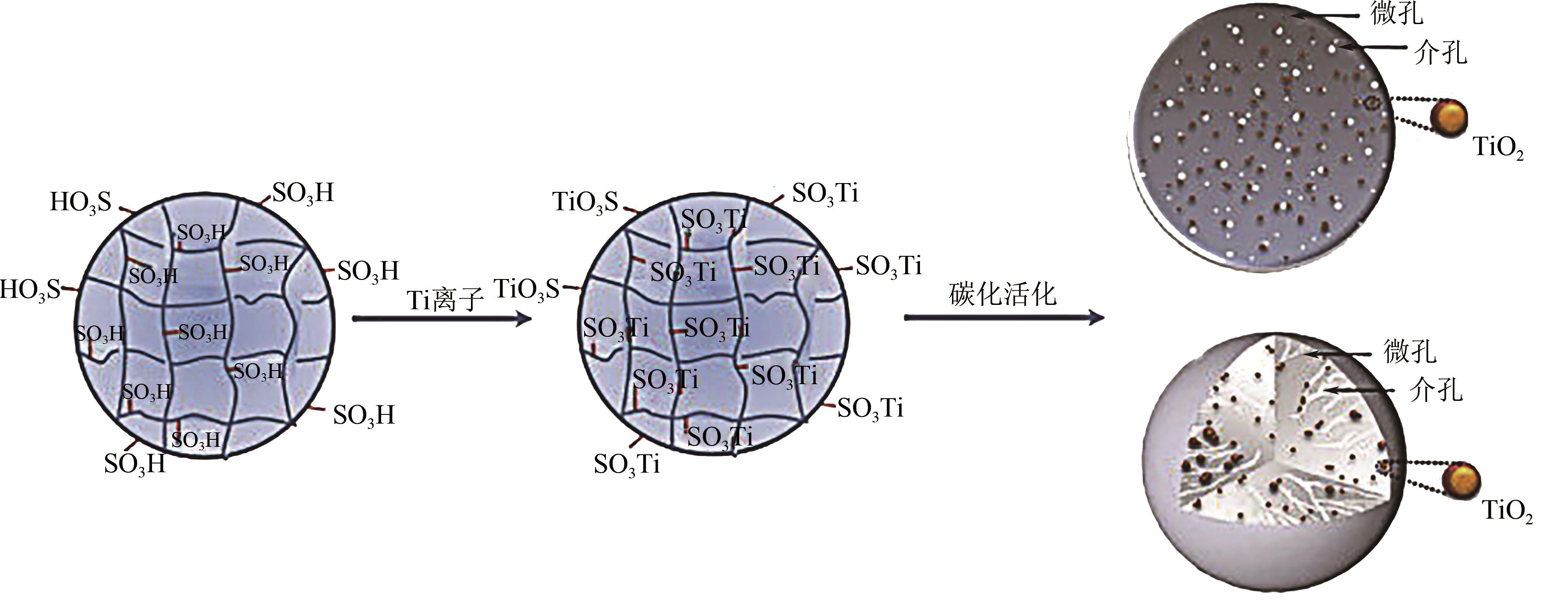
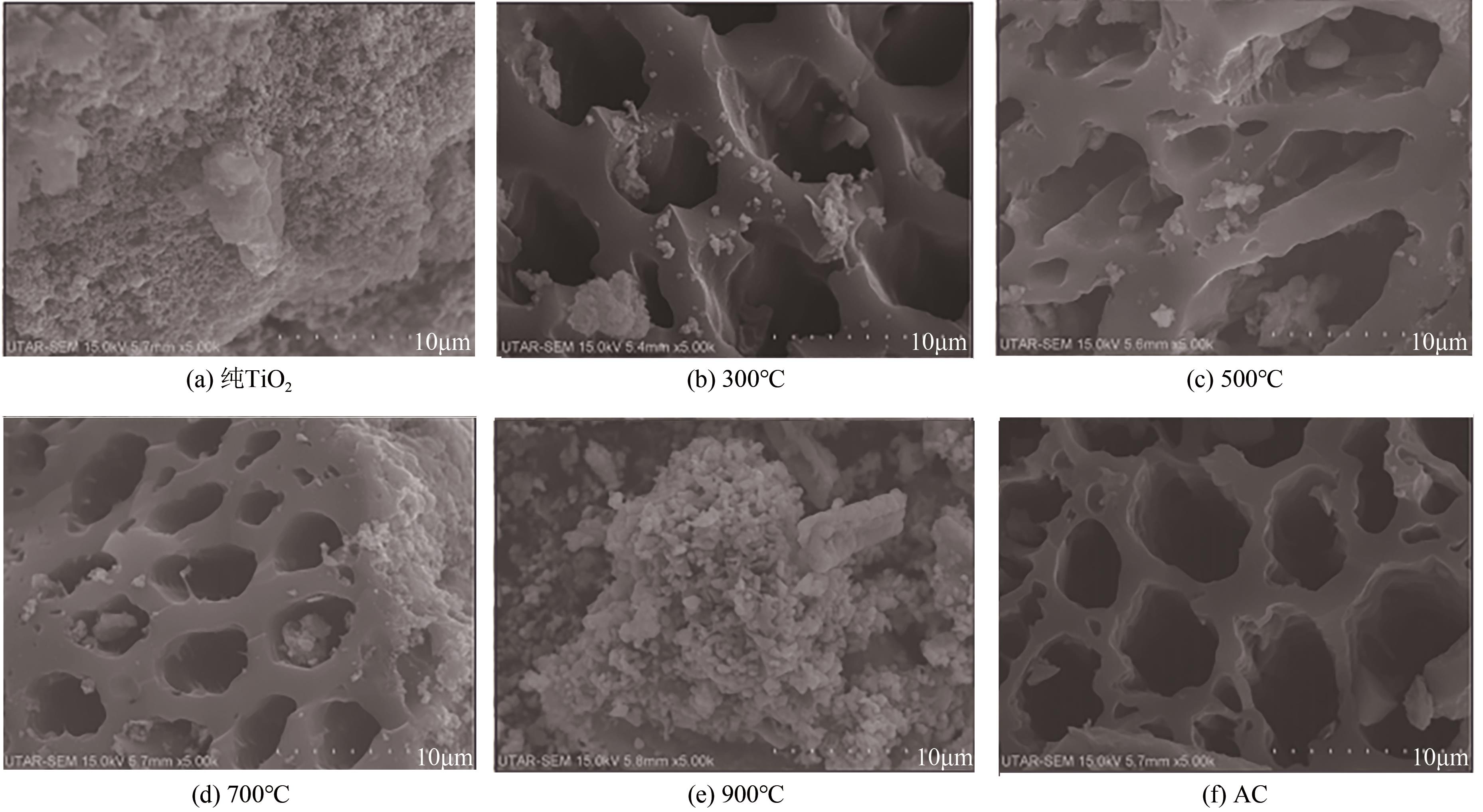
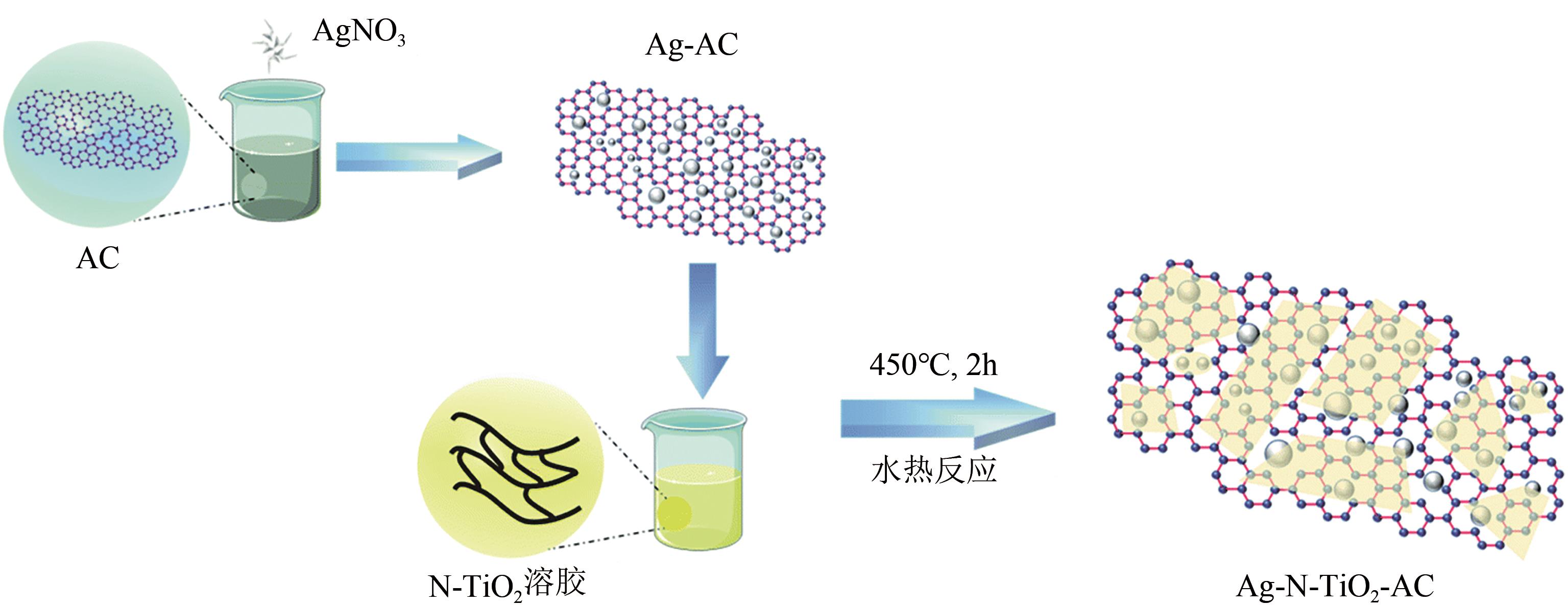
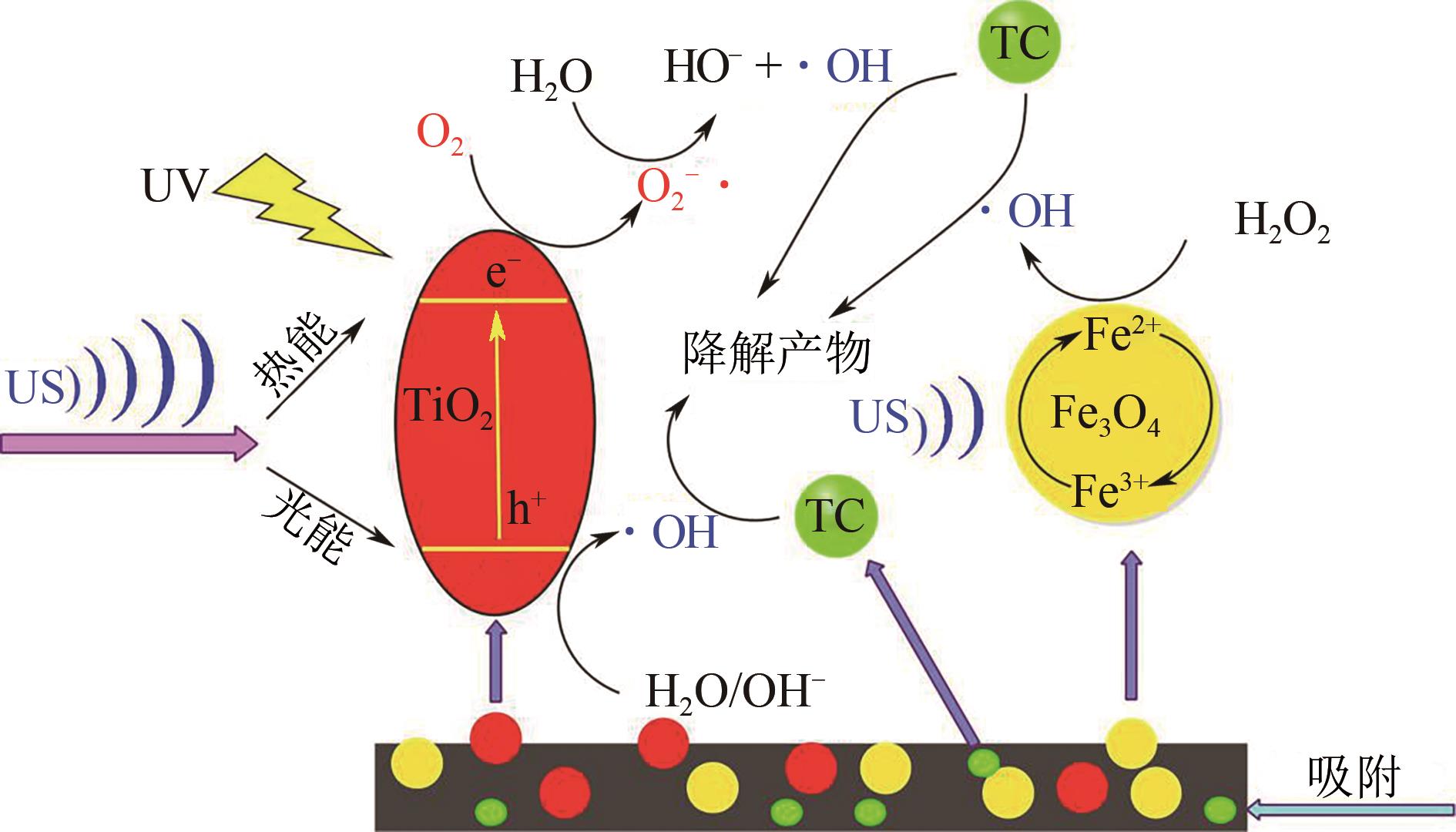
Chemical Industry and Engineering Progress ›› 2021, Vol. 40 ›› Issue (7): 3837-3846.DOI: 10.16085/j.issn.1000-6613.2020-1681
• Materials science and technology • Previous Articles Next Articles
Progress in preparation and modification of TiO2/AC composite photocatalysts for environmental purification
XIA Zhenguo( ), ZHU Yingying(
), ZHU Yingying( ), CHEN Geng, LU Yu, WANG Jiafeng
), CHEN Geng, LU Yu, WANG Jiafeng
- Faculty of Maritime and Transportation, Ningbo University, Ningbo 315211, Zhejiang, China
-
Received:2020-08-21Revised:2021-01-19Online:2021-07-19Published:2021-07-06 -
Contact:ZHU Yingying
用于环境净化的TiO2/AC复合材料的制备及其改性研究进展
- 宁波大学海运学院,浙江 宁波 315211
-
通讯作者:朱颖颖 -
作者简介:夏振国(1995—),男,硕士研究生,研究方向为新能源开发与环境保护。E-mail:15090091500@163.com 。 -
基金资助:国家自然科学基金(51406093);浙江省自然科学基金(LY20E060003);宁波市自然科学基金(2018A610281)
CLC Number:
Cite this article
XIA Zhenguo, ZHU Yingying, CHEN Geng, LU Yu, WANG Jiafeng. Progress in preparation and modification of TiO2/AC composite photocatalysts for environmental purification[J]. Chemical Industry and Engineering Progress, 2021, 40(7): 3837-3846.
夏振国, 朱颖颖, 陈耿, 卢宇, 王家锋. 用于环境净化的TiO2/AC复合材料的制备及其改性研究进展[J]. 化工进展, 2021, 40(7): 3837-3846.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2020-1681
| 改性方法 | 掺杂元素 | 禁带宽度/eV | 最大吸收波长/nm | 作用原理 | 参考文献 |
|---|---|---|---|---|---|
| 金属离子掺杂 | |||||
| 过渡金属离子 | Fe | 2.9 | 428 | Fe离子掺杂在TiO2晶格内部形成离子陷阱捕获电子和空穴,促进电子-空穴分离 | [ |
| W | 2.73 | 454 | W离子掺杂在TiO2带隙内引入杂质能级,使带隙变窄,吸收边缘向能量较低的方向移动 | [ | |
| Ag | — | — | Ag离子掺杂进入TiO2晶格,抑制了光生电子和空穴的复合, 提高光量子效率, 且TiO2光响应拓展至可见光区域 | [ | |
| 稀土金属离子 | Ce | — | — | Ce离子掺杂抑制TiO2晶粒生长,有效降低TiO2粒子半径,阻碍了TiO2由锐钛矿相向金红石相的转变 | [ |
| La | 2.85 | 435 | La元素具有较宽的光吸收带,其加入TiO2的效果与在反应溶液中添加光敏剂的效果类似,提升复合材料的光吸收能力 | [ | |
| 非金属离子掺杂 | N | 2.25 | 550 | N离子掺杂抑制TiO2晶体的生长,减小TiO2粒子半径;同时取代TiO2晶格中O的位置,使吸收光谱红移 | [ |
| F | — | — | F离子掺杂抑制光生电子与空穴的复合 | [ | |
| I | — | — | I离子掺杂使TiO2在较低温度下晶化并形成较大比表面积的介孔材料,减少高温煅烧产生的结构破坏。抑制 TiO2由锐钛矿相向金红石相的转变,减少粒子的团聚 | [ | |
| 多元素离子共掺杂 | |||||
| 非金属-非金属共掺杂 | C-N | 2.53 | 490 | C、N元素的掺杂拓宽光谱区域,增强了紫外光和可见光的吸收 | [ |
| I-N | 2.33 | 532 | I、N的掺杂使制备的复合材料在可见光区有更好的吸光性,电荷分离得到增强 | [ | |
| Br-N | — | — | N掺杂减小了TiO2的带隙,而Br的掺杂有利于产生电子和空穴的有效分离,从而增强光催化活性 | [ | |
| 金属-非金属共掺杂 | Cu-N | 2.47 | 502 | N、Cu共掺杂使得晶格中Ti4+和O2-离子重排,干扰了晶体的生长机制和相变 | [ |
| La-N | 2.20 | 564 | La离子和N离子均被纳入TiO2框架,使TiO2的带隙缩小,同时有效抑制电子和空穴的复合 | [ | |
| Ce-P-N | 2.51 | 494 | 有效缩减TiO2禁带宽度,抑制了光生电子和空穴的复合,延长载流子使用寿命 | [ | |
| 改性方法 | 掺杂元素 | 禁带宽度/eV | 最大吸收波长/nm | 作用原理 | 参考文献 |
|---|---|---|---|---|---|
| 金属离子掺杂 | |||||
| 过渡金属离子 | Fe | 2.9 | 428 | Fe离子掺杂在TiO2晶格内部形成离子陷阱捕获电子和空穴,促进电子-空穴分离 | [ |
| W | 2.73 | 454 | W离子掺杂在TiO2带隙内引入杂质能级,使带隙变窄,吸收边缘向能量较低的方向移动 | [ | |
| Ag | — | — | Ag离子掺杂进入TiO2晶格,抑制了光生电子和空穴的复合, 提高光量子效率, 且TiO2光响应拓展至可见光区域 | [ | |
