化工进展 ›› 2019, Vol. 38 ›› Issue (07): 3412-3424.DOI: 10.16085/j.issn.1000-6613.2018-1902
纳米零价铁的制备、改性及对废水中重金属和有机污染物的去除
- 1. 陕西师范大学地理科学与旅游学院,陕西 西安710119
2. 地理学国家级实验教学示范中心(陕西师范大学),陕西 西安710119
-
收稿日期:2018-09-20出版日期:2019-07-05发布日期:2019-07-05 -
通讯作者:李敏睿 -
作者简介:杨晓丹(1995—),女,硕士研究生,研究方向为纳米零价铁。E-mail:<email>yangxiaodan@snnu.edu.cn</email>。 -
基金资助:国家自然科学基金(51508317);中央高校基本科研业务费项目(GK201703044);中国博士后科学基金(2016M602762);陕西省博士后科研项目(2017BSHTDZZ09)
Preparation, modification of nanoscale zero valent iron and its application for the removal of heavy metals andorganic pollutants from wastewater
Xiaodan YANG1,2( ),Yuru WANG1,2,Minrui LI1,2(
),Yuru WANG1,2,Minrui LI1,2( )
)
- 1. School of Geography and Tourism, Shaanxi Normal University, Xi’an 710119, Shaanxi, China
2. National Demonstration Center for Experimental Geography Education, Shaanxi Normal University, Xi’an 710119, Shaanxi, China
-
Received:2018-09-20Online:2019-07-05Published:2019-07-05 -
Contact:Minrui LI
摘要:
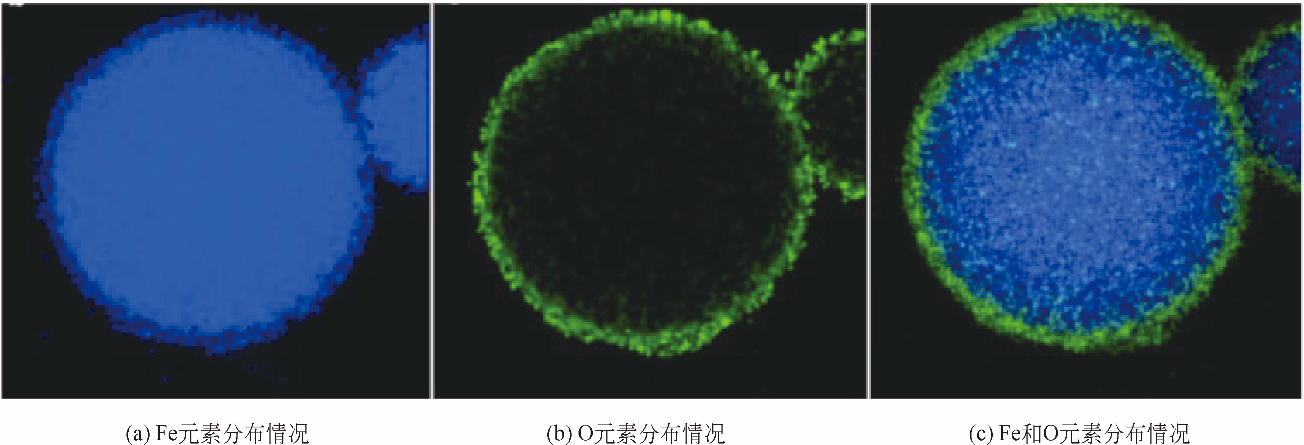
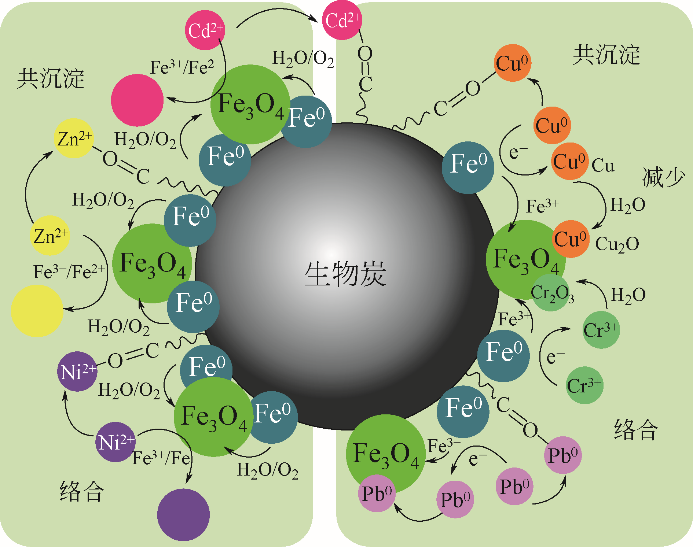
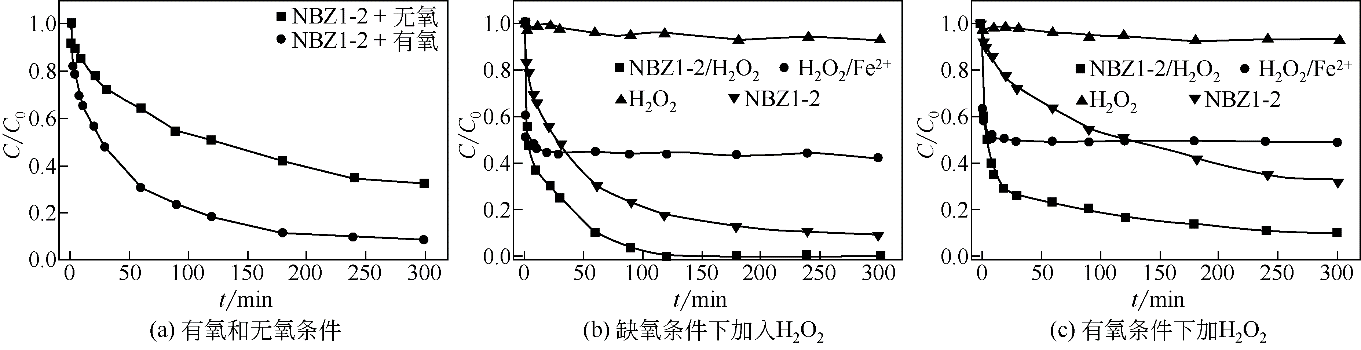
纳米零价铁(nZVI)结合了零价铁还原性强和纳米材料比表面积大的特点,能够高效去除水体中的重金属和有机污染物,是当前环境科学领域研究的热点之一。研究表明单一nZVI颗粒存在易团聚及表面易被氧化等问题,影响nZVI颗粒形态和对污染物去除效果,限制了其在环境修复中的应用。针对目前的研究现状,本文分析并总结了以下内容:①nZVI常用的制备方法;②提高nZVI活性与稳定性的改性方法,如合成时添加表面活性剂和负载材料;③nZVI去除废水中Cr、Cd、Cu和As等重金属和硝基苯、氯代芳烃、氯代脂肪烃等有机污染物的主要机理及影响因素;④应用于自然环境中的nZVI可能对环境产生的毒理学效应和在环境修复过程中存在的潜在风险及其评估;⑤对nZVI今后的研究重点和方向进行分析和展望。
中图分类号:
引用本文
杨晓丹, 王玉如, 李敏睿. 纳米零价铁的制备、改性及对废水中重金属和有机污染物的去除[J]. 化工进展, 2019, 38(07): 3412-3424.
