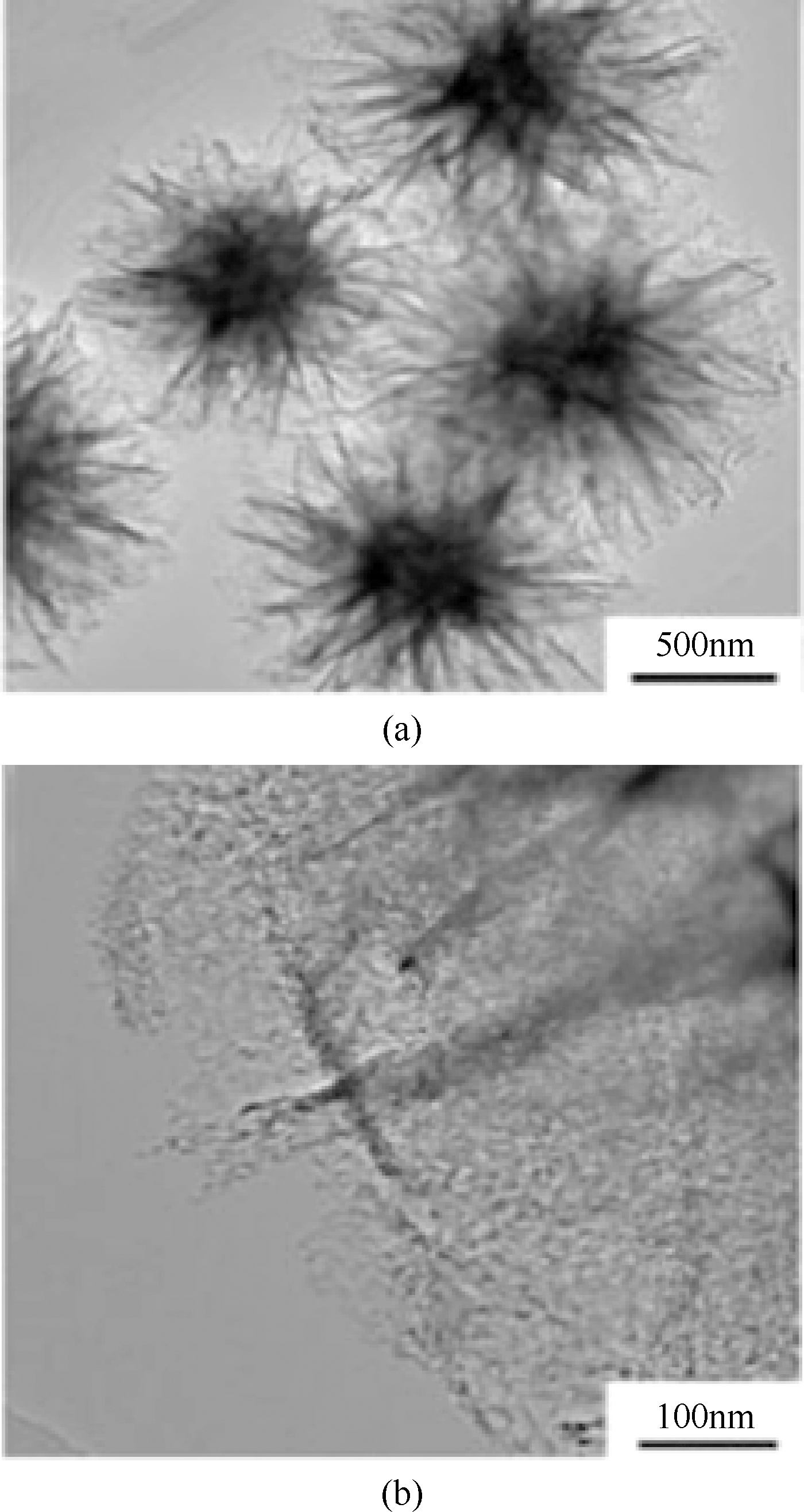
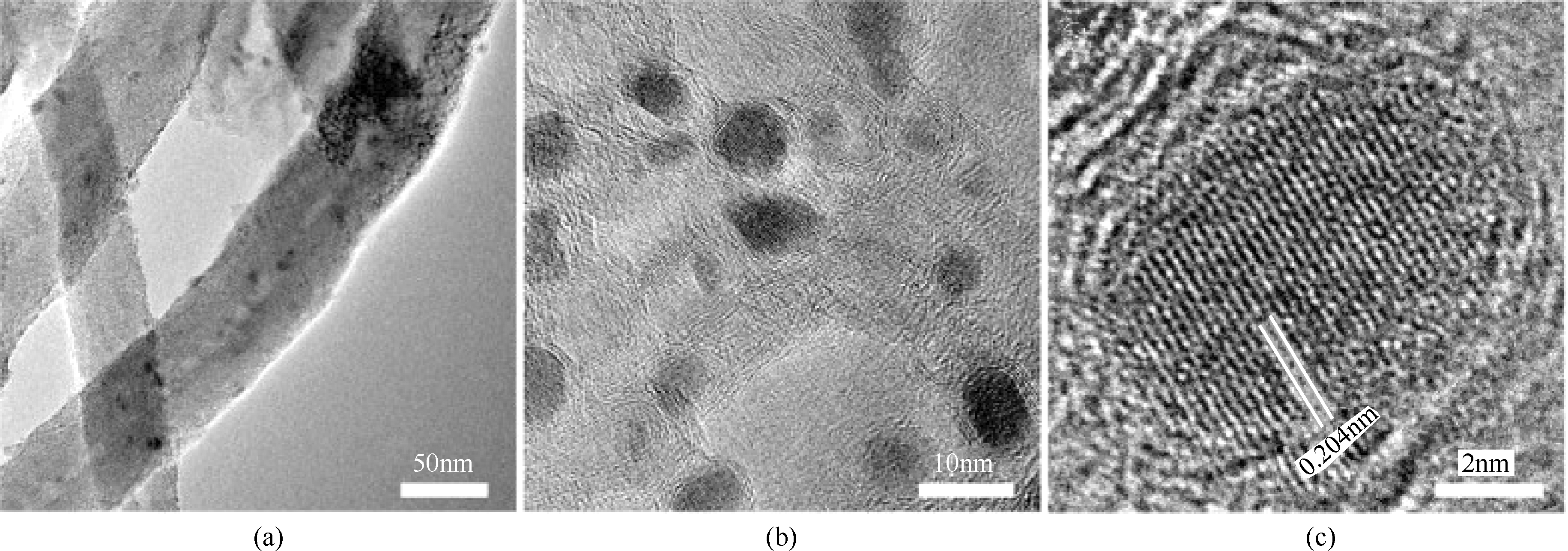
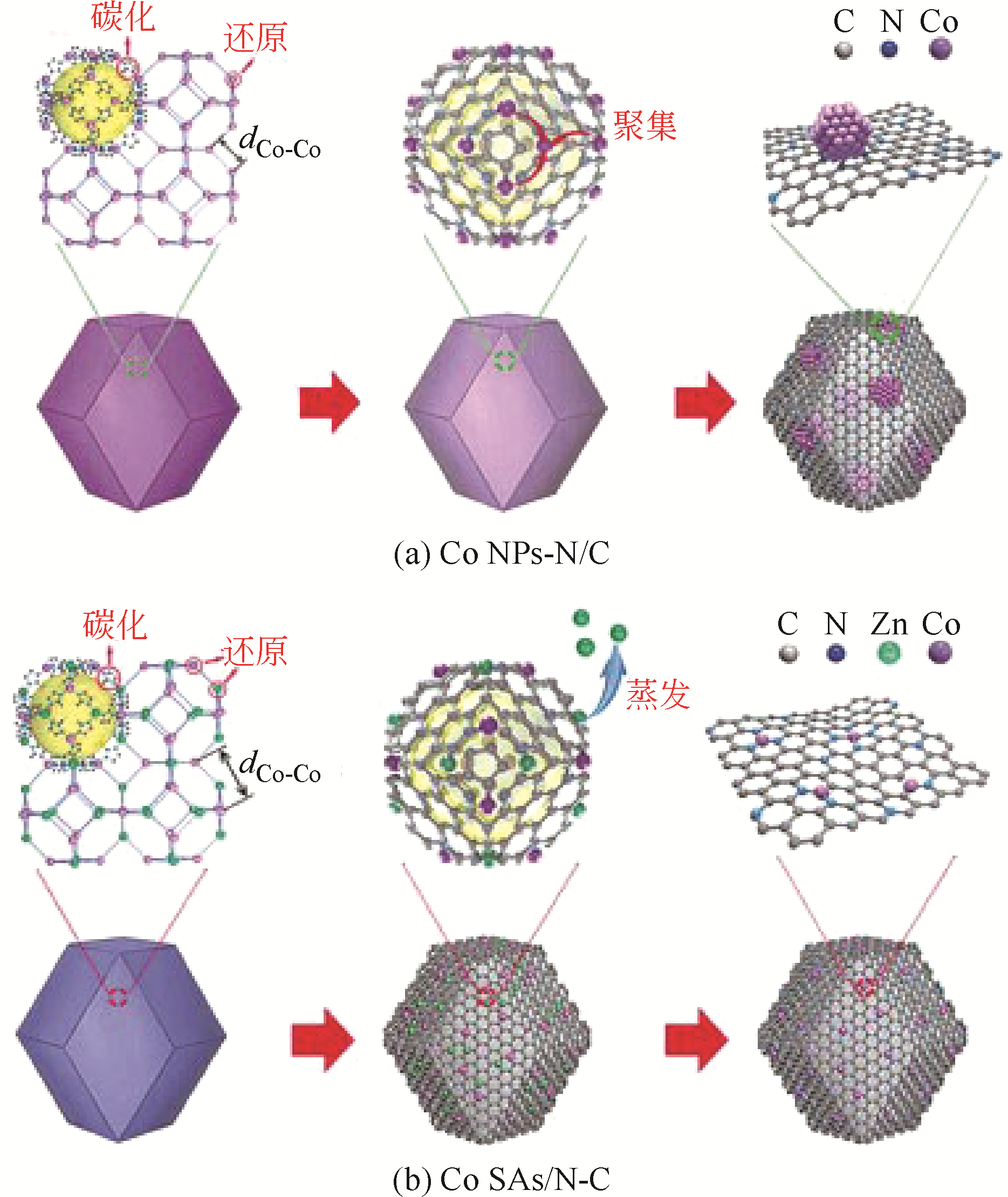
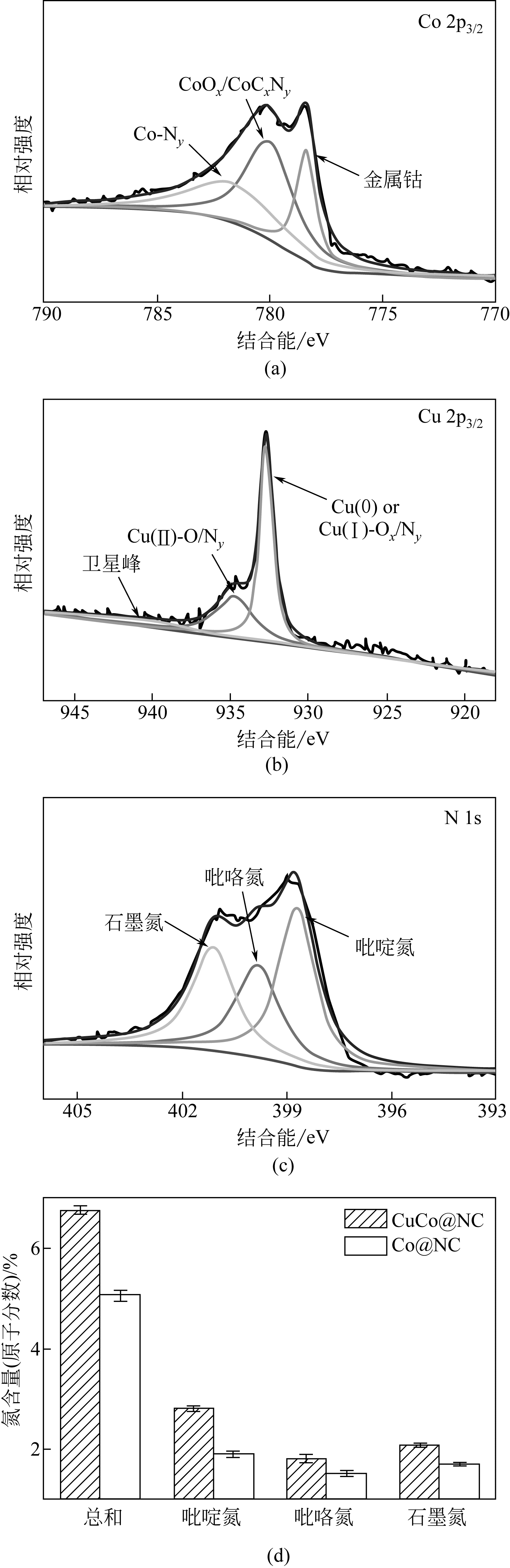
Chemical Industry and Engineering Progress ›› 2019, Vol. 38 ›› Issue (07): 3153-3162.DOI: 10.16085/j.issn.1000-6613.2018-1765
• Industrial catalysis • Previous Articles Next Articles
Research progress in non-platinum fuel cells electrocatalysts
Jian ZHANG( ),Dai DANG,Wenjin JI,Quanbing LIU,Yanxiong FANG,Yuying ZHENG(
),Dai DANG,Wenjin JI,Quanbing LIU,Yanxiong FANG,Yuying ZHENG( )
)
- College of Light Industry and Chemical Engineering,Guangdong University of Technology, Guangzhou 510006, Guangdong, China
-
Received:2018-09-03Online:2019-07-05Published:2019-07-05 -
Contact:Yuying ZHENG
非铂燃料电池电催化剂研究进展
- 广东工业大学轻工化工学院,广东 广州 510006
-
通讯作者:郑育英 -
作者简介:张健(1992—),男,硕士研究生,研究方向为燃料电池电催化剂。E-mail:<email>1456487841@qq.com</email>。 -
基金资助:广东省科技计划(2016A010103028)
CLC Number:
Cite this article
Jian ZHANG, Dai DANG, Wenjin JI, Quanbing LIU, Yanxiong FANG, Yuying ZHENG. Research progress in non-platinum fuel cells electrocatalysts[J]. Chemical Industry and Engineering Progress, 2019, 38(07): 3153-3162.
张健, 党岱, 姬文晋, 刘全兵, 方岩雄, 郑育英. 非铂燃料电池电催化剂研究进展[J]. 化工进展, 2019, 38(07): 3153-3162.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2018-1765
| 1 | YANG S B , FENG X L , WANG X C , et al . Graphene-based carbon nitride nanosheets as efficient metal-free electrocatalysts for oxygen reduction reactions[J]. Angew. Chem.: Int. Ed., 2011, 50(23): 5339-5343. |
| 2 | GONG K P , DU F , XIA Z H , et al . Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction[J]. Science, 2009, 323(5915): 760-764. |
| 3 | LEFEVRE M , PROIETTI E , JAOUEN F , et al . Iron-based catalysts with improved oxygen reduction activity in polymer electrolyte fuel cells[J]. Science, 2009, 324(5923): 71-74. |
| 4 | QIAO X C , PENG H L , YOU C H , et al . Nitrogen, phosphorus and iron doped carbon nanospheres with high surface area and hierarchical porous structure for oxygen reduction[J]. Journal of Power Sources, 2015, 288: 253-260. |
