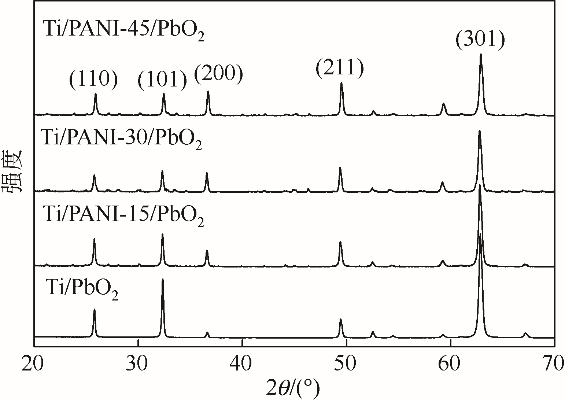
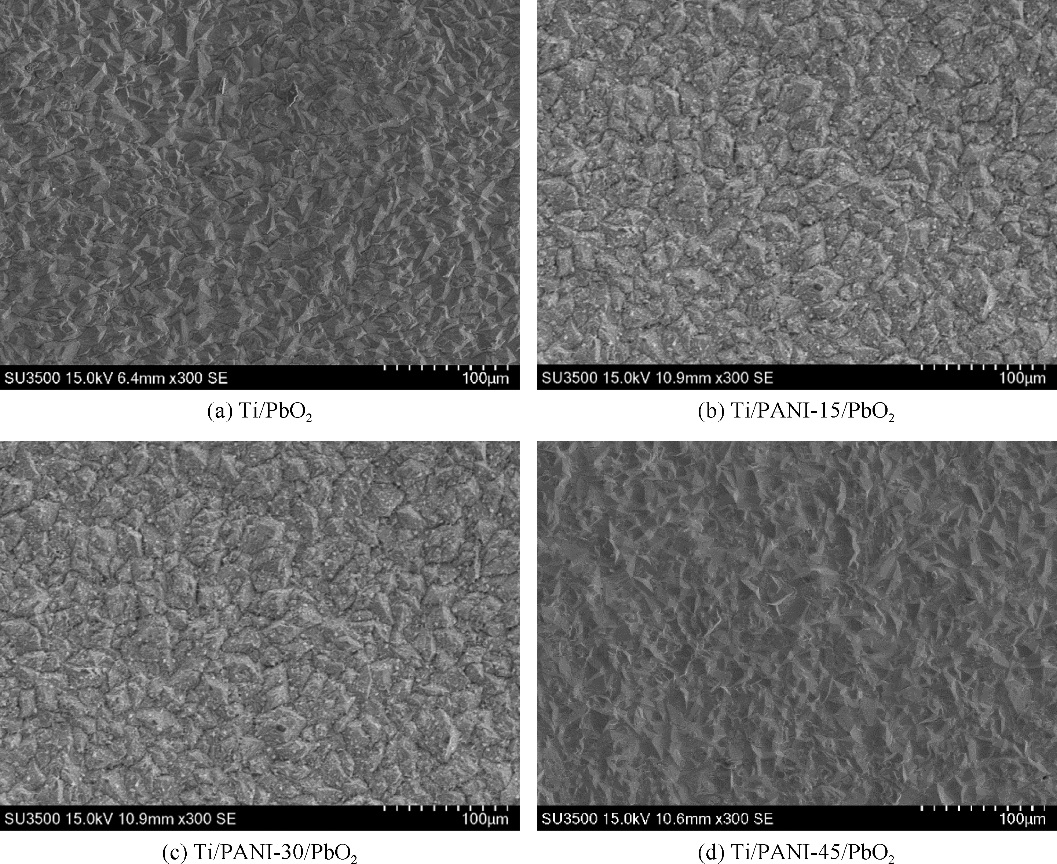
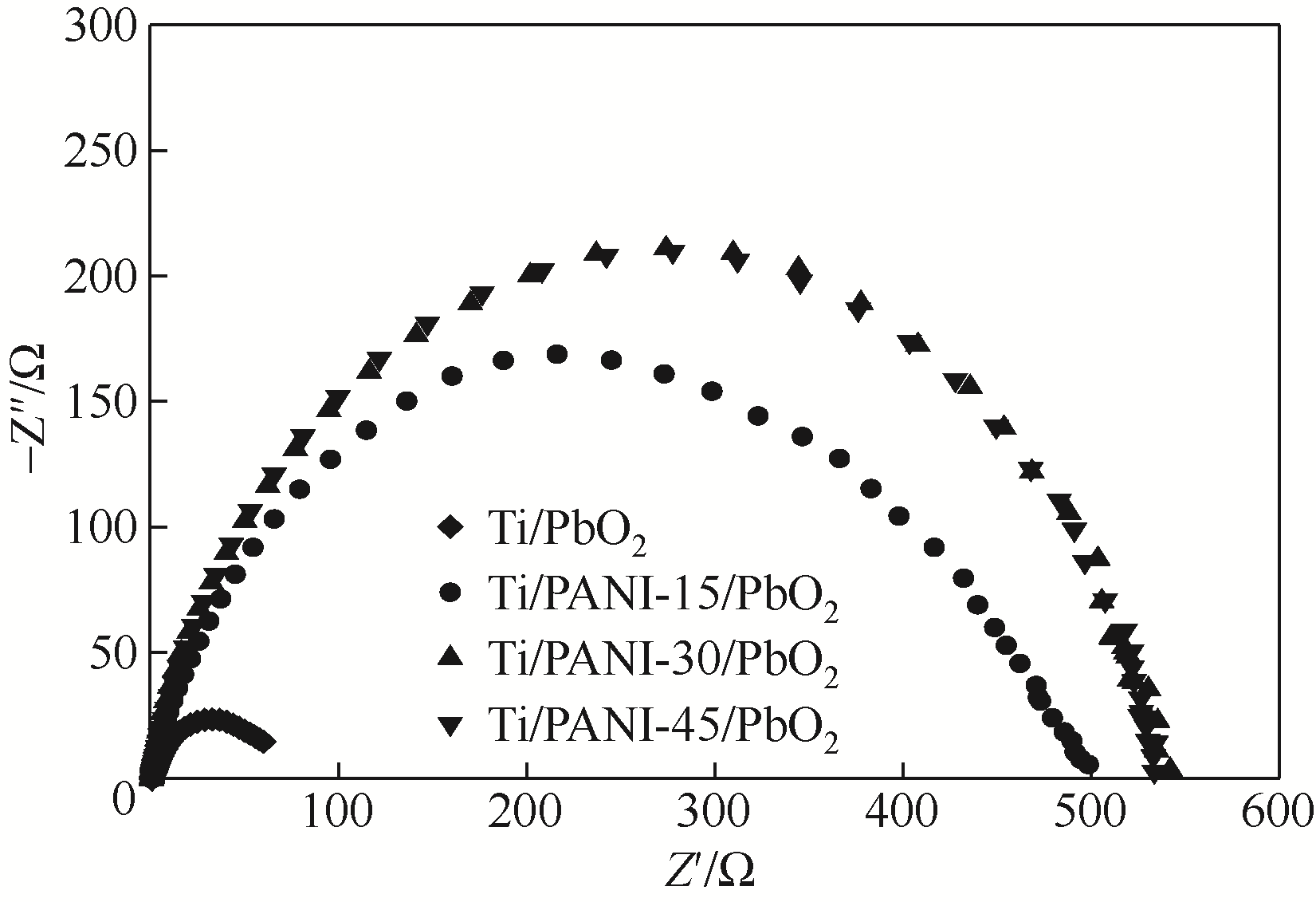
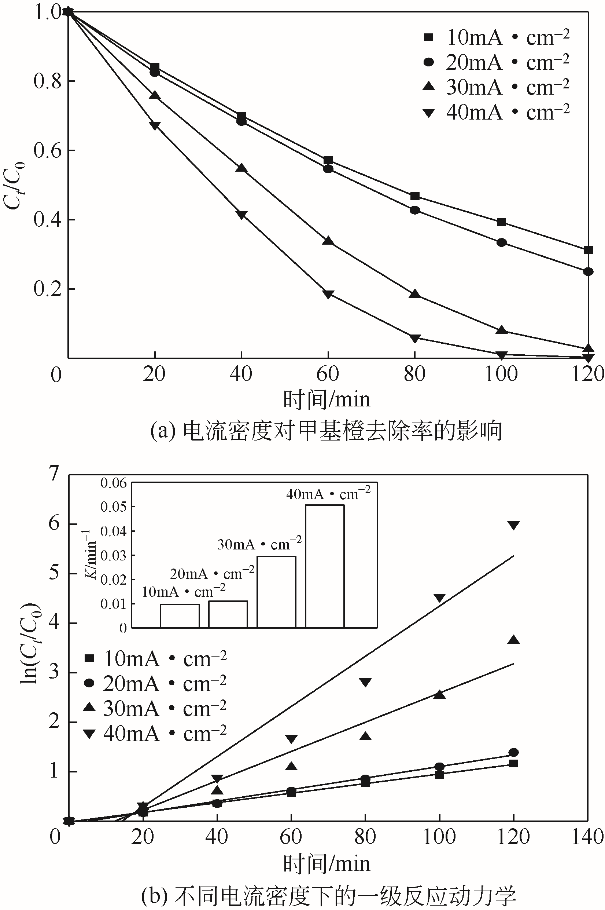
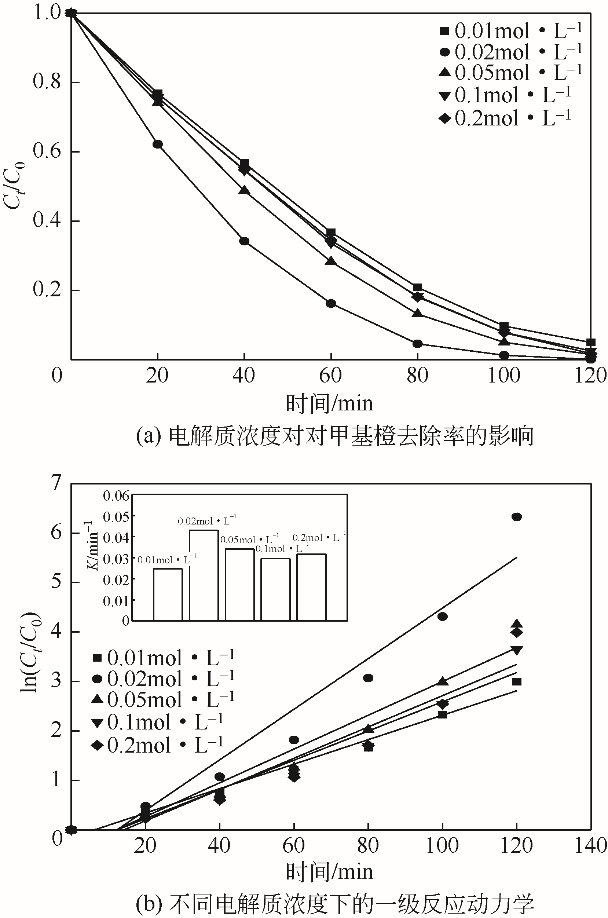
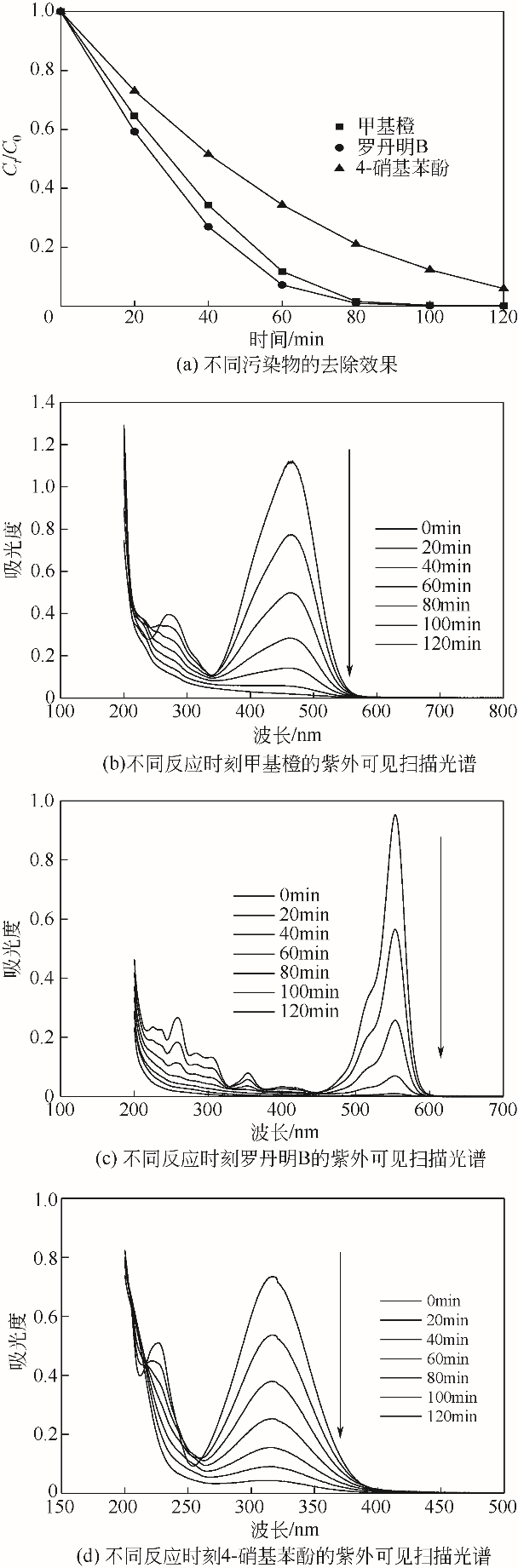
| 1 |
WEI Lingyong, GUO Shaohui, YAN Guangxu, et al. Electrochemical pretreatment of heavy oil refinery wastewater using a three-dimensional electrode reactor[J]. Electrochimica Acta, 2010, 55(28): 8615-8620.
|
| 2 |
ZHAO Huazhang, SUN Yan, XU Lina, et al. Removal of acid orange 7 in simulated wastewater using a three-dimensional electrode reactor: removal mechanisms and dye degradation pathway[J]. Chemosphere, 2010, 78(1): 46-51.
|
| 3 |
XU Li, LI Ming, XU Wei. Preparation and characterization of Ti/SnO2-Sb electrode with copper nanorods for AR 73 removal[J]. Electrochimica Acta, 2015, 166: 64-72.
|
| 4 |
WANG Chong, YIN Lifeng, XU Zesheng, et al. Electrochemical degradation of enrofloxacin by lead dioxide anode: kinetics, mechanism and toxicity evaluation[J]. Chemical Engineering Journal, 2017, 326: 911-920.
|
| 5 |
RAHMANI A R, NEMATOLLAHI D, SAMARGHANDI M R, et al. A combined advanced oxidation process: electrooxidation-ozonation for antibiotic ciprofloxacin removal from aqueous solution[J]. Journal of Electroanalytical Chemistry, 2018, 808: 82-89.
