Chemical Industry and Engineering Progress ›› 2019, Vol. 38 ›› Issue (05): 2339-2346.DOI: 10.16085/j.issn.1000-6613.2018-1349
• Industrial catalysis • Previous Articles Next Articles
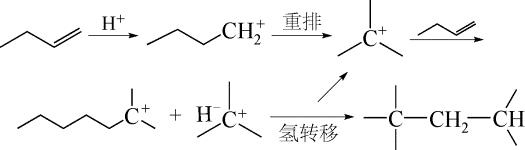
Progress in catalytic C4 alkylation with liquid acid coupled systems
Xiaodong TANG1,2( ),Zhiyu WANG2,Jingjing LI2,Dayong QING2,Manxi LENG3,Hongyu ZHANG3
),Zhiyu WANG2,Jingjing LI2,Dayong QING2,Manxi LENG3,Hongyu ZHANG3
- 1. State Key Laboratory of Oil and Gas Reservoir Geology and Exploitation, Southwest Petroleum University, Chengdu 610500, Sichuan, China
2. College of Chemistry and Chemical Engineering,Southwest Petroleum University, Chengdu 610500, Sichuan, China
3. College of Engineering, Southwest Petroleum University,Nanchong 637001, Sichuan, China
-
Received:2018-07-02Revised:2018-11-19Online:2019-05-05Published:2019-05-05
液体酸耦合体系催化C4烷基化研究进展
唐晓东1,2( ),王治宇2,李晶晶2,卿大咏2,冷曼希3,张洪宇3
),王治宇2,李晶晶2,卿大咏2,冷曼希3,张洪宇3
- 1. 西南石油大学油气藏地质及开发工程国家重点实验室,四川 成都 610500
2. 西南石油大学化学化工学院,四川 成都 610500
3. 西南石油大学工程学院,四川 南充 637001
-
作者简介:唐晓东 (1963—),男,教授,博士生导师,研究方向为石油天然气加工、稠油降黏与提高采收率等领域的基础理论及应用技术研究。E-mail:txd3079@163.com 。 -
基金资助:南充市校科技战略合作专项(NC17SY4023)
CLC Number:
Cite this article
Xiaodong TANG,Zhiyu WANG,Jingjing LI,Dayong QING,Manxi LENG,Hongyu ZHANG. Progress in catalytic C4 alkylation with liquid acid coupled systems[J]. Chemical Industry and Engineering Progress, 2019, 38(05): 2339-2346.
唐晓东,王治宇,李晶晶,卿大咏,冷曼希,张洪宇. 液体酸耦合体系催化C4烷基化研究进展[J]. 化工进展, 2019, 38(05): 2339-2346.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2018-1349
| 序号 | ILs | Acid | ILs∶ Acid (m/m) | | C8选择性(质量分数)/% | TMP /DMH | 参考文献 |
|---|---|---|---|---|---|---|---|
| 1 | [EMIM]HSO4 | TFOH | 11.1∶88.9 | >96.0 | 56.0 | 10.5 | [ |
| 2 | [OMIM]HSO4 | H2SO4 | 16.0∶84.0 | 98.0 | 51.3 | 6.6 | [ |
| 3 | [OMIM]HSO4 | TFOH | 23.7∶76.3 | 96.3 | 75.8 | 6.8 | [ |
| 4 | [MBSIM]OTf | H2SO4 | 46.7∶53.3 | 91.3 | 41.0 | 3.8 | [ |
| 5 | [MBSIM]OTf | TFOH | 48.3∶51.7 | 95.2 | 64.7 | 5.1 | [ |
| 6 | [MBSIM]OTf | CH3COOH | 60.0∶40.0 (v/v) | 91.8 | 67.1 | 4.1 | [ |
| 序号 | ILs | Acid | ILs∶ Acid (m/m) | | C8选择性(质量分数)/% | TMP /DMH | 参考文献 |
|---|---|---|---|---|---|---|---|
| 1 | [EMIM]HSO4 | TFOH | 11.1∶88.9 | >96.0 | 56.0 | 10.5 | [ |
| 2 | [OMIM]HSO4 | H2SO4 | 16.0∶84.0 | 98.0 | 51.3 | 6.6 | [ |
| 3 | [OMIM]HSO4 | TFOH | 23.7∶76.3 | 96.3 | 75.8 | 6.8 | [ |
| 4 | [MBSIM]OTf | H2SO4 | 46.7∶53.3 | 91.3 | 41.0 | 3.8 | [ |
| 5 | [MBSIM]OTf | TFOH | 48.3∶51.7 | 95.2 | 64.7 | 5.1 | [ |
| 6 | [MBSIM]OTf | CH3COOH | 60.0∶40.0 (v/v) | 91.8 | 67.1 | 4.1 | [ |
| 序号 | ILs | Acid | ILs∶Acid | (质量分数)/% | C8选择性 (质量分数)/% | TMP/DMH | RON | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| 1 | [EMIM]HSO4 | H2SO4 | 11.1∶88.9(质量比) | >96.0 | 56.8 | 11.3 | — | [ |
| 2 | [BMIM]HSO4 | H2SO4 | 11.1∶88.9(质量比) | >96.0 | 58.9 | 6.2 | — | [ |
| 3 | [OMIM]HSO4 | H2SO4 | 11.1∶88.9(质量比) | >96.0 | 55.2 | 6.7 | — | [ |