| 稀土金属离子 | Ce | — | — | Ce离子掺杂抑制TiO2晶粒生长,有效降低TiO2粒子半径,阻碍了TiO2由锐钛矿相向金红石相的转变 | [ |
| La | 2.85 | 435 | La元素具有较宽的光吸收带,其加入TiO2的效果与在反应溶液中添加光敏剂的效果类似,提升复合材料的光吸收能力 | [ | |
| 非金属离子掺杂 | N | 2.25 | 550 | N离子掺杂抑制TiO2晶体的生长,减小TiO2粒子半径;同时取代TiO2晶格中O的位置,使吸收光谱红移 | [ |
| F | — | — | F离子掺杂抑制光生电子与空穴的复合 | [ | |
| I | — | — | I离子掺杂使TiO2在较低温度下晶化并形成较大比表面积的介孔材料,减少高温煅烧产生的结构破坏。抑制 TiO2由锐钛矿相向金红石相的转变,减少粒子的团聚 | [ | |
| 多元素离子共掺杂 | |||||
| 非金属-非金属共掺杂 | C-N | 2.53 | 490 | C、N元素的掺杂拓宽光谱区域,增强了紫外光和可见光的吸收 | [ |
| I-N | 2.33 | 532 | I、N的掺杂使制备的复合材料在可见光区有更好的吸光性,电荷分离得到增强 | [ | |
| Br-N | — | — | N掺杂减小了TiO2的带隙,而Br的掺杂有利于产生电子和空穴的有效分离,从而增强光催化活性 | [ | |
| 金属-非金属共掺杂 | Cu-N | 2.47 | 502 | N、Cu共掺杂使得晶格中Ti4+和O2-离子重排,干扰了晶体的生长机制和相变 | [ |
| La-N | 2.20 | 564 | La离子和N离子均被纳入TiO2框架,使TiO2的带隙缩小,同时有效抑制电子和空穴的复合 | [ | |
| Ce-P-N | 2.51 | 494 | 有效缩减TiO2禁带宽度,抑制了光生电子和空穴的复合,延长载流子使用寿命 | [ | |
| 污染物种类 | 污染物 | 复合材料 | 污染物浓度 | 催化剂用量 | 光源 | 反应时间 | 降解率/% | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| 染料废水 | 亚甲基蓝 | Ce-TiO2/AC | 5.5mg/L | 1.5g/L | 40W紫外灯 | 60min | 72 | [ |
| 刚果红 | C-TiO2@Fe3O4/AC | 100mg/L | 1.0g/L | 200~800nm模拟太阳光 | 30min | 92.9 | [ | |
| 工厂印染废水 | Fe-N/TiO2/AC | 4823mg/L | 7.0g/L | 40W紫外灯 | 120min | 90.5 | [ | |
| 药物类 | 布洛芬 | TiO2/AC | 40mg/L | 2.0g/L | 18W紫外灯 | 180min | 85.6 | [ |
| 苯二氮卓类药物 | TiO2/AC | 100μg/L | 80mg/L | 300W模拟日光灯 | 60min | 97.5 | [ | |
| 抗生素 | TiO2/MAC | 10mg/L | 0.6g/L | 6W紫外灯+70W超声波辐射 | 180min | 93 | [ | |
| 酚类 | 苯酚 | TiO2/AC | 100mg/L | 1.2g/L | 太阳光 | 120min | 100 | [ |
| 双酚A | N-TiO2/AC | 36mg/L | 0.25g/L | 150W氙灯 | 8h | 90 | [ | |
| 挥发性有机化合物 | 甲苯 | Mn-TiO2/AC | 30mg/L | 1g | 两个4W紫外灯 | 2h | 86 | [ |
| 2-丙醇 | TiO2/AC | 2000mg/L | 200mg | 200W汞灯 | 1h | 75 | [ | |
| 甲醇 | TiO2/AC | 36.4mg/L | 7g | 8W紫外灯 | — | 90 | [ |
| 污染物种类 | 污染物 | 复合材料 | 污染物浓度 | 催化剂用量 | 光源 | 反应时间 | 降解率/% | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| 染料废水 | 亚甲基蓝 | Ce-TiO2/AC | 5.5mg/L | 1.5g/L | 40W紫外灯 | 60min | 72 | [ |
| 刚果红 | C-TiO2@Fe3O4/AC | 100mg/L | 1.0g/L | 200~800nm模拟太阳光 | 30min | 92.9 | [ | |
| 工厂印染废水 | Fe-N/TiO2/AC | 4823mg/L | 7.0g/L | 40W紫外灯 | 120min | 90.5 | [ | |
| 药物类 | 布洛芬 | TiO2/AC | 40mg/L | 2.0g/L | 18W紫外灯 | 180min | 85.6 | [ |
| 苯二氮卓类药物 | TiO2/AC | 100μg/L | 80mg/L | 300W模拟日光灯 | 60min | 97.5 | [ | |
| 抗生素 | TiO2/MAC | 10mg/L | 0.6g/L | 6W紫外灯+70W超声波辐射 | 180min | 93 | [ | |
| 酚类 | 苯酚 | TiO2/AC | 100mg/L | 1.2g/L | 太阳光 | 120min | 100 | [ |
| 双酚A | N-TiO2/AC | 36mg/L | 0.25g/L | 150W氙灯 | 8h | 90 | [ | |
| 挥发性有机化合物 | 甲苯 | Mn-TiO2/AC | 30mg/L | 1g | 两个4W紫外灯 | 2h | 86 | [ |
| 2-丙醇 | TiO2/AC | 2000mg/L | 200mg | 200W汞灯 | 1h | 75 | [ | |
| 甲醇 | TiO2/AC | 36.4mg/L | 7g | 8W紫外灯 | — | 90 | [ |
| 1 | FUJISHIMA A, HONDA K. Electrochemical photolysis of water at a semiconductor electrode[J]. Nature, 1972, 238(5358): 37-38. |
| 2 | SHEN S H, CHEN J, WANG M, et al. Titanium dioxide nanostructures for photoelectrochemical applications[J]. Progress in Materials Science, 2018, 98: 299-385. |