Xiaodan YANG, Yuru WANG, Minrui LI. Preparation, modification of nanoscale zero valent iron and its application for the removal of heavy metals andorganic pollutants from wastewater[J]. Chemical Industry and Engineering Progress, 2019, 38(07): 3412-3424.
| 方法 | 步骤 | 文献 |
|---|---|---|
| 气相法 | ||
| 等离子体法 | 在真空容器中,利用高温热源产生等离子体,加热熔化金属铁,高温下铁迅速蒸发,铁蒸气经循环泵输送到集粉器中冷凝沉积得到纳米铁粉 | [ |
| 爆炸丝法 | 置于惰性的反应器中金属丝在高压下加热熔断,熔断处高压放电使得金属变成蒸气状态形成纳米粒子,当介质温度降低时粒子迅速淬火形成分散纳米粒子 | [ |
| 溅射法 | 利用两块金属板作为两极,内部充氩气,铁金属作为阴极金属板,2kV电压释放出氩离子冲击溅射铁板,铁板表面蒸发出超微粒子 | [ |
| 液相法 | ||
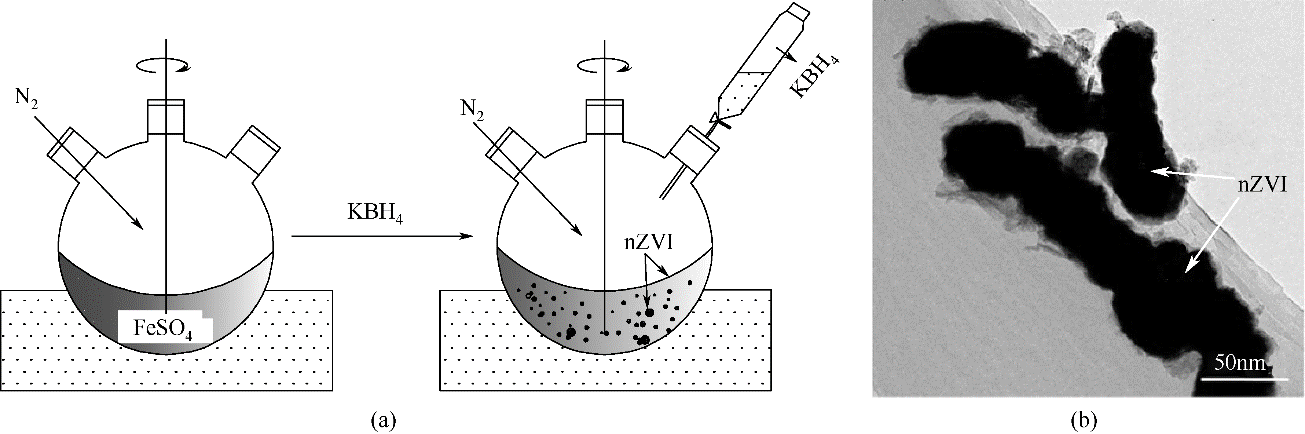
| 化学还原法 | 通过KBH4、NaBH4等强还原剂还原溶液中Fe2+、Fe3+为Fe0 | [ |
| 微乳液法 | 化学还原法基础上向反应相中加入表面活性剂等改性材料混合生成包覆型nZVI | [ |
| 电化学法 | 电解液中外加电压沉积纳米金属颗粒 | [ |
| 固相法 | ||
| 冷冻干燥法 | 保存于冷冻剂中的溶液喷雾,在真空中进行低温低压干燥,根据熔点不同升华冷冻剂便可得到金属纳米粒子 | [ |
| 深度塑性变法 | 外加高压使大块金属发生严重塑性变形,合成纳米金属 | [ |
| 高能球磨法 | 指在球磨机的机械驱动力作用下碾磨铁粉使得铁粉粒径不断减小形成纳米铁颗粒 | [ |
表1 制备纳米金属颗粒的常见方法与步骤
| 方法 | 步骤 | 文献 |
|---|---|---|
| 气相法 | ||
| 等离子体法 | 在真空容器中,利用高温热源产生等离子体,加热熔化金属铁,高温下铁迅速蒸发,铁蒸气经循环泵输送到集粉器中冷凝沉积得到纳米铁粉 | [ |
| 爆炸丝法 | 置于惰性的反应器中金属丝在高压下加热熔断,熔断处高压放电使得金属变成蒸气状态形成纳米粒子,当介质温度降低时粒子迅速淬火形成分散纳米粒子 | [ |
| 溅射法 | 利用两块金属板作为两极,内部充氩气,铁金属作为阴极金属板,2kV电压释放出氩离子冲击溅射铁板,铁板表面蒸发出超微粒子 | [ |
| 液相法 | ||
| 化学还原法 | 通过KBH4、NaBH4等强还原剂还原溶液中Fe2+、Fe3+为Fe0 | [ |
| 微乳液法 | 化学还原法基础上向反应相中加入表面活性剂等改性材料混合生成包覆型nZVI | [ |
| 电化学法 | 电解液中外加电压沉积纳米金属颗粒 | [ |
| 固相法 | ||
| 冷冻干燥法 | 保存于冷冻剂中的溶液喷雾,在真空中进行低温低压干燥,根据熔点不同升华冷冻剂便可得到金属纳米粒子 | [ |
| 深度塑性变法 | 外加高压使大块金属发生严重塑性变形,合成纳米金属 | [ |
| 高能球磨法 | 指在球磨机的机械驱动力作用下碾磨铁粉使得铁粉粒径不断减小形成纳米铁颗粒 | [ |
| 改性 | 粒径范围/nm | 比表面积/m2·g-1 | pH | 温度/℃ | 吸附容量/mg·g-1 | 污染物种类 | 去除效率/% | 文献 |
|---|---|---|---|---|---|---|---|---|
| — | 10~150 | 48.7 | — | 145.6 | 410 | Yb3+ | 99.9 | [ |
| 61 | La3+ | 99.9 | ||||||
| 20~200 | 26.3 | — | 147 | 769.2 | Cd2+ | — | [ | |
| 9.96 | 182.97 | 3 | 25 | 150 | Cr6+ | 73 | [ | |
| 20~80 | — | 4 | 30 | — | 甲基橙 | 100 | [ | |
| 二氧化硅 | 91 | 71 | — | 20 | — | 1,1,1-TCA | 70 | [ |
| 浮石 | 30.6 | 32.2 | 8.13 | 25 | 332.4 | Hg2+ | 99.1 | [ |
| 3.11 | 306.6 | Cr6+ | 96 | |||||
| 30.6 | 39.94 | 3.1 | 20 | 35.7 | As5+ | 99.99 | [ | |
| 海泡石 | 20~60 | 141.42 | 4~6 | 28 | 43.86 | Cr6+ | 98.5 | [ |
| 4~6 | 44.05 | Pb2+ | 99.9 | |||||
| 活性炭 | 30~70 | 32.9 | 3.0 | — | — | Cr6+ | 100 | [ |
| 有机膨润土 | 8 | 33.5 | 3.0 | 25 | — | 硝基苯 | 100 | [ |
| 硫化物 | 200 | — | 4~9 | 22 | 120 | Cd2+ | 97.85 | [ |
| 生物炭 | 2.14 | 84.82 | 3 | 25 | 84.4 | Cr6+ | 90 | [ |
| — | — | 6 | 25 | 363.63 | 环丙沙星 | 94 | [ | |
| 污泥活性炭 | 8.31 | 98.67 | — | — | — | Cr6+ | 90 | [ |
| — | — | — | Pb2+ | 82 | ||||
| 生物炭/HCl | — | 35 | 5 | 25±2 | — | Cr6+ | 35.29 | [ |
| 生物炭铜藻基 | 50~150 | — | 2 | 30 | — | Cr6+ | 100 | [ |
| 石墨烯 | 20~95 | 54.16 | 3 | 25 | 85 | 罗丹明B | 90 | [ |
| 石墨烯/过硫酸盐 | 300.15 | 19.05 | — | 20 | — | 三氯乙烯 | 99.7 | [ |
| Fe–Pd | 30~50 | 51.4 | 7.0 | 25 | — | 四氯化碳 | 81 | [ |
| Fe–Pd | 100~250 | 82.42 | 5.5 | 40 | — | 四氯化碳 | 94 | [ |
| Fe–Ni | 10~70 | 106.65 | — | 330 | — | 多氯联苯 | 89 | [ |
| Fe–Ni | 30~60 | — | 2 | 25 | — | Cr6+ | 96.33 | [ |
| 氢氧化物 | 10~100 | 42.2 | 3.0 | — | 60 | Cr6+ | 93.2 | [ |
| 乳化油 | 20~120 | 50 | 4 | 25 | — | 硝基苯 | 100 | [ |
| 聚乙烯吡咯烷酮–正硅酸乙酯 | 20~100 | 25~30 | 4.5 | 25 | 170 | Pb2+ | 99.9 | [ |
| 4.8 | 270 | Cu2+ | ||||||
| 5.0 | 110 | Cd2+ | ||||||
| 6.5 | 130 | 三氯乙烯 | ||||||
| 聚丙烯酸 | — | 43.67 | 9.72 | 20 | — | 亚甲基蓝 | 98.84 | [ |
| 草酸 | 80 | — | 3.18 | — | — | 五氯苯酚 | 89 | [ |
| 蒙脱石/海藻酸钠 | 3~4mm | 11.332 | 4.0 | 25 | 62.84 | Cu2+ | 92.11 | [ |
表2 近年来nZVI的改性及对污染物的去除效率
| 改性 | 粒径范围/nm | 比表面积/m2·g-1 | pH | 温度/℃ | 吸附容量/mg·g-1 | 污染物种类 | 去除效率/% | 文献 |
|---|---|---|---|---|---|---|---|---|
| — | 10~150 | 48.7 | — | 145.6 | 410 | Yb3+ | 99.9 | [ |
| 61 | La3+ | 99.9 | ||||||
| 20~200 | 26.3 | — | 147 | 769.2 | Cd2+ | — | [ | |
| 9.96 | 182.97 | 3 | 25 | 150 | Cr6+ | 73 | [ | |
| 20~80 | — | 4 | 30 | — | 甲基橙 | 100 | [ | |
| 二氧化硅 | 91 | 71 | — | 20 | — | 1,1,1-TCA | 70 | [ |
| 浮石 | 30.6 | 32.2 | 8.13 | 25 | 332.4 | Hg2+ | 99.1 | [ |
| 3.11 | 306.6 | Cr6+ | 96 | |||||
| 30.6 | 39.94 | 3.1 | 20 | 35.7 | As5+ | 99.99 | [ | |
| 海泡石 | 20~60 | 141.42 | 4~6 | 28 | 43.86 | Cr6+ | 98.5 | [ |
| 4~6 | 44.05 | Pb2+ | 99.9 | |||||
| 活性炭 | 30~70 | 32.9 | 3.0 | — | — | Cr6+ | 100 | [ |
| 有机膨润土 | 8 | 33.5 | 3.0 | 25 | — | 硝基苯 | 100 | [ |
| 硫化物 | 200 | — | 4~9 | 22 | 120 | Cd2+ | 97.85 | [ |