| 4 | [BMIM]HSO4 | H2SO4 | 20.9∶79.1(质量比) | 90.4 | 41.0 | 7.9 | — | [ |
| 5 | [OMIM]HSO4 | H2SO4 | 16.0∶84.0(质量比) | 98.0 | 51.3 | 6.6 | — | [ |
| 6 | [DMIPA]HSO4 | TFOH | 15.0∶85.0 (体积比) | ≈100.0 | 88.9 | 12.1 | 97.4 | [ |
| 7 | [MDEA]HSO4 | TFOH | 15.0∶85.0 (体积比) | ≈100.0 | 92.1 | 12.0 | 97.8 | [ |
| 8 | [DEEA]HSO4 | TFOH | 15.0∶85.0 (体积比) | ≈100.0 | 87.3 | 13.5 | 97.4 | [ |
| 9 | [DMEA]HSO4 | TFOH | 15.0∶85.0 (体积比) | ≈100.0 | 89.6 | 12.9 | 97.7 | [ |
| 10 | [MEA]HSO4 | TFOH | 25.0∶75.0 (体积比) | ≈100.0 | 87.9 | 10.4 | 97.0 | [ |
| 11 | [TEA]HSO4 | TFOH | 25.0:75.0 (体积比) | ≈100.0 | 91.5 | 13.5 | 98.0 | [ |
| 序号 | ILs | Acid | ILs∶Acid | (质量分数)/% | C8选择性 (质量分数)/% | TMP/DMH | RON | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| 1 | [EMIM]HSO4 | H2SO4 | 11.1∶88.9(质量比) | >96.0 | 56.8 | 11.3 | — | [ |
| 2 | [BMIM]HSO4 | H2SO4 | 11.1∶88.9(质量比) | >96.0 | 58.9 | 6.2 | — | [ |
| 3 | [OMIM]HSO4 | H2SO4 | 11.1∶88.9(质量比) | >96.0 | 55.2 | 6.7 | — | [ |
| 4 | [BMIM]HSO4 | H2SO4 | 20.9∶79.1(质量比) | 90.4 | 41.0 | 7.9 | — | [ |
| 5 | [OMIM]HSO4 | H2SO4 | 16.0∶84.0(质量比) | 98.0 | 51.3 | 6.6 | — | [ |
| 6 | [DMIPA]HSO4 | TFOH | 15.0∶85.0 (体积比) | ≈100.0 | 88.9 | 12.1 | 97.4 | [ |
| 7 | [MDEA]HSO4 | TFOH | 15.0∶85.0 (体积比) | ≈100.0 | 92.1 | 12.0 | 97.8 | [ |
| 8 | [DEEA]HSO4 | TFOH | 15.0∶85.0 (体积比) | ≈100.0 | 87.3 | 13.5 | 97.4 | [ |
| 9 | [DMEA]HSO4 | TFOH | 15.0∶85.0 (体积比) | ≈100.0 | 89.6 | 12.9 | 97.7 | [ |
| 10 | [MEA]HSO4 | TFOH | 25.0∶75.0 (体积比) | ≈100.0 | 87.9 | 10.4 | 97.0 | [ |
| 11 | [TEA]HSO4 | TFOH | 25.0:75.0 (体积比) | ≈100.0 | 91.5 | 13.5 | 98.0 | [ |
| 序号 | ILs | Acid | ILs∶ Acid (质量比) | (质量分数)/% | C8选择性 (质量分数)/% | TMP/DMH | RON | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| 1 | [BMIM]HSO4-CuSO4 | H2SO4 | 11.1∶88.9 | >96 | 77.8 | 4.8 | — | [ |
| 2 | [EMIM]HSO4-CuCl | H2SO4 | 11.1∶88.9 | >96 | 84.4 | 14.3 | 99.0 | [ |
| 3 | [Et3NH]HSO4-CuCl | H2SO4 | 11.1∶88.9 | >96 | 83.5 | 12.3 | 98.7 | [ |
| 4 | [EMIM]OTf-CuCl | TFOH | 11.1∶88.9 | >96 | 84.9 | 15.0 | 99.1 | [ |
| 5 | [EMIM]Tf2N-Cu(OTf)2 | TFOH | 11.1∶88.9 | >96 | 80.6 | 8.5 | 97.1 | [ |
| 6 | [Et3NH]Cl-1.8AlCl3-0.5CuCl | — | — | — | 71.4 | 5.5 | 93.1 | [ |
| 7 | [BMIM]Cl-1.8AlCl3-0.5CuCl | — | — | — | 76.6 | 6.8 | 94.8 | [ |
| 8 | NMA-AlCl3-CuCl | — | — | — | 94.65 | 15.0 | 98.4 | [ |
| 序号 | ILs | Acid | ILs∶ Acid (质量比) | (质量分数)/% | C8选择性 (质量分数)/% | TMP/DMH | RON | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| 1 | [BMIM]HSO4-CuSO4 | H2SO4 | 11.1∶88.9 | >96 | 77.8 | 4.8 | — | [ |
| 2 | [EMIM]HSO4-CuCl | H2SO4 | 11.1∶88.9 | >96 | 84.4 | 14.3 | 99.0 | [ |
| 3 | [Et3NH]HSO4-CuCl | H2SO4 | 11.1∶88.9 | >96 | 83.5 | 12.3 | 98.7 | [ |
| 4 | [EMIM]OTf-CuCl | TFOH | 11.1∶88.9 | >96 | 84.9 | 15.0 | 99.1 | [ |
| 5 | [EMIM]Tf2N-Cu(OTf)2 | TFOH | 11.1∶88.9 | >96 | 80.6 | 8.5 | 97.1 | [ |
| 6 | [Et3NH]Cl-1.8AlCl3-0.5CuCl | — | — | — | 71.4 | 5.5 | 93.1 | [ |
| 7 | [BMIM]Cl-1.8AlCl3-0.5CuCl | — | — | — | 76.6 | 6.8 | 94.8 | [ |
