Chemical Industry and Engineering Progress ›› 2022, Vol. 41 ›› Issue (4): 2090-2101.DOI: 10.16085/j.issn.1000-6613.2021-0740
• Resources and environmental engineering • Previous Articles Next Articles
Recent developments of phase-change absorption technology for CO2 capture from flue gas
ZHANG Weifeng( ), ZHOU Wu, WANG Qiuhua
), ZHOU Wu, WANG Qiuhua
- School of Civil Engineering and Architecture, East China Jiaotong University, Nanchang 330013, Jiangxi, China
-
Received:2021-04-08Revised:2021-06-23Online:2022-04-25Published:2022-04-23 -
Contact:ZHANG Weifeng
相变吸收捕集烟气中CO2技术的发展现状
- 华东交通大学土木建筑学院,江西 南昌 330013
-
通讯作者:张卫风 -
作者简介:张卫风(1977—),男,博士,副教授,研究方向为大气污染及其控制、温室气体CO2减排。E-mail:wfzhang2002@126.com 。
CLC Number:
Cite this article
ZHANG Weifeng, ZHOU Wu, WANG Qiuhua. Recent developments of phase-change absorption technology for CO2 capture from flue gas[J]. Chemical Industry and Engineering Progress, 2022, 41(4): 2090-2101.
张卫风, 周武, 王秋华. 相变吸收捕集烟气中CO2技术的发展现状[J]. 化工进展, 2022, 41(4): 2090-2101.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2021-0740
| 类型 | 体系 | 特点 |
|---|---|---|
| 单元基吸收剂 | MDEA+正丁醇+水 | 最优条件下吸收剂在膜器内停留时间仅为数秒,CO2的解吸率均高于80%,最高可达92%[ |
| MEA+叔丁醇+水 | 相比于质量分数为30%的MEA水溶液,MEA/叔丁醇/水相变吸收剂具有更高的循环负载和更少的解吸液量[ | |
| MEA+1-丙醇+水 | 与传统的质量分数为30%的MEA水溶液相比,初始吸收率更高,循环负载量增加了一半[ | |
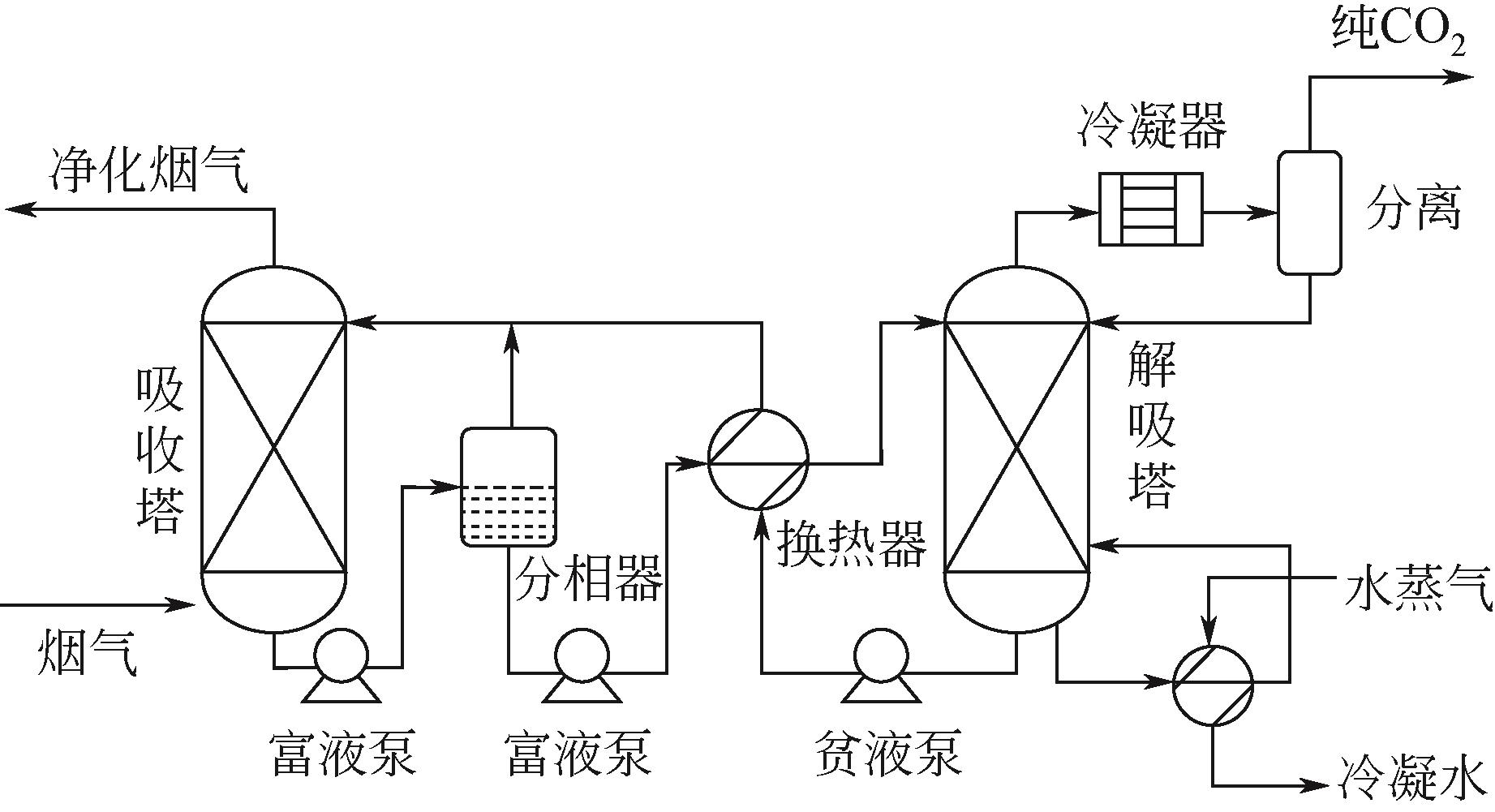
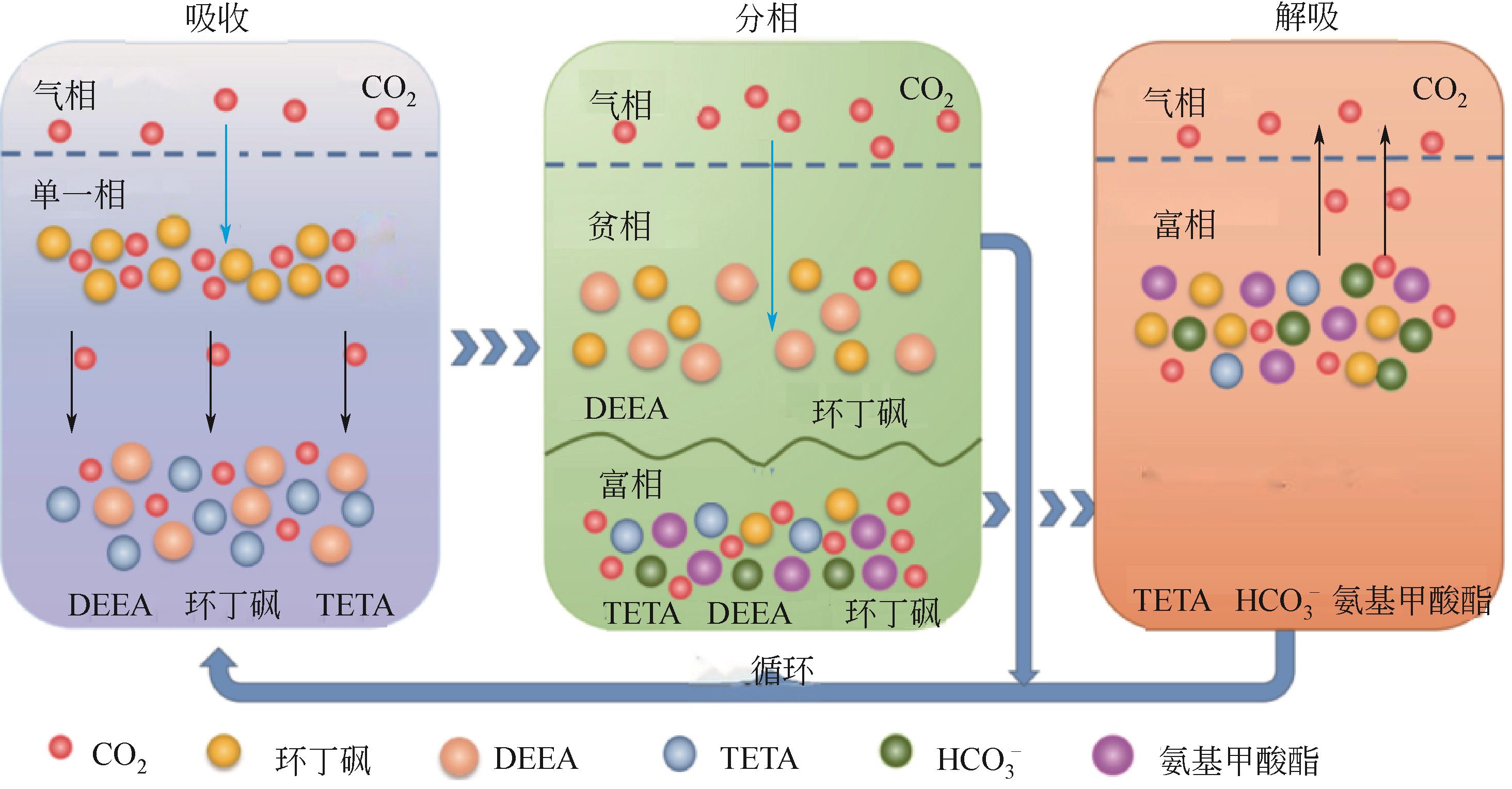
| 多元混合吸收剂 | TETA+DEEA | 下层相吸收CO2达到90%以上,合适的吸收和解吸温度分别为30℃和90℃[ |
| DMCA+TETA | 有较高的CO2吸收循环能力,良好的相分离行为和较低的再生热。与5mol/L MEA相比,TETA-DMCA的再生能耗可降低约40%[ | |
| MAPA+DEEA | 具有较低的挥发性,相比于质量分数为30%的MEA水溶液其再生能耗可以进一步降低[ | |
| DMCA+MCA+AMP | 有良好的相变温度,高CO2净负荷以及良好的抗氧化降解和热降解能力,且能耗可降至2.0GJ·(t CO2)-1 以下[ | |
| 无水吸收剂 | MEA+三甘醇 | 醇不参与反应,仅MEA和CO2反应,MEA浓度为5mol/L,吸收剂循环处理量高,能耗接近 2.0GJ·(t CO2)-1,优于MEA+水吸收剂[ |
| MEA+环丁砜 | MEA/环丁砜相变溶剂的总再生能耗为2.67GJ·(t CO2)-1,与传统的5mol/L MEA相比,降低了31%[ |
| 类型 | 体系 | 特点 |
|---|---|---|
| 单元基吸收剂 | MDEA+正丁醇+水 | 最优条件下吸收剂在膜器内停留时间仅为数秒,CO2的解吸率均高于80%,最高可达92%[ |
| MEA+叔丁醇+水 | 相比于质量分数为30%的MEA水溶液,MEA/叔丁醇/水相变吸收剂具有更高的循环负载和更少的解吸液量[ | |
| MEA+1-丙醇+水 | 与传统的质量分数为30%的MEA水溶液相比,初始吸收率更高,循环负载量增加了一半[ | |
| 多元混合吸收剂 | TETA+DEEA | 下层相吸收CO2达到90%以上,合适的吸收和解吸温度分别为30℃和90℃[ |
| DMCA+TETA | 有较高的CO2吸收循环能力,良好的相分离行为和较低的再生热。与5mol/L MEA相比,TETA-DMCA的再生能耗可降低约40%[ | |
| MAPA+DEEA | 具有较低的挥发性,相比于质量分数为30%的MEA水溶液其再生能耗可以进一步降低[ | |
| DMCA+MCA+AMP | 有良好的相变温度,高CO2净负荷以及良好的抗氧化降解和热降解能力,且能耗可降至2.0GJ·(t CO2)-1 以下[ | |
| 无水吸收剂 | MEA+三甘醇 | 醇不参与反应,仅MEA和CO2反应,MEA浓度为5mol/L,吸收剂循环处理量高,能耗接近 2.0GJ·(t CO2)-1,优于MEA+水吸收剂[ |
| MEA+环丁砜 | MEA/环丁砜相变溶剂的总再生能耗为2.67GJ·(t CO2)-1,与传统的5mol/L MEA相比,降低了31%[ |
| 类型 | 体系 | 特点 |
|---|---|---|
| 氨基酸盐溶液 | 牛磺酸钾 | 固液分离温度为40℃,与30% MEA基准相比,可将捕获过程的总能量降低15%[ |
| K2CO3溶液 | 高浓度K2CO3溶液 | 低成本、低毒性、不易挥发,能够吸收多杂质的CO2、SO x 和NO x 以及有价值的副产物的产生[ |
| K2CO3溶液+MEA | 向K2CO3中添加MEA会有效提高吸收速率[ | |
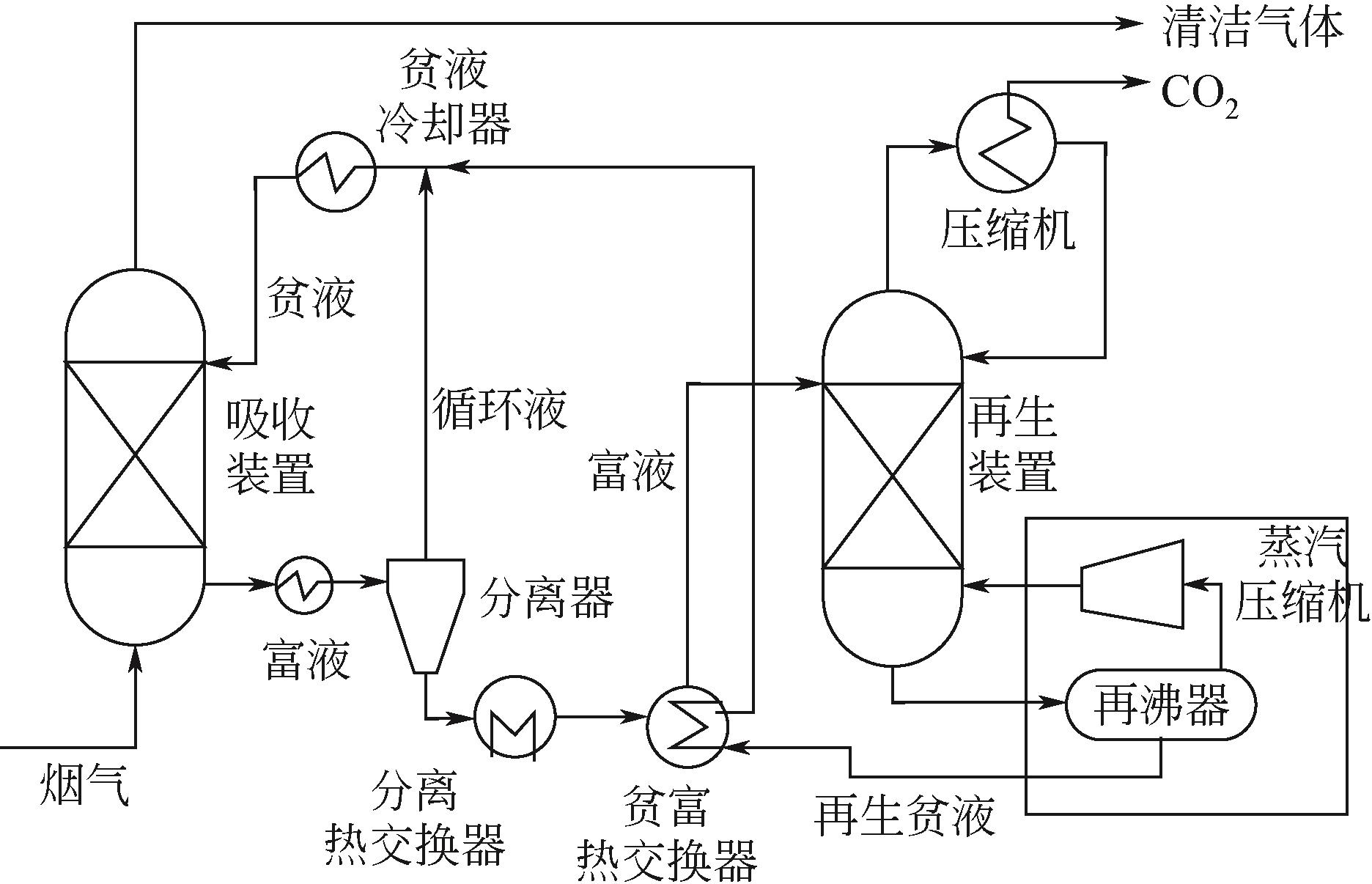
| 冷氨水溶液 | 冷氨水溶液 | 低成本,化学稳定和对氧气的高稳定性,较高的CO2负载能力[ |
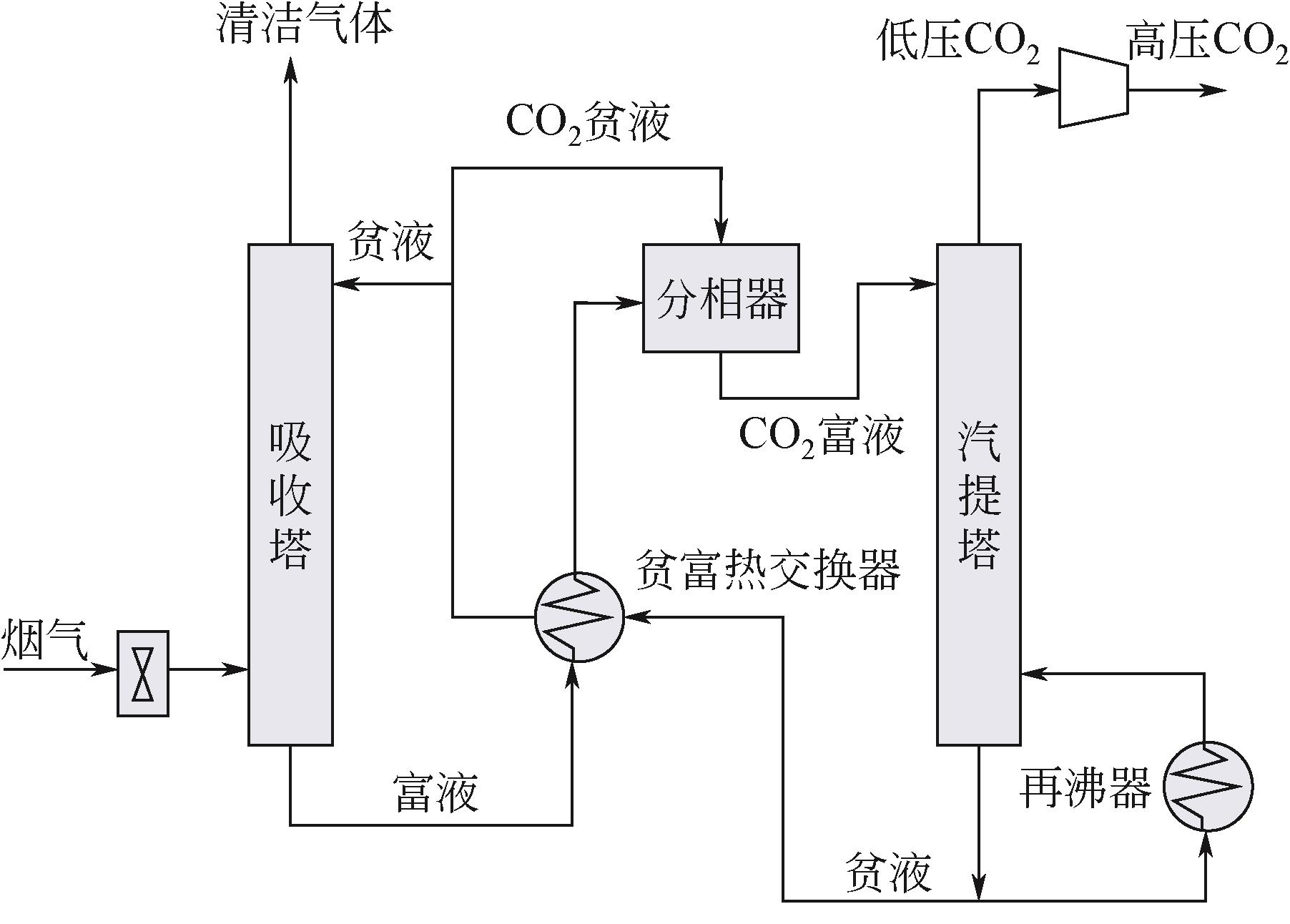
| 非水溶液吸收剂 | 脯氨酸钾+乙醇 | 与30% MEA水溶液相比,使用脯氨酸钾+乙醇溶液可以显著提高CO2的溶解度,尤其在低CO2负荷下[ |
| AMP/PZ/DME | 具有高吸收负荷和再生效率,吸收CO2后,富含CO2的下相体积占溶液总体积的43%,而约占总负载的94%[ |
| 类型 | 体系 | 特点 |
|---|---|---|
| 氨基酸盐溶液 | 牛磺酸钾 | 固液分离温度为40℃,与30% MEA基准相比,可将捕获过程的总能量降低15%[ |
