| 1 |
RYBTCHINSKI B, VIGALOK A, BEN-DAVID Y, et al. A room temperature direct metal insertion into a nonstrained carbon-carbon bond in solution. C H bond activation[J]. Journal of the American Chemical Society, 1996, 118(49): 12406-12415. H bond activation[J]. Journal of the American Chemical Society, 1996, 118(49): 12406-12415.
|
| 2 |
JUST-BARINGO X, LARROSA I. Ketone C—C bond activation meets the suzuki-miyaura cross-coupling[J]. Chem., 2018, 4(6): 1203-1204.
|
| 3 |
ARUN V, MAHANTY K, DE-SARKAR S. Nickel-catalyzed dehydrogenative couplings[J]. ChemCatChem, 2019, 11(9): 2243-2259.
|
| 4 |
OBLIGACION J V, CHIRIK P J. Earth-abundant transition metal catalysts for alkene hydrosilylation and hydroboration[J]. Nature Reviews Chemistry, 2018, 2(5): 15.
|
| 5 |
SAINT-DENIS T G, ZHU R Y, CHEN G, et al. Enantioselective C(sp3)—H bond activation by chiral transition metal catalysts[J]. Science, 2018, 359(6377): eaao4798.
|
| 6 |
WANG F, YU S, LI X. Transition metal-catalysed couplings between arenes and strained or reactive rings: combination of C H activation and ring scission[J]. Chemical Society Reviews, 2016, 45(23): 6462-6477. H activation and ring scission[J]. Chemical Society Reviews, 2016, 45(23): 6462-6477.
|
| 7 |
DONG Z, RENE Z, THOMPSON S J, et al. Transition-metal-catalyzed C H alkylation using alkenes[J]. Chemical Reviews, 2017, 117(13): 9333-9403. H alkylation using alkenes[J]. Chemical Reviews, 2017, 117(13): 9333-9403.
|
| 8 |
HATAZAWA M, YOSHIE N, SEINO H. Reversible hydride transfer to N,N′-diarylimidazolinium cations from hydrogen catalyzed by transition metal complexes mimicking the reaction of [Fe]-hydrogenase[J]. Inorganic Chemistry, 2017, 56(14): 8087-8099.
|
| 9 |
ESTESstes D P, SIDDIQI G, ALLOUCHE F, et al. C H activation on Co, O sites: isolated surface sites versus molecular analogs[J]. Journal of the American Chemical Society, 2016, 138(45): 14987-14997. H activation on Co, O sites: isolated surface sites versus molecular analogs[J]. Journal of the American Chemical Society, 2016, 138(45): 14987-14997.
|
| 10 |
PRATS H, GUTIERREZ R A, PIRRERO J J, et al. Room temperature methane capture and activation by Ni clusters supported on TiC (001): effects of metal-carbide interactions on the cleavage of the C H bond[J]. Journal of the American Chemical Society, 2019, 141(13): 5303-5313. H bond[J]. Journal of the American Chemical Society, 2019, 141(13): 5303-5313.
|
| 11 |
MARCINKOWSKI M D, DARBY M T, LIU J, et al. Pt/Cu single-atom alloys as coke-resistant catalysts for efficient C H activation[J]. Nature Chemistry, 2018, 10(3): 325. H activation[J]. Nature Chemistry, 2018, 10(3): 325.
|
| 12 |
COLLETTO C, PANIGRAHI A, FEMANDEZ-CASADO J, et al. Ag (i)  H activation enables near-room-temperature direct α-arylation of benzo [b] thiophenes[J]. Journal of the American Chemical Society, 2018, 140(30): 9638-9643. H activation enables near-room-temperature direct α-arylation of benzo [b] thiophenes[J]. Journal of the American Chemical Society, 2018, 140(30): 9638-9643.
|
| 13 |
JUN C H. Transition metal-catalyzed carbon-carbon bond activation[J]. Chemical Society Reviews, 2004, 33(9): 610-618.
|
| 14 |
LU T, ZHUANG X, LI Y, et al. C C bond cleavage of acetonitrile by a dinuclear copper (Ⅱ) cryptate[J]. Journal of the American Chemical Society, 2004, 126(15): 4760-4761. C bond cleavage of acetonitrile by a dinuclear copper (Ⅱ) cryptate[J]. Journal of the American Chemical Society, 2004, 126(15): 4760-4761.
|
| 15 |
LI S, WEI C, HU Y, et al. In situ synthesis and photocatalytic mechanism of a cyano bridged Cu (Ⅰ) polymer[J]. Inorganic Chemistry Frontiers, 2018, 5(6): 1282-1287.
|
| 16 |
CABALLERO A, DESPAGNET-AYOUB E, DIAZ-REQUEJO M M, et al. Silver-catalyzed CC bond formation between methane and ethyl diazoacetate in supercritical CO2[J]. Science, 2011, 332(6031): 835-838.