| 8 | NMA-AlCl3-CuCl | — | — | — | 94.65 | 15.0 | 98.4 | [ |
| 1 | 高步良 . 高辛烷值汽油组分生产技术[M]. 北京: 中国石化出版社,2006: 20-21. |
| GAO B L . High octane gasoline component production technology[M]. Beijing: China Petrochemical Press,2006: 20-21. | |
| 2 | 于兆臣, 田文君 . FCC汽油生产国Ⅵ汽油工艺路线的工业应用[J]. 炼油技术与工程, 2017, 47(8): 11-15. |
| YU Z C , TIAN W J . Industrial application of process route of FCC gasoline producing GUO Ⅵ gasoline[J]. Refining Technology and Engineering, 2017, 47(8): 11-15. | |
| 3 | 王傲运 . 离子材料协同酸催化C4烷基化反应研究[D]. 石家庄: 河北科技大学, 2016. |
| WANG A Y . Ionic liquid material and acid synergistically catalyzed C4 alkylation[D]. Shijiazhuang: Hebei University of Science and Technology, 2016. | |
| 4 | 杨为民 . 碳四烃转化与利用技术研究进展及发展前景[J]. 化工进展, 2015, 34(1): 1-9. |
| YANG W M . Progress and perspectives on conversion and utilization of C4 hydrocarbons[J]. Chemical Industry and Engineering Progress, 2015, 34(1): 1-9. | |
| 5 | 彭凯, 张成喜, 李永祥, 等 . 异丁烷/丁烯烷基化固体酸催化剂的再生方法研究进展[J]. 化工进展, 2015, 34(9): 3296-3302. |
| PENG K , ZHANG C X , LI Y X , et al . Advances in regeneration methods of solid acid catalyst for isobutane/butene alkylation[J]. Chemical Industry and Engineering Progress, 2015, 34(9): 1-9. | |
| 6 | BUI T L T, KORTH W , ASCHAUER S , et al . Alkylation of isobutane with 2-butene using ionic liquids as catalyst[J]. Green Chemistry, 2009, 11(12): 1961-1967. |
| 7 | 闵恩泽, 李成岳 . 绿色石化技术的科学与工程基础[M]. 北京: 中国石化出版社, 2002: 298-311. |
| MIN E Z , LI C Y . Science and engineering foundation of green petrochemical technology[M]. Beijing: China Petrochemical Press, 2002: 298-311. | |
| 8 | GAN P X , TANG S W . Research progress in ionic liquids catalyzed isobutane/butene alkylation[J]. Chinese Journal of Chemical Engineering, 2016, 24(11): 1497-1504. |
| 9 | OLAH G A , BATAMACK P , DEFFIEUX D , et al . Acidity dependence of the trifluoromethanesulfonic acid catalyzed isobutane/isobutylene alkylation modified with trifluoroacetic acid or water[J]. Applied Catalysis A: General, 1996, 146(1): 107-117. |
| 10 | REN H L , ZHAO G Y , ZHANG S J , et al . Triflic acid catalyzed isobutane alkylation with trifluoroethanol as a promoter[J]. Catalysis Communications, 2012, 18(18): 85-88. |
| 11 | ZHAO Z B , SUN W D , YANG X G , et al . Study of the catalytic behaviors of concentrated heteropolyacid solution.I. A novel catalyst for isobutane alkylation with butenes[J]. Catalysis Letters, 2000, 65(1/2/3): 115-121. |
| 12 | 赵振波 . 异丁烷-丁烯烷基化杂多酸-醋酸催化机理[J]. 物理化学学报, 2000, 16(7): 613-620. |
| ZHAO Z B . Active components and mechanism of Isobutane alkylation with butenes in the catalytic system of HPAs+AcOH[J]. Acta Physico-Chimica sinica, 2000, 16(7): 613-620. | |
| 13 | 杨美, 钟向宏, 陈群 . 离子液体催化二氧化碳合成环状碳酸酯的研究进展[J]. 化工进展, 2017, 36(9): 3300-3308. |
| YANG M , ZHONG X H , CHEN Q . Recent progress of the synthesis of cyclic carbonates from CO2 and epoxides catalyzed by ionic liquids[J]. Chemical Industry and Engineering Progress, 2017, 36(9): 3300-3308. | |
