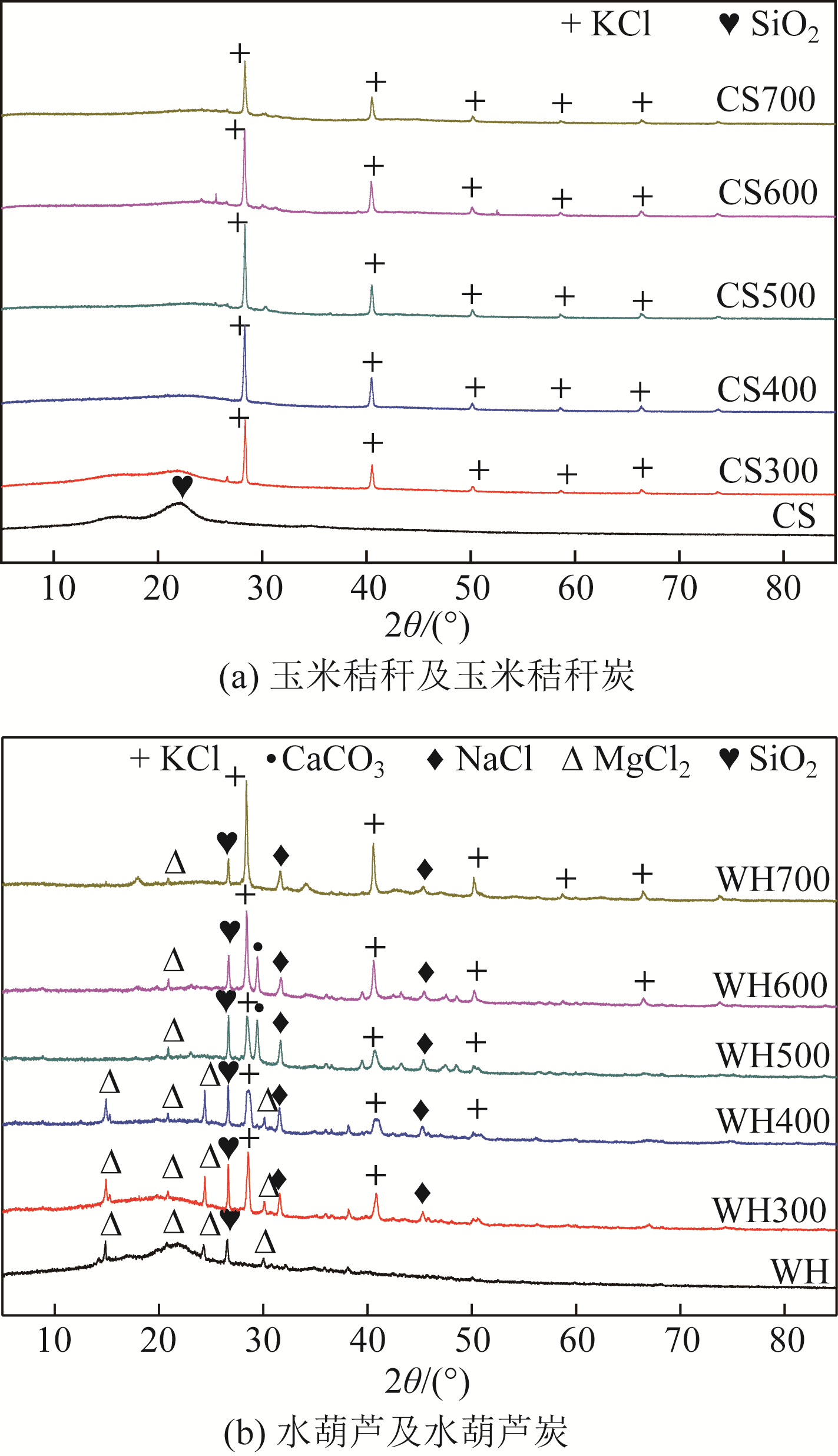
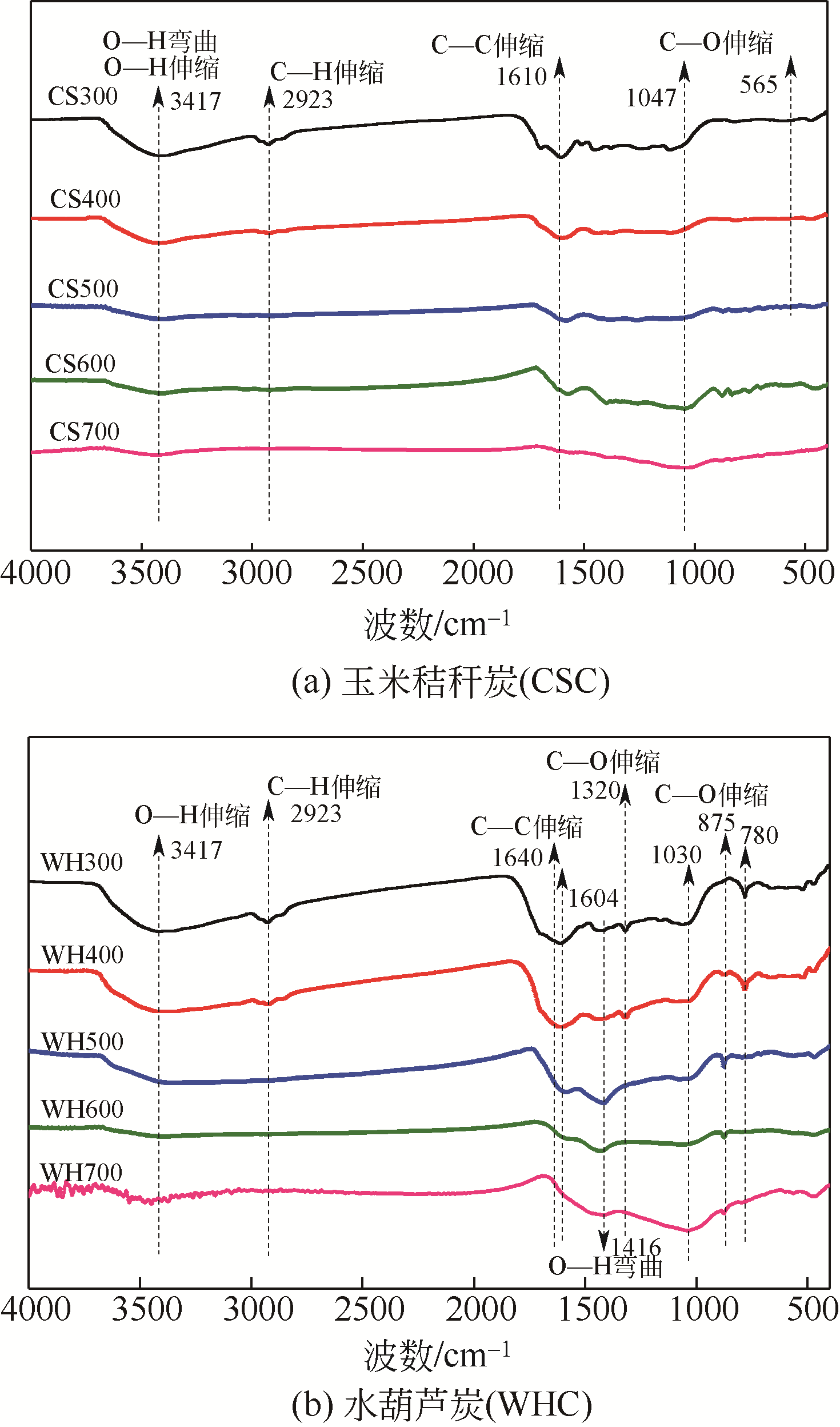
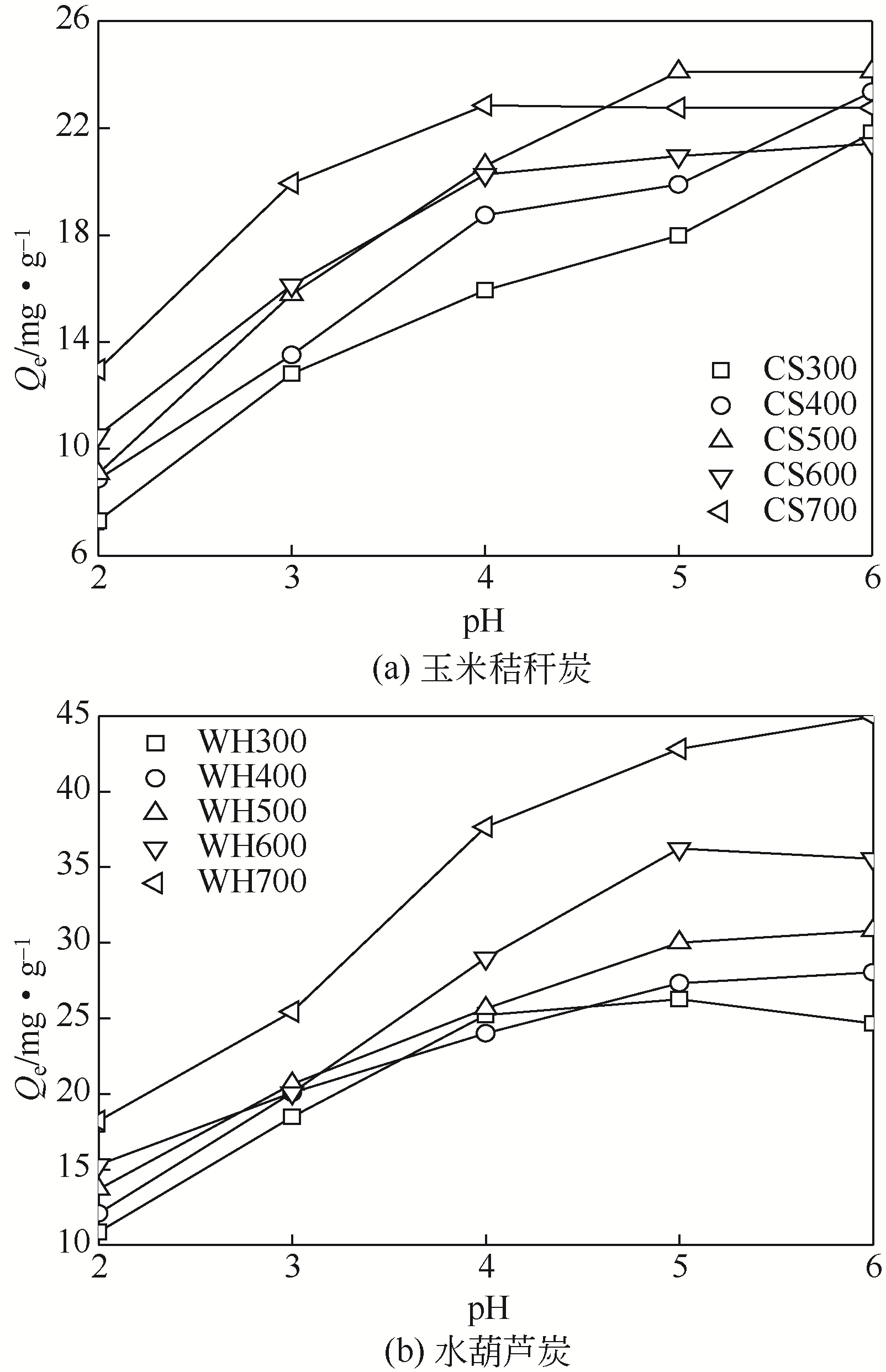
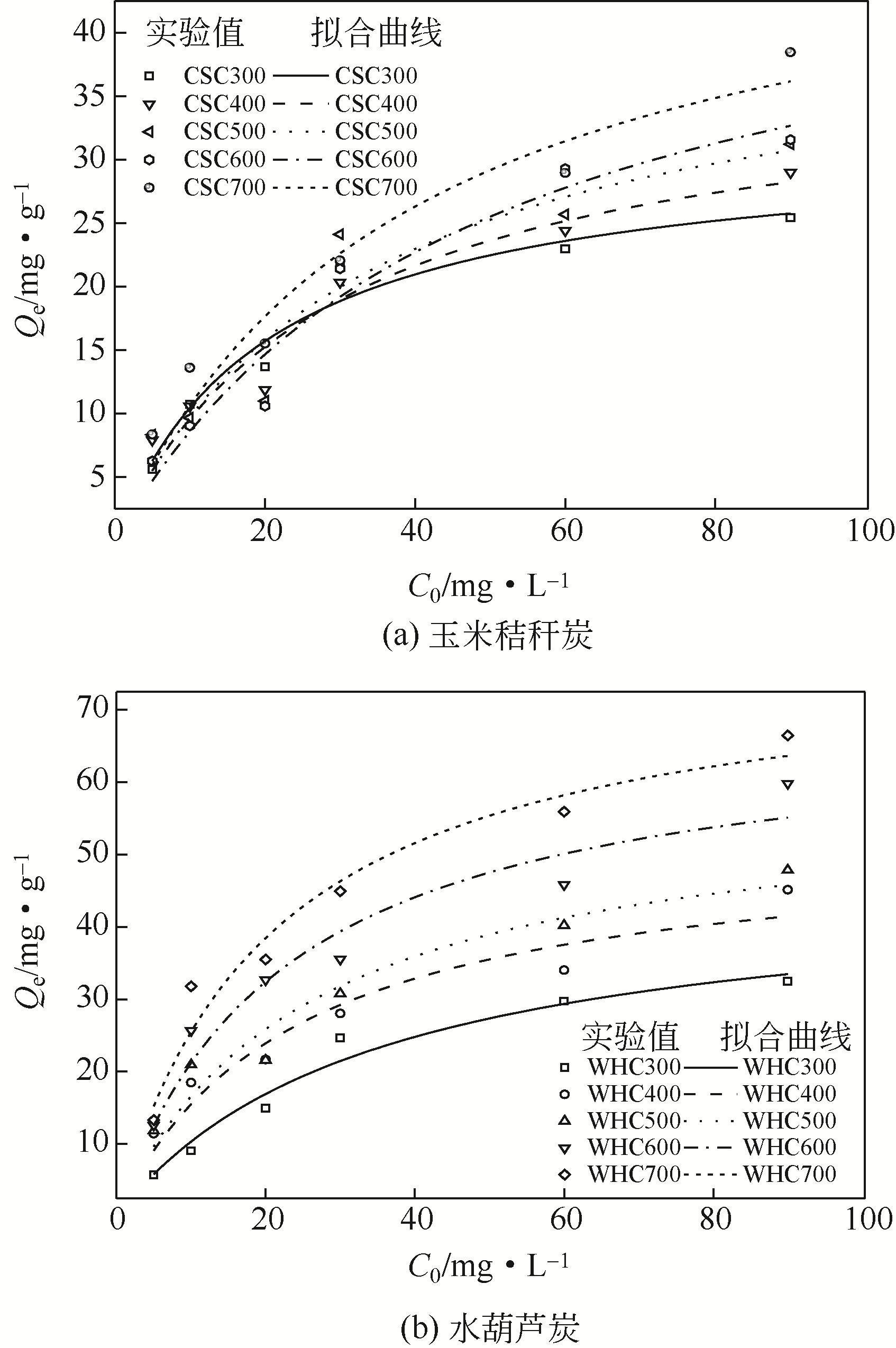
Chemical Industry and Engineering Progress ›› 2019, Vol. 38 ›› Issue (11): 5142-5150.DOI: 10.16085/j.issn.1000-6613.2019-0302
• Resources and environmental engineering • Previous Articles Next Articles
Adsorption and kinetics of heavy metal (Zn) over biochars in solution
Yuxuan WANG( ),Hong WANG,Ping LU(
),Hong WANG,Ping LU( )
)
- School of Energy and Mechanical Engineering, Nanjing Normal University, Nanjing 210023, Jiangsu, China
-
Received:2019-02-28Online:2019-11-05Published:2019-11-05 -
Contact:Ping LU
生物炭对重金属(Zn)的吸附特性及动力学
- 南京师范大学能源与机械工程学院,江苏 南京 210023
-
通讯作者:卢平 -
作者简介:王昱璇(1995—),女,硕士研究生,主要从事生物质和固体废弃物能源化与资源化利用技术的研究。E-mail:xgaza301@163.com 。 -
基金资助:国家自然科学基金(51476079)
CLC Number:
Cite this article
Yuxuan WANG,Hong WANG,Ping LU. Adsorption and kinetics of heavy metal (Zn) over biochars in solution[J]. Chemical Industry and Engineering Progress, 2019, 38(11): 5142-5150.