| 生物炭 | 2.14 | 84.82 | 3 | 25 | 84.4 | Cr6+ | 90 | [ |
| — | — | 6 | 25 | 363.63 | 环丙沙星 | 94 | [ | |
| 污泥活性炭 | 8.31 | 98.67 | — | — | — | Cr6+ | 90 | [ |
| — | — | — | Pb2+ | 82 | ||||
| 生物炭/HCl | — | 35 | 5 | 25±2 | — | Cr6+ | 35.29 | [ |
| 生物炭铜藻基 | 50~150 | — | 2 | 30 | — | Cr6+ | 100 | [ |
| 石墨烯 | 20~95 | 54.16 | 3 | 25 | 85 | 罗丹明B | 90 | [ |
| 石墨烯/过硫酸盐 | 300.15 | 19.05 | — | 20 | — | 三氯乙烯 | 99.7 | [ |
| Fe–Pd | 30~50 | 51.4 | 7.0 | 25 | — | 四氯化碳 | 81 | [ |
| Fe–Pd | 100~250 | 82.42 | 5.5 | 40 | — | 四氯化碳 | 94 | [ |
| Fe–Ni | 10~70 | 106.65 | — | 330 | — | 多氯联苯 | 89 | [ |
| Fe–Ni | 30~60 | — | 2 | 25 | — | Cr6+ | 96.33 | [ |
| 氢氧化物 | 10~100 | 42.2 | 3.0 | — | 60 | Cr6+ | 93.2 | [ |
| 乳化油 | 20~120 | 50 | 4 | 25 | — | 硝基苯 | 100 | [ |
| 聚乙烯吡咯烷酮–正硅酸乙酯 | 20~100 | 25~30 | 4.5 | 25 | 170 | Pb2+ | 99.9 | [ |
| 4.8 | 270 | Cu2+ | ||||||
| 5.0 | 110 | Cd2+ | ||||||
| 6.5 | 130 | 三氯乙烯 | ||||||
| 聚丙烯酸 | — | 43.67 | 9.72 | 20 | — | 亚甲基蓝 | 98.84 | [ |
| 草酸 | 80 | — | 3.18 | — | — | 五氯苯酚 | 89 | [ |
| 蒙脱石/海藻酸钠 | 3~4mm | 11.332 | 4.0 | 25 | 62.84 | Cu2+ | 92.11 | [ |
| 金属 | 来源 | 危害 | 文献 |
|---|---|---|---|
| Cu | 冶炼、化工、石油开采以及电子产业等 | Cu作为动植物生长的必需微量元素,缺Cu会引起贫血、腹泻等症状;过量的Cu通过食物链毒害动植物正常的生长发育 | [ |
| Cd | 蓄电池、印染、电镀等 | Cd易被植物富集,易造成植物在生长过程中植株短小、光合作用降低,通过食物链的富集放大作用,Cd在动物体尤其是人体的肝、肾等器官组织累积,造成内分泌失调、骨质疏松、引发心血管疾病和细胞癌变 | [ |
| Cr | 制革、电镀、冶金等 | 人体长期暴露于含铬环境中,易引起肝硬化、肺气肿、支气管扩张以及癌症等 | [ |
| Ni | 有色金属、采矿、涂料、电镀等 | 过量Ni会对生物生长发育产生毒害,影响植物各种酶系统、光合作用和呼吸作用的正常发育,影响动物DNA损伤,细胞坏死,引起组织病变及癌变 | [ |
| Pb | 蓄电池、印染、电镀等 | Pb被生物吸收后可以在人体和动植物体内蓄积,其主要毒性效应表现在导致神经失调影响,人体智力发育、肝肾和脑损伤 | [ |
| As | 农药生产、化学制药、采矿等 | As元素本身的毒性较低,而As的化合物普遍具有剧毒,砷化合物会对皮肤、食道和呼吸道等造成损伤,导致癌变。据报道,摄入As2O3剂量为70~180 mg·L-1便会使人致死 | [ |
表3 常见重金属来源及危害
| 金属 | 来源 | 危害 | 文献 |
|---|---|---|---|
| Cu | 冶炼、化工、石油开采以及电子产业等 | Cu作为动植物生长的必需微量元素,缺Cu会引起贫血、腹泻等症状;过量的Cu通过食物链毒害动植物正常的生长发育 | [ |
| Cd | 蓄电池、印染、电镀等 | Cd易被植物富集,易造成植物在生长过程中植株短小、光合作用降低,通过食物链的富集放大作用,Cd在动物体尤其是人体的肝、肾等器官组织累积,造成内分泌失调、骨质疏松、引发心血管疾病和细胞癌变 | [ |
| Cr | 制革、电镀、冶金等 | 人体长期暴露于含铬环境中,易引起肝硬化、肺气肿、支气管扩张以及癌症等 | [ |
| Ni | 有色金属、采矿、涂料、电镀等 | 过量Ni会对生物生长发育产生毒害,影响植物各种酶系统、光合作用和呼吸作用的正常发育,影响动物DNA损伤,细胞坏死,引起组织病变及癌变 | [ |
| Pb | 蓄电池、印染、电镀等 | Pb被生物吸收后可以在人体和动植物体内蓄积,其主要毒性效应表现在导致神经失调影响,人体智力发育、肝肾和脑损伤 | [ |
| As | 农药生产、化学制药、采矿等 | As元素本身的毒性较低,而As的化合物普遍具有剧毒,砷化合物会对皮肤、食道和呼吸道等造成损伤,导致癌变。据报道,摄入As2O3剂量为70~180 mg·L-1便会使人致死 | [ |
| 电极 | 电极反应 | 标准电极电位E ?(φ)/V |
|---|---|---|
| Pb/Pb2+ | | ? 0.1263 |
| Ni/Ni+ | | ? 0.25 |
| Cd/Cd2+ | | ? 0.4026 |
| Fe/Fe2+ | | ? 0.4402 |
| Cu/Cu2+ | | 0.337 |
| Hg/Hg2+ | | 0.854 |
| Ag/Ag+ | | 0.7996 |
| Cr2O7 2-/Cr3+ | | 1.33 |
表4 常见金属的氧化还原电极电位[72]
| 电极 | 电极反应 | 标准电极电位E ?(φ)/V |
|---|---|---|
| Pb/Pb2+ | | ? 0.1263 |
| Ni/Ni+ | | ? 0.25 |
| Cd/Cd2+ | | ? 0.4026 |
| Fe/Fe2+ | | ? 0.4402 |
| Cu/Cu2+ | | 0.337 |
| Hg/Hg2+ | | 0.854 |
| Ag/Ag+ | | 0.7996 |
| Cr2O7 2-/Cr3+ | | 1.33 |
| 1 | 李瑞农 . 中国环境统计年鉴-环境统计表[M]. 北京: 中国环境年鉴社, 2016: 633-638. |
| LI Ruinong . China statistical yearbook on environment-environmental statistics[R]. Beijing: Yearbook of China’s Environmental and Social, 2016: 633-638. | |
| 2 | GILLHAM R W , O, HANNSIN S F . Enhanced degradation of halogenated aliphatics by zero-valent iron[J]. Ground Water, 1994, 32(6): 958-967. |
| 3 | KORNILPVYCH B , WIREMAN M , UBALDINI S , et al . Uranium removal from groundwater by permeable reactive barrier with zero-valent iron and organic carbon mixtures: laboratory and field studies[J]. Metals, 2018, 8 (6): 1-15. |
| 4 | 王素娜, 江国庆, 白俊峰, 等 . 无机分子纳米材料的研究进展[J]. 无机化学学报, 2005, 21(1): 1-11. |
| WANG Suna , JIANG Guoqing , BAI Junfeng , et al . Research progress on inorganic molecular nanomaterials[J]. Chinese Journal of Inorganic Chemistry, 2005, 21(1): 1-11. | |
| 5 | CRANE R A , SAPSFORD D J . Sorption and fractionation of rare earth element ions onto nanoscale zerovalent iron particles[J]. Chemical Engineering Journal, 2018, 345: 126-137. |
| 6 | WANG C B , ZHANG W X . Synthesizing nanoscale iron particles for rapid and complete dechlorination of TCE and PCBs[J]. Environmental Science Technology, 1997, 31(7): 2154-2156. |
| 7 | ZHU N M , XU Y S , DAI L , et al . Application of sequential extraction analysis to Pb(Ⅱ) recovery by zerovalent iron-based particles[J]. Journal of Hazardous Materials, 2018, 351: 138-146. |
