Chemical Industry and Engineering Progress ›› 2019, Vol. 38 ›› Issue (08): 3701-3710.DOI: 10.16085/j.issn.1000-6613.2018-2121
• Industrial catalysis • Previous Articles Next Articles
Effect of surface defects density of carbon spheres on the catalytic performance of the supported Cu catalyst for oxidativecarbonylation of methanol
Dongsen JIA( ),Guoqiang ZHANG(
),Guoqiang ZHANG( ),Jiao YIN,Liangliang ZHANG,Dan ZHAO,Huayan ZHENG,Zhong LI(
),Jiao YIN,Liangliang ZHANG,Dan ZHAO,Huayan ZHENG,Zhong LI( )
)
- Key Laboratory of Coal Science and Technology of Ministry of Education and Shanxi Province, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
-
Received:2018-10-29Online:2019-08-05Published:2019-08-05 -
Contact:Guoqiang ZHANG,Zhong LI
碳球表面缺陷密度对其负载铜催化剂甲醇氧化羰基化反应性能的影响
贾东森( ),张国强(
),张国强( ),尹娇,张亮亮,赵丹,郑华艳,李忠(
),尹娇,张亮亮,赵丹,郑华艳,李忠( )
)
- 太原理工大学煤科学与技术教育部和山西省重点实验室,山西 太原 030024
-
通讯作者:张国强,李忠 -
作者简介:贾东森(1993—),男,硕士研究生,研究方向为甲醇高效转化合成碳酸二甲酯。E-mail:458603793@qq.com 。 -
基金资助:国家自然科学基金(U1510203);山西省青年科技研究基金(201701D221043)
CLC Number:
Cite this article
Dongsen JIA,Guoqiang ZHANG,Jiao YIN,Liangliang ZHANG,Dan ZHAO,Huayan ZHENG,Zhong LI. Effect of surface defects density of carbon spheres on the catalytic performance of the supported Cu catalyst for oxidativecarbonylation of methanol[J]. Chemical Industry and Engineering Progress, 2019, 38(08): 3701-3710.
贾东森,张国强,尹娇,张亮亮,赵丹,郑华艳,李忠. 碳球表面缺陷密度对其负载铜催化剂甲醇氧化羰基化反应性能的影响[J]. 化工进展, 2019, 38(08): 3701-3710.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2018-2121
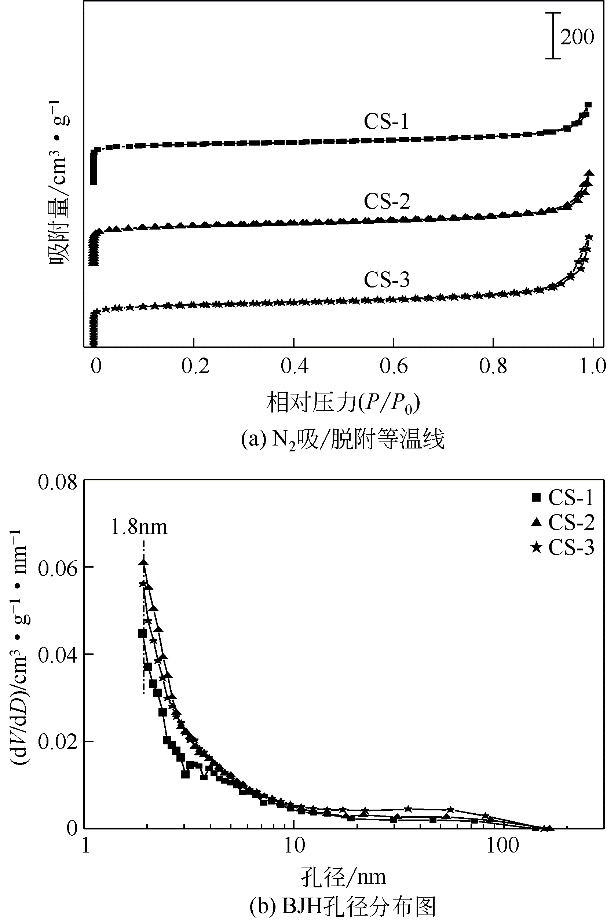
| 样品 | 比表面积/m2·g-1 | 孔体积/m3·g-1 | 最可几 孔径③/nm | ||
|---|---|---|---|---|---|
| 总比表面积 | 微孔比表面积① | 总孔体积 | 微孔体积② | ||
| CS-1 | 613 | 525 | 0.61 | 0.50 | 1.84 |
| CS-2 | 600 | 505 | 0.62 | 0.51 | 1.86 |
| CS-3 | 626 | 523 | 0.64 | 0.53 | 1.85 |
| Cu/CS-1 | 588 | 495 | 0.59 | 0.49 | 1.84 |
| Cu/CS-2 | 590 | 491 | 0.61 | 0.50 | 1.86 |
| Cu/CS-3 | 617 | 509 | 0.62 | 0.51 | 1.85 |
| 样品 | 比表面积/m2·g-1 | 孔体积/m3·g-1 | 最可几 孔径③/nm | ||
|---|---|---|---|---|---|
| 总比表面积 | 微孔比表面积① | 总孔体积 | 微孔体积② | ||
| CS-1 | 613 | 525 | 0.61 | 0.50 | 1.84 |
| CS-2 | 600 | 505 | 0.62 | 0.51 | 1.86 |
