化工进展 ›› 2021, Vol. 40 ›› Issue (12): 6807-6817.DOI: 10.16085/j.issn.1000-6613.2021-0680
微生物细胞工厂碳流调控进展
高聪1( ), 郭亮1, 胡贵鹏2, 陈修来1, 刘立明1(
), 郭亮1, 胡贵鹏2, 陈修来1, 刘立明1( )
)
- 1.江南大学食品科学与技术国家重点实验室,江苏 无锡 214122
2.江南大学药学院,江苏 无锡 214122
-
收稿日期:2021-04-10修回日期:2021-05-31出版日期:2021-12-05发布日期:2021-12-21 -
通讯作者:刘立明 -
作者简介:高聪(1991—),男,助理研究员,研究方向为微生物代谢工程。E-mail:conggao@jiangnan.edu.cn 。 -
基金资助:国家重点研发计划(2020YFA0908300);国家自然科学基金创新研究群体科学基金(32021005);国家自然科学基金(22008087)
Advances of metabolic flux regulation in microbial cell factories
GAO Cong1( ), GUO Liang1, HU Guipeng2, CHEN Xiulai1, LIU Liming1(
), GUO Liang1, HU Guipeng2, CHEN Xiulai1, LIU Liming1( )
)
- 1.State Key Laboratory of Food Science and Technology, Jiangnan University, Wuxi 214122, Jiangsu, China
