化工进展 ›› 2019, Vol. 38 ›› Issue (07): 3332-3340.DOI: 10.16085/j.issn.1000-6613.2019-0227
壳聚糖接枝聚丙烯酸/凹凸棒石复合材料对毒死蜱的吸附行为
- 仲恺农业工程学院化学化工学院,广东省普通高校农用绿色精细化学品重点实验室,广东 广州,510225
-
收稿日期:2019-02-18修回日期:2019-03-08出版日期:2019-07-05发布日期:2019-07-05 -
通讯作者:周新华 -
作者简介:徐华(1983—),男,博士,讲师。 -
基金资助:国家自然科学基金(21576303);广东省科技计划(2015A020209197);广东省教育厅特色创新项目(2014KQNCX163)
Adsorption performance of CTS-g-PAA/ATP composite on chlorpyrifos
Hua XU,Hongjian WEN,Jianda LIN,Hongjun ZHOU,Huayao CHEN,Xinhua ZHOU( )
)
- College of Chemistry and Chemical Engineering, Zhongkai College of Agriculture and Engineering, Key Laboratory of Agricultural Green Fine Chemicals of Guangdong Higher Education Institution, Guangzhou 510225, Guangdong, China
-
Received:2019-02-18Revised:2019-03-08Online:2019-07-05Published:2019-07-05 -
Contact:Xinhua ZHOU
摘要:
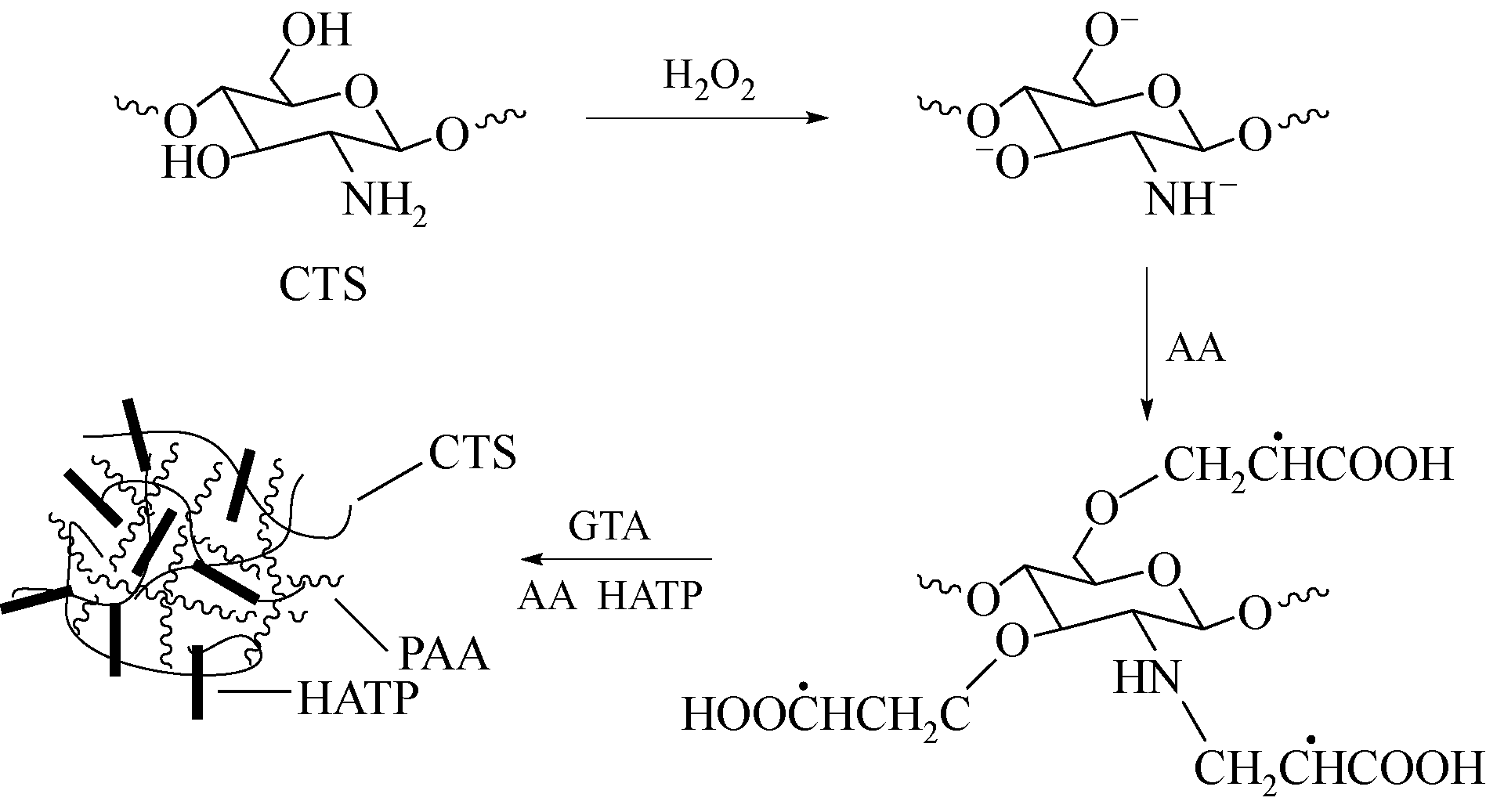
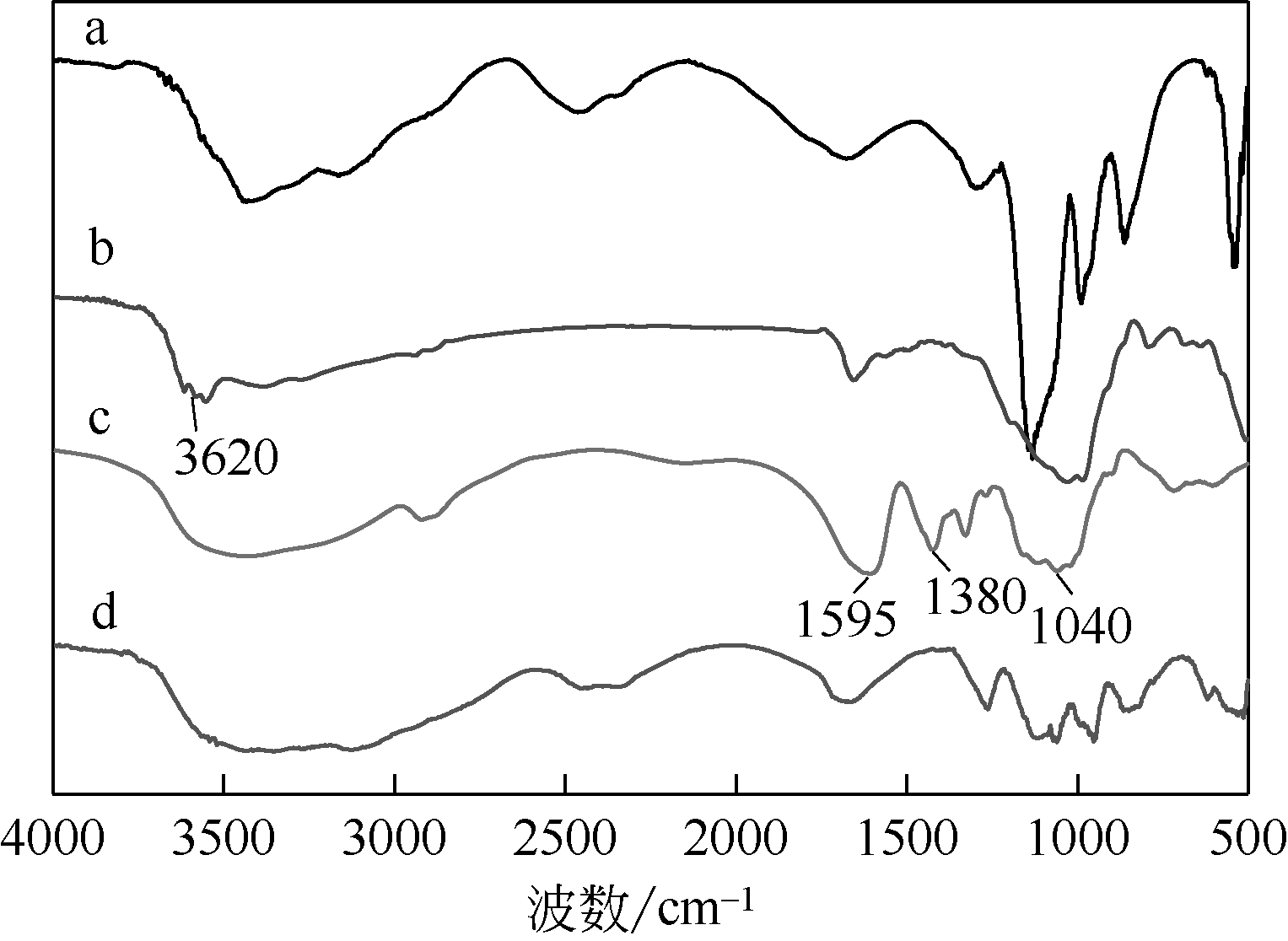
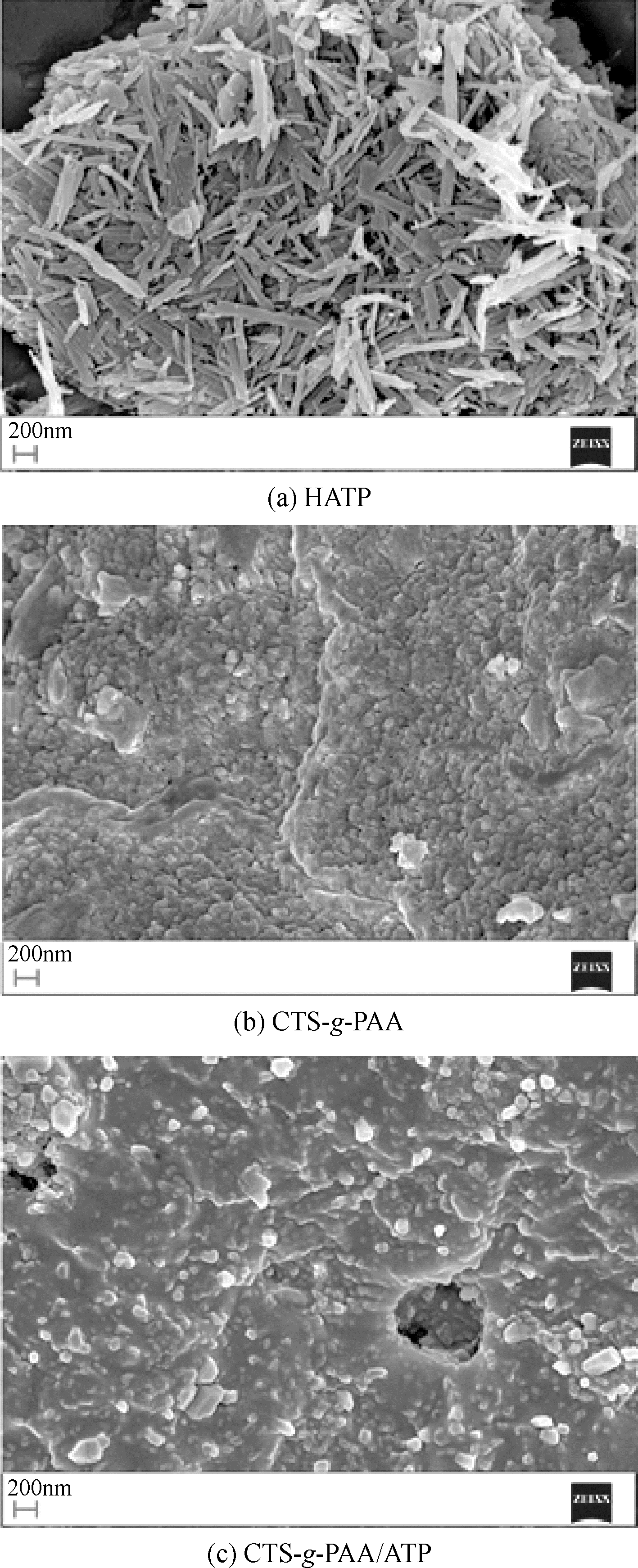
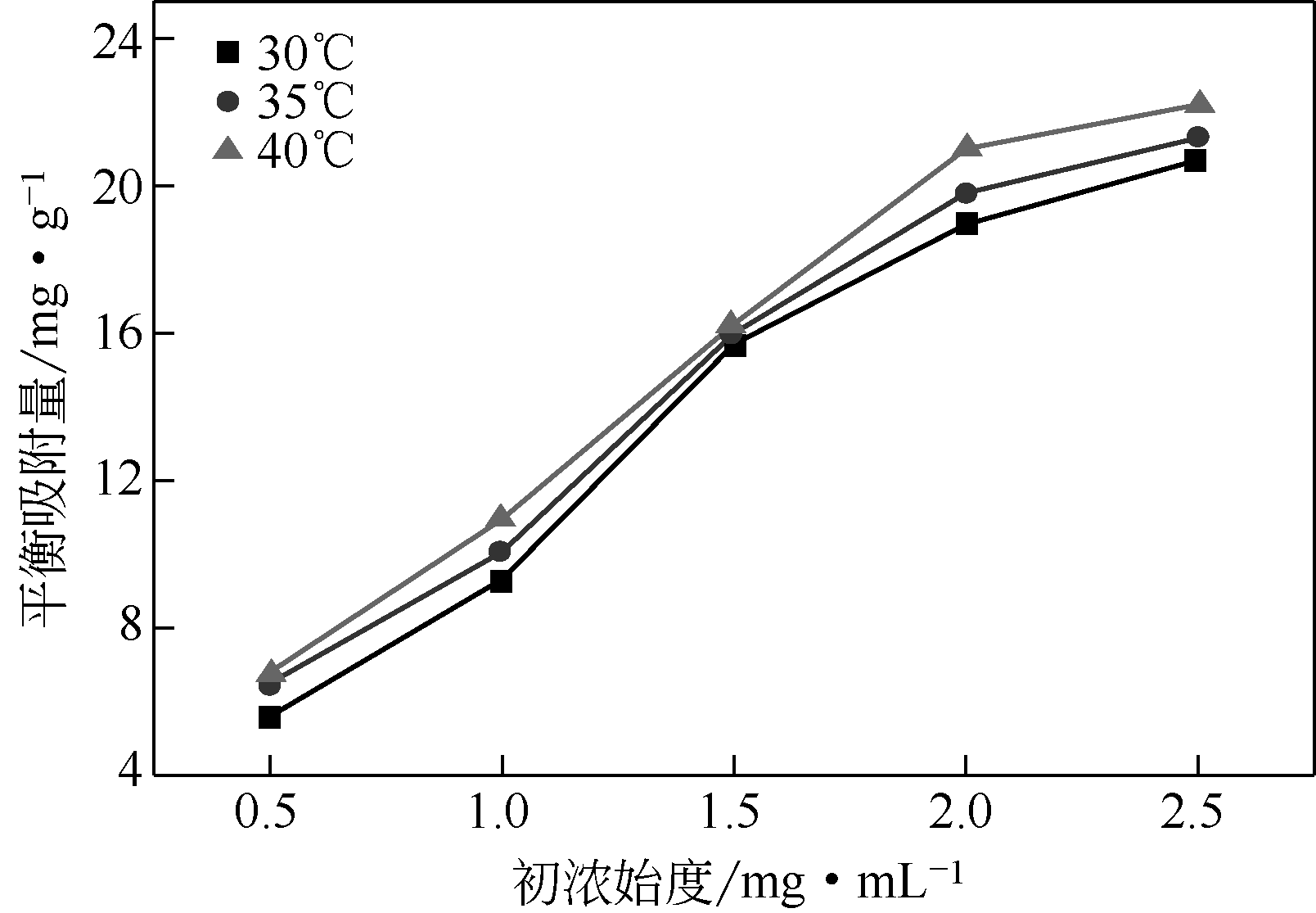
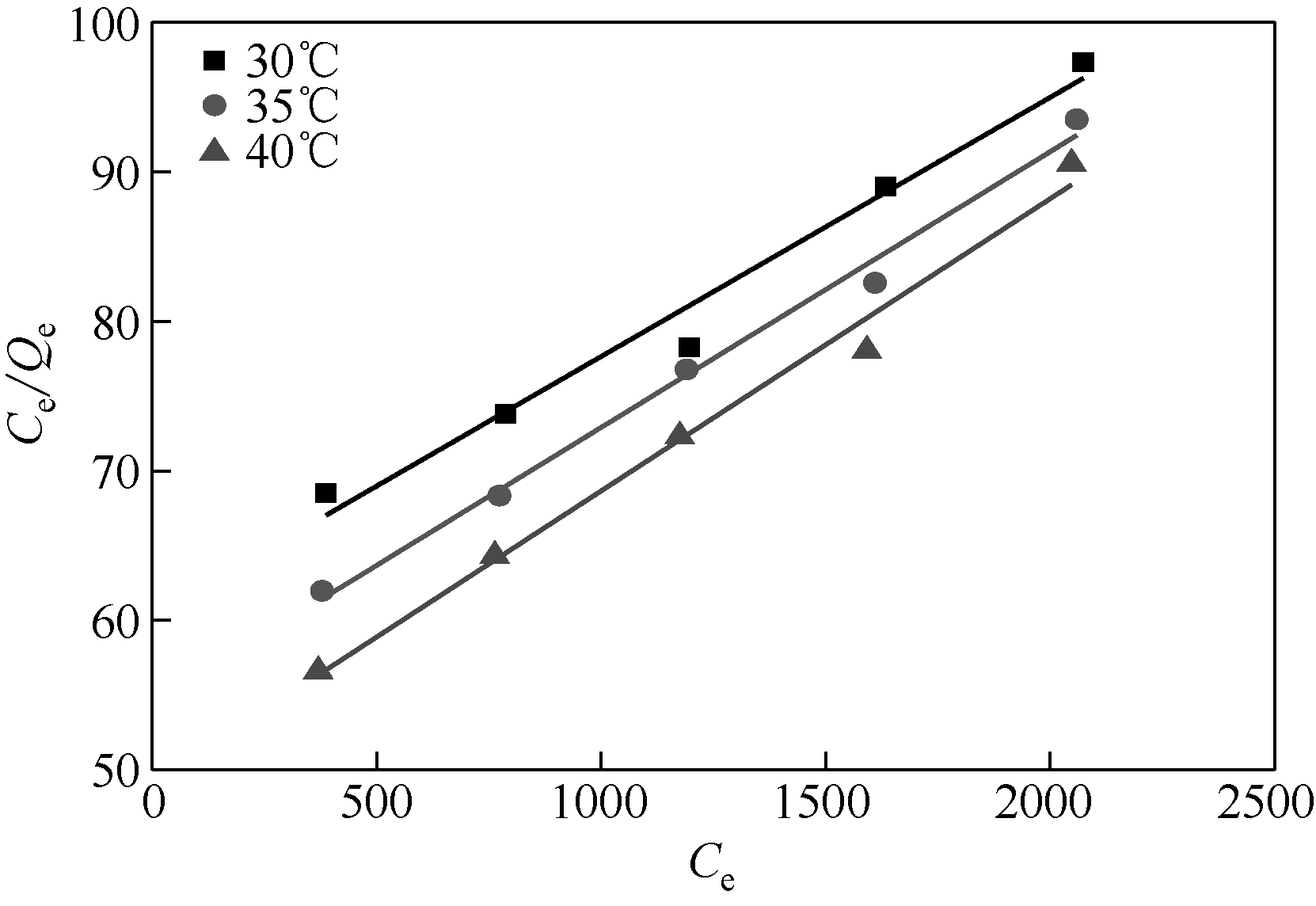
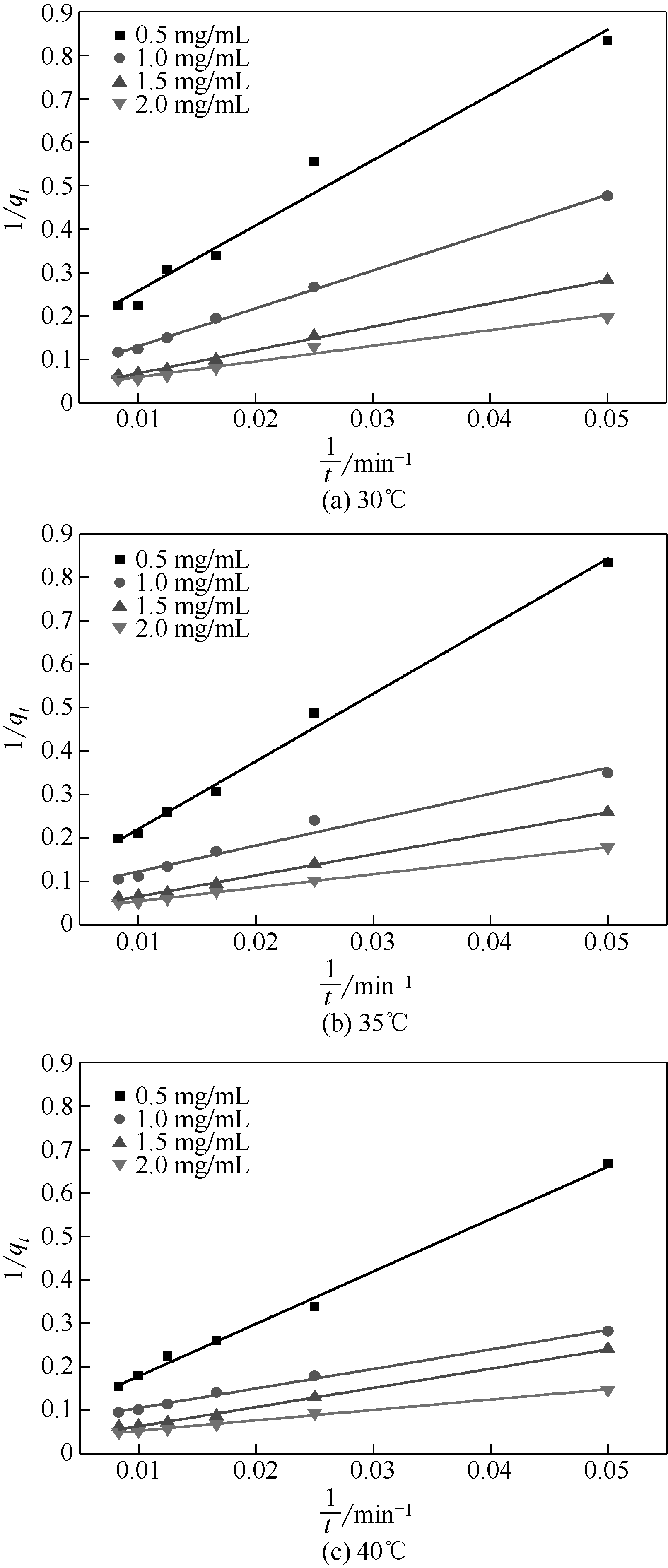
以壳聚糖(chitosan,CTS)、丙烯酸(acrylic acid,AA)和凹凸棒石(attapulgite,ATP)为原料,利用分散聚合法制备壳聚糖接枝聚丙烯酸/凹凸棒石(CTS-g-PAA/ATP)复合吸附剂,以毒死蜱(chlorpyrifos,CPF)为模型农药,研究了复合吸附剂CTS-g-PAA/ATP在不同温度、时间和不同毒死蜱浓度下对毒死蜱的吸附性能,并对实验结果进行吸附热力学与动力学拟合分析。结果表明,CTS-g-PAA/ATP和HATP(酸化凹凸棒石)在24h和30℃下对毒死蜱的吸附量分别为7.42mg/g和18.95mg/g,复合材料的吸附能力提高了180%;拟合结果分析表明,吸附过程遵循Langmuir单分子层等温吸附(R2≥0.97),在30℃、35℃、40℃下理论上最大吸附量分别为57.8mg/g、54.3mg/g、51.2mg/g;并与准二级动力学模型精准拟合(R2≥0.97),化学吸附是吸附过程的主要控制因素。
中图分类号:
引用本文
徐华, 温洪坚, 林建达, 周红军, 陈铧耀, 周新华. 壳聚糖接枝聚丙烯酸/凹凸棒石复合材料对毒死蜱的吸附行为[J]. 化工进展, 2019, 38(07): 3332-3340.
Hua XU, Hongjian WEN, Jianda LIN, Hongjun ZHOU, Huayao CHEN, Xinhua ZHOU. Adsorption performance of CTS-g-PAA/ATP composite on chlorpyrifos[J]. Chemical Industry and Engineering Progress, 2019, 38(07): 3332-3340.