| CS-3 | 626 | 523 | 0.64 | 0.53 | 1.85 |
| Cu/CS-1 | 588 | 495 | 0.59 | 0.49 | 1.84 |
| Cu/CS-2 | 590 | 491 | 0.61 | 0.50 | 1.86 |
| Cu/CS-3 | 617 | 509 | 0.62 | 0.51 | 1.85 |
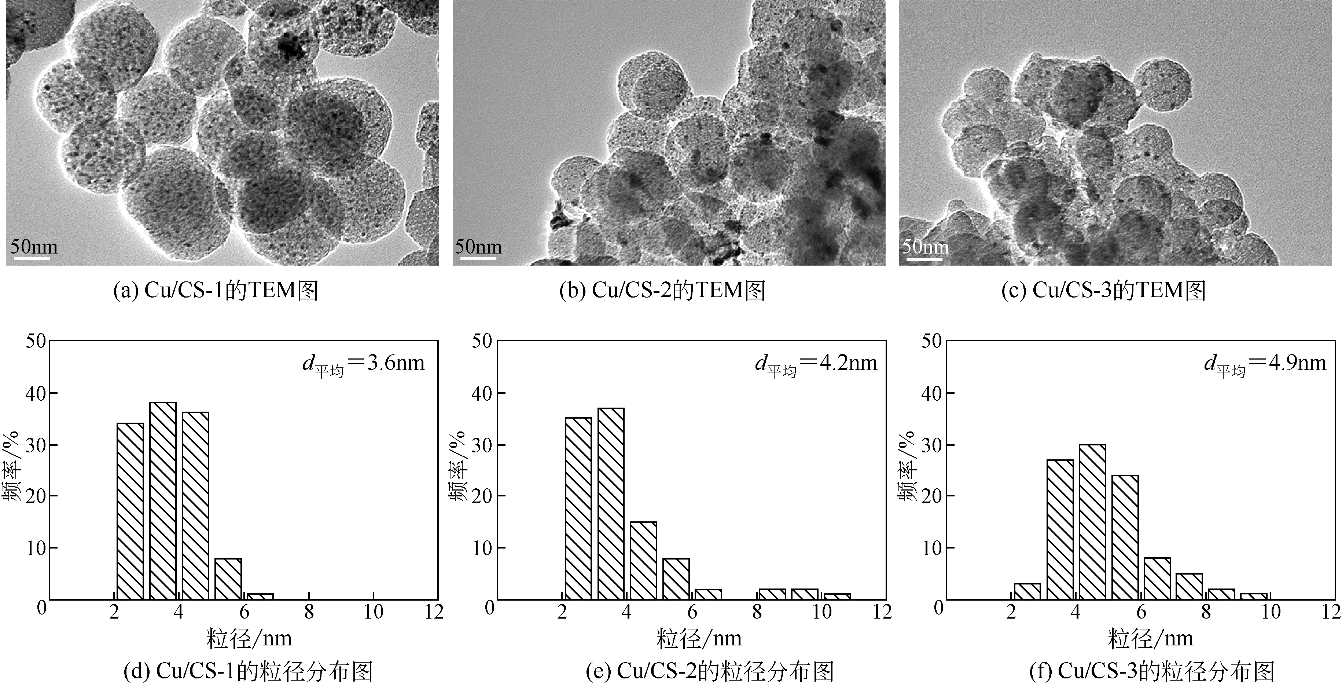
| 催化剂 | W Cu/% | d Cu ①/nm | S Cu ②/m2·g-1 | D Cu ③/% | C CH3OH/% | S DMC/% | STYDMC /mg·g-1·h-1 |
|---|---|---|---|---|---|---|---|
| Cu/CS-1 | 10 | 3.6 | 42.9 | 63.2 | 3.4 | 70.0 | 276.6 |
| Cu/CS-2 | 10 | 4.2 | 37.0 | 54.6 | 2.4 | 71.3 | 199.2 |
| Cu/CS-3 | 10 | 4.9 | 27.3 | 40.3 | 2.0 | 69.5 | 161.1 |
| Cu/AC[ | 12 | 32.0 | — | — | 6.0 | 62.1 | 156.0 |
| Cu/SCS[ | 10 | 9.9 | — | — | 0.7 | 52.7 | 47.4 |
| 催化剂 | W Cu/% | d Cu ①/nm | S Cu ②/m2·g-1 | D Cu ③/% | C CH3OH/% | S DMC/% | STYDMC /mg·g-1·h-1 |
|---|---|---|---|---|---|---|---|
| Cu/CS-1 | 10 | 3.6 | 42.9 | 63.2 | 3.4 | 70.0 | 276.6 |
| Cu/CS-2 | 10 | 4.2 | 37.0 | 54.6 | 2.4 | 71.3 | 199.2 |
| Cu/CS-3 | 10 | 4.9 | 27.3 | 40.3 | 2.0 | 69.5 | 161.1 |
| Cu/AC[ | 12 | 32.0 | — | — | 6.0 | 62.1 | 156.0 |
| Cu/SCS[ | 10 | 9.9 | — | — | 0.7 | 52.7 | 47.4 |
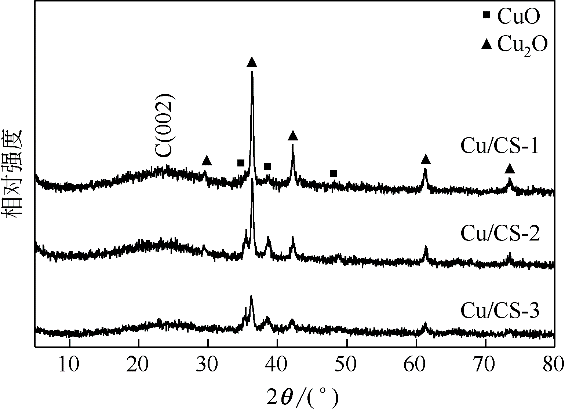
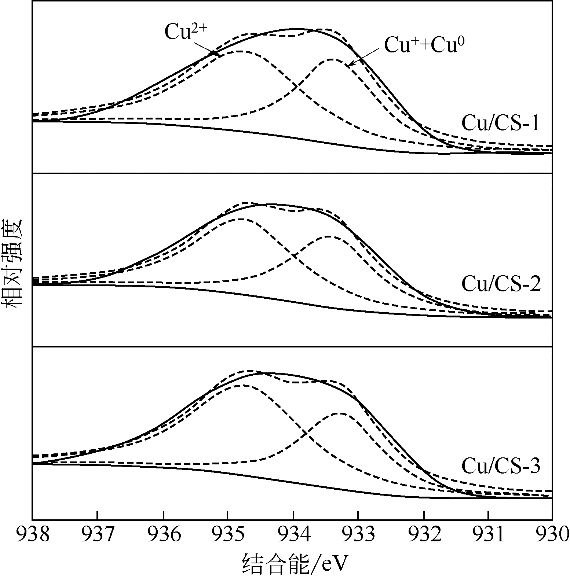
| 催化剂 | Cu2p3/2结合能/eV | 表面Cu物种的摩尔分数/% | ||
|---|---|---|---|---|
| Cu2+ | Cu++Cu0 | Cu2+ | Cu++Cu0 | |
| Cu/CS-1 | 934.7 | 933.3 | 54.3 | 45.7 |
| Cu/CS-2 | 934.8 | 933.4 | 56.3 | 43.7 |
| Cu/CS-3 | 934.7 | 933.2 | 62.4 | 37.6 |
| 反应后Cu/CS-1 | 934.9 | 933.1 | 57.0 | 43.0 |
| 反应后Cu/CS-2 | 934.8 | 933.1 | 60.8 | 39.2 |
| 反应后Cu/CS-3 | 935.7 | 933.5 | 80.3 | 19.7 |
| 催化剂 | Cu2p3/2结合能/eV | 表面Cu物种的摩尔分数/% | ||
|---|---|---|---|---|
| Cu2+ | Cu++Cu0 | Cu2+ | Cu++Cu0 | |
| Cu/CS-1 | 934.7 | 933.3 | 54.3 | 45.7 |
| Cu/CS-2 | 934.8 | 933.4 | 56.3 | 43.7 |
| Cu/CS-3 | 934.7 | 933.2 | 62.4 | 37.6 |
| 反应后Cu/CS-1 | 934.9 | 933.1 | 57.0 | 43.0 |
| 反应后Cu/CS-2 | 934.8 | 933.1 | 60.8 | 39.2 |
| 反应后Cu/CS-3 | 935.7 | 933.5 | 80.3 | 19.7 |
| 1 | FU Z H , ONO Y . Two-step synthesis of diphenyl carbonate from dimethyl carbonate and phenol using MoO3/SiO2 catalysts[J]. Journal of Molecular Catalysis A Chemical, 1997, 118(3): 293-299. |