| 14 | 李海方 . 离子液体酸催化异丁烷烷基化反应的研究[D]. 石家庄: 河北科技大学, 2013. |
| LI H F . Study on isobutane alkylation utilizing ionic liquid/acid as catalyst[D]. Shijiazhuang: Hebei University of Science and Technology, 2013. | |
| 15 | 孟祥海, 张睿, 刘海燕, 等 . 复合离子液体碳四烷基化技术开发与应用[J]. 中国科学: 化学, 2018 (4): 387-396. |
| MENG X H , ZHANG R , LIU H Y , et al . Development and application of composite ionic liquid catalyzed isobutane alkylation technology[J]. Scientia Sinica Chimica, 2018 (4): 387-396. | |
| 16 | 胡静, 张涛, 张帅, 等 . [BMim][HSO4]/H2SO4二元体系的黏度及其表面张力[J]. 石油化工, 2017, 46(9): 1161-1167. |
| HU J , ZHANG T , ZHANG S , et al . Viscosity and surface tensions of [BMim][HSO4]/H2SO4 binary system[J]. Petrochemical Technology, 2017, 46(9): 1161-1167. | |
| 17 | CONG Y , LIU Y , HU R . Isobutane/2-butene alkylation catalyzed by strong acids in the presence of ionic liquid additives[J]. Liquid Fuels Technology, 2014, 32(16): 1981-1987. |
| 18 | TANG S W , SCURTO A M , SUBRAMANIAM B . Improved 1-butene/isobutane alkylation with acidic ionic liquids and tunable acid/ionic liquid mixtures[J]. Journal of Catalysis, 2009, 268(2): 243-250. |
| 19 | 刘鹰, 胡瑞生, 刘贵丽, 等 . 酸性离子液体催化的异丁烷/丁烯烷基化反应研究[J]. 分子催化, 2010, 24(3): 217-221. |
| LIU Y , HU R S , LIU G L , et al . Study on the alkylation of isobutane/butene in acidic ionic liquids[J]. Journal of Molecular Catalysis, 2010, 24(3): 217-221. | |
| 20 | ZHANG Y , ZHANG T , GAN P X , et al . Solubility of isobutane in ionic liquids[BMIm][PF6], [BMIm][BF4], and [BMIm][Tf2N][J]. Journal of Chemical & Engineering Data, 2015, 60(6): 1709-1714. |
| 21 | CUI P , ZHAO G Y , REN H L , et al . Ionic liquid enhanced alkylation of iso-butane and 1-butene[J]. Catalysis Today, 2013, 200(1): 30-35. |
| 22 | ZHENG W Z , WANG H Y , XIE W X , et al . Understanding interfacial behaviors of isobutane alkylation with C4 olefin catalyzed by sulfuric acid or ionic liquids[J]. AIChE Journal, 2017, 64(3): 958-959. |
| 23 | WANG P , WANG D X , XU C M , et al . DFT calculations of the alkylation reaction mechanisms of isobutane and 2-butene catalyzed by Brönsted acids[J]. Applied Catalysis A: General, 2007, 332(1): 22-26. |
| 24 | XING X Q , ZHAO G Y , CUI J Z , et al . Isobutane alkylation using acidic ionic liquid catalysts[J]. Catalysis Communications, 2012, 26(26): 68-71. |
| 25 | 张锁江, 刘瑞霞, 赵国英, 等 . 一种硫酸和离子液体复合催化剂催化生产烷基化汽油的方法: CN106939173A[P].2017-07-11. |
| ZHANG S J , LIU R X , ZHAO G Y , et al . Method for catalyzing production of alkylated gasoline by using a composite catalyst of sulfuric acid and ionic liquid: CN106939173A[P]. 2017-07-11. | |
| 26 | ZHANG H H , LIU R X , YANG Z Q , et al . Alkylation of isobutane/butene promoted by fluoride-containing ionic liquids[J]. Fuel, 2018, 211: 233-240. |
| 27 | 张锁江, 周志茂, 杨飞飞, 等 . 一种含离子液体和硝酸的混酸体系催化合成烷基化油的方法: CN106010636A[P]. 2016-10-12. |