| 5 | SUN M , LIU H J , LIU Y , et al . Graphene-based transition metal oxide nanocomposites for the oxygen reduction reaction[J]. Nanoscale, 2015, 7(4): 1250-1269. |
| 6 | SUN M , ZHANG G , LIU H , et al . α- and γ-Fe2O3 nanoparticle/nitrogen doped carbon nanotube catalysts for high-performance oxygen reduction reaction[J]. Science China Materials, 2015, 58(9): 683-692. |
| 7 | ABROSHAN H , BOTHRA P , BACK S , et al . Ultrathin cobalt oxide overlayer promotes catalytic activity of cobalt nitride for the oxygen reduction reaction[J]. The Journal of Physical Chemistry C, 2018, 122(9): 4783-4791. |
| 8 | ALEXANDER A M , HARGREAVES J S J . Alternative catalytic materials: carbides, nitrides, phosphides and amorphous boron alloys[J]. Chemical Society Reviews, 2010, 39(11): 4388-4401. |
| 9 | XIE J F , XIE Y . Transition metal nitrides for electrocatalytic energy conversion: opportunities and challenges[J]. Chemistry, 2016, 22(11): 3588-3598. |
| 10 | DONG Y Y , DENG, Y J, ZENG J H , et al . A high-performance composite ORR catalyst based on the synergy between binary transition metal nitride and nitrogen-doped reduced graphene oxide[J]. Journal of Materials Chemistry A, 2017, 5(12): 5829-5837. |
| 11 | TANG H B , LUO J M , TIAN X L , et al . Template-free preparation of 3D porous co-doped VN nanosheet-assembled microflowers with enhanced oxygen reduction activity[J]. ACS Applied Materials & Interfaces, 2018, 10(14): 11604-11612. |
| 12 | SUN T , WU Q , CHE R C , et al . Alloyed Co-Mo nitride as high-performance electrocatalyst for oxygen reduction in acidic medium[J]. ACS Catalysis, 2015, 5(3): 1857-1862. |
| 13 | YU J M , GAO X P , CHEN G Z , et al . Electrocatalytic performance of commercial vanadium carbide for oxygen reduction reaction[J]. International Journal of Hydrogen Energy, 2016, 41(7): 4150-4158. |