|
| 17 |
SHELDRICK G M. SADABS[D]. Gottingen: University of Gottingen, 1996.
|
| 18 |
SHELDRICK G M. Crystal structure refinement with SHELXL[J]. Acta Crystallographica Section C: Structural Chemistry, 2015, 71(1): 3-8.
|
 ), Yuanhao HUANG1, Beiling LIAO2(
), Yuanhao HUANG1, Beiling LIAO2( )
)
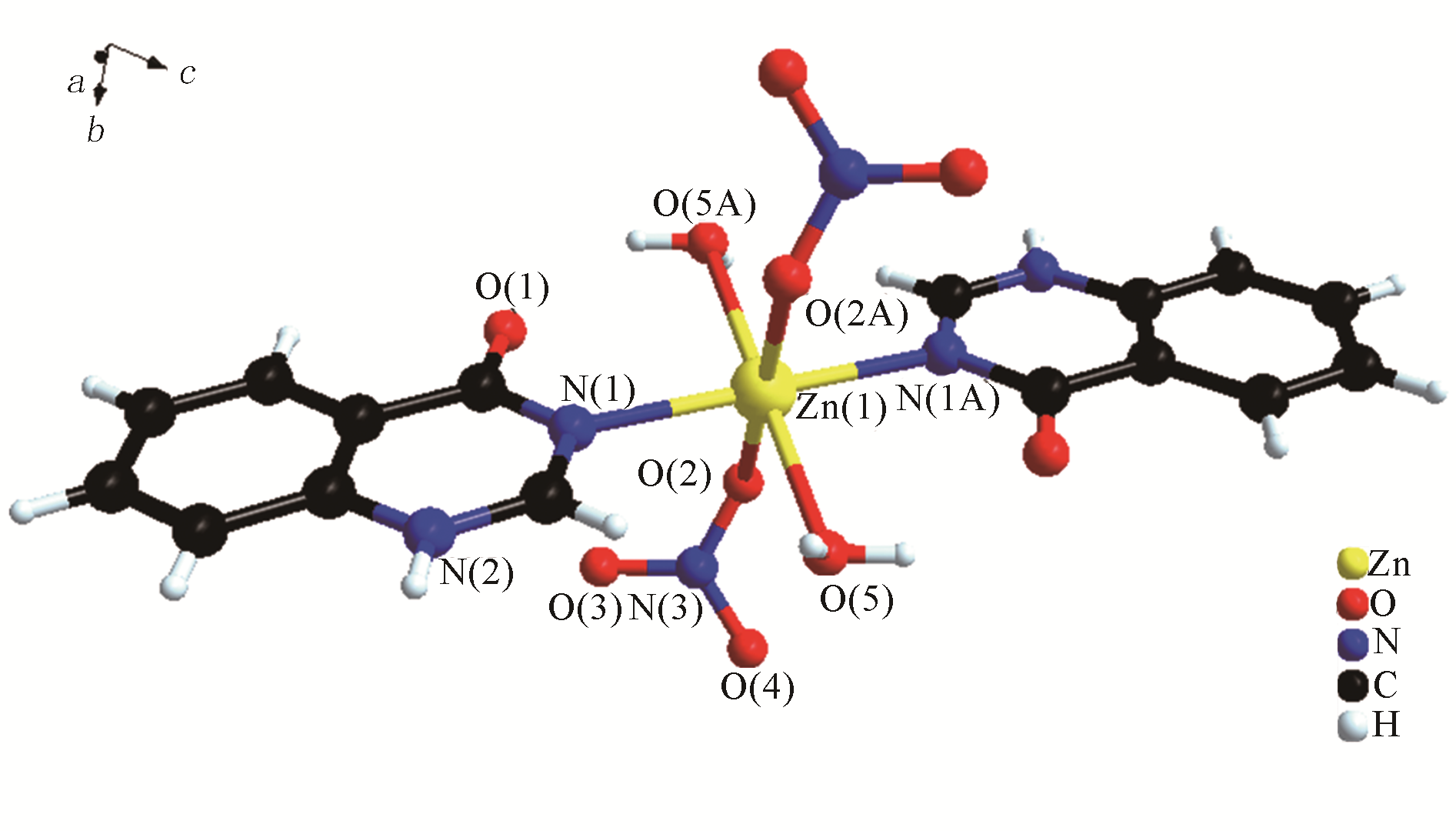
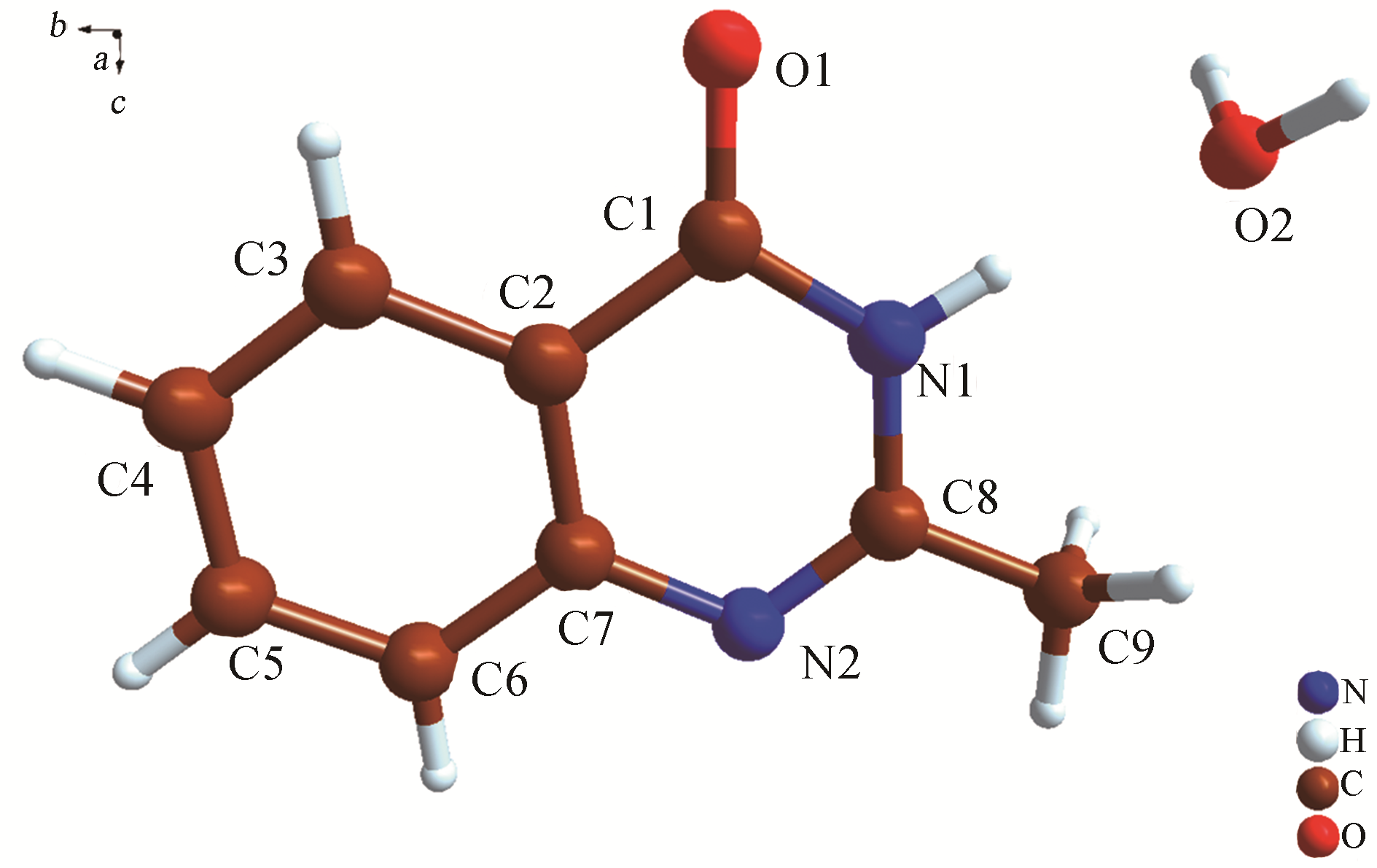
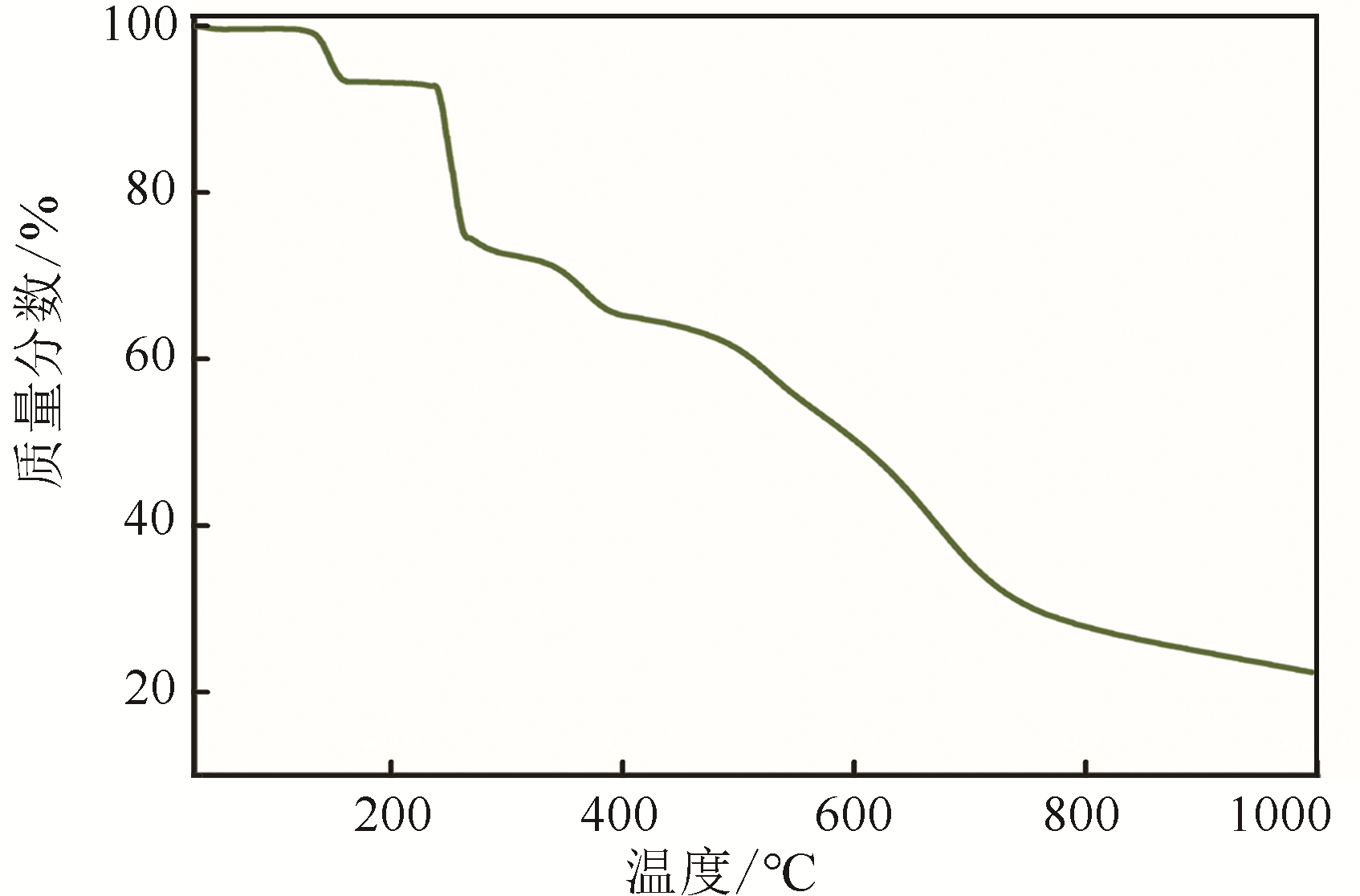


 H bond activation[J]. Journal of the American Chemical Society, 1996, 118(49): 12406-12415.
H bond activation[J]. Journal of the American Chemical Society, 1996, 118(49): 12406-12415. H activation and ring scission[J]. Chemical Society Reviews, 2016, 45(23): 6462-6477.