| K2CO3溶液 | 高浓度K2CO3溶液 | 低成本、低毒性、不易挥发,能够吸收多杂质的CO2、SO x 和NO x 以及有价值的副产物的产生[ |
| K2CO3溶液+MEA | 向K2CO3中添加MEA会有效提高吸收速率[ | |
| 冷氨水溶液 | 冷氨水溶液 | 低成本,化学稳定和对氧气的高稳定性,较高的CO2负载能力[ |
| 非水溶液吸收剂 | 脯氨酸钾+乙醇 | 与30% MEA水溶液相比,使用脯氨酸钾+乙醇溶液可以显著提高CO2的溶解度,尤其在低CO2负荷下[ |
| AMP/PZ/DME | 具有高吸收负荷和再生效率,吸收CO2后,富含CO2的下相体积占溶液总体积的43%,而约占总负载的94%[ |
| 试剂 | 浓度 | 相变 形式 | 再生能耗 /MJ·(kg CO2)-1 | 参考 文献 |
|---|---|---|---|---|
| MEA | 30% | — | 4.22 | [ |
| MEA/1-丙醇/水 | 30% | 液-液 | 2.40 | [ |
| MEA/环丁砜 | 4mol/L | 液-液 | 2.67 | [ |
| TETA/DEEA | 5mol/L | 液-液 | 2.7 | [ |
| TETA/DMCA | 4mol/L | 液-液 | 2.6 | [ |
| MAPA/DEEA | 7mol/L | 液-液 | 2.2~2.4 | [ |
| AEEA/DEEA | 20%+60% | 液-液 | 2.46 | [ |
| DMCA/MCA/AMP | (3+1+1)mol/L | 液-液 | 2.0 | [ |
| 脯氨酸钾/乙醇 | 3.3~5.9mol/kg乙醇 | 液-固 | 2.0~2.5 | [ |
| 牛磺酸钾溶液 | 4mol/L | 液-固 | 3.25 | [ |
| AMP/PZ/DME | 1mol/kg | 液-固 | 1.61 | [ |
| K2CO3溶液 | 20%~40% | 液-固 | 2.0~2.5 | [ |
| 冷氨水溶液 | 30% | 液-固 | 2.5 | [ |
| 试剂 | 浓度 | 相变 形式 | 再生能耗 /MJ·(kg CO2)-1 | 参考 文献 |
|---|---|---|---|---|
| MEA | 30% | — | 4.22 | [ |
| MEA/1-丙醇/水 | 30% | 液-液 | 2.40 | [ |
| MEA/环丁砜 | 4mol/L | 液-液 | 2.67 | [ |
| TETA/DEEA | 5mol/L | 液-液 | 2.7 | [ |
| TETA/DMCA | 4mol/L | 液-液 | 2.6 | [ |
| MAPA/DEEA | 7mol/L | 液-液 | 2.2~2.4 | [ |
| AEEA/DEEA | 20%+60% | 液-液 | 2.46 | [ |
| DMCA/MCA/AMP | (3+1+1)mol/L | 液-液 | 2.0 | [ |
| 脯氨酸钾/乙醇 | 3.3~5.9mol/kg乙醇 | 液-固 | 2.0~2.5 | [ |
| 牛磺酸钾溶液 | 4mol/L | 液-固 | 3.25 | [ |
| AMP/PZ/DME | 1mol/kg | 液-固 | 1.61 | [ |
| K2CO3溶液 | 20%~40% | 液-固 | 2.0~2.5 | [ |
| 冷氨水溶液 | 30% | 液-固 | 2.5 | [ |
| 69 | GAO Hongxia, RONGWONG Wichitpan, PENG Chao, et al. Thermal and oxidative degradation of aqueous N,N-diethylethanolamine (DEEA) at stripping conditions for CO2 capture[J]. Energy Procedia, 2014, 63: 1911-1918. |
| 70 | 刘畅, 陈旭, 杨江. CO2腐蚀及其缓蚀剂应用研究进展[J]. 化工进展, 2021, 39(6): 1-13. |
| LIU Chang, CHEN Xu, YANG Jiang. Progress in CO2 corrosion and application of corrosion inhibitors[J]. Chemical Industry and Engineering Progress, 2021, 39(6): 1-13. | |
| 71 | 杨钧晗. 醇胺水溶液吸收CO2过程的腐蚀特性研究[D]. 北京: 华北电力大学, 2018. |
| YANG Junhan. Study on corrosion characteristics of CO2 absorption of amine aqueous solution[D]. Beijing: North China Electric Power University, 2018. | |
| 72 | TZIRAKIS F, TSIVINTZELIS I, PAPADOPOULOS A I, et al. Experimental measurement and assessment of equilibrium behaviour for phase change solvents used in CO2 capture[J]. Chemical Engineering Science, 2019, 199: 20-27. |
| 73 | 方童波, 赵兵涛, 王大淇, 等. 二元复合溶液脱除烟气中CO2的过程模拟与评价[J]. 化工进展, 2019, 38(3): 1561-1566. |
| FANG Tongbo, ZHAO Bingtao, WANG Daqi, et al. Process simulation and evaluation of CO2 removal from flue gas by binary compound solutions[J]. Chemical Industry and Engineering Progress, 2019, 38(3): 1561-1566. | |
| 1 | 乔明, 李雪静, 周笑洋. 新形势下石油公司二氧化碳减排策略的变化[J]. 化工进展, 2018, 37(1): 1-6. |
| QIAO Ming, LI Xuejing, ZHOU Xiaoyang. Transformation of carbon emission reduction strategy of oil petroleum companies under the new situation[J]. Chemical Industry and Engineering Progress, 2018, 37(1): 1-6. | |
| 2 | SPECHT E, REDEMANN T, LORENZ N. Simplified mathematical model for calculating global warming through anthropogenic CO2 [J]. International Journal of Thermal Sciences, 2016, 102: 1-8. |