| 3 | 张巧玲, 秦钊, 刘有智, 等. 氧化石墨烯-TiO2复合材料对三种染料的吸附动力学及光催化性能[J]. 化工进展, 2019, 38(6): 2870-2879. |
| ZHANG Qiaoling, QIN Zhao, LIU Youzhi, et al. Adsorption kinetics and photocatalytic activity of grapheneoxide-TiO2 composites for three dyes[J]. Chemical Industry and Engineering Progress, 2019, 38(6): 2870-2879. | |
| 4 | 刘文慧, 赵志换, 薛永强, 等. 纳米TiO2/粉煤灰微珠光催化复合材料制备方法的比较[J]. 化工新型材料, 2018, 46(5): 121-125. |
| LIU Wenhui, ZHAO Zhihuan, XUE Yongqiang, et al. Comparision of preparation of nanometer TiO2/FACs photocatalytic composite material[J]. New Chemical Materials, 2018, 46(5): 121-125. | |
| 5 | DARAEE M, GHASEMY E, RASHIDI A. Effective adsorption of hydrogen sulfide by intercalation of TiO2 and N-doped TiO2 in graphene oxide[J]. Journal of Environmental Chemical Engineering, 2020, 8(4): 103836. |
| 6 | 胡小龙, 孙青, 徐春宏, 等. 纳米TiO2/沸石复合材料光催化降解苯酚的性能[J]. 化工进展, 2016, 35(5): 1519-1523. |
| HU Xiaolong, SUN Qing, XU Chunhong, et al. Photocatalytic degradation of phenol with nano-TiO2/zeolite composite material[J]. Chemical Industry and Engineering Progress, 2016, 35(5): 1519-1523. | |
| 7 | WANG L, GUO J, DANG J, et al. Comparison of the photocatalytic performance of TiO2/AC and TiO2/CNT nanocomposites for methyl orange photodegradation[J]. Water Science and Technology, 2018, 78(5): 1082-1093. |
| 8 | LEARY R, WESTWOOD A. Carbonaceous nanomaterials for the enhancement of TiO2 photocatalysis[J]. Carbon, 2011, 49(3): 741-772. |
| 9 | 陈玉娟, 胡中华, 王晓静, 等. 活性炭孔径和比表面积对TiO2/AC光催化性能的影响[J]. 物理化学学报, 2008, 24(9): 1589-1596. |
| CHEN Yujuan, HU Zhonghua, WANG Xiaojing, et al. Influence of pore size and surface area of activated carbon on the performance of TiO2/AC photocatalyst[J]. Acta Physico-Chimica Sinica, 2008, 24(9): 1589-1596. | |
| 10 | KONSTANTINOS V P, ATHINA T, ANASTASIOS J K. Enhanced photo-catalytic performance of activated carbon fibers for water treatment[J]. Water, 2019, 11(9): 1794-1806. |
| 11 | 钟永科, 周斌, 张成江, 等. 活性炭表面含氧官能团对TiO2/AC的吸附和光催化活性的影响[J]. 新型炭材料, 2017, 32(5): 460-466. |
| ZHONG Yongke, ZHOU Bin, ZHANG Chengjiang, et al. Effects of surface oxygen functional groups on activated carbon on the adsorption and photocatalytic degradation activities of a TiO2-AC hybrid for Methylene Blue and methylene orange[J]. New Carbon Materials, 2017, 32(5): 460-466. | |
| 12 | XING B, SHI C, ZHANG C, et al. Preparation of TiO2/activated carbon composites for photocatalytic degradation of RhB under UV light irradiation[J]. Journal of Nanomaterials, 2016, 2016: 1-10. |
| 13 | RAGUPATHY S, RAGHU K, PRABU P. Synthesis and characterization of TiO2 loaded cashew nut shell activated carbon and photocatalytic activity on BG and MB dyes under sunlight radiation[J]. Spectrochimica Acta A: Molecular and Biomolecular Spectroscopy, 2015, 138: 314-320. |