| 2 | ONO Y . Catalysis in the production and reactions of dimethyl carbonate, an environmentally benign building block[J]. Applied Catalysis A: General, 1997, 155(2): 133-166. |
| 3 | WANG Y J , ZHAO X Q , YUAN B G , et al . Synthesis of dimethyl carbonate by gas-phase oxidative carbonylation of methanol on the supported solid catalyst Ⅰ. Catalyst preparation and catalytic properties[J]. Applied Catalysis A: General, 1998, 171(2): 255-260. |
| 4 | FIORANI G , PEROSA A , SELVA M . Dimethyl carbonate: a versatile reagent for a sustainable valorization of renewables[J]. Green Chemistry, 2017, 20(2): 288-322. |
| 5 | TUNDO P , SELVA M . The chemistry of dimethyl carbonate[J]. ACC Chem. Res., 2002, 35(9): 706-716. |
| 6 | 宋一兵, 罗爱国, 杜玉海, 等 . 甲醇直接气相氧化羰基化合成碳酸二甲酯[J]. 化学进展, 2008, 20(2): 221-226. |
| SONG Y B , LUO A G , DU Y H , et al . Synthesis of dimethyl carbonate by direct vapor-phase oxycarbonylation of methanol[J]. Progress of Chemistry, 2008, 20(2): 221-226. | |
| 7 | WANG R Y , LI Z , ZHENG H Y . Preparation of chlorine-free Cu/AC catalyst and its catalytic properties for vapor phase oxidative carbonylation of methanol[J]. Chinese Journal of Catalysis, 2010, 31(7): 851-856. |
| 8 | LI Z , WEN C M , WANG R Y , et al . Chloride-free Cu2O/AC catalyst prepared by pyrolysis of copper acetate and catalytic oxycarbonylation[J]. Chemical Journal of Chinese Universities, 2009, 30(10): 2024-2031. |
| 9 | REN J , WANG W , WANG D L , et al . A theoretical investigation on the mechanism of dimethyl carbonate formation on Cu/AC catalyst[J]. Applied Catalysis A: General, 2014, 472(472): 47-52. |
| 10 | ZHANG R G , SONG L Z , WANG B J , et al . A density functional theory investigation on the mechanism and kinetics of dimethyl carbonate formation on Cu2O catalyst.[J]. Journal of Computational Chemistry, 2012, 33(11): 1101-1110. |
| 11 | FU T J , WANG X , ZHENG H Y , et al . Effect of Cu location and dispersion on carbon sphere supported Cu catalysts for oxidative carbonylation of methanol to dimethyl carbonate[J]. Carbon, 2017, 115: 363-374. |
| 12 | ZHANG G Q , YAN J F , WANG J J , et al . Effect of carbon support on the catalytic performance of Cu-based nanoparticles for oxidative carbonylation of methanol[J]. Applied Surface Science, 2018, 455: 696-704. |