| 3 | 康丽娜, 尚会建, 郑学明. CO2的捕集封存技术进展及在我国的应用前景[J]. 化工进展, 2010, 29(S1): 24-27. |
| KANG Lina, SHANG Huijian, ZHENG Xueming. Progress in CO2 capture and storage technology and its application prospect in China[J]. Chemical Industry and Engineering Progress, 2010, 29(S1): 24-27. | |
| 4 | 白宏山, 赵东亚, 田群宏, 等. CO2捕集、运输、驱油与封存全流程随机优化[J]. 化工进展, 2019, 38(11): 4911-4920. |
| BAI Hongshan, ZHAO Dongya, TIAN Qunhong, et al. Stochastic optimization of the whole process of CO2 capture, transportation, utilization and sequestration[J]. Chemical Industry and Engineering Progress, 2019, 38(11): 4911-4920. | |
| 5 | 彭召静, 赵彦杰, 黄成德, 等. 用于燃烧后CO2捕集系统的胺基固态吸附材料研究进展[J]. 化工进展, 2018, 37(2): 610-620. |
| PENG Zhaojing, ZHAO Yanjie, HUANG Chengde, et al. Recent advances in amine-based solid sorbents for post-combustion CO2 capture system[J]. Chemical Industry and Engineering Progress, 2018, 37(2): 610-620. | |
| 6 | 张卫风, 李娟, 王秋华, 等. 燃煤烟气中CO2膜吸收分离技术的膜浸润特性述评[J]. 化工进展, 2019, 38(8): 3866-3873. |
| ZHANG Weifeng, LI Juan, WANG Qiuhua, et al. Review on membrane wettability of membrane CO2 absorption method from coal-fired flue gas[J]. Chemical Industry and Engineering Progress, 2019, 38(8): 3866-3873. | |
| 7 | WANG M H, JOEL A S, RAMSHAW C, et al. Process intensification for post-combustion CO2 capture with chemical absorption:a critical review[J]. Applied Energy, 2015, 158: 275-291. |
| 8 | SHAVALIEVA G, KAZEPIDIS P, PAPADOPOULOS A I, et al. Environmental, health and safety assessment of post-combustion CO2 capture processes with phase-change solvents[J]. Sustainable Production and Consumption, 2021, 25: 60-76. |
| 9 | PINTO D D D, KNUUTILA H, FYTIANOS G, et al. CO2 post combustion capture with a phase change solvent. Pilot plant campaign[J]. International Journal of Greenhouse Gas Control, 2014, 31: 153-164. |
| 10 | 赵文波, 李广振, 许胜超, 等. 相变吸收酸性气体的发展现状[J]. 化工进展, 2021, 40(1): 401-414. |
| ZHAO Wenbo, LI Guangzhen, XU Shengchao, et al. Recent developments of acid gas absorption by phase-change[J]. Chemical Industry and Engineering Progress, 2021, 40(1): 401-414. | |
| 11 | PAPADOPOULOS A I, PERDOMO F A, TZIRAKIS F, et al. Molecular engineering of sustainable phase-change solvents: from digital design to scaling-up for CO2 capture[J]. Chemical Engineering Journal, 2021, 420: 127624. |
| 12 | 张亚萍, 刘建周, 季芹芹, 等. 醇胺法捕集燃煤烟气CO2工艺模拟及优化[J]. 化工进展, 2013, 32(4): 930-935. |
| ZHANG Yaping, LIU Jianzhou, JI Qinqin, et al. Process simulation and optimization of flue gas CO2 capture by the alkanolamine solutions[J]. Chemical Industry and Engineering Progress, 2013, 32(4): 930-935. | |
| 13 | ZHANG J F, NWANI O, TAN Y, et al. Carbon dioxide absorption into biphasic amine solvent with solvent loss reduction[J]. Chemical Engineering Research and Design, 2011, 89(8): 1190-1196. |
| 14 | ZHANG Jiafei, QIAO Yu, WANG Wanzhong, et al. Development of an energy-efficient CO2 capture process using thermomorphic biphasic solvents[J]. Energy Procedia, 2013, 37: 1254-1261. |
| 15 | ZHANG Jiafei, QIAO Yu, AGAR D W. Intensification of low temperature thermomorphic biphasic amine solvent regeneration for CO2 capture[J]. Chemical Engineering Research and Design, 2012, 90(6): 743-749. |