| 热力学模型 | 方程式 | |
|---|---|---|
| Langmuir model | (3) | |
| Freundlich model | (4) | |
| Tempkin model | (5) |
表1 吸附热力学模型方程
| 热力学模型 | 方程式 | |
|---|---|---|
| Langmuir model | (3) | |
| Freundlich model | (4) | |
| Tempkin model | (5) |
| 温度/℃ | Langmuir等温吸附方程 | Freundlich等温吸附方程 | Tempkin等温吸附方程 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Qmax/mg·g-1 | KL/mg·g-1 | R2 | n | KF/mg·g-1·(L·mg-1)1/n | R2 | KT/L·mg-1 | B | bT/kJ·mol-1 | R2 | |
| 30 | 57.8 | 2.86×10-4 | 0.9724 | 1.21 | 4.15×10-2 | 0.9721 | 0.833 | 9.52 | 0.264 | 0.9539 |
| 35 | 54.3 | 3.38×10-4 | 0.9908 | 1.34 | 7.75×10-2 | 0.9777 | 0.829 | 9.28 | 0.276 | 0.9493 |
| 40 | 51.2 | 3.97×10-4 | 0.9863 | 1.37 | 8.99×10-2 | 0.9844 | 0.828 | 9.56 | 0.272 | 0.9532 |
表2 Langmuir、Freundlich和Tempkin等温吸附方程拟合参数
| 温度/℃ | Langmuir等温吸附方程 | Freundlich等温吸附方程 | Tempkin等温吸附方程 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Qmax/mg·g-1 | KL/mg·g-1 | R2 | n | KF/mg·g-1·(L·mg-1)1/n | R2 | KT/L·mg-1 | B | bT/kJ·mol-1 | R2 | |
| 30 | 57.8 | 2.86×10-4 | 0.9724 | 1.21 | 4.15×10-2 | 0.9721 | 0.833 | 9.52 | 0.264 | 0.9539 |
| 35 | 54.3 | 3.38×10-4 | 0.9908 | 1.34 | 7.75×10-2 | 0.9777 | 0.829 | 9.28 | 0.276 | 0.9493 |
| 40 | 51.2 | 3.97×10-4 | 0.9863 | 1.37 | 8.99×10-2 | 0.9844 | 0.828 | 9.56 | 0.272 | 0.9532 |
| 动力学模型 | 方程式 | |
|---|---|---|
| 准一级模型 | (6) | |
| 准二级模型 | (7) | |
| Elovich方程 | (8) | |
| 内扩散模型 | (9) | |
| Bangham扩散模型 | (10) |
表3 吸附动力学模型方程
| 动力学模型 | 方程式 | |
|---|---|---|
| 准一级模型 | (6) | |
| 准二级模型 | (7) | |
| Elovich方程 | (8) | |
| 内扩散模型 | (9) | |
| Bangham扩散模型 | (10) |
| 浓度/mg·mL-1(温度/℃) | 准一级动力学 | 准二级动力学 | |||||
|---|---|---|---|---|---|---|---|
| qe,cal | qe,exp | k1 | R2 | qe | k2 | R2 | |
| 0.5 (30) | 5.60 | 6.03 | -0.0141 | 0.9417 | 9.30 | 7.70×10-4 | 0.9868 |