王昱璇,王红,卢平. 生物炭对重金属(Zn)的吸附特性及动力学[J]. 化工进展, 2019, 38(11): 5142-5150.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2019-0302
| 样品 | 工业分析/% | 元素分析/% | Q net,ad | H/C | O/C | (O+N)/C | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mad | Vad | FCad | Aad | Cad | Had | Oad ① | Nad | Sad | /MJ·kg-1 | ||||||
| CS | 13.14 | 64.68 | 15.82 | 6.36 | 40.71 | 5.64 | 33.59 | 0.50 | 0.06 | 14.11 | 0.14 | 0.83 | 0.84 | ||
| CSC300 | — | 42.67 | 43.57 | 13.76 | 58.24 | 4.70 | 22.75 | 0.49 | 0.06 | 22.76 | 0.08 | 0.39 | 0.40 | ||
| CSC400 | — | 23.08 | 59.78 | 17.13 | 64.89 | 4.01 | 13.48 | 0.48 | 0.01 | 20.58 | 0.06 | 0.21 | 0.22 | ||
| CSC500 | — | 13.09 | 66.23 | 20.68 | 67.77 | 3.06 | 8.01 | 0.47 | 0.01 | 22.84 | 0.05 | 0.12 | 0.13 | ||
| CSC600 | — | 8.41 | 70.36 | 21.23 | 68.96 | 2.56 | 6.78 | 0.46 | 0.01 | 25.37 | 0.04 | 0.10 | 0.11 | ||
| CSC700 | — | 6.99 | 70.34 | 22.68 | 69.20 | 1.84 | 5.79 | 0.43 | 0.06 | 24.73 | 0.03 | 0.08 | 0.09 | ||
| WH | 13.93 | 60.79 | 12.84 | 12.45 | 36.94 | 5.63 | 29.56 | 1.33 | 0.16 | 10.52 | 0.15 | 0.80 | 0.84 | ||
| WHC300 | — | 45.55 | 33.12 | 21.32 | 50.15 | 4.34 | 21.86 | 2.22 | 0.11 | 19.20 | 0.09 | 0.44 | 0.48 | ||
| WHC400 | — | 29.67 | 42.59 | 27.75 | 52.31 | 3.72 | 13.96 | 2.19 | 0.07 | 19.71 | 0.07 | 0.27 | 0.31 | ||
| WHC500 | — | 18.93 | 48.50 | 32.57 | 51.89 | 2.79 | 10.59 | 2.05 | 0.11 | 18.95 | 0.05 | 0.20 | 0.24 | ||
| WHC600 | — | 13.66 | 51.13 | 35.20 | 53.24 | 1.98 | 7.65 | 1.92 | 0.01 | 19.21 | 0.04 | 0.14 | 0.18 | ||
| WHC700 | — | 9.22 | 54.82 | 35.96 | 55.57 | 1.66 | 5.20 | 1.51 | 0.10 | 19.35 | 0.03 | 0.09 | 0.12 | ||
| 样品 | 工业分析/% | 元素分析/% | Q net,ad | H/C | O/C | (O+N)/C | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mad | Vad | FCad | Aad | Cad | Had | Oad ① | Nad | Sad | /MJ·kg-1 | ||||||
| CS | 13.14 | 64.68 | 15.82 | 6.36 | 40.71 | 5.64 | 33.59 | 0.50 | 0.06 | 14.11 | 0.14 | 0.83 | 0.84 | ||
| CSC300 | — | 42.67 | 43.57 | 13.76 | 58.24 | 4.70 | 22.75 | 0.49 | 0.06 | 22.76 | 0.08 | 0.39 | 0.40 | ||
| CSC400 | — | 23.08 | 59.78 | 17.13 | 64.89 | 4.01 | 13.48 | 0.48 | 0.01 | 20.58 | 0.06 | 0.21 | 0.22 | ||
| CSC500 | — | 13.09 | 66.23 | 20.68 | 67.77 | 3.06 | 8.01 | 0.47 | 0.01 | 22.84 | 0.05 | 0.12 | 0.13 | ||
| CSC600 | — | 8.41 | 70.36 | 21.23 | 68.96 | 2.56 | 6.78 | 0.46 | 0.01 | 25.37 | 0.04 | 0.10 | 0.11 | ||
| CSC700 | — | 6.99 | 70.34 | 22.68 | 69.20 | 1.84 | 5.79 | 0.43 | 0.06 | 24.73 | 0.03 | 0.08 | 0.09 | ||
| WH | 13.93 | 60.79 | 12.84 | 12.45 | 36.94 | 5.63 | 29.56 | 1.33 | 0.16 | 10.52 | 0.15 | 0.80 | 0.84 | ||
| WHC300 | — | 45.55 | 33.12 | 21.32 | 50.15 | 4.34 | 21.86 | 2.22 | 0.11 | 19.20 | 0.09 | 0.44 | 0.48 | ||
| WHC400 | — | 29.67 | 42.59 | 27.75 | 52.31 | 3.72 | 13.96 | 2.19 | 0.07 | 19.71 | 0.07 | 0.27 | 0.31 | ||
| WHC500 | — | 18.93 | 48.50 | 32.57 | 51.89 | 2.79 | 10.59 | 2.05 | 0.11 | 18.95 | 0.05 | 0.20 | 0.24 | ||
| WHC600 | — | 13.66 | 51.13 | 35.20 | 53.24 | 1.98 | 7.65 | 1.92 | 0.01 | 19.21 | 0.04 | 0.14 | 0.18 | ||
| WHC700 | — | 9.22 | 54.82 | 35.96 | 55.57 | 1.66 | 5.20 | 1.51 | 0.10 | 19.35 | 0.03 | 0.09 | 0.12 | ||
| 样品 | S BET /m2·g-1 | S D-R /m2·g-1 | V T /mm3·g-1 | V M /mm3·g-1 | X /% | D a /nm |
|---|---|---|---|---|---|---|
| CS | 1.54 | 2.39 | 2.9 | 0.9 | 31.30 | 7.53 |
| CS300 | 6.80 | 11.34 | 9.4 | 4.3 | 45.99 | 5.53 |
| CS400 | 10.51 | 16.57 | 11.7 | 5.9 | 50.43 | 4.45 |
| CS500 | 13.88 | 20.82 | 13.1 | 7.4 | 56.49 | 3.78 |