| 8 | 陈海军, 黄舒怡, 张志宾, 等 . 功能性纳米零价铁的构筑及其对环境放射性核素铀的富集应用研究进展[J]. 化学学报, 2017, 75(6): 560-574. |
| CHEN Haijun , HUANG Shuyi , ZHANG Zhibin , et al . Synthesis of functional nanoscale zero-valent iron composites for the application of radioactive uranium enrichment from environment: a review[J]. Acta Chimica Sinica, 2017, 75(6): 560-574. | |
| 9 | CAO Z , LIU X , XU J , et al . Removal of antibiotic florfenicol by sulfide-modified nanoscale zero-valent iron[J]. Environmental Science Technology, 2017, 51(19): 11269-11277. |
| 10 | SHI X D , RUAN W Q , HU J W , et al . Optimizing the removal of Rhodamine B in aqueous solutions by reduced graphene oxide-supported nanoscale zerovalent iron (nZVI/rGO) using an artificial neural network-genetic algorithm (ANN-GA)[J]. Nanomaterials, 2017, 7(6): 134-152. |
| 11 | LI S L , WANG W , LIANG F P , et al . Heavy metal removal using nanoscale zero-valent iron (nZVI): theory and application[J]. Journal of Hazardous Materials, 2017, 322: 163-171. |
| 12 | ZHANG S H , WU M F , TANG T T , et al . Mechanism investigation of anoxic Cr(Ⅵ) removal by nano zero-valent iron based on XPS analysis in time scale[J]. Chemical Engineering Journal, 2018, 335: 945-953. |
| 13 | TANG L , FENG H , TANG J , et al . Treatment of arsenic in acid wastewater and river sediment by Fe@Fe2O3 nanobunches: the effect of environmental conditions and reaction mechanism[J]. Water Research, 2017, 117: 175-186. |
| 14 | 赵旭飞, 魏才倢, 张瑾, 等 . 不同粒径零价铁颗粒降解地下水中卤代烃的动力学研究[J]. 北京大学学报(自然科学版), 2018, 54(2): 435-442. |
| ZHAO Xufei , WEI Caijie , ZHANG Jin ,et al . Reductive removal of four halogenated organic compounds by different sizes of zero-valent-iron[J]. Acta Scientiarum Naturalium Universitatis Pekinensis, 2018, 54(2): 435-442. | |
| 15 | OUYANG H , WEI C Y , SHI H Y . Short-chain organic acids increase the reactivity of zerovalent iron nanoparticles toward polychlorinated aromatic pollutants[J]. Chemical Engineering Journal, 2016, 284: 372-379. |
| 16 | LI H , QIU Y F , WANG X L , et al . Biochar supported Ni/Fe bimetallic nanoparticles to remove 1,1,1-trichloroethane under various reaction conditions[J]. Chemosphere, 2017, 169: 534-541. |
| 17 | 冯婧微 . 纳米零价铁及铁(氢)氧化物去除水中Cr和Cu的机制研究[D]. 沈阳: 沈阳工业大学, 2012: 25-29. |
| FENG Jingwei . Mechanisms of chromium and copper removal with nanoscale zero-valent iron and iron oxides/hydroxides[D]. Shenyang: Shenyang University of Technology, 2012: 25-29. | |
| 18 | 秦伯雄, 曾悦坚, 张炳范, 等 . 等离子体制纳米铁粉技术[J]. 天津大学学报(自然科学与工程技术版), 1996, 29(2): 315-318. |
| QIN Boxiong , ZENG Yuejian , ZHANG Bingfan , et al . Study on developing iron ultrafine powders[J]. Journal of Tianjin University (Science and Technology), 1996, 29(2): 315-318. | |
| 19 | SEYED S M , RABIEE H , SHAHABADI S , et al . Synthesis of zero-valent iron nanoparticles via electrical wire explosion for efficient removal of heavy metals[J]. CSAWAC, 2017, 45(3): 139-148. |
| 20 | 潘成福, 侯登录, 张民 . 纳米Fe微粒的溅射制备及粒度计算[J]. 磁记录材料, 1999(2): 8-10. |
| PAN Chengfu , HOU Denglu , ZHANG Min . Sputtering preparation and particle size calculation of nano-Fe particles[J]. Information Recording Materials, 1999(2): 8-10. | |
| 21 | ZHOU S M , LI Y , CHEN J T , et al . Enhanced Cr(Ⅵ) removal from aqueous solutions using Ni/Fe bimetallic nanoparticles: characterization, kinetics and mechanism[J]. RSC Advances, 2014, 4: 50699-50707. |
| 22 | 董洋, 温春宇, 于锦秋, 等 . 乳化纳米铁修复硝基苯污染含水层研究[J]. 中国环境科学, 2017, 37(12): 4541-4548. |
| DONG Yang , WEN Chunyu , YU Jinqiu , et al . Laboratory evaluation of degradation of nitrobenzene in aquifer using emulsified nanoscale zero-valent iron[J]. China Environmental Science, 2017, 37(12): 4541-4548. | |
| 23 | 尹秉胜, 马厚义, 陈慎豪 . 电化学技术制备纳米材料研究的新进展[J]. 化学进展, 2004, 16(2): 196-203. |
| YI Binsheng , Houyi MA , CHEN Shenhao . New progress in electrochemical preparation of nanomaterials[J]. Progress in Chemistry ,2004, 16(2): 196-203. | |
| 24 | LI S L , YAN W L , ZHANG W . Solvent-free production of nanoscale zero-valent iron (nZVI) with precision milling[J]. Green Chemical, 2009, 11: 1618-1626. |
| 25 | XU C , WANG X , AN Y , et al . Preparation of organosolv lignin-stabilized nano zero-valent iron and its application as granular electrode in the tertiary treatment of pulp and paper wastewater[J]. Chemical Engineering Journal , 2018, 331: 317-325. |