| 16 | ZHOU Haicheng, XU Xin, CHEN Xiaochun, et al. Novel ionic liquids phase change solvents for CO2 capture[J]. International Journal of Greenhouse Gas Control, 2020, 98: 103068. |
| 17 | 金显杭. 面向CO2捕集的相变吸收剂开发及应用研究[D]. 北京: 北京化工大学, 2017. |
| JIN Xianhang. The development and application of phase change absorbents for CO2 capture[D]. Beijing: Beijing University of Chemical Technology, 2017. | |
| 18 | 涂巍巍, 方佳伟, 李竹石, 等. 基于MEA的CO2相变化吸收剂的开发[J]. 中国科学: 化学, 2018, 48(6): 641-647. |
| TU Weiwei, FANG Jiawei, LI Zhushi, et al. Development of CO2 phase change absorbent based on MEA[J]. Scientia Sinica (Chimica), 2018, 48(6): 641-647. | |
| 19 | ZHANG Weidong, JIN Xianhang, TU Weiwei, et al. A novel CO2 phase change absorbent: MEA/1-propanol/H2O[J]. Energy & Fuels, 2017, 31(4): 4273-4279. |
| 20 | LI Yaoyao, LIU Changjun, PARNAS Richard, et al. The CO2 absorption and desorption performance of the triethylenetetramine + N,N-diethylethanolamine + H2O system[J]. Chinese Journal of Chemical Engineering, 2018, 26(11): 2351-2360. |
| 21 | ZHANG Shihan, SHEN Yao, SHAO Peijing, et al. Kinetics, thermodynamics, and mechanism of a novel biphasic solvent for CO2 capture from flue gas[J]. Environmental Science & Technology, 2018, 52(6) : 3660-3668. |
| 22 | KIERZKOWSKA-PAWLAK H, SOABLA K. Heat of absorption of CO2 in aqueous solutions of DEEA and DEEA + MAPA blends—A new approach to measurement methodology[J]. International Journal of Greenhouse Gas Control, 2020, 100: 103102. |
| 23 | TAN J, SHAO H W, XU J H, et al. Mixture absorption system of monoethanolamine-triethylene glycol for CO2 capture[J]. Industrial & Engineering Chemistry Research, 2011, 50(7): 3966-3976. |
| 24 | WANG Lidong, ZHANG Yifeng, WANG Rujie, et al. Advanced monoethanolamine absorption using sulfolane as a phase splitter for CO2 capture[J]. Environmental Science & Technology, 2018, 52(24) : 14556-14563. |
| 25 | YE Qing, WANG Xinlei, LU Yongqi. Screening and evaluation of novel biphasic solvents for energy-efficient post-combustion CO2 capture[J]. International Journal of Greenhouse Gas Control, 2015, 39: 205-214. |
| 26 | RAYNAL L, ALIX P, BOUILLON P A, et al. The DMX™ process:an original solution for lowering the cost of post-combustion carbon capture[J]. Energy Procedia, 2011, 4: 779-786. |
| 27 | ALEIXO M, PRIGENT M, GIBERT A, et al. Physical and chemical properties of DMXTM solvents[J]. Energy Procedia, 2011, 4: 148-155. |
| 28 | RAYNAL L, BRIOT P, DREILLARD M, et al. Evaluation of the DMX process for industrial pilot demonstration-methodology and results[J]. Energy Procedia, 2014, 63: 6298-6309. |
| 29 | 安山龙, 汪黎东, 于松华, 等. 相变溶剂捕集CO2技术的研究进展[J]. 化工环保, 2017, 37(1): 31-37. |
| AN Shanlong, WANG Lidong, YU Songhua, et al. Research progresses in CO2 capture technology using phase change solvents[J]. Environmental Protection of Chemical Industry, 2017, 37(1): 31-37. | |
| 30 | CIFTJA A F, HARTONO A, SVENDSEN H F. Experimental study on phase change solvents in CO2 capture by NMR spectroscopy[J]. Chemical Engineering Science, 2013, 102: 378-386. |