| 14 | WANG X, LIU Y, HU Z, et al. Degradation of methyl orange by composite photocatalysts nano-TiO2 immobilized on activated carbons of different porosities[J]. Journal of Hazardous Materials, 2009, 169(1/2/3): 1061-1067. |
| 15 | TAOUFIK N, ELMCHAOURI A, ANOUAR F, et al. Improvement of the adsorption properties of an activated carbon coated by titanium dioxide for the removal of emerging contaminants[J]. Journal of Water Process Engineering, 2019, 31: 100876. |
| 16 | BAEK M H, JUNG W C, YOON J W, et al. Preparation, characterization and photocatalytic activity evaluation of micro- and mesoporous TiO2/spherical activated carbon[J]. Journal of Industrial and Engineering Chemistry, 2013, 19(2): 469-477. |
| 17 | PANG Y L, LIM S, LEE R K L, et al. Enhancement of sonocatalytic degradation of organic dye by using titanium dioxide (TiO2)/activated carbon (AC) derived from oil palm empty fruit bunch[J]. Environmental Science and Pollution Research, 2020, 27(28): 34638-34652. |
| 18 | 徐鑫, 王晓静, 胡中华, 等. 溶胶-凝胶和浸渍-水热制备方法对TiO2/AC光催化剂结构和性能的影响[J]. 物理化学学报, 2010, 26(1): 79-86. |
| XU Xin, WANG Xiaojing, HU Zhonghua, et al. Influence of sol-gel and dip-hydrothermal preparation methods on structure and performance of TiO2/AC photocatalysts[J]. Acta Physico-Chimica Sinica, 2010, 26(1): 79-86. | |
| 19 | PEÑAS-GARZÓN M, GÓMEZ-AVILÉSA, BEDIA J, et al. Effect of activating agent on the properties of TiO2/activated carbon heterostructures for solar photocatalytic degradation of acetaminophen[J]. Materials, 2019, 12(3): 378-394. |
| 20 | SUN S, ZHAO R, XIE Y, et al. Photocatalytic degradation of aflatoxin B1 by activated carbon supported TiO2 catalyst[J]. Food Control, 2019, 100: 183-188. |
| 21 | TIAN F, WU Z, CHEN Q, et al. Microwave-induced crystallization of AC/TiO2 for improving the performance of Rhodamine B dye degradation[J]. Applied Surface Science, 2015, 351: 104-112. |
| 22 | TIAN F, WU Z, YAN Y, et al. Photodegradation of formaldehyde by activated carbon loading TiO2 synthesized via microwave irradiation[J]. Korean Journal of Chemical Engineering, 2015, 32(7): 1333-1339. |
| 23 | PEÑAS-GARZÓN M, GÓMEZ-AVILÉS A, BELVER C, et al. Degradation pathways of emerging contaminants using TiO2-activated carbon heterostructures in aqueous solution under simulated solar light[J]. Chemical Engineering Journal, 2020, 392: 124867. |
| 24 | ANTOLINI E. Photo-assisted methanol oxidation on Pt-TiO2 catalysts for direct methanol fuel cells: a short review[J]. Applied Catalysis B: Environmental, 2018, 237: 491-503. |
| 25 | LOW J, YU J, JARONIEC M, et al. Heterojunction photocatalysts[J]. Advanced Materials, 2017, 29: 1601694. |
| 26 | HARUNA A, ALAN J M, REBECCA S F T, et al. Simultaneous photocatalytic removal of nitrate and oxalic acid over Cu2O/TiO2 and Cu2O/TiO2-AC composites[J]. Applied Catalysis B: Environmental, 2017, 217: 181-191. |