| ZHANG S J , ZHOU Z M , YANG F F , et al . Method for synthesizing alkylated oil by mixed acid system containing ionic liquid and nitric acid: CN106010636A[P]. 2016-10-12. | |
| 28 | WANG A Y , ZHAO G Y , LIU F F , et al . Anionic clusters enhanced catalytic performance of protic acid ionic liquids for isobutane alkylation[J]. Industrial & Engineering Chemistry Research, 2016, 55(30): 8271-8280. |
| 29 | HUANG Q , ZHAO G Y , ZHANG S J , et al . Improved catalytic lifetime of H2SO4 for isobutane alkylation with trace amount of ionic liquids buffer[J]. Industrial & Engineering Chemistry Research, 2015, 54(5): 1464-1469. |
| 30 | ZHENG W Z , HUANG C Z , SUN W Z ,et al . Microstructures of the sulfonic-acid-functionalized ionic liquid/sulfuric acid and their interactions: a perspective from the isobutane alkylation[J]. Journal of Physical Chemistry B, 2018, 122(4): 1460-1470. |
| 31 | 卢丹, 赵国英, 任保增, 等 . 醚基功能化离子液体合成及催化烷基化反应[J]. 化工学报, 2015, 66(7): 2481-2487. |
| LU D , ZHAO G Y , REN B Z , et al . Isobutane alkylation catalyzed by ether functionalized ionic liquids[J]. CIESC Journal, 2015, 66(7): 2481-2487. | |
| 32 | YU F L , LI G X , GU Y L , et al . Preparation of alkylate gasoline in polyether-based acidic ionic liquids[J]. Catalysis Today, 2018, 310: 141-145. |
| 33 | 刘鹰, 孙宏娟, 丛迎楠, 等 . Cu对离子液体异丁烷/丁烯烷基化反应选择性的影响研究[J]. 燃料化学学报, 2014, 42(8): 1003-1009. |
| LIU Y , SUN H J , CONG Y N , et al . Study on the selectivity of isobutane/2-butene alkylation catalyzed by ionic liquid with Cu compound[J]. Journal of Fuel Chemistry and Technology, 2014, 42(8): 1003-1009. | |
| 34 | HU P C , WANG Y D , MENG X H , et al . Isobutane alkylation with 2-butene catalyzed by amide-AlCl3-based ionic liquid analogues[J]. Fuel, 2017, 189: 203-209. |
| 35 | LIU Y , LI R , SUN H J , et al . Effects of catalyst composition on the ionic liquid catalyzed isobutane/2-butene alkylation[J]. Journal of Molecular Catalysis A Chemical, 2015, 398: 133-139. |
| 36 | MA H, ZHANG R , MENG X H , et al . Solid formation during composite-ionic-liquid-catalyzed isobutane alkylation[J]. Energy & Fuels, 2014, 28(8): 5389-5395. |
| 37 | WANG H , MENG X Z , ZHAO G Y , et al . Isobutane/butene alkylation catalyzed by ionic liquids: a more sustainable process for clean oil production[J]. Green Chemistry, 2017, 19(6): 1462-1489. |
| [1] | GAO Yufei, LU Jinfeng. Mechanism of heterogeneous catalytic ozone oxidation:A review [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 430-438. |
| [2] | XU Ruosi, TAN Wei. Flow field simulation and fluid-structure coupling analysis of C-tube pool boiling two-phase flow model [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 47-55. |
| [3] | ZHOU Longda, ZHAO Lixin, XU Baorui, ZHANG Shuang, LIU Lin. Advances in electrostatic-cyclonic coupling enhanced multiphase media separation research [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3443-3456. |