| 1.0 (30) | 9.32 | 11.32 | -0.0215 | 0.9600 | 23.32 | 2.11×10-4 | 0.9983 |
| 1.5 (30) | 15.95 | 26.48 | -0.0328 | 0.8836 | 69.59 | 3.85×10-5 | 0.9968 |
| 2.0 (30) | 18.95 | 27.67 | -0.0304 | 0.9282 | 42.28 | 1.56×10-4 | 0.9732 |
| 0.5 (35) | 6.50 | 6.92 | -0.0129 | 0.9857 | 15.34 | 2.73×10-4 | 0.9932 |
| 1.0 (35) | 10.06 | 13.01 | -0.0239 | 0.9290 | 15.77 | 6.74×10-4 | 0.9763 |
| 1.5 (35) | 16.43 | 24.05 | -0.0296 | 0.9305 | 58.48 | 6.03×10-5 | 0.9972 |
| 2.0 (35) | 19.80 | 31.60 | -0.0345 | 0.9085 | 42.37 | 1.79×10-4 | 0.9984 |
| 0.5 (40) | 6.80 | 9.11 | -0.0229 | 0.8413 | 17.39 | 2.74×10-4 | 0.9949 |
| 1.0 (40) | 10.95 | 13.52 | -0.0255 | 0.9515 | 16.57 | 8.11×10-4 | 0.9948 |
| 1.5 (40) | 16.70 | 23.89 | -0.0304 | 0.9475 | 52.91 | 8.09×10-5 | 0.9960 |
| 2.0 (40) | 21.00 | 27.18 | -0.0284 | 0.9513 | 34.61 | 3.48×10-4 | 0.9941 |
表4 准一级动力学和准二级动力学方程拟合参数
| 浓度/mg·mL-1(温度/℃) | 准一级动力学 | 准二级动力学 | |||||
|---|---|---|---|---|---|---|---|
| qe,cal | qe,exp | k1 | R2 | qe | k2 | R2 | |
| 0.5 (30) | 5.60 | 6.03 | -0.0141 | 0.9417 | 9.30 | 7.70×10-4 | 0.9868 |
| 1.0 (30) | 9.32 | 11.32 | -0.0215 | 0.9600 | 23.32 | 2.11×10-4 | 0.9983 |
| 1.5 (30) | 15.95 | 26.48 | -0.0328 | 0.8836 | 69.59 | 3.85×10-5 | 0.9968 |
| 2.0 (30) | 18.95 | 27.67 | -0.0304 | 0.9282 | 42.28 | 1.56×10-4 | 0.9732 |
| 0.5 (35) | 6.50 | 6.92 | -0.0129 | 0.9857 | 15.34 | 2.73×10-4 | 0.9932 |
| 1.0 (35) | 10.06 | 13.01 | -0.0239 | 0.9290 | 15.77 | 6.74×10-4 | 0.9763 |
| 1.5 (35) | 16.43 | 24.05 | -0.0296 | 0.9305 | 58.48 | 6.03×10-5 | 0.9972 |
| 2.0 (35) | 19.80 | 31.60 | -0.0345 | 0.9085 | 42.37 | 1.79×10-4 | 0.9984 |
| 0.5 (40) | 6.80 | 9.11 | -0.0229 | 0.8413 | 17.39 | 2.74×10-4 | 0.9949 |
| 1.0 (40) | 10.95 | 13.52 | -0.0255 | 0.9515 | 16.57 | 8.11×10-4 | 0.9948 |
| 1.5 (40) | 16.70 | 23.89 | -0.0304 | 0.9475 | 52.91 | 8.09×10-5 | 0.9960 |