| 26 | LIU X , CAO Z , YUAN Z L , et al . Insight into the kinetics and mechanism of removal of aqueous chlorinated nitroaromatic antibiotic chloramphenicol by nanoscale zero-valent iron[J]. Chemical Engineering Journal, 2017, 334: 508-518. |
| 27 | ZHANG Y L , SUA Y M, ZHOU X F , et al . A new insight on the core-shell structure of zerovalent iron nanoparticles and its application for Pb(II) sequestration[J]. Journal of Hazardous Materials, 2013, 263: 685-693. |
| 28 | ZOU Y D , WANG X X , KHAN A , et al . Environmental remediation and application of nanoscale zero valent iron and its composites for the removal of heavy metal ions: a review[J]. Environmental Science Technology, 2016, 50: 7290-7304. |
| 29 | ZHU N M , LI Y , ZHANG F S . Catalytic dechlorination of polychlorinated biphenyls in subcritical water by Ni/Fe nanoparticles[J]. Chemical Engineering Journal, 2011, 171: 919-925. |
| 30 | SOHN K , KANG S , AHN S, et al . Fe(0) nanoparticles for nitrate reduction: stability, reactivity, and transformation[J]. Environmental Science Technology, 2006, 40: 5514-5519. |
| 31 | LING L , HUANG X R , LI M Y , et al . Mapping the reactions in a single zero-valent iron nanoparticle[J]. Environmental Science Technology, 2017, 51: 14293-14300. |
| 32 | HUANG D L , HU Z X , PENG Z W , et al . Cadmium immobilization in river sediment using stabilized nanoscale zero-valent iron with enhanced transport by polysaccharide coating[J]. Journal of Environmental Management, 2018, 210: 191-200. |
| 33 | MU Y , JIA F L , AI Z H , et al . Iron oxide shell mediated environmental remediation properties of nano zero-valent iron[J]. Environmental Science Nano, 2017, 4: 27-45. |
| 34 | PENG Z L , XIONG C M , WANG W , et al . Facile modification of nanoscale zero-valent iron with high stability for Cr(Ⅵ) remediation[J]. Science of the Total Environment, 2017, 596/597: 266-273. |
| 35 | 邱心泓, 方战强 . 修饰型纳米零价铁降解有机卤化物的研究[J]. 化学进展, 2010, 22 (2/3): 291-297. |
| QIU Xinhong , FANG Zhanqiang . Degradation of halogenated organic compoundsby modified nano zero-valent iron[J]. Progress in Chemistry, 2010, 22(2/3): 291-297. | |
| 36 | BOPARAI H K , JOSEPH M , O'Carroll, et al . Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles[J]. Journal of Hazardous Materials, 2011, 186(1): 458-465. |
| 37 | FAN J , GUO Y H , WANG J J , et al . Rapid decolorization of azo dye methyl orange in aqueous solution by nanoscale zerovalent iron particles[J]. Journal of Hazardous Materials, 2009, 166 (2/3): 904-910. |
| 38 | CHEN S , BEDIA J , LI H , et al . Nanoscale zero-valent iron@mesoporous hydrated silica core-shell particles with enhanced dispersibility, transportability and degradation of chlorinated aliphatic hydrocarbons[J]. Chemical Engineering Journal, 2018, 343: 619-628. |
| 39 | LIU T Y , WANG Z L , YAN X X , et al . Removal of mercury (Ⅱ) and chromium (Ⅵ) from wastewater using a new and effective composite: pumice-supported nanoscale zero-valent iron[J]. Chemical Engineering Journal, 2014, 245: 34-40. |
| 40 | 杨艺琳, 周孜迈, 邓文娜, 等 . 浮石负载纳米零价铁去除水相中的砷(Ⅴ)[J]. 环境化学, 2017, 36(3): 598-607. |
| YANG Yilin , ZHOU Zimai , DENG Wenna , et al . Removal of arsenic(Ⅴ) from aqueous solutions using improved nanoscale zero-valent iron on pumice[J]. Environmental Chemistry, 2017, 36(3): 598-607. | |
| 41 | FU R , YANG Y , XU Z , et al . The removal of chromium(Ⅵ) and lead(Ⅱ) from groundwater using sepiolite-supported nanoscale zero-valent iron (S-NZVI) [J]. Chemosphere, 2015, 138: 726-734. |
| 42 | QU G Z , KOU L Q , WANG T , et al . Evaluation of activated carbon fiber supported nanoscale zero-valent iron for chromium(Ⅵ) removal from groundwater in a permeable reactive column[J]. Journal of Environmental Management, 2017, 201: 378-387. |
| 43 | 胡六江, 李益民 . 有机膨润土负载纳米铁去除废水中硝基苯[J]. 环境科学学报, 2008, 28 (6): 1107-1112. |
| HU Liujiang , LI Yimin . Removal of nitrobenzene from synthetic wastewater by nanoscalezero-valent iron supported onorgan obentonite[J]. Acta Scientiae Circumstantiae, 2008, 28(6): 1107-1112. | |