2.School of Pharmaceutical Sciences, Jiangnan University, Wuxi 214122, Jiangsu, China
-
Received:2021-04-10Revised:2021-05-31Online:2021-12-05Published:2021-12-21 -
Contact:LIU Liming
摘要:
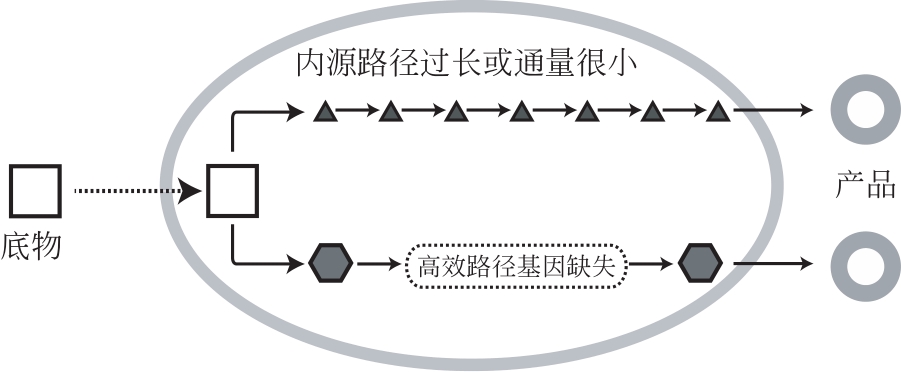
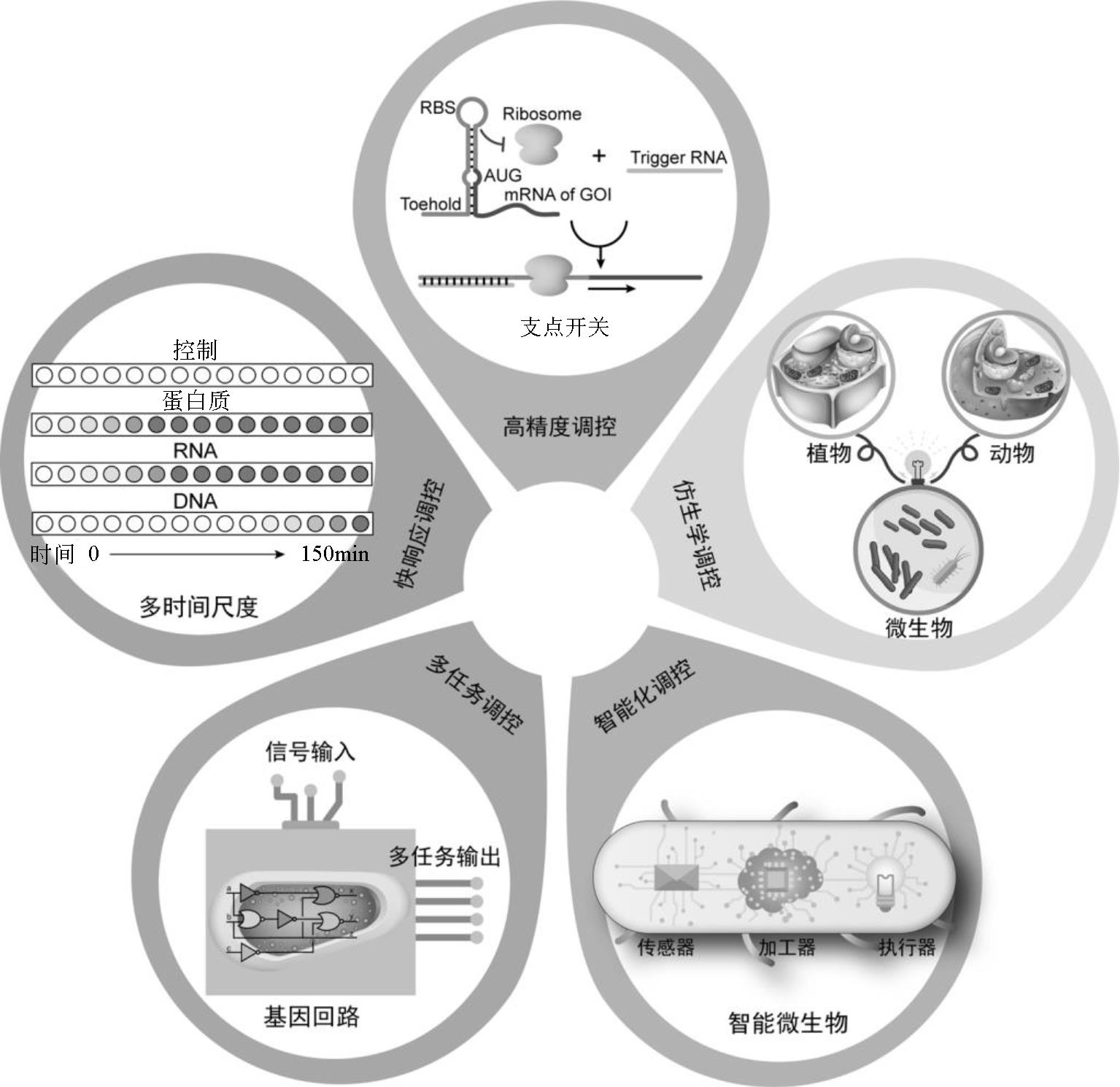
随着代谢工程技术的进步,越来越多微生物细胞工厂可用于化学品发酵生产。微生物细胞生产化学品具有生产条件温和、环境友好等优势,是实现化学品绿色可持续生产的重要手段。为了提高微生物细胞工厂的产量、得率和生产强度,传统代谢工程手段主要采用基因过表达或基因敲除方式增大目标代谢路径碳代谢流。然而由于代谢流调控精度不足,易导致细胞生产能力下降。本文主要针对微生物细胞工厂碳流调控中存在的瓶颈问题,从代谢流改造靶点选择、细胞生长与产物合成碳流平衡、副产物路径与产物合成竞争、产物合成效率强化四个角度,系统综述微生物细胞工厂碳代谢流调控的最新进展。并从高精度、仿生学、智能化、多任务、快响应调控工具的设计出发,对未来微生物细胞工厂的发展趋势进行展望。
中图分类号:
引用本文
高聪, 郭亮, 胡贵鹏, 陈修来, 刘立明. 微生物细胞工厂碳流调控进展[J]. 化工进展, 2021, 40(12): 6807-6817.