| 2.0 (40) | 21.00 | 27.18 | -0.0284 | 0.9513 | 34.61 | 3.48×10-4 | 0.9941 |
| 浓度/mg·mL-1(温度/℃) | Elovich动力学 | 颗粒内扩散方程 | Bangham动力学 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| a | b | R2 | kid | C | R2 | k3 | α | R2 | |
| 0.5 (30) | 0.0402 | 0.513 | 0.9166 | 0.433 | -0.407 | 0.9360 | 3.17×10-3 | 0.822 | 0.9708 |
| 1.0 (30) | 0.0224 | 0.272 | 0.9603 | 0.830 | -0.700 | 0.9600 | 3.27×10-3 | 0.800 | 0.9892 |
| 1.5 (30) | 0.0102 | 0.139 | 0.9696 | 1.545 | -1.605 | 0.9424 | 2.85×10-3 | 0.919 | 0.9878 |
| 2.0 (30) | 0.0099 | 0.122 | 0.9487 | 1.825 | -1.591 | 0.9479 | 3.26×10-3 | 0.824 | 0.9743 |
| 0.5 (35) | 0.0332 | 0.446 | 0.9598 | 0.487 | -0.494 | 0.9459 | 3.00×10-3 | 0.880 | 0.9917 |
| 1.0 (35) | 0.0224 | 0.256 | 0.9391 | 0.903 | -0.696 | 0.9641 | 3.75×10-3 | 0.750 | 0.9869 |
| 1.5 (35) | 0.0109 | 0.139 | 0.9773 | 1.577 | -1.444 | 0.9522 | 3.14×10-3 | 0.879 | 0.9847 |
| 2.0 (35) | 0.0110 | 0.122 | 0.9766 | 1.908 | -1.296 | 0.9710 | 3.76×10-3 | 0.767 | 0.9897 |
| 0.5 (40) | 0.0296 | 0.375 | 0.9493 | 0.593 | -0.550 | 0.9530 | 3.48×10-3 | 0.854 | 0.9901 |
| 1.0 (40) | 0.0278 | 0.251 | 0.9797 | 0.994 | -0.401 | 0.9900 | 4.75×10-3 | 0.663 | 0.9967 |
| 1.5 (40) | 0.0116 | 0.139 | 0.9835 | 1.614 | -1.277 | 0.9594 | 3.42×10-3 | 0.844 | 0.9783 |
| 2.0 (40) | 0.0138 | 0.126 | 0.9855 | 1.960 | -0.797 | 0.9859 | 4.59×10-3 | 0.678 | 0.9886 |
表5 Elovich动力学模型、颗粒内扩散方程和Bangham动力学模型拟合参数
| 浓度/mg·mL-1(温度/℃) | Elovich动力学 | 颗粒内扩散方程 | Bangham动力学 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| a | b | R2 | kid | C | R2 | k3 | α | R2 | |
| 0.5 (30) | 0.0402 | 0.513 | 0.9166 | 0.433 | -0.407 | 0.9360 | 3.17×10-3 | 0.822 | 0.9708 |
| 1.0 (30) | 0.0224 | 0.272 | 0.9603 | 0.830 | -0.700 | 0.9600 | 3.27×10-3 | 0.800 | 0.9892 |
| 1.5 (30) | 0.0102 | 0.139 | 0.9696 | 1.545 | -1.605 | 0.9424 | 2.85×10-3 | 0.919 | 0.9878 |