| 44 | SU Y M , SADELEYE A , KELLER A A , et al . Magnetic sulfide-modified nanoscale zerovalent iron (S-nZVI) for dissolved metal ion removal[J]. Water Research, 2015, 74: 47-57. |
| 45 | WU B , PENG D , HOU S , et al . Dynamic study of Cr(Ⅵ) removal performance and mechanism from water using multilayer material coated nanoscale zerovalent iron[J]. Environmental Pollution, 2018, 240: 717-724. |
| 46 | SHAO Y L , ZHAO P , YUE Q Y , et al . Preparation of wheat straw-supported nanoscale zero-valent iron and its removal performance on ciprofloxacin[J]. Ecotoxicology and Environmental Safety, 2018, 158: 100-107. |
| 47 | DIAO Z H , DU J J , JIANG D , et al . Insights into the simultaneous removal of Cr6+ and Pb2+ by a novel sewage sludge-derived biochar immobilized nanoscale zero valent iron: coexistence effect and mechanism[J]. Science of the Total Environment, 2018, 642: 505-515. |
| 48 | DONG H R , DENG J M , XIE Y K , et al . Stabilization of nanoscale zero-valent iron (nZVI) with modified biochar for Cr(Ⅵ) removal from aqueous solution[J]. Journal of Hazardous Materials, 2017, 332: 79-86. |
| 49 | 曾淦宁, 武晓, 郑林, 等 . 负载纳米零价铁铜藻基活性炭的制备及其去除水中Cr(Ⅵ)的研究[J]. 环境科学, 2015, 30(2): 530-536. |
| ZENG Jinning , WU Xiao , ZHENG Lin , et al . Preparation of nano zero-valent iron/sargassum horneri based activated carbon for removal of Cr(Ⅵ) from aqueous solution[J]. Environmental Science, 2015, 30(2): 530-536. | |
| 50 | GU M , FAROOQ U , LU S , et al . Degradation of trichloroethylene in aqueous solution by rGO supported nZVI catalyst under several oxic environments[J]. Journal of Hazardous Materials, 2018, 349: 35-44. |
| 51 | WANG X Y , CHEN C , CHANG Y , et al . Dechlorination of chlorinated methanes by Pd/Fe bimetallic nanoparticles[J]. Journal of Hazardous Materials, 2009, 161: 815-823. |
| 52 | MA Y Y, LV X F , YANG Q , et al . Reduction of carbon tetrachloride by nanoscale palladized zero-valent iron@ graphene composites: kinetics, activation energy, effects of reaction conditions and degradation mechanism[J]. Applied Catalysis A: Environmental, 2017, 542: 252-261. |
| 53 | SHENG G D , HU J , LI H . Enhanced sequestration of Cr(Ⅵ) by nanoscale zero-valent iron supported on layered double hydroxide by batch and XAFS study[J]. Chemosphere, 2016, 148: 227-232. |
| 54 | 董军, 王敏, 刘登峰, 等 . 地下水化学成分对植物油改性纳米铁还原硝基苯影响[J]. 中国环境科学, 2014, 34(7): 1769-1775. |
| DONG Jun , WANG Min , LIU Dengfeng , et al . Effects of groundwater chemical composition on nitrobenzene reduction by vegetable oil modified nano-iron[J]. China Environmental Science, 2014, 34(7): 1769-1775. | |
| 55 | EGLAL M M , RAMAMURHY A S . Removal of Pb(Ⅱ), Cd(Ⅱ), Cu(Ⅱ) and trichloroethylene from water by nanofer ZVI[J]. Journal of Environmental Science and Health Part A, 2015, 50: 901-912. |
| 56 | 和婧, 王向宇, 王培, 等 . PAA改性纳米铁强化还原降解水中亚甲基蓝[J]. 环境科学, 2015, 36 (3): 980-988. |
| HE Jing , WANG Xiangyu , WANG Bei , et al . Enhanced reductive decoloration of methylene blue by polyacrylic acid modified zero-valent iron nanoparticles[J]. Environmental Science, 2015, 36(3): 980-988. | |
| 57 | 肖燕萍, 宋新山, 赵志淼, 等 . 海藻酸钠/蒙脱石联合负载型纳米Fe对Cu(Ⅱ) 的去除研究[J]. 环境科学学报, 2017, 37(1): 227-233. |
| XIAO Yanping , SONG Xinshan , ZHAO Zhimiao , et al . Removal of Cu(Ⅱ) using nanoscale zero-valent iron supported on sodium alginate and montmorillonite[J]. Acta Scientiae Circumstantiae, 2017, 37(1): 227-233. | |
| 58 | JAMEI M R , KHOSRAVI M R , ANVARIPOUR B . A novel ultrasound assisted method in synthesis of NZVI particles[J]. Ultrasonics Sonochemistry, 2014, 21: 226–233. |
| 59 | 边森, 钱枫, 王志楠, 等 . 超声波-零价铁粉还原降解含氯有机物的研究[J]. 环境科学与技术, 2010, 33(s2): 137-140. |
| BIAN Sen , QIAN Feng , WANG Zhinan , et al . Reductive dechlorination of chlorinated organic compounds by ultrasound with zero valent iron[J]. Environmental Science & Technology, 2010, 33(s2): 137-140. | |
| 60 | 汪涛, 张贺, 张沙, 等 . 超声波联用技术在污水处理中的研究进展[J]. 现代化工, 2015, 35(7): 10-13. |
| WANG Tao , ZHANG He , ZHANG Sha , et al . Research progress of ultrasonic coupling technique for wastewater treatment[J]. Modern Chemical Industry, 2015, 35(7): 10-13. | |
| 61 | LIU J , LIU A R , ZHANG W X . The influence of polyelectrolyte modification on nanoscale zero-valent iron (nZVI): aggregation, sedimentation, and reactivity with Ni(Ⅱ) in water[J]. Chemical Engineering Journal, 2016, 303: 268-274. |