| 14 | YANG W X , LIU X J , YUE X Y , et al . Bamboo-like carbon nanotube/Fe3C nanoparticle hybrids and their highly efficient catalysis for oxygen reduction[J]. J. Am. Chem. Soc., 2015, 137(4): 1436-1439. |
| 15 | FAN X J , PENG Z W , YE R Q , et al . M3C (M: Fe, Co, Ni) nanocrystals encased in graphene nanoribbons: an active and stable bifunctional electrocatalyst for oxygen reduction and hydrogen evolution reactions[J]. ACS Nano, 2015, 9(7): 7407-7418. |
| 16 | XIAO M L , ZHU J B , FENG L G , et al . Meso/macroporous nitrogen-doped carbon architectures with iron carbide encapsulated in graphitic layers as an efficient and robust catalyst for the oxygen reduction reaction in both acidic and alkaline solutions[J]. Adv. Mater., 2015, 27(15): 2521-2527. |
| 17 | REN G Y , LU X Y , LI Y N , et al . Porous core-shell Fe3C embedded n-doped carbon nanofibers as an effective electrocatalysts for oxygen reduction reaction[J]. ACS Appl. Mater. Interfaces, 2016, 8(6): 4118-4125. |
| 18 | SUN M , DAVENPORT D , LIU H J , et al . Highly efficient and sustainable non-precious-metal Fe-N-C electrocatalysts for the oxygen reduction reaction[J]. Journal of Materials Chemistry A, 2018, 6(6): 2527-2539. |
| 19 | KIM J H, SA Y J, JEONG H Y , et al . Roles of Fe-N x and Fe-Fe3C@c species in Fe-N/C electrocatalysts for oxygen reduction reaction[J]. ACS Applied Materials & Interfaces, 2017, 9(11): 9567-9575. |
| 20 | KWAK D H , HAN S B , LEE Y W, et al . Fe/N/S-doped mesoporous carbon nanostructures as electrocatalysts for oxygen reduction reaction in acid medium[J]. Applied Catalysis B: Environmental, 2017, 203: 889-898. |
| 21 | JIANG W J , GU L , LI L , et al . Understanding the high activity of Fe-N-C electrocatalysts in oxygen reduction: Fe/Fe3C nanoparticles boost the activity of Fe-N(x)[J]. J. Am. Chem. Soc., 2016, 138(10): 3570-3587. |
| 22 | LEE S, KWAK D H , HAN S B , et al . Bimodal porous iron/nitrogen-doped highly crystalline carbon nanostructure as a cathode catalyst for the oxygen reduction reaction in an acid medium[J]. ACS Catalysis, 2016, 6(8): 5095-5102. |