| 2.0 (30) | 0.0099 | 0.122 | 0.9487 | 1.825 | -1.591 | 0.9479 | 3.26×10-3 | 0.824 | 0.9743 |
| 0.5 (35) | 0.0332 | 0.446 | 0.9598 | 0.487 | -0.494 | 0.9459 | 3.00×10-3 | 0.880 | 0.9917 |
| 1.0 (35) | 0.0224 | 0.256 | 0.9391 | 0.903 | -0.696 | 0.9641 | 3.75×10-3 | 0.750 | 0.9869 |
| 1.5 (35) | 0.0109 | 0.139 | 0.9773 | 1.577 | -1.444 | 0.9522 | 3.14×10-3 | 0.879 | 0.9847 |
| 2.0 (35) | 0.0110 | 0.122 | 0.9766 | 1.908 | -1.296 | 0.9710 | 3.76×10-3 | 0.767 | 0.9897 |
| 0.5 (40) | 0.0296 | 0.375 | 0.9493 | 0.593 | -0.550 | 0.9530 | 3.48×10-3 | 0.854 | 0.9901 |
| 1.0 (40) | 0.0278 | 0.251 | 0.9797 | 0.994 | -0.401 | 0.9900 | 4.75×10-3 | 0.663 | 0.9967 |
| 1.5 (40) | 0.0116 | 0.139 | 0.9835 | 1.614 | -1.277 | 0.9594 | 3.42×10-3 | 0.844 | 0.9783 |
| 2.0 (40) | 0.0138 | 0.126 | 0.9855 | 1.960 | -0.797 | 0.9859 | 4.59×10-3 | 0.678 | 0.9886 |
| 1 | 季静, 肖斌, 李杨, 等. 两种不同剂型毒死蜱对四种环境生物的毒性评价[J]. 农业环境科学学报, 2010, 29(9):1681-1686. |
| JIJing, XIAOBin, LIYang, et al. Toxicity assessment of two different formulations of chlorpyrifos to four environmental organisms[J].Journal of Agro-Environment Science, 2010,29(9): 1681-1686. | |
| 2 | 单正军, 陈祖义. 农药对陆生环境生物的污染影响及污染控制技术 [J]. 农药科学与管理, 2007, 28(11):18-26. |
| SHANZhengjun, CHENZuyi. Pollution effects of pesticides on terrestrial environmental organisms and pollution controlled techniques [J]. Pesticide Science and Administration, 2007, 28(11):18-26. | |
| 3 | 赵华, 李康, 吴声敢, 等. 毒死蜱对环境生物的毒性与安全性评价[J]. 浙江农业学报, 2004, 16(5): 292-298. |
| ZHAOHua, LIKang, WUShenggan,et al. Evaluation on toxicity and safety of chlorpyrifos to environmental organisms[J]. Acta Agriculturae Zhejiangensis, 2004, 16(5):292-298. | |
| 4 | 阎会平. 山西省芹菜农残检测结果分析及治理对策[J]. 农药科学与管理, 2014, 35(9):37-39. |