| 62 | ARUNKUMAR M , BAE S, HANC S , et al . Reductive dechlorination of trichloroethylene by polyvinylpyrrolidone stabilized nanoscale zerovalent iron particles with Ni[J]. Journal of Hazardous Materials, 2017, 340: 399-406. |
| 63 | SHI L X , CHEN J J , WANG Q W , et al . Effects of carrier on the transport and DDT removal performance of nano-zerovalent iron in packed sands[J]. Chemosphere, 2018, 209: 489-495. |
| 64 | TAN L , LIANG B , FANG Z , et al . Effect of humic acid and transition metal ions on the debromination of decabromodiphenyl by nano zero-valent iron: kinetics and mechanisms[J]. Journal of Nanoparticle Research, 2014, 16: 1-13. |
| 65 | LI J X , ZHANG X Y , SUN Y K , et al . Advances in sulfidation of zerovalent iron for water decontamination[J]. Environmental Science Technology, 2017, 51: 13533-13544. |
| 66 | LIU T Y , WANG Z L , ZHAO L , et al . Enhanced chitosan/Fe0-nanoparticles beads for hexavalent chromium removal from wastewater[J]. Chemical Engineering Journal, 2012, 189/190: 196-202. |
| 67 | 陈喆, 房丽莎, 谭韵盈, 等 . CMC-nZVI 对高硫矿山土壤中铜的固定效果及机理[J]. 环境科学学报, 2017, 37(11): 4336-4343. |
| CHEN Zhe , FANG Lisha , TAN Yunying , et al . Immobilization of Cu in high sulfate mine soil using stabilized nonoscale zero-valent iron[J]. Acta Scientiae Circumstantiae, 2017, 37(11): 4336-4343. | |
| 68 | JABEEN H , KEMP K C , CHANDRA V J . Synthesis of nano zerovalent iron nanoparticles-Graphene composite for the treatment of lead contaminated water[J]. Journal of Environmental Management, 2013, 130: 429-435. |
| 69 | ZHU S S , HO S H, HUANG X C , et al . Magnetic nanoscale zerovalent iron assisted biochar: interfacial chemical behaviors and heavy metals remediation performance[J]. Sustainable Chemical Engineer, 2017, 5: 9673-9682. |
| 70 | BHATNAGAR A , MINOCHA A K . Biosorption optimization of nickel removal from water using Punica granatum peel waste[J]. Colloids and Surfaces B: Biointerfaces, 2010, 76 (2): 544-548. |
| 71 | LI X Q , ZHANG W X . Sequestration of metal cations with zerovalent iron nanoparticless: a study with high resolution X-ray photoelectron spectroscopy(HR-XPS)[J]. Journal of Physical Chemistry C, 2007, 111: 6939-6946. |
| 72 | ANTHELMAN M S , HARRIS F J . The encyclopedia of chemical electrode potentials[M].New York: Plenum Press, 1982: 50-88. |
| 73 | CRANEA R A , SAPSFORD D J . Selective formation of copper nanoparticles from acid mine drainage using nanoscale zerovalent iron particles[J]. Journal of Hazardous Materials, 2018, 347: 252-265. |
| 74 | MOHAN D , Jr PITTMAN C U . Activated carbons and low cost adsorbents for remediation of tri- and hexavalent chromium from water(review)[J]. Journal of Hazardous Materials B, 2006, 137: 762-811. |
| 75 | 秦泽敏, 董黎明, 刘平, 等 . 零价纳米铁吸附去除水中六价铬的研究[J]. 中国环境科学, 2014, 34 (12): 3106-3111. |
| QIN Zemin , DONG Liming , LIU Ping , et al . Removal Cr6+ from water using nanoscale zero-valent iron[J]. China Environmental Science, 2014, 34(12): 3106-3111. | |
| 76 | 周孜迈, 邓文娜, 杨艺琳, 等 . 零价铁活化过硫酸钠去除废水中的砷(Ⅴ)[J]. 环境科学, 2017, 11(38): 4623-4631. |
| ZHOU Zimai , DENG Wenna , YANG Yilin , et al . Zero-valent iron(ZVI) activation of persulfate (PS) for oxidation of arsenic (Ⅴ) form aqueous solutions[J]. Environmental Science, 2017, 11(3): 4623-4631. | |
| 77 | 吕国晓, 尹军, 刘蕾, 等 . pH值对零价铁还原降解模拟地下水中硝基苯的影响[J]. 环境化学, 2009, 28(3): 355-359. |
| Guoxiao LÜ , YIN Jun , LIU Lei , et al . Effect of pH on the degradation of zero-valent iron to simulate nitrobenzene in groundwater[J]. Environmental Chemistry, 2009, 28(3): 355-359. | |
| 78 | SCHERER M M , JOHNSON K . M, WESTALL J C,et al. Mass transport effects on the kinetics of nitrobenzene reduction by iron metal[J]. Environmental Science Technology, 2001, 35: 2804-2811. |
| 79 | LI L X , ZHANG S S , LU B , et al . Nitrobenzene reduction using nanoscale zero-valent iron supported by polystyrene microspheres with different surface functional groups[J]. Environmental Science and Pollution Research, 2018, 25: 7916-7923. |
| 80 | WANG C B , ZHANG W X . Synthesizing nanoscale iron particles for rapid and complete dechlorination of TCE and PCBs[J]. Environmental Science Technology, 1997, 31(7): 2154-2156. |