| 23 | YANG J , ZHANG F J , LU H Y , et al . Hollow Zn/Co ZIF particles derived from core-shell ZIF-67@ZIF-8 as selective catalyst for the semi-hydrogenation of acetylene[J]. Angew. Chem.: Int. Ed., 2015, 54(37): 10889-10893. |
| 24 | YIN P Q , YAO T , WU Y , et al . Single cobalt atoms with precise N-coordination as superior oxygen reduction reaction catalysts[J]. Angew. Chem.: Int. Ed., 2016, 55(36): 10800-10805. |
| 25 | KUANG M , WANG Q , HAN P , et al . Cu, Co-embedded N-enriched mesoporous carbon for efficient oxygen reduction and hydrogen evolution reactions[J]. Advanced Energy Materials, 2017, 7(17): 1700193. |
| 26 | LI Z T , SUN H D , WEI L Q , et al . Lamellar metal organic framework-derived Fe-N-C non-noble electrocatalysts with bimodal porosity for efficient oxygen reduction[J]. ACS Applied Materials & Interfaces, 2017, 9(6): 5272-5278. |
| 27 | TANG C , ZHANG Q . Nanocarbon for oxygen reduction electrocatalysis: dopants, edges, and defects[J]. Adv. Mater., 2017, 29(13): 1604103. |
| 28 | YANG S B , ZHI L J , TANG K , et al . Efficient synthesis of heteroatom (N or S)-doped graphene based on ultrathin graphene oxide-porous silica sheets for oxygen reduction reactions[J]. Advanced Functional Materials, 2012, 22(17): 3634-3640. |
| 29 | DAI L M , XUE Y H , QU L T , et al . Metal-free catalysts for oxygen reduction reaction[J]. Chem. Rev., 2015, 115(11): 4823-4892. |
| 30 | LIU Z W , PENG F , WANG H J , et al . Phosphorus-doped graphite layers with high electrocatalytic activity for the O2 reduction in an alkaline medium[J]. Angew. Chem.: Int. Ed., 2011, 50(14): 3257-3261. |
| 31 | SHAO Y Y , ZHANG S , ENGELHARD M H , et al . Nitrogen-doped graphene and its electrochemical applications[J]. Journal of Materials Chemistry, 2010, 20(35): 7491. |
| 32 | YANG D S , BHATTACHARJYA D , INAMDAR S , et al . Phosphorus-doped ordered mesoporous carbons with different lengths as efficient metal-free electrocatalysts for oxygen reduction reaction in alkaline media[J]. J. Am Chem. Soc., 2012, 134(39): 16127-16130. |