| YANHuiping. Results analysis and countermeasures of celery pesticide residue testing in Shanxi province[J]. Pesticide Science and Administration, 2014,35(9): 37-52. | |
| 5 | 王绪芬, 王振华, 冯爱丽, 等. 绿叶菜类蔬菜农药残留分布与原因分析[J]. 北方园艺, 2011(15):76-77. |
| 6 | WANGXufen, WANGZhenhua, FENGAili, et al. Analysis of the causeand distributing of greenery vegetable peticide remnant[J]. Northern Horticulture, 2011(15):77-78. |
| 7 | ATABILAA , PHUNGD T , HOGARHJ N , et al. Dermal exposure of applicators to chlorpyrifos on rice farms in Ghana[J]. Chemosphere, 2017, 178:350-358. |
| 8 | 王爱勤, 王文波, 郑易安, 等. 凹凸棒石棒晶束解离及其纳米功能复合材料[M]. 北京:科学出版社, 2014:1-4. |
| WANGAiqin, WANGWenbo, ZHENGYi’an, et al. Attapulgite rod crystal beam dissociation and its nano-functional composite [M]. Beijing: Science Press, 2014:1-4. | |
| 9 | RAFATULLAHM, SULAIMANO, HASHIMR, et al. Adsorption of methylene blue on low-cost adsorbents: a review[J]. J. Hazard Mater., 2010,177(1/2/3):70-80. |
| 10 | LIUY, KANGY R, ZHANGG L, et al. Attapulgite/bentonite interactions for methylene blue adsroption characteristics from aqueous solutio[J]. Chem. Eng. J., 2014, 237(1):403-410. |
| 11 | XUJ X, WANGW B, WANGA Q. Influence of anions on the electrokinetic and colloidal properties of palygorskite clay via high-pressure homogenization[J]. J. Chem. Eng. Date, 2013, 58(3):764-772. |
| 12 | HEX, YANGH M. Au nanoparticles assembled on palygorskite: enhanced catalytic property and Au-Au2O3 coexistence[J]. J. Mol. Catal A: Chem., 2013,379(15):219-224. |
| 13 | 徐继红, 许少薇, 李慧玲, 等. LS-g-PAA/AMPS/APT树脂对亚甲基蓝吸附性能[J]. 精细化工, 2016, 33(5):497-503. |
| XUJihong, XUShaowei, LIHuiling, et al. Adsorption performance of methylene blue dye by LS-g-PAA / AMPS / APT resin[J]. Fine Chemicals, 2016, 33(5): 497-503. | |