| CS600 | 60.50 | 81.25 | 61.2 | 25.1 | 41.01 | 4.05 |
| CS700 | 20.92 | 30.35 | 21.1 | 10.8 | 51.18 | 4.03 |
| WH | 2.26 | 3.36 | 5.8 | 1.2 | 20.83 | 10.27 |
| WH300 | 2.79 | 5.33 | 8.2 | 1.9 | 23.17 | 11.76 |
| WH400 | 3.80 | 5.69 | 8.3 | 2.0 | 24.10 | 8.74 |
| WH500 | 8.19 | 12.13 | 16.9 | 4.2 | 24.85 | 8.24 |
| WH600 | 37.46 | 52.85 | 39.2 | 18.8 | 47.96 | 4.19 |
| WH700 | 53.74 | 78.14 | 43.6 | 27.8 | 63.76 | 3.25 |
| 样品 | S BET /m2·g-1 | S D-R /m2·g-1 | V T /mm3·g-1 | V M /mm3·g-1 | X /% | D a /nm |
|---|---|---|---|---|---|---|
| CS | 1.54 | 2.39 | 2.9 | 0.9 | 31.30 | 7.53 |
| CS300 | 6.80 | 11.34 | 9.4 | 4.3 | 45.99 | 5.53 |
| CS400 | 10.51 | 16.57 | 11.7 | 5.9 | 50.43 | 4.45 |
| CS500 | 13.88 | 20.82 | 13.1 | 7.4 | 56.49 | 3.78 |
| CS600 | 60.50 | 81.25 | 61.2 | 25.1 | 41.01 | 4.05 |
| CS700 | 20.92 | 30.35 | 21.1 | 10.8 | 51.18 | 4.03 |
| WH | 2.26 | 3.36 | 5.8 | 1.2 | 20.83 | 10.27 |
| WH300 | 2.79 | 5.33 | 8.2 | 1.9 | 23.17 | 11.76 |
| WH400 | 3.80 | 5.69 | 8.3 | 2.0 | 24.10 | 8.74 |
| WH500 | 8.19 | 12.13 | 16.9 | 4.2 | 24.85 | 8.24 |
| WH600 | 37.46 | 52.85 | 39.2 | 18.8 | 47.96 | 4.19 |
| WH700 | 53.74 | 78.14 | 43.6 | 27.8 | 63.76 | 3.25 |
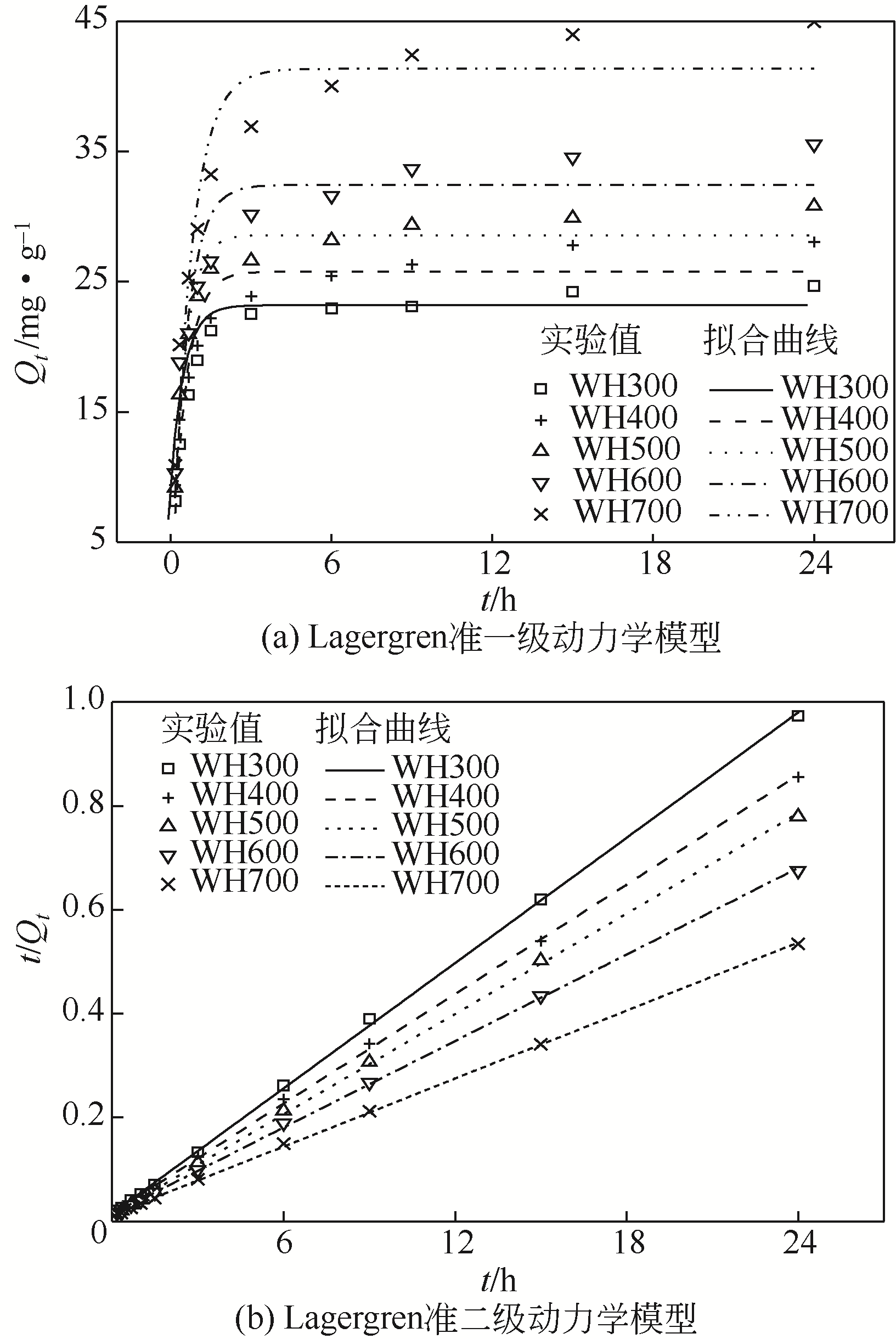
| 生物炭 | C 0 /mg·g-1 | 实验平衡吸附量Q e1/mg·g-1 | 一级动力学吸附模型 | 二级动力学吸附模型 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Q e2 /mg·g-1 | K 1 /h-1 | R 2 | Q e2 /mg·g-1 | K 2 /g·mg-1·h-1 | R 2 | ||||
| CS300 | 30 | 21.82 | 19.44 | 1.458 | 0.9026 | 22.12 | 0.064 | 0.9983 | |
| CS400 | 30 | 20.35 | 17.99 | 1.877 | 0.8773 | 20.57 | 0.077 | 0.9978 | |
| CS500 | 30 | 24.12 | 22.34 | 2.104 | 0.9060 | 23.69 | 0.073 | 0.9979 | |
| CS600 | 30 | 21.41 | 20.47 | 2.979 | 0.9748 | 21.47 | 0.214 | 0.9998 | |
| CS700 | 30 | 22.06 | 20.88 | 3.639 | 0.9738 | 21.97 | 0.215 | 0.9994 | |
| WH300 | 30 | 24.66 | 23.19 | 2.018 | 0.9588 | 24.88 | 0.107 | 0.9997 | |
| WH400 | 30 | 28.05 | 25.77 | 1.924 | 0.8934 | 28.48 | 0.075 | 0.9996 | |
| WH500 | 30 | 30.80 | 28.58 | 2.124 | 0.9488 | 31.05 | 0.080 | 0.9996 | |
| WH600 | 30 | 35.55 | 32.42 | 1.764 | 0.8782 | 36.03 | 0.053 | 0.9995 | |
| WH700 | 30 | 44.95 | 41.35 | 1.409 | 0.9220 | 45.84 | 0.036 | 0.9997 | |
| 生物炭 | C 0 /mg·g-1 | 实验平衡吸附量Q e1/mg·g-1 | 一级动力学吸附模型 | 二级动力学吸附模型 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Q e2 /mg·g-1 | K 1 /h-1 | R 2 | Q e2 /mg·g-1 | K 2 /g·mg-1·h-1 | R 2 | ||||
| CS300 | 30 | 21.82 | 19.44 | 1.458 | 0.9026 | 22.12 | 0.064 | 0.9983 | |
| CS400 | 30 | 20.35 | 17.99 | 1.877 | 0.8773 | 20.57 | 0.077 | 0.9978 | |
| CS500 | 30 | 24.12 | 22.34 | 2.104 | 0.9060 | 23.69 | 0.073 | 0.9979 | |
| CS600 | 30 | 21.41 | 20.47 | 2.979 | 0.9748 | 21.47 | 0.214 | 0.9998 | |
| CS700 | 30 | 22.06 | 20.88 | 3.639 | 0.9738 | 21.97 | 0.215 | 0.9994 | |
| WH300 | 30 | 24.66 | 23.19 | 2.018 | 0.9588 | 24.88 | 0.107 | 0.9997 | |
| WH400 | 30 | 28.05 | 25.77 | 1.924 | 0.8934 | 28.48 | 0.075 | 0.9996 | |
| WH500 | 30 | 30.80 | 28.58 | 2.124 | 0.9488 | 31.05 | 0.080 | 0.9996 | |
| WH600 | 30 | 35.55 | 32.42 | 1.764 | 0.8782 | 36.03 | 0.053 | 0.9995 | |
| WH700 | 30 | 44.95 | 41.35 | 1.409 | 0.9220 | 45.84 | 0.036 | 0.9997 | |
| 1 | LIANG J Y , LIU Y Y , ZOU J , et al . Inhibitory effect of zinc on human prostatic carcinoma cell growth[J]. The Prostate, 1999, 40(3): 200-207. |
| 2 | ALQADAMI A A , NAUSHAD M , ABDALLA M A , et al . Efficient removal of toxic metal ions from wastewater using a recyclable nanocomposite: a study of adsorption parameters and interaction mechanism[J]. Journal of Cleaner Production, 2017, 156: 426-436. |