| 33 | DAEMS N , SHENG X , VANKELECOM I F J , et al . Metal-free doped carbon materials as electrocatalysts for the oxygen reduction reaction[J]. Journal of Materials Chemistry A, 2014, 2(12): 4085-4110. |
| 34 | WANG D W , SU D S . Heterogeneous nanocarbon materials for oxygen reduction reaction[J]. Energy & Environmental Science, 2014, 7(2): 576-591. |
| 35 | ZHANG J T , ZHAO Z H , XIA Z H , et al . A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions[J]. Nature Nanotechnology, 2015, 10(5): 444-452. |
| 36 | JEON I Y , ZHANG S , ZHANG L P , et al . Edge-selectively sulfurized graphene nanoplatelets as efficient metal-free electrocatalysts for oxygen reduction reaction: the electron spin effect[J]. Advanced Materials, 2013, 25(42): 6138-6145. |
| 37 | LAI L F , POTTS J R , ZHAN D , et al . Exploration of the active center structure of nitrogen-doped graphene-based catalysts for oxygen reduction reaction[J]. Energy & Environmental Science, 2012, 5(7): 7936. |
| 38 | GUO D H , SHIBUYA R , AKIBA C , et al . Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts[J]. Science, 2016, 351(6271): 361-365. |
| 39 | YU H J , SHANG L , BIAN T , et al . Nitrogen-doped porous carbon nanosheets templated from g-C3N4 as metal-free electrocatalysts for efficient oxygen reduction reaction[J]. Adv. Mater., 2016, 28(25): 5080-5086. |
| 40 | YANG Z , YAO Z , LI G F , et al . Sulfur-doped graphene as an efficient metal-free cathode catalyst for oxygen reduction[J]. ACS Nano, 2012, 6(1): 205-211. |
| 41 | QIAO X C , LIAO S J , YOU C H , et al . Phosphorus and nitrogen dual doped and simultaneously reduced graphene oxide with high surface area as efficient metal-free electrocatalyst for oxygen reduction[J]. Catalysts, 2015, 5(2): 981-991. |
| 42 | MA T Y, RAN J R , DAI S , et al . Phosphorus-doped graphitic carbon nitrides grown in situ on carbon-fiber paper: flexible and reversible oxygen electrodes[J]. Angew. Chem.: Int. Ed., 2015, 54(15): 4646-4650. |