GAO Cong, GUO Liang, HU Guipeng, CHEN Xiulai, LIU Liming. Advances of metabolic flux regulation in microbial cell factories[J]. Chemical Industry and Engineering Progress, 2021, 40(12): 6807-6817.
| 1 | HAN T, KIM G B, LEE S Y. Glutaric acid production by systems metabolic engineering of an l-lysine-overproducing Corynebacterium glutamicum[J]. Proceedings of the National Academy of Sciences of the United States of America, 2020, 117(48): 30328-30334. |
| 2 | WANG J, SHEN X L, YUAN Q P, et al. Microbial synthesis of pyrogallol using genetically engineered Escherichia coli[J]. Metabolic Engineering, 2018, 45: 134-141. |
| 3 | WANG C L, PFLEGER B F, KIM S W. Reassessing Escherichia coli as a cell factory for biofuel production[J]. Current Opinion in Biotechnology, 2017, 45: 92-103. |
| 4 | KO Y S, KIM J W, LEE J A, et al. Tools and strategies of systems metabolic engineering for the development of microbial cell factories for chemical production[J]. Chemical Society Reviews, 2020, 49(14): 4615-4636. |
| 5 | LIU H, QI Y, ZHOU P, et al. Microbial physiological engineering increases the efficiency of microbial cell factories[J]. Critical Reviews in Biotechnology, 2021, 41(3): 339-354. |
| 6 | WANG G L, SHI T, CHEN T, et al. Integrated whole-genome and transcriptome sequence analysis reveals the genetic characteristics of a riboflavin-overproducing Bacillus subtilis[J]. Metabolic Engineering, 2018, 48: 138-149. |
| 7 | MEWALAL R, RAI D K, KAINER D, et al. Plant-derived terpenes: a feedstock for specialty biofuels[J]. Trends in Biotechnology, 2017, 35(3): 227-240. |
| 8 | QIAO K J, WASYLENKO T M, ZHOU K, et al. Lipid production in Yarrowia lipolytica is maximized by engineering cytosolic redox metabolism[J]. Nature Biotechnology, 2017, 35(2): 173-177. |
| 9 | HARDER B J, BETTENBROCK K, KLAMT S. Model-based metabolic engineering enables high yield itaconic acid production by Escherichia coli[J]. Metabolic Engineering, 2016, 38: 29-37. |
| 10 | YE C, LUO Q L, GUO L, et al. Improving lysine production through construction of an Escherichia coli enzyme-constrained model[J]. Biotechnology and Bioengineering, 2020, 117(11): 3533-3544. |
| 11 | YE C, XU N, GAO C, et al. Comprehensive understanding of Saccharomyces cerevisiae phenotypes with whole-cell model WM_S288C[J]. Biotechnology and Bioengineering, 2020, 117(5): 1562-1574. |
| 12 | DING Q, LUO Q L, ZHOU J, et al. Enhancing l-malate production of Aspergillus oryzae FMME218-37 by improving inorganic nitrogen utilization[J]. Applied Microbiology and Biotechnology, 2018, 102(20): 8739-8751. |
| 13 | CHOU H H, KEASLING J D. Programming adaptive control to evolve increased metabolite production[J]. Nature Communications, 2013, 4: 2595. |
| 14 | JAKOČIŪNAS T, JENSEN M K, KEASLING J D. CRISPR/Cas9 advances engineering of microbial cell factories[J]. Metabolic Engineering, 2016, 34: 44-59. |
| 15 | WANG S H, HOU Y R, CHEN X L, et al. Kick-starting evolution efficiency with an autonomous evolution mutation system[J]. Metabolic Engineering, 2019, 54: 127-136. |
| 16 | MAHR R, GÄTGENS C, GÄTGENS J, et al. Biosensor-driven adaptive laboratory evolution of l-valine production in Corynebacterium glutamicum[J]. Metabolic Engineering, 2015, 32: 184-194. |