| 3 | COLLA T S , ANDREAZZA R , BUCKER F , et al . Bioremediation assessment of diesel-biodiesel-contaminated soil using an alternative bioaugmentation strategy[J]. Environmental Science and Pollution Research, 2014, 21(4): 2592-2602. |
| 4 | ZHANG Y , LUO W . Adsorptive removal of heavy metal from acidic wastewater with biochar produced from anaerobically digested residues: kinetics and surface complexation modeling[J]. Bioresources, 2014, 9(2): 2484-2499. |
| 5 | INYANG M , GAO B , YAO Y , et al . A review of biochar as a low-cost adsorbent for aqueous heavy metal removal[J]. Critical Reviews in Environmental Science and Technology, 2016, 46(4): 406-433. |
| 6 | 王怀臣, 冯雷雨, 陈银广 . 废物资源化制备生物质炭及其应用的研究进展[J]. 化工进展, 2012, 31(4): 907-914. |
| WANG H C , FENG L Y , CHEN Y G . Advances in biochar production from wastes and its applications[J]. Chemical Industry and Engineering Progress, 2012, 31(4): 907-914. | |
| 7 | GUL S, WHALEN J K , THOMAS B W , et al . Physico-chemical properties and microbial responses in biochar-amended soils: mechanisms and future directions[J]. Agriculture, Ecosystems & Environment, 2015, 206: 46-59. |
| 8 | ZGANG G , GUO X , ZHAO Z , et al . Effects of biochars on the availability of heavy metals to ryegrass in an alkaline contaminated soil[J]. Environmental Pollution, 2016, 218: 513-522. |
| 9 | TAN X , LIU Y , ZENG G , et al . Application of biochar for the removal of pollutants from aqueous solutions[J]. Chemosphere, 2015,125: 70-85. |
| 10 | 王重庆, 王晖, 江小燕, 等 . 生物炭吸附重金属离子的研究进展[J]. 化工进展, 2019, 38(1): 692-706. |
| WANG C Q , WANG H , JIANG X Y , et al . Research advances on adsorption of heavy metals by biochar[J]. Chemical Industry and Engineering Progress, 2019, 38(1): 692-706. | |
| 11 | CHEN X , CHEN G , CHEN L , et al . Adsorption of copper and zinc by biochars produced from pyrolysis of hardwood and corn straw in aqueous solution[J]. Bioresource Technology, 2011, 102(19): 8877-8884. |
| 12 | 谢伟雪, 刘孝敏, 李小东, 等 . 废毛发生物炭的特性及其对Ni(Ⅱ)和Zn(Ⅱ)的吸附研究[J]. 环境工程技术学报, 2018, 8(6): 656-662. |
| XIE W X , LIU X M , LI X D , et al . Characteristics of waste hair biochar and its adsorption to Ni(Ⅱ) and Zn(Ⅱ)[J]. Journal of Environmental Engineering Technology, 2018, 8(6): 656-662. | |
| 13 | 王震宇, 刘国成, XING Monica , 等 .不同热解温度生物炭对Cd(Ⅱ)的吸附特性 [J]. 环境科学, 2014, 35(12): 4735-4744. |
| WANG Z Y , LIU G C , XING M , et al . Adsorption of Cd(Ⅱ) varies with biochars derived at different pyrolysis temperatures[J]. Environmental Science, 2014, 35(12): 4735-4744. | |
| 14 | LI Q , TANG L , HU J , et al . Removal of toxic metals from aqueous solution by biochars derived from long-root Eichhornia crassipes[J]. Royal Society Open Science, 2018, 5(10): 180966. |
| 15 | QIAN T , WU P , QIN Q , et al . Screening of wheat straw biochars for the remediation of soils polluted with Zn (Ⅱ) and Cd (Ⅱ)[J]. Journal of Hazardous Materials, 2019, 365: 311-317. |
| 16 | JIANG S S , HUANG L B , NGUYEN T A , et al . Copper and zinc adsorption by softwood and hardwood biochars under elevated sulphate-induced salinity and acidic pH conditions[J]. Chemosphere, 2016, 142: 64-71. |
| 17 | XU X , CAO X , ZHAO L , et al . Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar[J]. Environmental Science and Pollution Research, 2013, 20(1): 358-368. |