| 31 | 王敏. 液-液相变溶剂吸收CO2关键设备设计与数值模拟研究[D]. 青岛: 青岛科技大学, 2019. |
| WANG Min. Design and numerical simulation of key equipment for CO2 absorption by liquid-liquid phase change solvents [D]. Qingdao: Qingdao University of Science and Technology, 2019. | |
| 32 | SANCHEZ-FERNANDEZ E, HEFFERNAN K, VAN DER HAM L, et al. Analysis of process configurations for CO2 capture by precipitating amino acid solvents[J]. Industrial & Engineering Chemistry Research, 2014, 53(6): 2348-2361. |
| 33 | SMITH K, XIAO G K, MUMFORD K, et al. Demonstration of a concentrated potassium carbonate process for CO2 capture[J]. Energy & Fuels, 2014, 28(1): 299-306. |
| 34 | THEE H, SURYAPUTRADINATA Y A, MUMFORD K A, et al. A kinetic and process modeling study of CO2 capture with MEA-promoted potassium carbonate solutions[J]. Chemical Engineering Journal, 2012, 210: 271-279. |
| 35 | GAZZANI M, SUTTER D, MAZZOTTI M. Improving the efficiency of a chilled ammonia CO2 capture plant through solid formation: a thermodynamic analysis[J]. Energy Procedia, 2014, 63: 1084-1090. |
| 36 | BIAN Yangyang, SHEN Shufeng. CO2 absorption into a phase change absorbent: water-lean potassium prolinate/ethanol solution[J].Chinese Journal of Chemical Engineering, 2018, 26(11): 2318-2326. |
| 37 | CHEN Zhibiao, JING Guohua, Bihong LYU, et al. An efficient solid-liquid biphasic solvent for CO2 capture: crystalline powder product and low heat duty[J]. ACS Sustainable Chemistry & Engineering, 2020, 8: 14493-14503. |
| 38 | SANCHEZ-FERNANDEZ E, MERCADER F D M, MISIAK K, et al. New process concepts for CO2 capture based on precipitating amino acids[J]. Energy Procedia, 2013, 37: 1160-1171. |
| 39 | SANCHEZ-FERNANDEZ E, HEFFERNAN K, VAN DER HAM L, et al. Precipitating amino acid solvents for CO2 capture. opportunities to reduce costs in post combustion capture[J]. Energy Procedia, 2014, 63: 727-738. |
| 40 | WANG Xianfeng, AKHMEDOV N G, HOPKINSON D, et al. Phase change amino acid salt separates into CO2-rich and CO2-lean phases upon interacting with CO2 [J]. Applied Energy, 2016, 161: 41-47. |
| 41 | MOIOLI S, HO M T, WILEY D E, et al. Thermodynamic modeling of the system of CO2 and potassium taurate solution for simulation of the carbon dioxide capture process[J]. Chemical Engineering Research and Design, 2018, 136: 834-845. |
| 42 | MOIOLI S, HO M T, WILEY D E, et al. Assessment of carbon dioxide capture by precipitating potassium taurate solvent[J]. International Journal of Greenhouse Gas Control, 2019, 87: 159-169. |
| 43 | HO M T, GARCIA-CALVO CONDE E, MOIOLI S, et al Wiley. The effect of different process configurations on the performance and cost of potassium taurate solvent absorption[J]. International Journal of Greenhouse Gas Control, 2019, 81: 1-10. |
| 44 | DARDE V, THOMSEN K, WELL W J M VAN, et al. Chilled ammonia process for CO2 capture[J]. Energy Procedia, 2009, 1(1): 1035-1042. |
| 45 | PARK J Y, YOON S J, LEE H. Effect of steric hindrance on carbon dioxide absorption into new amine solutions: thermodynamic and spectroscopic verification through solubility and NMR analysis[J]. Environmental Science & Technology, 2003, 37(8): 1670-1675. |