| 13 | DESHMUKH A A , MHLANGA S D , COVILLE N J . Carbon spheres[J]. Materials Science & Engineering R, 2010, 70(1/2):1-28. |
| 14 | MORENO C C . Colloidal and micro-carbon spheres derived from low-temperature polymerization reactions[J]. Advances in Colloid & Interface Science, 2016, 236: 113-141. |
| 15 | WANG J , HAO P P , SHI R N , et al . Fabrication of yolk-shell Cu@C nanocomposites as high-performance catalysts in oxidative carbonylation of methanol to dimethyl carbonate[J]. Nanoscale Research Letters, 2017, 12(1): 481. |
| 16 | HAO P P , REN J , YANG L L , et al . Direct and generalized synthesis of carbon-based yolk-shell nanocomposites from metal-oleate precursor[J]. Chemical Engineering Journal, 2016, 283: 113-141. |
| 17 | LI H X , ZHAO J X , SHI R N , et al . Remarkable activity of nitrogen-doped hollow carbon spheres encapsulated Cu on synthesis of dimethyl carbonate: role of effective nitrogen[J]. Applied Surface Science, 2018, 436: 803-813. |
| 18 | FANG Y , GU D , ZOU Y , et al . A low-concentration hydrothermal synthesis of biocompatible ordered mesoporous carbon nanospheres with tunable and uniform size[J]. Angewandte Chemie, 2010, 49(43): 7987-7991. |
| 19 | SING K S W , WILLIAMS R T . Physisorption hysteresis loops and the characterization of nanoporous materials[J]. Adsorption Science & Technology, 2004, 22(10): 773-782. |
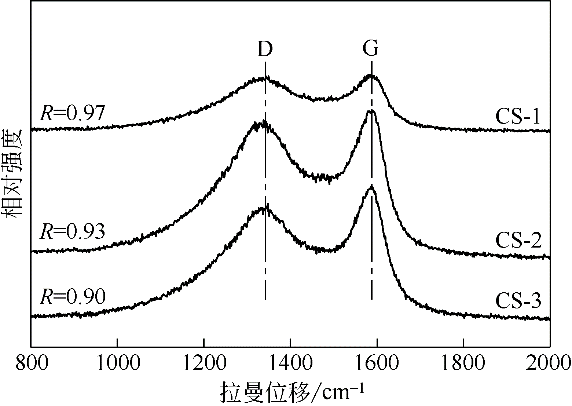
| 20 | SONG S , JIANG S . Selective catalytic oxidation of ammonia to nitrogen over CuO/CNTs: the promoting effect of the defects of CNTs on the catalytic activity and selectivity[J]. Applied Catalysis B: Environmental, 2012, 117/118(1): 346-350. |
| 21 | SUZUKI S , HIBINO H . Characterization of doped single-wall carbon nanotubes by Raman spectroscopy[J]. Carbon, 2011, 49(7): 2264-2272. |
| 22 | LIN C R , SU C H , CHANG C Y , et al . Synthesis of nanosized flake carbons by RF-chemical vapor method[J]. Surface & Coatings Technology, 2006, 200(10): 3190-3193. |