| 17 | DING Q, DIAO W W, GAO C, et al. Microbial cell engineering to improve cellular synthetic capacity[J]. Biotechnology Advances, 2020, 45: 107649. |
| 18 | CHO J S, CHOI K R, PRABOWO C P S, et al. CRISPR/Cas9-coupled recombineering for metabolic engineering of Corynebacterium glutamicum[J]. Metabolic Engineering, 2017, 42: 157-167. |
| 19 | GUO L, DIAO W W, GAO C, et al. Engineering Escherichia coli lifespan for enhancing chemical production[J]. Nature Catalysis, 2020, 3(3): 307-318. |
| 20 | GAO C, WANG S H, HU G P, et al. Engineering Escherichia coli for malate production by integrating modular pathway characterization with CRISPRi-guided multiplexed metabolic tuning[J]. Biotechnology and Bioengineering, 2018, 115(3): 661-672. |
| 21 | FANG Y, WANG J L, MA W J, et al. Rebalancing microbial carbon distribution for L-threonine maximization using a thermal switch system[J]. Metabolic Engineering, 2020, 61: 33-46. |
| 22 | PIRANER D I, ABEDI M H, MOSER B A, et al. Tunable thermal bioswitches for in vivo control of microbial therapeutics[J]. Nature Chemical Biology, 2017, 13(1): 75-80. |
| 23 | SNOEK T, ROMERO-SUAREZ D, ZHANG J, et al. An orthogonal and pH-tunable sensor-selector for muconic acid biosynthesis in yeast[J]. ACS Synthetic Biology, 2018, 7(4): 995-1003. |
| 24 | HWANG H J, KIM J W, JU S Y, et al. Application of an oxygen-inducible nar promoter system in metabolic engineering for production of biochemicals in Escherichia coli[J]. Biotechnology and Bioengineering, 2017, 114(2): 468-473. |
| 25 | ZHAO E M, ZHANG Y F, MEHL J, et al. Optogenetic regulation of engineered cellular metabolism for microbial chemical production[J]. Nature, 2018, 555(7698): 683-687. |
| 26 | LALWANI M A, IP S S, CARRASCO-LÓPEZ C, et al. Optogenetic control of the lac operon for bacterial chemical and protein production[J]. Nature Chemical Biology, 2021, 17(1): 71-79. |
| 27 | AUBRY M, WANG W A, GUYODO Y, et al. Engineering E. coli for magnetic control and the spatial localization of functions[J]. Acs Synthetic Biology, 2020, 9(11): 3030-3041. |
| 28 | XU X H, LI X L, LIU Y F, et al. Pyruvate-responsive genetic circuits for dynamic control of central metabolism[J]. Nature Chemical Biology, 2020, 16(11): 1261-1268. |
| 29 | ZHOU L, REN J, LI Z, et al. Characterization and engineering of a clostridium glycine riboswitch and its use to control a novel metabolic pathway for 5-aminolevulinic acid production in Escherichia coli[J]. ACS Synthetic Biology, 2019, 8(10): 2327-2335. |
| 30 | XU P, LI L, ZHANG F, et al. Improving fatty acids production by engineering dynamic pathway regulation and metabolic control[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(31): 11299-11304. |
| 31 | GUPTA A, BROCKMAN REIZMAN I M, REISCH C R, et al. Dynamic regulation of metabolic flux in engineered bacteria using a pathway-independent quorum-sensing circuit[J]. Nature Biotechnology, 2017, 35(3): 273-279. |
| 32 | TSAO C Y, HOOSHANGI S, WU H C, et al. Autonomous induction of recombinant proteins by minimally rewiring native quorum sensing regulon of E. coli[J]. Metabolic Engineering, 2010, 12(3): 291-297. |
