| 11 | 陈小娟, 张伟庆, 余小岚, 等. 适用于本科教学的BET比表面测定实验[J]. 大学化学, 2017, 32(7): 60-67. |
| CHEN Xiaojuan, ZHANG Weiqing, YU Xiaolan, et al. Measuring BET specific surface area: a new experiment for undergraduate teaching[J]. University Chemistry, 2017, 32(7): 60-67. | |
| 12 | CYCHOSZ STRUCKHOFF K, THOMMES M, SARKISOV L. On the universality of capillary condensation and adsorption hysteresis phenomena in ordered and crystalline mesoporous materials[J]. Advanced Materials Interfaces, 2020, 7(12): 2000184. |
| 13 | 王芳平, 马婧, 李小亚, 等. 板栗壳生物炭高性能对称性超级电容器电极材料的制备及性能[J]. 化工进展, 2021, 40(8): 4381-4387. |
| WANG Fangping, MA Jing, LI Xiaoya, et al. Preparation and properties of chestnut shell-based biochar electrode material for high-performance symmetrical supercapacitor[J]. Chemical Industry and Engineering Progress, 2021, 40(8): 4381-4387. | |
| 14 | 屈笑笑, 邢宝林, 康伟伟, 等. 玉米芯电容炭的制备及其电化学性能[J]. 化工进展, 2018, 37(6): 2340-2346. |
| QU Xiaoxiao, XING Baolin, KANG Weiwei, et al. Preparation and electrochemical performance of capacitive carbon derived from corncob[J]. Chemical Industry and Engineering Progress, 2018, 37(6): 2340-2346. | |
| 15 | 赵思宇, 李曜, 樊晗晗, 等. 蔗渣基活性炭孔结构的调控及其对超级电容器电化学性能的影响研究[J]. 中国造纸学报, 2021, 36(2): 39-49. |
| ZHAO Siyu, LI Yao, FAN Hanhan, et al. Regulation of the pore structure of bagasse-based activated carbon and its effect on the electrochemical properties of supercapacitor[J]. Transactions of China Pulp and Paper, 2021, 36(2): 39-49. | |
| 16 | SETIARSO P, SARI N P. Graphene oxide-paraffin-nanobentonite as working electrode for cyclic voltammetry analysis for nicotinic acid[J]. Asian Journal of Chemistry, 2021, 33(4): 757-761. |
| 17 | WADHAI S, JADHAV Y, THAKUR P. Synthesis of metal-free phosphorus doped graphitic carbon nitride-P25 (TiO2) composite: characterization, cyclic voltammetry and photocatalytic hydrogen evolution[J]. Solar Energy Materials and Solar Cells, 2021, 223: 110958. |
| 18 | KILIC A, EROGLU D. Characterization of the effect of cell design on Li-S battery resistance using electrochemical impedance spectroscopy[J]. ChemElectroChem, 2021, 8(5): 963-971. |
| [1] | ZHANG Lihong, JIN Yaoru, CHENG Fangqin. Resource utilization of coal gasification slag [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4447-4457. |
| [2] | ZHANG Yaojie, ZHANG Chuanxiang, SUN Yue, ZENG Huihui, JIA Jianbo, JIANG Zhendong. Application of coal-based graphene quantum dots in supercapacitors [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4340-4350. |
| [3] | XING Xianjun, LUO Tian, BU Yuzheng, MA Peiyong. Preparation of biochar from walnut shells activated by H3PO4 and its application in Cr(Ⅵ) adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1527-1539. |
| [4] | LIU Yajuan. Research status of membrane fouling mitigation by PAC in submerged PAC-AMBRs [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 457-468. |
| [5] | LIU Nan, HU Yiming, YANG Ying, LI Hongjin, GAO Zhuqing, HAO Xiuli. Microwave assisted co-pyrolysis of waste polypropylene /activated carbon to produce combustible pyrolysis gas and light pyrolysis oil [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 150-159. |
| [6] | CHEN Xiaoyun, GUO Yadong, DI Lu, BI Dongmei, LI Kaikai, LIN Xiaona. Catalytic co-pyrolysis of biomass and plastic for aromatics production with boron doped activated carbon catalyst [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 199-209. |
| [7] | ZHANG Xinhai, ZHAO Sichen, ZHU Hui, WANG Kai, ZHANG Shoushi. Application of activated carbon fiber supported desulfurizer in mine gas environment [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 415-423. |
| [8] | ZHANG Xinhai, ZHAO Sichen, ZHU Hui, ZHANG Shoushi, WANG Kai. Comparative study on desulfurization performance of various carbon materials combined with sodium carbonate [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 424-435. |
| [9] | QI Yuan, XU Xinrong, RUAN Wei, WU Hao, WU Ke, ZHOU Yaming, YANG Hongmin. Characterization of aniline adsorption by modified activated carbon fiber [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 622-630. |
| [10] | SUN Xianhang, REN Zhu, ZHANG Guojun, SUN Yuan, FAN Kaifeng, HUANG Weiqiu. Study on the desorption mechanism of toluene in activated carbon under supercritical CO2 [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 631-636. |
| [11] | HE Chenlu, QIU Chenxi, FANG Juan, YANG Xuan, LAI Jianjun, ZHENG Xinyu, LYU Jianhua, CHEN Yandan, HUANG Biao. Nitrogen-doped supercapacitor carbon based on eutectic solvent system [J]. Chemical Industry and Engineering Progress, 2022, 41(9): 4946-4953. |
| [12] | XIONG Yongzhi, LIU Yanyan, CHEN Xiaohong, LU Beili, HUANG Biao, LIN Guanfeng. Preparation and electrochemical performance of bagasse-based phosphorus-doped activated carbon [J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4397-4405. |
| [13] | HUANG Ping’an, XU Jun, YANG Yuxuan, PAN Yuhan, WANG Xinwen, HUANG Qunxing. Ball milled modified pyrolysis carbon adsorb sulfamethoxazole [J]. Chemical Industry and Engineering Progress, 2022, 41(7): 3784-3793. |
| [14] | CAI Sichao, ZHOU Jing, DU Jinze, LI Fangzhou, LI Yuansen, HE Lin, LI Xingang, WANG Chengyang. Process analysis of resource utilization of phenol-based distillation residue from coal chemical industry [J]. Chemical Industry and Engineering Progress, 2022, 41(6): 3360-3371. |
| [15] | CHEN Yong, MA Yannan, XU Cheng. Electrochemical performance of activated carbon electrode adsorbing electroactive dyes [J]. Chemical Industry and Engineering Progress, 2022, 41(5): 2537-2545. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||