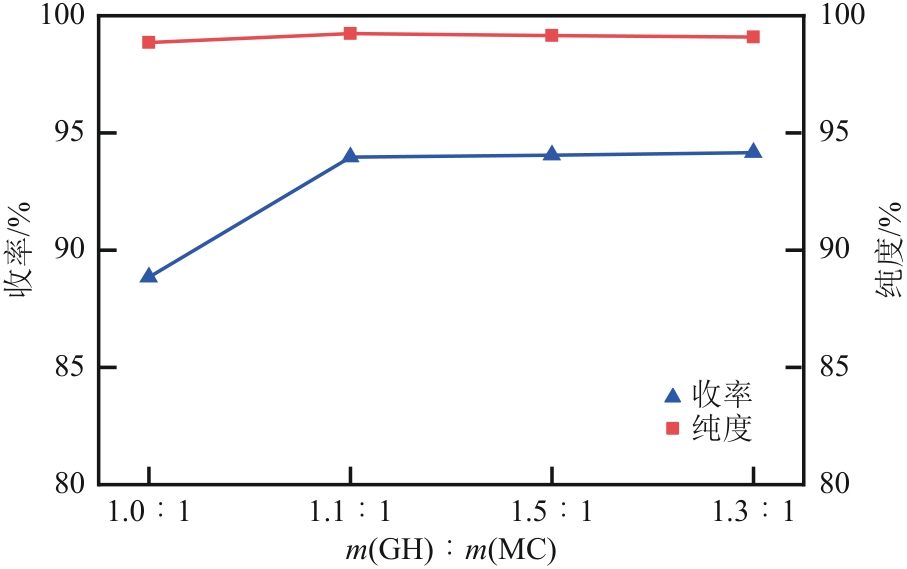
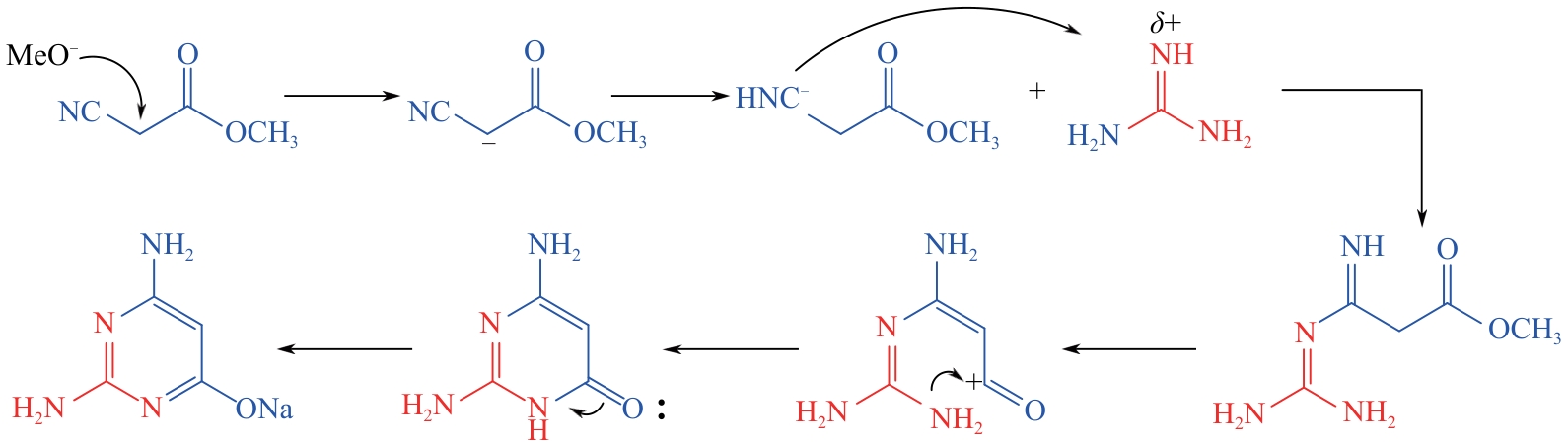
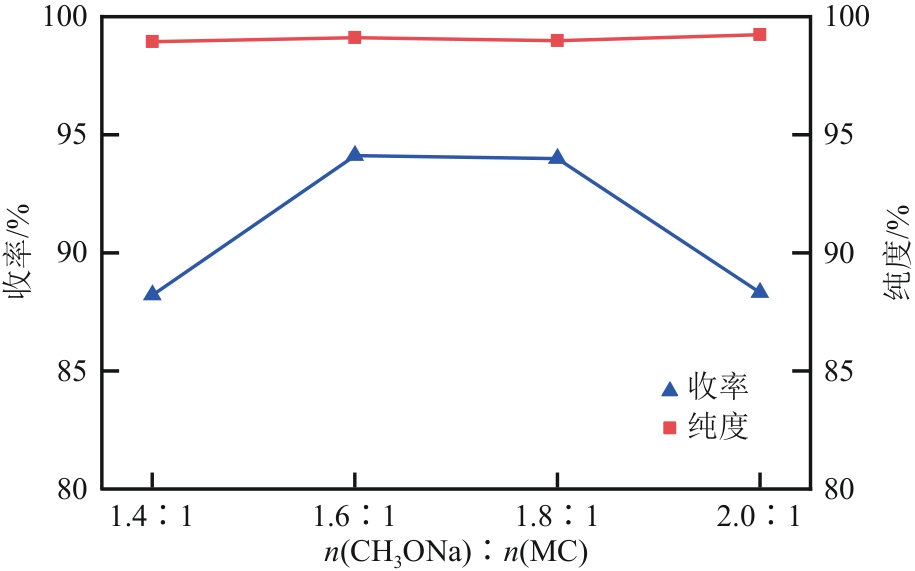
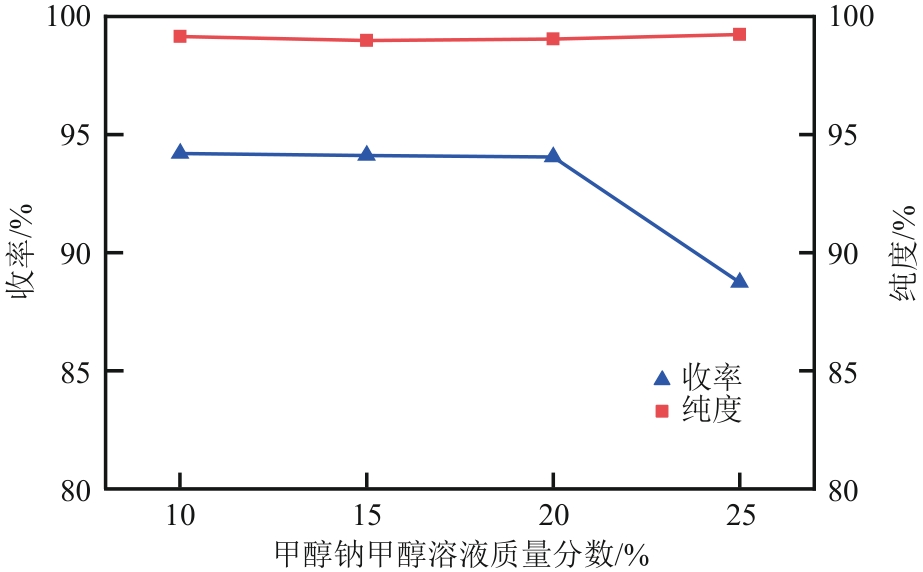
| 1 |
ANGELASTRO Antonio, DAWSON William M, Louis Y P LUK, et al. Chemoenzymatic assembly of isotopically labeled folates[J]. Journal of the American Chemical Society, 2017, 139(37): 13047-13054.
|
| 2 |
陈新, 王颖. 鸟嘌呤的化学合成[J]. 中国医药工业杂志, 1992, 23(1): 32-33.
|
|
CHEN Xin, WANG Ying. Synthesis of guanine[J]. Chinese Journal of Pharmaceuticals, 1992, 23(1): 32-33.
|
| 3 |
李坚军, 鞠金军, 蒲通, 等. 阿昔洛韦的合成工艺改进[J]. 化工生产与技术, 2012, 18(2): 9-11, 33.
|
|
LI Jianjun, JU Jinjun, PU Tong, et al. Improvement on the synthesis process of acyclovir[J]. Chemical Production and Technology, 2012, 18(2): 9-11, 33.
|
| 4 |
DERUDAS Marco, CARTA Davide, BRANCALE Andrea, et al. The application of phosphoramidate protide technology to acyclovir confers anti-HIV inhibition[J]. Journal of Medicinal Chemistry, 2009, 52(17): 5520-5530.
|
| 5 |