|
| 6 |
WU Weiyi, HUANG Zhaohong, Teikthye LIM. Recent development of mixed metal oxide anodes for electrochemical oxidation of organic pollutants in water[J]. Applied Catalysis A: General, 2014, 480: 58-78.
|
| 7 |
ZHANG Chao, JIANG Yonghai, LI Yunlin, et al. Three-dimensional electrochemical process for wastewater treatment: a general review[J]. Chemical Engineering Journal, 2013, 228: 455-467.
|
| 8 |
HE Yapeng, WANG Xue, HUANG Weimin, et al. Hydrophobic networked PbO2 electrode for electrochemical oxidation of paracetamol drug and degradation mechanism kinetics[J]. Chemosphere, 2018, 193: 89-99.
|
| 9 |
CHEN Fengtao, YU Sanchuan, DONG Xiaoping, et al. Preparation and characterization of PbO2 electrode and its application in electro-catalytic degradation of o-aminophenol in aqueous solution assisted by CuO-Ce2O3/gamma-Al2O3 catalyst[J]. Journal of Hazardous Materials, 2013, 260: 747-753.
|
| 10 |
ZHANG Qianli, GUO Xinyan, CAO Xiaodan, et al. Facile preparation of a Ti/α-PbO2/β-PbO2 electrode for the electrochemical degradation of 2-chlorophenol[J]. Chinese Journal of Catalysis, 2015, 36(7): 975-981.
|
| 11 |
XU Zesheng, YU Yanxin, LIU Han, et al. Highly efficient and stable Zr-doped nanocrystalline PbO2 electrode for mineralization of perfluorooctanoic acid in a sequential treatment system[J]. Science of the Total Environment, 2017, 579: 1600-1607.
|
| 12 |
宋秀丽, 杨慧敏, 梁镇海. 钛基氧化物阳极中间层的研究进展[J].电化学, 2013, 19(4): 313-321.
|
|
SONG Xiuli, YANG Huimin, LIANG Zhenhai. Research progress of intermediate layers in Ti-based oxide anodes[J]. Journal of Electrochemistry, 2013, 19(4): 313-321.
|
| 13 |
DUAN Xiaoyue, ZHAO Cuimei, LIU Wei, et al. Fabrication of a novel PbO2 electrode with a graphene nanosheet interlayer for electrochemical oxidation of 2-chlorophenol[J]. Electrochimica Acta, 2017, 240: 424-436.
|
| 14 |
Sambhu BHADRA, Dipak KHASTGIR, SINGHA Nikhil K, et al. Progress in preparation, processing and applications of polyaniline[J]. Progress in Polymer Science, 2009, 34(8): 783-810.
|
| 15 |
Ju-Won JEON, MA Yuguang, MIKE Jared F, et al. Oxidatively stable polyaniline:polyacid electrodes for electrochemical energy storage[J]. Physical Chemistry Chemical Physics, 2013, 15(24): 9654-9662.
|
| 16 |
Ali OLAD, Rahimeh NOSRATI. Preparation and corrosion resistance of nanostructured PVC/ZnO-polyaniline hybrid coating[J]. Progress in Organic Coatings, 2013, 76(1): 113-118.
|
| 17 |
Anongnad THIANGKAEW, Khamsone KEOTHONGKHAM, Wasan MAIAUGREE, et al. One-step electrochemically-codeposited polyaniline-platinum for dye-sensitized solar cell applications[J]. Journal of the Korean Physical Society, 2014, 64(9): 1356-1362.
|
| 18 |
ASADOLLAHI A, SOHRABNEZHAD S, ANSARI R, et al. p-n Heterojuction in organic (polyaniline)-inorganic (Ag2CO3) polymer-based heterojuction photocatalyst[J]. Materials Science in Semiconductor Processing, 2018, 87: 119-125.
|
| 19 |
SHEN Junjie, Salman SHAHID, Adem SARIHAN, et al. Effect of polyacid dopants on the performance of polyaniline membranes in organic solvent nanofiltration[J]. Separation and Purification Technology, 2018, 204: 336-344.