| 18 | KOLODYŃSKA D , KRUKOWSKA J , THOMAS P . Comparison of sorption and desorption studies of heavy metal ions from biochar and commercial active carbon [J]. Chemical Engineering Journal, 2017, 307: 353-363. |
| 19 | 国家质量技术监督局 . 木质活性炭试验方法pH值的测定:GB/T 12496.7―1999 [S]. 北京:中国标准出版社, 2000. |
| State Bureau of Quality Technical . Test methods of wooden activated carbon-Determination of pH: GB/T 12496.7—1999 [S]. Beijing: Standards Press of China, 2000. | |
| 20 | MASULILIA, UTOMO W H , SYECHFANI M S , et al . Rice husk biochar for rice based cropping system in acid soil 1. The characteristics of rice husk biochar and its influence on the properties of acid sulfate soils and rice growth in West Kalimantan, Indonesia[J]. The Journal of Agricultural Science, 2010, 2(1): 39-47. |
| 21 | HO Y S, MCKAY G . Pseudo-second order model for sorption processes[J]. Process Biochemistry, 1999, 34(5): 451-465. |
| 22 | 安增莉, 侯艳伟, 蔡超, 等 . 水稻秸秆生物炭对Pb(Ⅱ)的吸附特性[J]. 环境化学, 2011, 30(11): 1851-1857. |
| AN Z L , HOU Y W , CAI C , et al . Lead (Ⅱ) adsorption characteristics on different biochars derived from rice straw[J]. Environmental Chemistry, 2011, 30(11): 1851-1857. | |
| 23 | HO Y S . Review of second-order models for adsorption systems[J]. Journal of Hazardous Materials, 2006, 136(3): 681-689. |
| 24 | NOVAK J M , LIMA I , XING B , et al . Characterization of designer biochar produced at different temperatures and their effects on a Loamy sand[J]. Annals of Environmental Science, 2009, 3: 195-206. |
| 25 | GASKIN J W , STEINER C , HARRIS K , et al . Effect of low-temperature pyrolysis conditions on biochar for agricultural use[J]. Transactions of the ASABE, 2008, 51(6): 2061-2069. |
| 26 | AHMAD M , RAJAPAKSHA A U , LIM J E, et al . Biochar as a sorbent for contaminant management in soil and water: a review[J]. Chemosphere, 2014, 99: 19-33. |
| 27 | 谢超然, 王兆炜, 朱俊民, 等 . 核桃青皮生物炭对重金属铅、铜的吸附特性研究[J]. 环境科学学报, 2016, 36(4): 1190-1198. |
| XIE C R , WANG Z W , ZHU J M , et al . Adsorption of lead and copper from aqueous solutions on biochar produced from walnut[J]. Acta Scientiae Circumstantiae, 2016, 36(4): 1190-1198. | |
| 28 | 郭素华, 许中坚, 李方文,等 . 生物炭对水中Pb(Ⅱ)和Zn(Ⅱ)的吸附特征[J]. 环境工程学报, 2015, 9(7): 3215-3222. |
| GUO S H , XU Z J , LI F W , et al . Adsorption of Pb (Ⅱ) , Zn (Ⅱ) from aqueous solution by biochars[J]. Chinese Journal of Environmental Engineering, 2015, 9(7): 3215-3222. | |
| 29 | ZHU Q , WU J , WANG L , et al . Adsorption characteristics of Pb2+ onto wine lees-derived biochar[J]. Bulletin of Environmental Contamination and Toxicology, 2016, 97(2): 294-299. |
| 30 | EL-ASHTOUKHY E S Z , AMIN N K , ABDELWAHAB O . Removal of lead (Ⅱ) and copper (Ⅱ) from aqueous solution using pomegranate peel as a new adsorbent [J]. Desalination, 2008, 223(1/2/3): 162-173. |
| 31 | 邓金环,郜礼阳,周皖婉,等 . 不同温度制备香根草生物炭对 Cd2+的吸附特性与机制[J]. 农业环境科学学报, 2018, 37(2): 340-349. |
| DENG J H , GAO L Y , ZHOU W W , et al . Adsorption characteristics and mechanisms of Cd2+ in biochar derived from vetiver grass under different pyrolysis temperatures[J]. Journal of Agro-Environment Science, 2018, 37(2): 340-349. | |
| 32 | CAO X , MA L , GAO B , et al . Dairy-manure derived biochar effectively sorbs lead and atrazine[J]. Environmental Science & Technology, 2009, 43(9): 3285-3291. |
| 33 | LI H , DONG X , SILVA E B , et al . Mechanisms of metal sorption by biochars: biochar characteristics and modifications[J]. Chemosphere, 2017,178: 466-478. |