| 43 | LI R , WEI Z D , GOU X L . Nitrogen and phosphorus dual-doped graphene/carbon nanosheets as bifunctional electrocatalysts for oxygen reduction and evolution[J]. ACS Catalysis, 2015, 5(7): 4133-4142. |
| 44 | BORGHEI M , LAOCHAROEN N , KIBENA-PÕLDSEPP E , et al . Porous N,P-doped carbon from coconut shells with high electrocatalytic activity for oxygen reduction: alternative to Pt-C for alkaline fuel cells[J]. Applied Catalysis B: Environmental, 2017, 204: 394-402. |
| 45 | LI J J , ZHANG Y M , ZHANG X H , et al . S, N dual-doped graphene-like carbon nanosheets as efficient oxygen reduction reaction electrocatalysts[J]. ACS Appl. Mater. Interfaces, 2017, 9(1): 398-405. |
| 46 | DONG D , LIU Y , LI J H . Co3O4 hollow polyhedrons as bifunctional electrocatalysts for reduction and evolution reactions of oxygen[J]. Particle & Particle Systems Characterization, 2016, 33(12): 887-895. |
| 47 | WU C , LIU D , LI H , et al . Molybdenum carbide-decorated metallic cobalt@nitrogen-doped carbon polyhedrons for enhanced electrocatalytic hydrogen evolution[J]. Small, 2018, 14(16): e1704227. |
| 48 | SUN M , LIU H J , QU J H , et al . Earth-rich transition metal phosphide for energy conversion and storage[J]. Advanced Energy Materials, 2016, 6(13): 1600087. |
| 49 | LIU M J , LI J H . Cobalt phosphide hollow polyhedron as efficient bifunctional electrocatalysts for the evolution reaction of hydrogen and oxygen[J]. ACS Applied Materials & Interfaces, 2016, 8(3): 2158-2165. |
| 50 | LI S P , ZHANG G , TU X M , et al . Polycrystalline CoP/CoP2 structures for efficient full water splitting[J]. ChemElectroChem, 2018, 5(4): 701-707. |
| 51 | ZHANG G , LI J . Tailoring oxygen vacancy on Co3O4 nanosheets with high surface area for oxygen evolution reaction[J]. Chinese Journal of Chemical Physics, 2018, 31(4): 517-522. |
| 52 | ZHANG G , WANG G C , LIU H J , et al . Rapidly catalysis of oxygen evolution through sequential engineering of vertically layered FeNi structure[J]. Nano Energy, 2018, 43: 359-367. |