| 23 | SONG S , YANG H , RAO R , et al . Defects of multi-walled carbon nanotubes as active sites for benzene hydroxylation to phenol in the presence of HO[J]. Catalysis Communications, 2010, 11(8): 783-787. |
| 24 | RODRÍGUEZMANZO J A , CRETU O , BANHART F . Trapping of metal atoms in vacancies of carbon nanotubes and graphene[J]. ACS Nano, 2010, 4(6): 3422-3428. |
| 25 | SONG S , JIANG S , RAO R , et al . Bicomponent VO2-defects/MWCNT catalyst for hydroxylation of benzene to phenol: promoter effect of defects on catalytic performance[J]. Applied Catalysis A: General, 2011, 401(1): 215-219. |
| 26 | GROBMANN D , DREIER A , LEHMANN C W , et al . Encapsulation of copper and zinc oxide nanoparticles inside small diameter carbon nanotubes[J]. Microporous & Mesoporous Materials, 2015, 202(4): 189-197. |
| 27 | YAN B , HUANG S S , WANG S P , et al . Catalytic oxidative carbonylation over Cu2O nanoclusters supported on carbon materials: the role of the carbon support[J]. Chemcatchem, 2014, 6(9): 2671-2679. |
| 28 | SONG S Q , RAO R C , YANG H X , et al . Cu2O/MWCNTs prepared by spontaneous redox: growth mechanism and superior catalytic activity[J]. Journal of Physical Chemistry C, 2010, 114(33): 13998-14003. |
| 29 | ZHANG Z L , CHE H W , WANG Y L , et al . Template-free synthesis of Cu@Cu2O core-shell microspheres and their application as copper-based catalysts for dimethyldichlorosilane synthesis[J]. Chemical Engineering Journal, 2012, 211(47): 421-431. |
| 30 | ZHANG G Q , LI Z , ZHENG H Y , et al . Influence of the surface oxygenated groups of activated carbon on preparation of a nano Cu/AC catalyst and heterogeneous catalysis in the oxidative carbonylation of methanol[J]. Applied Catalysis B: Environmental, 2015, 179: 95-105. |
| 31 | HU Q , FAN G L , ZHANG S Y , et al . Gas phase hydrogenation of dimethyl-1,4-cyclohexane dicarboxylate over highly dispersed and stable supported copper-based catalysts[J]. Journal of Molecular Catalysis A: Chemical, 2015, 397: 134-141. |
| 32 | CHAKRABORTY A K , WOOLLEY R A J , BUTENKO Y V , et al . A photoelectron spectroscopy study of ion-irradiation induced defects in single-wall carbon nanotubes[J]. Carbon, 2007, 45(14): 2744-2750. |