| 27 | OTIENO B O, APOLLO S O, NAIDOO B E, et al. Photodecolorisation of melanoidins in vinasse with illuminated TiO2-ZnO/activated carbon composite[J]. Journal of Environmental Science and Health A, 2017, 52(7): 616-623. |
| 28 | WANG N, LI X, YANG Y, et al. Two-stage calcination composite of Bi2O3-TiO2 supported on powdered activated carbon for enhanced degradation of sulfamethazine under solar irradiation[J]. Journal of Water Process Engineering, 2020, 35: 101220. |
| 29 | BAI H, JUAY J, LIU Z, et al. Hierarchical SrTiO3/TiO2 nanofibers heterostructures with high efficiency in photocatalytic H2 generation[J]. Applied Catalysis B: Environmental, 2012, 125: 367-374. |
| 30 | PANG X, CHEN C, JI H, et al. Unraveling the photocatalytic mechanisms on TiO2 surfaces using the oxygen-18 isotopic label technique[J]. Molecules, 2014,19(10): 16291-16311. |
| 31 | LI Z, LUAN Y, QU Y, et al. Modification strategies with inorganic acids for efficient photocatalysts by promoting the adsorption of O2[J]. ACS Appl. Mater. Interfaces, 2015,7(41): 22727-22740. |
| 32 | JING L, CAO Y, CUI H, et al. Acceleration effects of phosphate modification on the decay dynamics of photo-generated electrons of TiO2 and its photocatalytic activity[J]. Chemical Communications, 2012, 48(87): 10775-10777. |
| 33 | ALI S, LI Z, CHEN S, et al. Synthesis of activated carbon-supported TiO2-based nano-photocatalysts with well recycling for efficiently degrading high-concentration pollutants[J]. Catalysis Today, 2019, 335: 557-564. |
| 34 | SATHISHKUMAR P, ANANDAN S, MARUTHAMUTHU P, et al. Synthesis of Fe3+ doped TiO2 photocatalysts for the visible assisted degradation of an azo dye[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2011, 375(1/2/3): 231-236. |
| 35 | ESHAGHI A, MORADI H. Optical and photocatalytic properties of the Fe-doped TiO2 nanoparticles loaded on the activated carbon[J]. Advanced Powder Technology, 2018, 29(8): 1879-1885. |
| 36 | LI Y, ZHOU X, CHEN W, et al. Photodecolorization of Rhodamine B on tungsten-doped TiO2/activated carbon under visible-light irradiation[J]. Journal of Hazardous Materials, 2012, 227/228: 25-33. |
| 37 | 康宏平, 孙振亚, 刘建永, 等. Ag+-TiO2 /AC复合材料的可见光吸附-光催化协同作用[J]. 环境工程学报, 2015, 9(4): 1620-1624. |
| KANG Hongping, SUN Zhenya, LIU Jianyong, et al. Adsorption-visible photocatalytic synergistic of Ag+-TiO2 /AC composite[J]. Chinese Journal of Environmental Engineering, 2015, 9(4): 1620-1624. | |
| 38 | 陈伟, 李佑稷, 李雷勇, 等. 外负载Ce-TiO2/活性炭复合体对亚甲基蓝光催化[J]. 环境工程学报, 2012, 6(6): 1895-1900. |
| CHEN Wei, LI Youji, LI Leiyong, et al. Photocatalysis of Methylene Blue by composites of cerium doped TiO2-outerloaded activated carbon[J]. Chinese Journal of Environmental Engineering, 2012, 6(6): 1895-1900. | |