| 46 | 张政. 有机胺非水体系相变吸收CO2研究[D]. 昆明: 昆明理工大学, 2016. |
| ZHANG Zheng. Study on CO2 absorption by phase change in non-aqueous organic amines[D]. Kunming: Kunming University of Science and Technology, 2016. | |
| 47 | ALIVAND M S, MAZAHERI O, WU Y, et al. Data in brief on CO2 absorption-desorption of aqueous-based amino acid solvents with phase change behaviour.[J]. Data in Brief, 2019, 27: 104741. |
| 48 | LI Yannan, CHENG Jun, HU Leiqing, et al. Regulating crystal structures of EDA-carbamates in solid-liquid phase- changing CO2 capture solutions[J]. Fuel, 2019, 252(9): 47-54. |
| 49 | 张克舫, 刘中良, 王远亚, 等. 化学吸收法CO2捕集解吸能耗的分析计算[J]. 化工进展, 2013, 32(12): 3008-3014. |
| ZHANG Kefang, LIU Zhongliang, WANG Yuanya, et al. Analysis and calculation of the desorption energy consumption of CO2 capture process by chemical absorption method[J]. Chemical Industry and Engineering Progress, 2013, 32(12): 3008-3014. | |
| 50 | 柳康, 许世森, 李广宇, 等. 基于整体煤气化联合循环的燃烧前CO2捕集工艺及系统分析[J]. 化工进展, 2018, 37(12): 4897-4907. |
| LIU Kang, XU Shisen, LI Guangyu, et al. Technological process and system analysis of pre-combustion CO2 capture based on IGCC[J]. Chemical Industry and Engineering Progress, 2018, 37(12): 4897-4907. | |
| 51 | 陆诗建, 高丽娟, 王家凤, 等. 烟气CO2捕集热能梯级利用节能工艺耦合优化[J]. 化工进展, 2020, 39(2): 728-737. |
| LU Shijian, GAO Lijuan, WANG Jiafeng, et al. Coupling optimization of energy-saving technology for cascade utilization of flue gas CO2 capture system[J]. Chemical Industry and Engineering Progress, 2020, 39(2): 728-737. | |
| 52 | MÄNNISTÖ M, UUSI-KYYNY P, RICHON D, et al. Study of CO2 absorption into phase change solvents MAPA and DEEA[J]. Journal of Chemical & Data, 2017, 62(8): 2261-2271. |
| 53 | LIU Fei, FANG Mengxiang, YI Ningtong, et al. Biphasic behaviors and regeneration energy of a 2-(diethylamino)-ethanol and 2-((2-aminoethyl)amino) ethanol blend for CO2 capture[J]. Sustainable Energy & Fuels, 2019, 3(12): 3594-3602. |
| 54 | 彭艳娇, 汤谧琼. 国际领先“相变型”二氧化碳捕集工业装置在华能运行成功[N]. 中国电力报, 2020-11-27. |
| PENG Yanjiao, TANG Miqiong. The world’s leading “phase-variant” carbon dioxide capture industrial plant has been successfully run in China[N]. China Electric Power News, 2020-11-27. | |
| 55 | WANG Lidong, AN Shanlong, YU Songhua, et al. Mass transfer characteristics of CO2 absorption into a phase-change solvent in a wetted-wall column[J]. International Journal of Greenhouse Gas Control, 2017, 64: 276-283. |
| 56 | LEE J, HONG Y K, YOU J K. Phase separation characteristics in biphasic solvents based on mutually miscible amines for energy efficient CO2 capture[J]. Korean Journal of Chemical Engineering, 2017, 34(6) : 1840-1845. |
| 57 | WANG Lidong, LIU Shanshan, WANG Rujie, et al. Regulating phase separation behavior of a DEEA-TETA biphasic solvent using sulfolane for energy-saving CO2 capture[J]. Environmental Science and Technology, 2019, 53(21) : 12873-12881. |
| 58 | YE Jiexu, JIANG Chenkai, CHEN Han, et al. Novel biphasic solvent with tunable phase separation for CO2 capture: role of water content in mechanism, kinetics, and energy penalty[J]. Environmental Science & Technology, 2019, 53(8) : 4470-4479. |