| 53 | AIJAZ A , MASA J , ROSLER C , et al . Co@Co3O4 encapsulated in carbon nanotube-grafted nitrogen-doped carbon polyhedra as an advanced bifunctional oxygen electrode[J]. Angew. Chem.: Int. Ed., 2016, 55(12): 4087-4091. |
| 54 | WU C , ZHANG Y H , DONG D , et al . Co9S8 nanoparticles anchored on nitrogen and sulfur dual-doped carbon nanosheets as highly efficient bifunctional electrocatalyst for oxygen evolution and reduction reactions[J]. Nanoscale, 2017, 9(34): 12432-12440. |
| 55 | HU B C , WU Z Y , CHU S Q , et al . SiO2-protected shell mediated templating synthesis of Fe-N-doped carbon nanofibers and their enhanced oxygen reduction reaction performance[J]. Energy & Environmental Science, 2018, 11(8): 2208-2215. |
| [1] | CHEN Kuangyin, LI Ruilan, TONG Yang, SHEN Jianhua. Structure design of gas diffusion layer in proton exchange membrane fuel cell [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 246-259. |
| [2] | ZHANG Mingyan, LIU Yan, ZHANG Xueting, LIU Yake, LI Congju, ZHANG Xiuling. Research progress of non-noble metal bifunctional catalysts in zinc-air batteries [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 276-286. |
| [3] | SHI Yongxing, LIN Gang, SUN Xiaohang, JIANG Weigeng, QIAO Dawei, YAN Binhang. Research progress on active sites in Cu-based catalysts for CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 287-298. |
| [4] | XIE Luyao, CHEN Songzhe, WANG Laijun, ZHANG Ping. Platinum-based catalysts for SO2 depolarized electrolysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 299-309. |
| [5] | YANG Xiazhen, PENG Yifan, LIU Huazhang, HUO Chao. Regulation of active phase of fused iron catalyst and its catalytic performance of Fischer-Tropsch synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 310-318. |
| [6] | XU Jiaheng, LI Yongsheng, LUO Chunhuan, SU Qingquan. Optimization of methanol steam reforming process [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 41-46. |