| 39 | XING J, SUN X, QIU J. Preparation, characterization, and photocatalytic activity of La-doped TiO2 supported on activated carbon at the decomposition of methylene orange[J]. Russian Journal of Physical Chemistry A, 2015, 89(6): 1108-1114. |
| 40 | YAP P S, LIM T T, LIM M, et al. Synthesis and characterization of nitrogen-doped TiO2/AC composite for the adsorption-photocatalytic degradation of aqueous Bisphenol-A using solar light[J]. Catalysis Today, 2010, 151: 8-13. |
| 41 | PUDWAT S, KEAWJAIJONG P, AKKANIT L, et al. Decolorization of MB by F-TiO2/AC in a dynamic reactor[J]. Materials Today: Proceedings, 2018, 5(7): 14807-14812. |
| 42 | 秦亚菲, 陈杰, 刘瑶, 等. 活性炭负载I-TiO2对气体中甲苯的吸附[J]. 环境工程学报, 2016, 10(12): 7147-7155. |
| QIN Yafei, CHEN Jie, LIU Yao, et al. Adsorption of toluene in gas using loaded I-TiO2 activated carbon[J]. Chinese Journal of Environmental Engineering, 2016, 10(12): 7147-7155. | |
| 43 | ZHOU J, LI F, DU C, et al. Photodegradation performance and recyclability of a porous nitrogen and carbon co-doped TiO2/activated carbon composite prepared by an extremely fast one-step microwave method[J]. RSC Advances, 2016, 6(87): 84457-84463. |
| 44 | WANG X, SONG J, HUANG J, et al. Activated carbon-based magnetic TiO2 photocatalyst co-doped with iodine and nitrogen for organic pollution degradation[J]. Applied Surface Science, 2016, 390: 190-201. |
| 45 | 王佳忆, 王学江, 黄嘉瑜, 等. Br-N共掺杂TiO2/磁性炭复合材料的制备及其可见光催化性能[J]. 复合材料学报, 2017, 34(4): 662-670. |
| WANG Jiayi, WANG Xuejiang, HUANG Jiayu, et al. Preparation and photocatalytic performance of Br-N codoped TiO2/magnetic carbon composites[J]. Acta Materiae Compositae Sinica, 2017, 34(4): 662-670. | |
| 46 | TIAN F, WU Z, YAN Y, et al. Synthesis of visible-light-responsive Cu and N-codoped AC/TiO2 photocatalyst through microwave irradiation[J]. Nanoscale Research Letters, 2016, 11(1): 292-292. |
| 47 | LIU D, WU Z, TIAN F, et al. Synthesis of N and La co-doped TiO2/AC photocatalyst by microwave irradiation for the photocatalytic degradation of naphthalene[J]. Journal of Alloys and Compounds, 2016, 676: 489-498. |
| 48 | LIU D, LIU Y, WU Z, et al. Enhancement of photodegradation of Ce, N, and P tri-doped TiO2/AC by microwave radiation with visible light response for naphthalene[J]. Journal of the Taiwan Institute of Chemical Engineers, 2016, 68: 506-513. |
| 49 | SU E, HUANG B, LIU C, et al. Photocatalytic conversion of simulated EDTA wastewater to hydrogen by pH-resistant Pt/TiO2 activated carbon photocatalysts[J]. Renewable Energy, 2015, 75: 266-271. |
| 50 | 阳鹏飞, 孙允凯, 柯国军. 双效功能型复合光催化剂Ag/TiO2/AC的制备及光催化活性[J]. 精细化工, 2016, 33(12): 1393-1397. |
| YANG Pengfei, SUN Yunkai, KE Guojun. Preparation and photocatalytic activity of Ag/TiO2/AC photo-catalyst with double function[J]. Fine Chemical Industry, 2016, 33(12): 1393-1397. | |
| 51 | LIU W, LANG Z. The structure and self-regeneration performance of Salix psammophila-activated carbon modified by Ag and N co-doped TiO2[J]. RSC Advances, 2020, 10(7): 3844-3852. |