| 59 | LIU Fei, FANG Mengxiang, YI Ningtong, et al. Research on alkanolamine-based physical-chemical solutions as biphasic solvents for CO2 capture[J]. Energy & Fuels, 2019, 33(11): 11389-11398. |
| 60 | 周小斌, 荆国华, 周作明, 等. 2-氨基-2-甲基-1-丙醇(AMP)活化相变吸收剂捕集CO2特性[C]//2017中国环境科学学会科学与技术年会论文集. 厦门, 2017: 876-883. |
| ZHOU Xiaobin, JING Guohua, ZHOU Zuoming, et al. Capture of CO2 with 2-amino-2-methyl-1-propanol (AMP) activated phase change absorber[C]//2017 Chinese Society for Environmental Science Science Annual Conference Proceedings Xiamen, 2017: 876-883. | |
| 61 | 孙亚伟, 谢美连, 刘庆岭, 等. 膜法分离燃煤电厂烟气中CO2的研究现状及进展[J]. 化工进展, 2017, 36(5): 1880-1889. |
| SUN Yawei, XIE Meilian, LIU Qingling, et al. Membrane-based carbon dioxide separation from flue gases of coal-fired power plant—current status and developments[J]. Chemical Industry and Engineering Progress, 2017, 36(5): 1880-1889. | |
| 62 | YI Ningtong, FANG Mengxiang, DI Wentao, et al. Aerosol emissions of amine-based CO2 absorption system: effects of condensation nuclei and operating conditions.[J]. Environmental Science & Technology, 2021, 55(8): 5152-5160. |
| 63 | 杨林军, 张琳, 孙莹. 燃煤净烟气中共存杂质对膜法捕集CO2影响现状[J]. 化工进展, 2019, 38(4): 1996-2002. |
| YANG Linjun, ZHANG Lin, SUN Ying. Present situation of the effect of coexisting impurities in coal fired flue gas on CO2 capture by membranes[J]. Chemical Industry and Engineering Progress, 2019, 38(4): 1996-2002. | |
| 64 | NGUYEN T, HILLIARD M, ROCHELLE G T. Amine volatility in CO2 capture[J]. International Journal of Greenhouse Gas Control, 2010, 4(5) : 707-715. |
| 65 | HILLIARD M D. A predictive thermodynamic model for an aqueous blend of potassium carbonate, piperazine, and monoethanolamine for carbon dioxide capture from flue gas[J]. Carbon Dioxide—Absorption and Adsorption, 2008: 1083. |
| 66 | LIU F, ROCHELLE G T, FANG M X, et al. Volatility of 2-(diethylamino)-ethanol and 2-((2-aminoethyl) amino) ethanol, a biphasic solvent for CO2 capture[J]. International Journal of Greenhouse Gas Control, 2021, 106 : 103257. |
| 67 | 李红, 吉轲, 齐天勤机, 等. 复配醇胺溶液对CO2的吸收解吸性能及其降解性[J]. 化工进展, 2021, 45(5): 1-17. |
| LI Hong, JI Ke, qinji QITIAN, et al. CO2 absorption and desorption properties of mixed alcohol amine solution[J]. Chemical Industry and Engineering Progress, 2021, 45(5): 1-17. | |
| 68 | 储可弘, 陈绍云, 李强, 等. 基于N-乙基乙醇胺非水CO2吸收剂的抗氧化剂[J]. 化工进展, 2019, 38(12): 5565-5571. |
| CHU Kehong, CHEN Shaoyun, LI Qiang, et al. Oxidation inhibitor for thylethanolamine based non-aqueous CO2 absorbent[J]. Chemical Industry and Engineering Progress, 2019, 38(12): 5565-5571. |
| [1] | DAI Huantao, CAO Lingyu, YOU Xinxiu, XU Haoliang, WANG Tao, XIANG Wei, ZHANG Xueyang. Adsorption properties of CO2 on pomelo peel biochar impregnated by lignin [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 356-363. |
| [2] | CHEN Chongming, CHEN Qiu, GONG Yunqian, CHE Kai, YU Jinxing, SUN Nannan. Research progresses on zeolite-based CO2 adsorbents [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 411-419. |
| [3] | WANG Shengyan, DENG Shuai, ZHAO Ruikai. Research progress on carbon dioxide capture technology based on electric swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 233-245. |