| [4] | WU Heping, CAO Ning, XU Yuanyuan, CAO Yunbo, LI Yudong, YANG Qiang, LU Hao. Rapid separation of hydrofluoric acid and alkylated oil [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2845-2853. |
| [5] | XIA Shaobo, DUAN Lu, WANG Jianpeng, JI Renshan. Effect of water content of fly ash on the performance of coupling reinforced electrostatic-fabric integrated precipitator [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2101-2108. |
| [6] | WU Weixiong, XIE Shiwei, MA Ruixin, LIU Jizhen, WANG Shuangfeng, RAO Zhonghao. Research progress of solid-liquid/gas-liquid multiphase coupling thermal control technology [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1143-1154. |
| [7] | GUO Zhipeng, BU Xianbiao, LI Huashan, GONG Yulie, WANG Lingbao. Numerical simulation of heat extraction in single-well enhanced geothermal system based on thermal-hydraulic-chemical coupling model [J]. Chemical Industry and Engineering Progress, 2023, 42(2): 711-721. |
| [8] | LIU Yanhui, ZHOU Mingfang, MA Ming, WANG Kai, TAN Tianwei. Recent advances on the bio-fixation of CO2 driven by renewable energy [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 1-15. |
| [9] | CHEN Yu, LIU Chong, QIU Yuhui, BI Zixin, MU Tiancheng. Ionic liquids and deep eutectic solvents for green recycle of spent lithium-ion batteries [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 485-496. |
| [10] | DONG Kun, ZHAO Xu, YANG Fuxin, TAN Houzhang, LEI Yanzhou, CHEN Zhanjun. Effects of calcium-based additives on sulfur release during decoupling combustion of different coals [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 595-605. |
| [11] | SHI Xuan, YANG Dongyuan, HU Haobin, WANG Jiaofei, ZHANG Zhuangzhuang, HE Jianxun, DAI Chengyi, MA Xiaoxun. One-step preparation of toluene/xylene from benzene and syngas over ZnAlCrO x &HZSM-5 bifunctional catalyst [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 247-259. |
| [12] | HAN Xuan, WANG Lihong, BAI Xueyuan, YI Weiming, LI Yongjun, LI Zhihe, ZHANG Andong. Preparation of dealkalized red mud catalysts and its effect on bio-oil composition of corn straw catalytic pyrolysis [J]. Chemical Industry and Engineering Progress, 2022, 41(9): 4723-4732. |
| [13] | BAI Jingang, YUAN Zhengji, LIU Yu, ZHANG Yishi, LYU Xifeng. Fabrication and thermal properties of decanoic acid-paraffin/graphene aerogel form-stable phase change materials [J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4441-4448. |
| [14] | ZHU Tingting, SU Zhongxian, ZHAO Tianhang, LIU Yiwen. Treatment of antibiotic wastewater enhanced by zero-valent iron and its coupling technology [J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4513-4529. |
| [15] | SHAN Qingwen, ZHANG Juan, WANG Yajuan, LIU Wenqiang. Synthesis of polymeric ionic liquid and its performance on adsorption desulfurization [J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4571-4579. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||