| 33 | JIANG W, HE X, LUO Y, et al. Two completely orthogonal quorum sensing systems with self-produced autoinducers enable automatic delayed cascade control[J]. ACS Synthetic Biology, 2020, 9(9): 2588-2599. |
| 34 | GAO C, GUO L, DING Q, et al. Dynamic consolidated bioprocessing for direct production of xylonate and shikimate from xylan by Escherichia coli[J]. Metabolic Engineering, 2020, 60: 128-137. |
| 35 | MAEDA M, SHIMADA T, ISHIHAMA A. Strength and regulation of seven rRNA promoters in Escherichia coli[J]. PLoS One, 2015, 10(12): e0144697. |
| 36 | BESHAY U, MIKSCH G, FRIEHS K, et al. Increasing the secretion ability of the kil gene for recombinant proteins in Escherichia coli by using a strong stationary-phase promoter[J]. Biotechnology Letters, 2007, 29(12): 1893-1901. |
| 37 | GAO C, HOU J S, XU P, et al. Programmable biomolecular switches for rewiring flux in Escherichia coli[J]. Nature Communications, 2019, 10: 3751. |
| 38 | HE X Y, CHEN Y, LIANG Q F, et al. Autoinduced AND gate controls metabolic pathway dynamically in response to microbial communities and cell physiological state[J]. ACS Synthetic Biology, 2017, 6(3): 463-470. |
| 39 | ALPER H, MIYAOKU K, STEPHANOPOULOS G. Construction of lycopene-overproducing E. coli strains by combining systematic and combinatorial gene knockout targets[J]. Nature Biotechnology, 2005, 23(5): 612-616. |
| 40 | DONG X X, CHEN X L, QIAN Y Y, et al. Metabolic engineering of Escherichia coli W3110 to produce L-malate[J]. Biotechnology and Bioengineering, 2017, 114(3): 656-664. |
| 41 | LIAN J Z, HAMEDIRAD M, HU S M, et al. Combinatorial metabolic engineering using an orthogonal tri-functional CRISPR system[J]. Nature Communications, 2017, 8: 1688. |
| 42 | ZHAO M, HUANG D X, ZHANG X J, et al. Metabolic engineering of Escherichia coli for producing adipic acid through the reverse adipate-degradation pathway[J]. Metabolic Engineering, 2018, 47: 254-262. |
| 43 | GUO L, ZHANG F, ZHANG C, et al. Enhancement of malate production through engineering of the periplasmic rTCA pathway in Escherichia coli[J]. Biotechnology and Bioengineering, 2018, 115(6): 1571-1580. |
| 44 | QI L S, LARSON M H, GILBERT L A, et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression[J]. Cell, 2013, 152(5): 1173-1183. |
| 45 | KIM S K, SEONG W, HAN G H, et al. CRISPR interference-guided multiplex repression of endogenous competing pathway genes for redirecting metabolic flux in Escherichia coli[J]. Microbial Cell Factories, 2017, 16(1): 188. |
| 46 | NA D, YOO S M, CHUNG H, et al. Metabolic engineering of Escherichia coli using synthetic small regulatory RNAs[J]. Nature Biotechnology, 2013, 31(2): 170-174. |
| 47 | YANG Y P, LIN Y H, LI L Y, et al. Regulating malonyl-CoA metabolism via synthetic antisense RNAs for enhanced biosynthesis of natural products[J]. Metabolic Engineering, 2015, 29: 217-226. |
| 48 | FERNANDEZ-RODRIGUEZ J, VOIGT C A. Post-translational control of genetic circuits using Potyvirus proteases[J]. Nucleic Acids Research, 2016, 44(13): 6493-6502. |
| 49 | EWEN CAMERON D, COLLINS J J. Tunable protein degradation in bacteria[J]. Nature Biotechnology, 2014, 32(12): 1276-1281. |