| 33 | ESPINÓS J P , MORALES J , BARRANCO A , et al . Interface effects for Cu, CuO, and Cu2O deposited on SiO2 and ZrO2. XPS determination of the valence state of copper in Cu/SiO2 and Cu/ZrO2 catalysts[J]. Journal of Physical Chemistry B, 2002, 106(27): 6921-6929. |
| 34 | ZHANG G Q , LI Z , ZHENG H Y , et al . Influence of surface oxygenated groups on the formation of active Cu species and the catalytic activity of Cu/AC catalyst for the synthesis of dimethyl carbonate[J]. Applied Surface Science, 2016, 390: 68-77. |
| 35 | HANSEN T W , DELARIVA A T , CHALLA S R , et al . Sintering of catalytic nanoparticles: particle migration or Ostwald ripening[J]. Accounts of Chemical Research, 2013, 46(8): 1720-1730. |
| 36 | YE R P , LIN L , LI Q H , et al . Recent progress in improving the stability of copper-based catalysts for hydrogenation of carbon-oxygen bonds[J]. Catalysis Science & Technology, 2018, 8(14): 3428-3449. |
| [1] | ZHANG Mingyan, LIU Yan, ZHANG Xueting, LIU Yake, LI Congju, ZHANG Xiuling. Research progress of non-noble metal bifunctional catalysts in zinc-air batteries [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 276-286. |
| [2] | SHI Yongxing, LIN Gang, SUN Xiaohang, JIANG Weigeng, QIAO Dawei, YAN Binhang. Research progress on active sites in Cu-based catalysts for CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 287-298. |
| [3] | XIE Luyao, CHEN Songzhe, WANG Laijun, ZHANG Ping. Platinum-based catalysts for SO2 depolarized electrolysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 299-309. |
| [4] | YANG Xiazhen, PENG Yifan, LIU Huazhang, HUO Chao. Regulation of active phase of fused iron catalyst and its catalytic performance of Fischer-Tropsch synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 310-318. |
| [5] | WANG Lele, YANG Wanrong, YAO Yan, LIU Tao, HE Chuan, LIU Xiao, SU Sheng, KONG Fanhai, ZHU Canghai, XIANG Jun. Influence of spent SCR catalyst blending on the characteristics and deNO x performance for new SCR catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 489-497. |