| 52 | ZHU L, KONG X, YANG C, et al. Fabrication and characterization of the magnetic separation photocatalyst C-TiO2@Fe3O4/AC with enhanced photocatalytic performance under visible light irradiation[J]. Journal of Hazardous Materials, 2020, 381: 120910. |
| 53 | ZHOU J, ZHU B, WANG L, et al. Enhanced photocatalytic activity of Fe-doped TiO2 coated on N-doped activated carbon composites for photocatalytic degradation of dyeing wastewater[J]. AIP Conference Proceedings, 2017, 1890(1): 5541-5557. |
| 54 | 闫春燕, 伊文涛, 李法强. TiO2/AC复合催化剂光催化降解布洛芬[J]. 化工环保, 2014, 34(3): 281-286. |
| YAN Chunyan, YI Wentao, LI Faqiang. Photocatalytic degradation of ibuprofen by TiO2/AC composite catalyst[J]. Environmental Protection of Chemical Industry, 2014, 34(3): 281-286. | |
| 55 | CUNHA D L, KUZNETSOV A, ARAUJO J R, et al. Optimization of benzodiazepine drugs removal from water by heterogeneous photocatalysis using TiO2/activated carbon composite[J]. Water, Air and Soil Pollution, 2019, 230(7): 141-157. |
| 56 | KAKAVANDI B, BAHARI N, KALANTARY R R, et al. Enhanced sono-photocatalysis of tetracycline antibiotic using TiO2 decorated on magnetic activated carbon (MAC@T) coupled with US and UV: a new hybrid system[J]. Ultrasonics Sonochemistry, 2019, 55: 75-85. |
| 57 | GAR A M, TAWFIK A, OOKAWARA S. Solar photocatalytic degradation of phenol by TiO2/AC prepared by temperature impregnation method[J]. Desalination and Water Treatment, 2016, 57(2): 835-844. |
| 58 | YAP P S, LIM T T. Effect of aqueous matrix species on synergistic removal of Bisphenol-A under solar irradiation using nitrogen-doped TiO2/AC composite[J]. Applied Catalysis B: Environmental, 2011, 101(3/4): 709-717. |
| 59 | SHU Y, XU Y, HUANG H, et al. Catalytic oxidation of VOCs over Mn/TiO2/activated carbon under 185nm VUV irradiation[J]. Chemosphere, 2018, 208: 550-558. |
| 60 | HORIKOSHI S, SAKAMOTO S, SERPONE N. Formation and efficacy of TiO2/AC composites prepared under microwave irradiation in the photoinduced transformation of the 2-propanol VOC pollutant in air[J]. Applied Catalysis B: Environmental, 2013, 140/141: 646-651. |
| 61 | TAO Y, WU C-Y, MAZYCK D W. Removal of methanol from pulp and paper mills using combined activated carbon adsorption and photocatalytic regeneration[J]. Chemosphere, 2006, 65(1): 35-42. |
| [1] | ZHANG Mingyan, LIU Yan, ZHANG Xueting, LIU Yake, LI Congju, ZHANG Xiuling. Research progress of non-noble metal bifunctional catalysts in zinc-air batteries [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 276-286. |
| [2] | SHI Yongxing, LIN Gang, SUN Xiaohang, JIANG Weigeng, QIAO Dawei, YAN Binhang. Research progress on active sites in Cu-based catalysts for CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 287-298. |