| 50 | OLIVARES A O, BAKER T A, SAUER R T. Mechanistic insights into bacterial AAA+ proteases and protein-remodelling machines[J]. Nature Reviews Microbiology, 2016, 14(1): 33-44. |
| 51 | COOKSON N A, MATHER W H, DANINO T, et al. Queueing up for enzymatic processing: correlated signaling through coupled degradation[J]. Molecular Systems Biology, 2011, 7: 561. |
| 52 | WU Y Q, WANG Y X. Protein circuits reprogram cells[J]. Nature Chemical Biology, 2019, 15(2): 96-97. |
| 53 | LO T M, CHNG S H, TEO W S, et al. A two-layer gene circuit for decoupling cell growth from metabolite production[J]. Cell Systems, 2016, 3(2): 133-143. |
| 54 | DOONG S J, GUPTA A, PRATHER K L J. Layered dynamic regulation for improving metabolic pathway productivity in Escherichia coli[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(12): 2964-2969. |
| 55 | WU J J, BAO M J, DUAN X G, et al. Developing a pathway-independent and full-autonomous global resource allocation strategy to dynamically switching phenotypic states[J]. Nature Communications, 2020, 11: 5521. |
| 56 | GAO C, XU P, YE C, et al. Genetic circuit-assisted smart microbial engineering[J]. Trends in Microbiology, 2019, 27(12): 1011-1024. |
| 57 | CHEN X L, DONG X X, WANG Y C, et al. Mitochondrial engineering of the TCA cycle for fumarate production[J]. Metabolic Engineering, 2015, 31: 62-73. |
| 58 | GREWAL P S, SAMSON J A, BAKER J J, et al. Peroxisome compartmentalization of a toxic enzyme improves alkaloid production[J]. Nature Chemical Biology, 2021, 17(1): 96-103. |
| 59 | SRINIVASAN P, SMOLKE C D. Biosynthesis of medicinal tropane alkaloids in yeast[J]. Nature, 2020, 585(7826): 614-619. |
| 60 | THOMIK T, WITTIG I, CHOE J Y, et al. An artificial transport metabolon facilitates improved substrate utilization in yeast[J]. Nature Chemical Biology, 2017, 13(11): 1158-1163. |
| 61 | MOON T S, DUEBER J E, SHIUE E, et al. Use of modular, synthetic scaffolds for improved production of glucaric acid in engineered E. coli[J]. Metabolic Engineering, 2010, 12(3): 298-305. |
| 62 | AVALOS J L, FINK G R, STEPHANOPOULOS G. Compartmentalization of metabolic pathways in yeast mitochondria improves the production of branched-chain alcohols[J]. Nature Biotechnology, 2013, 31(4): 335-341. |
| 63 | QIN J F, ZHOU Y J, KRIVORUCHKO A, et al. Modular pathway rewiring of Saccharomyces cerevisiae enables high-level production of L -ornithine[J]. Nature Communications, 2015, 6: 8224. |
| 64 | CHEN X Z, ZHOU J B, ZHANG L H, et al. Development of an Escherichia coli-based biocatalytic system for the efficient synthesis of N-acetyl-D-neuraminic acid[J]. Metabolic Engineering, 2018, 47: 374-382. |
| 65 | XU J Z, YANG H K, LIU L M, et al. Rational modification of Corynebacterium glutamicum dihydrodipicolinate reductase to switch the nucleotide-cofactor specificity for increasing l-lysine production[J]. Biotechnology and Bioengineering, 2018, 115(7): 1764-1777. |
| 66 | LI X, ZHANG C, XU X, et al. A single-component light sensor system allows highly tunable and direct activation of gene expression in bacterial cells[J]. Nucleic Acids Research, 2020, 48(6): e33. |
| 67 | YOO S M, JUNG S W, YEOM J, et al. Tunable gene expression system independent of downstream coding sequence[J]. ACS Synthetic Biology, 2020, 9(11): 2998-3007. |