| [6] | DENG Liping, SHI Haoyu, LIU Xiaolong, CHEN Yaoji, YAN Jingying. Non-noble metal modified vanadium titanium-based catalyst for NH3-SCR denitrification simultaneous control VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 542-548. |
| [7] | CHENG Tao, CUI Ruili, SONG Junnan, ZHANG Tianqi, ZHANG Yunhe, LIANG Shijie, PU Shi. Analysis of impurity deposition and pressure drop increase mechanisms in residue hydrotreating unit [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4616-4627. |
| [8] | WANG Peng, SHI Huibing, ZHAO Deming, FENG Baolin, CHEN Qian, YANG Da. Recent advances on transition metal catalyzed carbonylation of chlorinated compounds [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4649-4666. |
| [9] | ZHANG Qi, ZHAO Hong, RONG Junfeng. Research progress of anti-toxicity electrocatalysts for oxygen reduction reaction in PEMFC [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4677-4691. |
| [10] | GE Quanqian, XU Mai, LIANG Xian, WANG Fengwu. Research progress on the application of MOFs in photoelectrocatalysis [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4692-4705. |
| [11] | WANG Weitao, BAO Tingyu, JIANG Xulu, HE Zhenhong, WANG Kuan, YANG Yang, LIU Zhaotie. Oxidation of benzene to phenol over aldehyde-ketone resin based metal-free catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4706-4715. |
| [12] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| [13] | WU Haibo, WANG Xilun, FANG Yanxiong, JI Hongbing. Progress of the development and application of 3D printing catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 3956-3964. |
| [14] | XIANG Yang, HUANG Xun, WEI Zidong. Recent progresses in the activity and selectivity improvement of electrocatalytic organic synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4005-4014. |
| [15] | WANG Yaogang, HAN Zishan, GAO Jiachen, WANG Xinyu, LI Siqi, YANG Quanhong, WENG Zhe. Strategies for regulating product selectivity of copper-based catalysts in electrochemical CO2 reduction [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4043-4057. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||