| 81 | ZHANG W , XIAO T , LIU N , et al . The removal of chlorinated aliphatic hydrocarbons from water using reactive activated carbon: the influence of synthesis factors and reaction environments[J]. Environmental Technology, 2018, 39(10): 1328-1339. |
| 82 | 吴德礼, 王红武, 马鲁铭 . 催化铁还原去除含氯有机物生物毒性的研究[C]//持久性有机污染物论坛 2 0 0 6 暨第一届持久性有机污染物全国学术研讨会. 北京: 中国化学会, 2006: 219-223. |
| WU Deli , WANG Hongwu , Luming MA . Biotoxicity removal of chlorinated organic compounds by catalytic iron reduction[C]//Forum on Persistent Organic Pollutants 2006 and the First National Symposium on Persistent Organic Pollutants. Beijing: Chinese Chemical Society, 2006: 219-223. | |
| 83 | ARNOLD W A , ROBERTS A L . Pathways of chlorinated ethylene and chlorinated acetylene reaction with Zn(0)[J]. Environmental Science Technology, 1998, 32: 3017-3025. |
| 84 | MATHESON L J , TRATNYEK P G . Reductive dehalogenation of chlorinated methanes by iron metal[J]. Environmental Science Technology, 1994, 28: 2045-2053. |
| 85 | CHUANG F W , LARSON R , WESSMAN M . Zero-valent iron-promoted dechlorination of polychlorinated biphenyls[J]. Environmental Science Technology, 1995, 29: 2460-2463. |
| 86 | DONG H R , JIANG Z , ZHANG C , et al . Removal of tetracycline by Fe/Ni bimetallic nanoparticles in aqueous solution[J]. Journal of Colloid and Interface Science, 2018, 513: 117-125. |
| 87 | 成岳, 焦创, 樊文井, 等 . 包覆型纳米零价铁的制备及其去除水中的活性艳蓝[J]. 环境工程学报, 2013,7(1): 53-57. |
| CHENG Yue , JIAO Chuang , FAN Wenjing , et al . Preparation of coated nanoscale zero-valent iron and its application in removing KN-R from wastewater[J]. Chinese Journal of Environmental Engineering, 2013, 7(1): 53-57. | |
| 88 | LIANG J , XIA X , YUAN L , et al . The reproductive responses of earthworms (Eisenia fetida) exposed to nanoscale zero-valent iron(nZVI) in the presence of decabromodiphenyl ether(BDE209)[J]. Environmental Pollution, 2018, 237: 784-791. |
| 89 | LEE C, KIM J Y, LEE W I, et al . Bactericidal effect of zero-valent iron nanoparticles on Escherichia coli [J]. Environmental Science Technology, 2008, 42(13): 4927–4933. |
| 90 | QIU X , FANG Z , YAN X , et al . Chemical stability and toxicity of nanoscale zero-valent iron in the remediation of chromium-contaminated watershed[J]. Chemical Engineering Journal, 2013, 220: 61-66. |
| 91 | NGUYEN N H A , VON M N R, SLAVEYKVAl V I , et al. Biological effects of four iron-containing nano remediation materials on the green alga Chlamydomonas sp.[J]. Ecotoxicology and Environmental Safety, 2018, 154: 36-44. |
| 92 | NGUYEN N H A , ŠP NEK R , KASALICK V , et al. Different effects of nano-scale and micro-scale zero-valent iron particles on planktonic microorganisms from natural reservoir water [J]. Environmental Science Nano, 2018, 5(5): 1117-1129. |
| 93 | 王学, 李勇超, 李铁龙, 等 . 零价纳米铁对大肠杆菌的毒性效应[J]. 生态毒理学报, 2012, 7(1): 49-56. |
| WANG Xue , LI Yongchao , LI Tielong . et al . Toxicity effects of nano-Fe0 on Escherichia coli [J]. Asian Journal of Ecotoxicology, 2012, 7(1): 49-56. |
| [1] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [2] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [3] | 李世霖, 胡景泽, 王毅霖, 王庆吉, 邵磊. 电渗析分离提取高值组分的研究进展[J]. 化工进展, 2023, 42(S1): 420-429. |
| [4] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [5] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [6] | 赵景超, 谭明. 表面活性剂对电渗析减量化工业含盐废水的影响[J]. 化工进展, 2023, 42(S1): 529-535. |
| [7] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [8] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [9] | 葛全倩, 徐迈, 梁铣, 王凤武. MOFs材料在光电催化领域应用的研究进展[J]. 化工进展, 2023, 42(9): 4692-4705. |
| [10] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [11] | 王晨, 白浩良, 康雪. 大功率UV-LED散热与纳米TiO2光催化酸性红26耦合系统性能[J]. 化工进展, 2023, 42(9): 4905-4916. |
| [12] | 李卫华, 于倩雯, 尹俊权, 吴寅凯, 孙英杰, 王琰, 王华伟, 杨玉飞, 龙於洋, 黄启飞, 葛燕辰, 何依洋, 赵灵燕. 酸雨环境下填埋飞灰吨袋破损后重金属的溶出行为[J]. 化工进展, 2023, 42(9): 4917-4928. |
| [13] | 李志远, 黄亚继, 赵佳琪, 于梦竹, 朱志成, 程好强, 时浩, 王圣. 污泥与聚氯乙烯共热解重金属特性[J]. 化工进展, 2023, 42(9): 4947-4956. |
| [14] | 王琦, 寇丽红, 王冠宇, 王吉坤, 刘敏, 李兰廷, 王昊. 焦化废水生物出水中可溶解性有机物的分子识别[J]. 化工进展, 2023, 42(9): 4984-4993. |
| [15] | 史天茜, 石永辉, 武新颖, 张益豪, 秦哲, 赵春霞, 路达. Fe2+对厌氧氨氧化EGSB反应器运行性能的影响[J]. 化工进展, 2023, 42(9): 5003-5010. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||