| 14 | ZHENGY, ZHANGJ, WANGA Q. Fast removal of ammonium nitrogen from aqueous solution using chitosan-g-ploy(acrylic acid)/attapulgite composite[J]. Chem. Eng. J., 2009, 155(1/2):215-222. |
| 15 | WANGL, ZHANGJ, WANGA. Fast removal of methylene blue from aqueous solution by adsorption onto chitosan-g-poly(acrylic acid)/attapulgite composite[J]. Desalination, 2011, 266(1/2/3):33-39. |
| 16 | WANGX, WANGA. Adsorption characteristics of chitosan-g-poly(acrylic acid)/attapulgite hydrogel composite for Hg(Ⅱ) ions from aqueous solution[J]. Sep. Sci. Technol., 2010, 45(14): 2086-2094. |
| 17 | WANGL, ZHANGJ, WANGA. East removal of metlhyene blue from aqueous solution by adsorption onto chitosan-g-poly(acrylic acid)/attapulgite composite[J]. Desalination, 2011, 266(1-3):33-39 |
| 18 | 范荣玉, 郑细鸣. 铅(Ⅱ)离子印迹复合膜对重金属离子的吸附热力学与吸附动力学[J]. 化工学报, 2013, 64(5):1651-1659. |
| FANRongyu, ZHENGXiming. Thermodynamics and dynamics for adsorption of heavy metal ions on Pb(Ⅱ) ion-imprinted composite membrane[J]. CIESC Journal, 2013, 64(5):1651-1659. | |
| 19 | FREUNDLICHH, HELLERW. The adsorption of cis- and trans-azobenzene[J]. J. Am. Chem. Soc., 1939, 61: 2228-2230. |
| 20 | MANEV S, MALLI D, SRIVASTAVAV C. Kinetic and equilibrium isotherm studies for the adsorptive removal of Brilliant Green dye from aqueous solution by rice husk ash[J]. J. Environ. Mange., 2007, 84(4):390-400. |
| 21 | KIRANB, KAUSHIKA. Chromium binding capacity of Lyngbya putealis exopolysaccharides[J]. Biochem. Eng. J., 2008, 38(1): 47-54. |
| 22 | SKODRASG, DIAMANTOPOULOUI, PANTOLEONTOSG, et al. Kinetic studies of elemental mercury adsorption in activated carbon fixed bed reactor[J] . Journal of Hazardous Materials, 2008, 158(1): 1-13. |
| 23 | WANGS B, LIH T. Dye adsorption on unburned carbon: kinetics and equilibrium[J]. Journal of Hazardous Materials, 2005, 16(1/2/3): 71-77. |