| [3] | XIE Luyao, CHEN Songzhe, WANG Laijun, ZHANG Ping. Platinum-based catalysts for SO2 depolarized electrolysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 299-309. |
| [4] | YANG Xiazhen, PENG Yifan, LIU Huazhang, HUO Chao. Regulation of active phase of fused iron catalyst and its catalytic performance of Fischer-Tropsch synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 310-318. |
| [5] | WANG Lele, YANG Wanrong, YAO Yan, LIU Tao, HE Chuan, LIU Xiao, SU Sheng, KONG Fanhai, ZHU Canghai, XIANG Jun. Influence of spent SCR catalyst blending on the characteristics and deNO x performance for new SCR catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 489-497. |
| [6] | DENG Liping, SHI Haoyu, LIU Xiaolong, CHEN Yaoji, YAN Jingying. Non-noble metal modified vanadium titanium-based catalyst for NH3-SCR denitrification simultaneous control VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 542-548. |
| [7] | CHENG Tao, CUI Ruili, SONG Junnan, ZHANG Tianqi, ZHANG Yunhe, LIANG Shijie, PU Shi. Analysis of impurity deposition and pressure drop increase mechanisms in residue hydrotreating unit [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4616-4627. |
| [8] | WANG Peng, SHI Huibing, ZHAO Deming, FENG Baolin, CHEN Qian, YANG Da. Recent advances on transition metal catalyzed carbonylation of chlorinated compounds [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4649-4666. |
| [9] | GAO Yanjing. Analysis of international research trend of single-atom catalysis technology [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4667-4676. |
| [10] | ZHANG Qi, ZHAO Hong, RONG Junfeng. Research progress of anti-toxicity electrocatalysts for oxygen reduction reaction in PEMFC [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4677-4691. |
| [11] | GE Quanqian, XU Mai, LIANG Xian, WANG Fengwu. Research progress on the application of MOFs in photoelectrocatalysis [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4692-4705. |
| [12] | WANG Weitao, BAO Tingyu, JIANG Xulu, HE Zhenhong, WANG Kuan, YANG Yang, LIU Zhaotie. Oxidation of benzene to phenol over aldehyde-ketone resin based metal-free catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4706-4715. |
| [13] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| [14] | SHAO Zhiguo, REN Wen, XU Shipei, NIE Fan, XU Yu, LIU Longjie, XIE Shuixiang, LI Xingchun, WANG Qingji, XIE Jiacai. Influence of final temperature on the distribution and characteristics of oil-based drilling cuttings pyrolysis products [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4929-4938. |
| [15] | XIANG Yang, HUANG Xun, WEI Zidong. Recent progresses in the activity and selectivity improvement of electrocatalytic organic synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4005-4014. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||