H activation and ring scission[J]. Chemical Society Reviews, 2016, 45(23): 6462-6477. H alkylation using alkenes[J]. Chemical Reviews, 2017, 117(13): 9333-9403.
H alkylation using alkenes[J]. Chemical Reviews, 2017, 117(13): 9333-9403. H activation on Co, O sites: isolated surface sites versus molecular analogs[J]. Journal of the American Chemical Society, 2016, 138(45): 14987-14997.
H activation on Co, O sites: isolated surface sites versus molecular analogs[J]. Journal of the American Chemical Society, 2016, 138(45): 14987-14997. H bond[J]. Journal of the American Chemical Society, 2019, 141(13): 5303-5313.
H bond[J]. Journal of the American Chemical Society, 2019, 141(13): 5303-5313. H activation[J]. Nature Chemistry, 2018, 10(3): 325.
H activation[J]. Nature Chemistry, 2018, 10(3): 325. H activation enables near-room-temperature direct α-arylation of benzo [b] thiophenes[J]. Journal of the American Chemical Society, 2018, 140(30): 9638-9643.
H activation enables near-room-temperature direct α-arylation of benzo [b] thiophenes[J]. Journal of the American Chemical Society, 2018, 140(30): 9638-9643. C bond cleavage of acetonitrile by a dinuclear copper (Ⅱ) cryptate[J]. Journal of the American Chemical Society, 2004, 126(15): 4760-4761.
C bond cleavage of acetonitrile by a dinuclear copper (Ⅱ) cryptate[J]. Journal of the American Chemical Society, 2004, 126(15): 4760-4761.