| [1] | ZHANG Mingyan, LIU Yan, ZHANG Xueting, LIU Yake, LI Congju, ZHANG Xiuling. Research progress of non-noble metal bifunctional catalysts in zinc-air batteries [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 276-286. |
| [2] | DAI Huantao, CAO Lingyu, YOU Xinxiu, XU Haoliang, WANG Tao, XIANG Wei, ZHANG Xueyang. Adsorption properties of CO2 on pomelo peel biochar impregnated by lignin [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 356-363. |
| [3] | CUI Shoucheng, XU Hongbo, PENG Nan. Simulation analysis of two MOFs materials for O2/He adsorption separation [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 382-390. |
| [4] | CHEN Chongming, CHEN Qiu, GONG Yunqian, CHE Kai, YU Jinxing, SUN Nannan. Research progresses on zeolite-based CO2 adsorbents [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 411-419. |
| [5] | LI Shilin, HU Jingze, WANG Yilin, WANG Qingji, SHAO Lei. Research progress in separation and extraction of high value components by electrodialysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 420-429. |
| [6] | XU Chunshu, YAO Qingda, LIANG Yongxian, ZHOU Hualong. Research progress on functionalization strategies of covalent organic frame materials and its adsorption properties for Hg(Ⅱ) and Cr(Ⅵ) [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 461-478. |
| [7] | GU Yongzheng, ZHANG Yongsheng. Dynamic behavior and kinetic model of Hg0 adsorption by HBr-modified fly ash [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 498-509. |
| [8] | WANG Peng, ZHANG Yang, FAN Bingqiang, HE Dengbo, SHEN Changshuai, ZHANG Hedong, ZHENG Shili, ZOU Xing. Process and kinetics of hydrochloric acid leaching of high-carbon ferrochromium [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 510-517. |
| [9] | GUO Qiang, ZHAO Wenkai, XIAO Yonghou. Numerical simulation of enhancing fluid perturbation to improve separation of dimethyl sulfide/nitrogen via pressure swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 64-72. |
| [10] | WANG Shengyan, DENG Shuai, ZHAO Ruikai. Research progress on carbon dioxide capture technology based on electric swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 233-245. |
| [11] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| [12] | YANG Bin, WANG Xiaodong, WANG Yan, YI Guiyun, WANG Tielang, SHI Chuang, ZHANG Zhanying. Preparation of nano-Pt/ZnO heterostructures and gas sensitive properties [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4817-4827. |
| [13] | LI Weihua, YU Qianwen, YIN Junquan, WU Yinkai, SUN Yingjie, WANG Yan, WANG Huawei, YANG Yufei, LONG Yuyang, HUANG Qifei, GE Yanchen, HE Yiyang, ZHAO Lingyan. Leaching behavior of heavy metals from broken ton bags filled with fly ash in acid rain environment [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4917-4928. |
| [14] | LI Zhiyuan, HUANG Yaji, ZHAO Jiaqi, YU Mengzhu, ZHU Zhicheng, CHENG Haoqiang, SHI Hao, WANG Sheng. Characterization of heavy metals during co-pyrolysis of sludge with PVC [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4947-4956. |
| [15] | YANG Ying, HOU Haojie, HUANG Rui, CUI Yu, WANG Bing, LIU Jian, BAO Weiren, CHANG Liping, WANG Jiancheng, HAN Lina. Coal tar phenol-based carbon nanosphere prepared by Stöber method for adsorption of CO2 [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 5011-5018. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||