| 68 | GAO X J, CHONG L S, KIM M S, et al. Programmable protein circuits in living cells[J]. Science, 2018, 361(6408): 1252-1258. |
| 69 | GLEIZER S, BEN-NISSAN R, BAR-ON Y M, et al. Conversion of Escherichia coli to generate all biomass carbon from CO2[J]. Cell, 2019, 179(6): 1255-1263.e12. |
| 70 | DING Q, MA D, LIU G Q, et al. Light-powered Escherichia coli cell division for chemical production[J]. Nature Communications, 2020, 11(1): 2262. |
| 71 | GASSLER T, SAUER M, GASSER B, et al. The industrial yeast Pichia pastoris is converted from a heterotroph into an autotroph capable of growth on CO2[J]. Nature Biotechnology, 2020, 38(2): 210-216. |
| 72 | CHEN F Y H, JUNG H W, TSUEI C Y, et al. Converting Escherichia coli to a synthetic methylotroph growing solely on methanol[J]. Cell, 2020, 182(4): 933-946.e14. |
| 73 | KIM S, LINDNER S N, ASLAN S, et al. Growth of E. coli on formate and methanol via the reductive glycine pathway[J]. Nature Chemical Biology, 2020, 16(5): 538-545. |
| 74 | ZHOU K, QIAO K J, EDGAR S, et al. Distributing a metabolic pathway among a microbial consortium enhances production of natural products[J]. Nature Biotechnology, 2015, 33(4): 377-383. |
| 75 | SHAHAB R L, BRETHAUER S, DAVEY M P, et al. A heterogeneous microbial consortium producing short-chain fatty acids from lignocellulose[J]. Science, 2020, 369(6507): 1073.. |
| 76 | NISHIDA K, ARAZOE T, YACHIE N, et al. Targeted nucleotide editing using hybrid prokaryotic and vertebrate adaptive immune systems[J]. Science, 2016, 353(6305): aaf8279. |
| 77 | GAUDELLI N M, KOMOR A C, REES H A, et al. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage[J]. Nature, 2017, 551(7681): 464-471. |
| 78 | ZHAO D D, LI J, LI S W, et al. Glycosylase base editors enable C-to-A and C-to-G base changes[J]. Nature Biotechnology, 2021, 39(1): 35-40. |
| 79 | GREEN A A, SILVER P A, COLLINS J J, et al. Toehold switches: de-novo-designed regulators of gene expression[J]. Cell, 2014, 159(4): 925-939. |
| 80 | BAUMSCHLAGER A, RULLAN M, KHAMMASH M. Exploiting natural chemical photosensitivity of anhydrotetracycline and tetracycline for dynamic and setpoint chemo-optogenetic control[J]. Nature Communications, 2020, 11: 3834. |
| 81 | LI Y, JIN M, O'LAUGHLIN R, et al. Multigenerational silencing dynamics control cell aging[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(42): 11253-11258. |
| 82 | MILLER T E, BENEYTON T, SCHWANDER T, et al. Light-powered CO2 fixation in a chloroplast mimic with natural and synthetic parts[J]. Science, 2020, 368(6491): 649-654. |
| 83 | LEE K Y, PARK S J, LEE K A, et al. Photosynthetic artificial organelles sustain and control ATP-dependent reactions in a protocellular system[J]. Nature Biotechnology, 2018, 36(6): 530-535. |
| 84 | CERONI F, BOO A, FURINI S, et al. Burden-driven feedback control of gene expression[J]. Nature Methods, 2018, 15(5): 387-393. |
| 85 | PHAM H L, WONG A, CHUA N, et al. Engineering a riboswitch-based genetic platform for the self-directed evolution of acid-tolerant phenotypes[J]. Nature Communications, 2017, 8: 411. |