| [7] | WANG Lele, YANG Wanrong, YAO Yan, LIU Tao, HE Chuan, LIU Xiao, SU Sheng, KONG Fanhai, ZHU Canghai, XIANG Jun. Influence of spent SCR catalyst blending on the characteristics and deNO x performance for new SCR catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 489-497. |
| [8] | DENG Liping, SHI Haoyu, LIU Xiaolong, CHEN Yaoji, YAN Jingying. Non-noble metal modified vanadium titanium-based catalyst for NH3-SCR denitrification simultaneous control VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 542-548. |
| [9] | CHENG Tao, CUI Ruili, SONG Junnan, ZHANG Tianqi, ZHANG Yunhe, LIANG Shijie, PU Shi. Analysis of impurity deposition and pressure drop increase mechanisms in residue hydrotreating unit [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4616-4627. |
| [10] | WANG Peng, SHI Huibing, ZHAO Deming, FENG Baolin, CHEN Qian, YANG Da. Recent advances on transition metal catalyzed carbonylation of chlorinated compounds [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4649-4666. |
| [11] | ZHANG Qi, ZHAO Hong, RONG Junfeng. Research progress of anti-toxicity electrocatalysts for oxygen reduction reaction in PEMFC [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4677-4691. |
| [12] | GE Quanqian, XU Mai, LIANG Xian, WANG Fengwu. Research progress on the application of MOFs in photoelectrocatalysis [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4692-4705. |
| [13] | WANG Weitao, BAO Tingyu, JIANG Xulu, HE Zhenhong, WANG Kuan, YANG Yang, LIU Zhaotie. Oxidation of benzene to phenol over aldehyde-ketone resin based metal-free catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4706-4715. |
| [14] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| [15] | YANG Ying, HOU Haojie, HUANG Rui, CUI Yu, WANG Bing, LIU Jian, BAO Weiren, CHANG Liping, WANG Jiancheng, HAN Lina. Coal tar phenol-based carbon nanosphere prepared by Stöber method for adsorption of CO2 [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 5011-5018. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||