| 24 | OZACARM, SENGILI A. A kinetic study of metal complex dye sorption onto pine sawdust[J]. Process Biochem., 2005, 40(2): 565-572. |
| 25 | SUMANJITK, SEEMAR, MAHAJANR K, et al. Synthesis and adsorption properties of mesoporous material for theremoval of dye safranin: kinetics, equilibrium, and thermodynamics[J]. Journal of Industrial and Engineering Chemistry, 2015, 22:19-27. |
| 26 | 周强, 段钰锋, 冒咏秋, 等. 活性炭汞吸附动力学及吸附机制研究[J]. 中国电机工程学报, 2013, 33(29):10-17. |
| ZHOUQiang, DUANYufeng, MAOYongqiu,et al. Kinetics and mechanism of activated carbon adsorption for mercury removal[J]. Proceedings of the CSEE, 2013, 33(29): 10-17. |
| [1] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [2] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [3] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [4] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [5] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [6] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [7] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [8] | 王少凡, 周颖, 郝康安, 黄安荣, 张如菊, 吴翀, 左晓玲. 具有pH响应性的自愈合蓝光水凝胶[J]. 化工进展, 2023, 42(9): 4837-4846. |
| [9] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| [10] | 张振, 李丹, 陈辰, 吴菁岚, 应汉杰, 乔浩. 吸附树脂对唾液酸的分离纯化[J]. 化工进展, 2023, 42(8): 4153-4158. |
| [11] | 姜晶, 陈霄宇, 张瑞妍, 盛光遥. 载锰生物炭制备及其在环境修复中应用研究进展[J]. 化工进展, 2023, 42(8): 4385-4397. |
| [12] | 于静文, 宋璐娜, 刘砚超, 吕瑞东, 武蒙蒙, 冯宇, 李忠, 米杰. 一种吲哚基超交联聚合物In-HCP对水中碘的吸附作用[J]. 化工进展, 2023, 42(7): 3674-3683. |
| [13] | 李艳玲, 卓振, 池亮, 陈曦, 孙堂磊, 刘鹏, 雷廷宙. 氮掺杂生物炭的制备与应用研究进展[J]. 化工进展, 2023, 42(7): 3720-3735. |
| [14] | 白亚迪, 邓帅, 赵睿恺, 赵力, 杨英霞. 变温吸附碳捕集机组标准化测试方案探讨及性能实验[J]. 化工进展, 2023, 42(7): 3834-3846. |
| [15] | 张雪伟, 黄亚继, 许月阳, 程好强, 朱志成, 李金壘, 丁雪宇, 王圣, 张荣初. 碱性吸附剂对燃煤烟气中SO3的吸附特性[J]. 化工进展, 2023, 42(7): 3855-3864. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||