| [4] | YANG Ying, HOU Haojie, HUANG Rui, CUI Yu, WANG Bing, LIU Jian, BAO Weiren, CHANG Liping, WANG Jiancheng, HAN Lina. Coal tar phenol-based carbon nanosphere prepared by Stöber method for adsorption of CO2 [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 5011-5018. |
| [5] | WANG Xin, WANG Bingbing, YANG Wei, XU Zhiming. Anti-scale and anti-corrosion properties of PDA/PTFE superhydrophobic coating on metal surface [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4315-4321. |
| [6] | BAI Yadi, DENG Shuai, ZHAO Ruikai, ZHAO Li, YANG Yingxia. Exploration on standardized test scheme and experimental performance of temperature swing adsorption carbon capture unit [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3834-3846. |
| [7] | LU Yang, ZHOU Jinsong, ZHOU Qixin, WANG Tang, LIU Zhuang, LI Bohao, ZHOU Lingtao. Leaching mechanism of Hg-absorption products on CeO2/TiO2 sorbentsin syngas [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3875-3883. |
| [8] | WU Zhanhua, SHENG Min. Pitfalls of accelerating rate calorimeter for reactivity hazard evaluation and risk assessment [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3374-3382. |
| [9] | XIE Zhiwei, WU Zhangyong, ZHU Qichen, JIANG Jiajun, LIANG Tianxiang, LIU Zhenyang. Viscosity properties and magnetoviscous effects of Ni0.5Zn0.5Fe2O4 vegetable oil-based magnetic fluid [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3623-3633. |
| [10] | YANG Jingying, SHI Wansheng, HUANG Zhenxing, XIE Lijuan, ZHAO Mingxing, RUAN Wenquan. Research progress on the preparation of modified nano zero-valent iron materials [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2975-2986. |
| [11] | LU Shijian, ZHANG Yuanyuan, WU Wenhua, YANG Fei, LIU Ling, KANG Guojun, LI Qingfang, CHEN Hongfu, WANG Ning, WANG Feng, ZHANG Juanjuan. Health risk assessment of nitrosamine pollutant diffusion in a million ton CO2 capture project [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3209-3216. |
| [12] | WANG Jiuheng, RONG Nai, LIU Kaiwei, HAN Long, SHUI Taotao, WU Yan, MU Zhengyong, LIAO Xuqing, MENG Wenjia. Enhanced CO2 capture performance and strength of cellulose-templated CaO-based pellets with steam reactivation [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3217-3225. |
| [13] | GU Shiya, DONG Yachao, LIU Linlin, ZHANG Lei, ZHUANG Yu, DU Jian. Design and optimization of pipeline system for carbon capture considering intermediate nodes [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2799-2808. |
| [14] | YANG Yang, SUN Zhigao, LI Cuimin, LI Juan, HUANG Haifeng. Promotion on the formation of HCFC-141b hydrate under static conditions by surfactant OP-13 [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2854-2859. |
| [15] | LI Ling, MA Chaofeng, LU Chunshan, YU Wanjin, SHI Nengfu, JIN Jiamin, ZHANG Jianjun, LIU Wucan, LI Xiaonian. Progress on the synthesis of 1,1,2-trifluoroethene and the catalysts [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 1822-1831. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||