| 86 | XIAO Y, BOWEN C H, LIU D, et al. Exploiting nongenetic cell-to-cell variation for enhanced biosynthesis[J]. Nature Chemical Biology, 2016, 12(5): 339-344. |
| 87 | CHEN Y, ZHANG S, YOUNG E M, et al. Genetic circuit design automation for yeast[J]. Nature Microbiology, 2020, 5(11): 1349-1360. |
| 88 | NIELSEN A A K, DER B S, SHIN J, et al. Genetic circuit design automation[J]. Science, 2016, 352(6281): aac7341. |
| 89 | SHIN J, ZHANG S, DER B S, et al. Programming Escherichia coli to function as a digital display[J]. Molecular Systems Biology, 2020, 16(3): e9401. |
| 90 | ZHANG J, PETERSEN S D, RADIVOJEVIC T, et al. Combining mechanistic and machine learning models for predictive engineering and optimization of tryptophan metabolism[J]. Nature Communications, 2020, 11: 4880. |
| 91 | WIJNANDS S P W, ENGELEN W, LAFLEUR R P M, et al. Controlling protein activity by dynamic recruitment on a supramolecular polymer platform[J]. Nature Communications, 2018, 9: 65. |
| 92 | ELOWITZ M B, LEIBLER S. A synthetic oscillatory network of transcriptional regulators[J]. Nature, 2000, 403(6767): 335-338. |
| 93 | GORDLEY R M, WILLIAMS R E, BASHOR C J, et al. Engineering dynamical control of cell fate switching using synthetic phospho-regulons[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(47): 13528-13533. |
| 94 | XUE P, SI T, MISHRA S, et al. A mass spectrometry-based high-throughput screening method for engineering fatty acid synthases with improved production of medium-chain fatty acids[J]. Biotechnology and Bioengineering, 2020, 117(7): 2131-2138. |
| 95 | HAMEDIRAD M, CHAO R, WEISBERG S, et al. Towards a fully automated algorithm driven platform for biosystems design[J]. Nature Communications, 2019, 10: 5150. |
| 96 | CHEN X, GAO C, GUO L, et al. DCEO biotechnology: tools to design, construct, evaluate, and optimize the metabolic pathway for biosynthesis of chemicals[J]. Chemical Reviews, 2018, 118(1): 4-72. |
| [1] | 高聪, 陈城虎, 陈修来, 刘立明. 代谢工程改造微生物合成生物基单体的进展与挑战[J]. 化工进展, 2023, 42(8): 4123-4135. |
| [2] | 郭峰, 张尚杰, 蒋羽佳, 姜万奎, 信丰学, 章文明, 姜岷. 一碳资源在酵母中的利用与转化[J]. 化工进展, 2023, 42(1): 30-39. |
| [3] | 陶雨萱, 张尚杰, 景艺文, 信丰学, 董维亮, 周杰, 蒋羽佳, 章文明, 姜岷. 甲基营养型大肠杆菌构建策略的研究进展[J]. 化工进展, 2021, 40(7): 3932-3941. |
| [4] | 孙文涛, 李春. 微生物合成植物天然产物的细胞工厂设计与构建[J]. 化工进展, 2021, 40(3): 1202-1214. |
| [5] | 郭亮, 高聪, 张丽, 陈修来, 刘立明. 人工代谢路径适配性的研究进展[J]. 化工进展, 2021, 40(3): 1252-1261. |
| [6] | 王颖, 曲俊泽, 梁楠, 郝鹤, 元英进. 合成类胡萝卜素细胞工厂的快速构建和定向进化[J]. 化工进展, 2021, 40(3): 1187-1201. |
| [7] | 刘卫兵, 叶邦策. 放线菌聚酮类化合物的合成生物学研究及生物制造[J]. 化工进展, 2021, 40(3): 1226-1237. |
| [8] | 马悦原, 陈金春, 陈国强. 嗜盐微生物底盘细胞:应用和前景[J]. 化工进展, 2021, 40(3): 1178-1186. |
| [9] | 王琛, 赵猛, 丁明珠, 王颖, 姚明东, 肖文海. 生物支架系统在合成生物学中的应用[J]. 化工进展, 2020, 39(11): 4557-4567. |
| [10] | 常鹏程, 于洋, 王颖, 李春. 酿酒酵母高效合成萜类化合物的组合调控策略[J]. 化工进展, 2019, 38(01): 598-605. |
| [11] | 张正晖, 曹铭铭, 李珺, 李春, 刘护. 微生物高效分泌蛋白质的策略与应用[J]. 化工进展, 2018, 37(08): 3129-3137. |
| [12] | 樊婧婧, 赵雨佳, 王晨, 李春, 周晓宏. 酿酒酵母乙酰辅酶A精细调控合成萜类化合物研究进展[J]. 化工进展, 2018, 37(07): 2773-2779. |
| [13] | 杨坤, 王颖, 李春. 细胞转运蛋白及其工程化应用[J]. 化工进展, 2017, 36(04): 1410-1417. |
| [14] | 刘丁玉, 孟娇, 王智文, 陈涛, 赵学明. 多元模块工程在代谢工程中的应用与研究进展[J]. 化工进展, 2016, 35(11): 3619-3626. |
| [15] | 肖文海, 周嗣杰, 王颖, 元英进. 如何工程化生物学[J]. 化工进展, 2016, 35(06): 1827-1836. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||