|
| 20 |
YUAN Xueya, XU Yan, MENG Hao, et al. Fabrication of ternary polyaniline-graphene oxide-TiO2 hybrid films with enhanced activity for photoelectrocatalytic hydrogen production[J]. Separation and Purification Technology, 2018, 193: 358-367.
|
| 21 |
XU Zesheng, LIU Han, NIU Junfeng, et al. Hydroxyl multi-walled carbon nanotube-modified nanocrystalline PbO2 anode for removal of pyridine from wastewater[J]. Journal of Hazardous Materials, 2017, 327: 144-152.
|
| 22 |
SONG Shuang, FAN Jiaqi, HE Zhiqiao, et al. Electrochemical degradation of azo dye C.I. reactive red 195 by anodic oxidation on Ti/SnO2-Sb/PbO2 electrodes[J]. Electrochimica Acta, 2010, 55(11): 3606-3613.
|
| 23 |
GURUNG K, NCIBI M C, SHESTAKOVA M, et al. Removal of carbamazepine from MBR effluent by electrochemical oxidation (EO) using a Ti/Ta2O5-SnO2 electrode[J]. Applied Catalysis B: Environmental, 2018, 221: 329-338.
|
| 24 |
QIAO Qicheng, Seema SINGH, Shanglien LO, et al. Electrochemical oxidation of acid orange 7 dye with Ce, Nd, and Co-modified PbO2 electrodes: preparation, characterization, optimization, and mineralization[J]. Journal of the Taiwan Institute of Chemical Engineers, 2018, 84: 110-122.
|
| 25 |
DAI Qizhou, ZHOU Jiazhong, MENG Xiaoyang, et al. electrochemical oxidation of cinnamic acid with Mo modified PbO2 electrode: electrode characterization, kinetics and degradation pathway[J]. Chemical Engineering Journal, 2016, 289: 239-246.
|
| 26 |
CHEN Jianmeng, XIA Yijing, DAI Qizhou. Electrochemical degradation of chloramphenicol with a novel Al doped PbO2 electrode: performance, kinetics and degradation mechanism[J]. Electrochimica Acta, 2015, 165: 277-287.
|
| 27 |
LI Xiaolin, LI Xueming, YANG Wenjing, et al. Preparation of 3D PbO2 nanospheres@SnO2 nanowires/Ti electrode and its application in methyl orange degradation[J]. electrochimica Acta, 2014, 146: 15-22.
|
| 28 |
王春英, 朱清江, 谷传涛, 等. 稀土Ce3+掺杂Bi2WO6光催化降解罗丹明B的研究[J]. 中国环境科学, 2015, 35(9): 2682-2689.
|
|
WANG Chunying, ZHU Qingjiang, GU Chuantao, et al. Investigation of rhodamine B photocatalytic degradation by Ce3+ doped Bi2WO6[J]. China Environmental Science, 2015, 35(9): 2682-2689.
|
| 29 |
YU Chongfei, YANG Pengyan, Luna TIE, et al. One-pot fabrication of β-Bi2O3@Bi2S3 hierarchical hollow spheres with advanced sunlight photocatalytic RhB oxidation and Cr(Ⅵ) reduction activities[J]. Applied Surface Science, 2018, 455: 8-17.
|
 ),Wenjing LIU1,2,Pei DONG1,2,Liang ZHANG1,2,Zhengwei YANG1,2,Chaocheng ZHAO1,2(
),Wenjing LIU1,2,Pei DONG1,2,Liang ZHANG1,2,Zhengwei YANG1,2,Chaocheng ZHAO1,2( )
)
 ),刘文静1,2,董培1,2,张亮1,2,杨政伟1,2,赵朝成1,2(
),刘文静1,2,董培1,2,张亮1,2,杨政伟1,2,赵朝成1,2( )
)