刘小溪. 鸟嘌呤化学发光体系的优化及其分析应用[D]. 西安: 陕西师范大学, 2018.
|
|
LIU Xiaoxi. Optimization of guanine chemiluminescence system and its analytical application[D]. Xi’an: Shaanxi Normal University, 2018.
|
| 6 |
LA ROSA Giuseppe, ZACHARIAS Martin. Global deformation facilitates flipping of damaged 8-oxo-guanine and guanine in DNA[J]. Nucleic Acids Research, 2016, 44(20): 9591-9599.
|
| 7 |
BALANIKAS Evangelos, BANYASZ Akos, DOUKI Thierry, et al. Guanine radicals induced in DNA by low-energy photoionization[J]. Accounts of Chemical Research, 2020, 53(8): 1511-1519.
|
| 8 |
Natalie FARDIAN-MELAMED, KATRIVAS Liat, ROTEM Dvir, et al. Electronic level structure of novel guanine octuplex DNA single molecules[J]. Nano Letters, 2021, 21(21): 8987-8992.
|
| 9 |
LIAU Chun-Eng, YAMASHITA Kyohei, MATSUI Masanao. A new synthetic method of 6-hydroxypurines[J]. Agricultural and Biological Chemistry, 1962, 26(9): 624-629.
|
| 10 |
朱文佳, 王海波, 李嫦, 等. 一种鸟嘌呤的合成方法: CN112522351A[P]. 2021-03-19.
|
|
ZHU Wenjia, WANG Haibo, LI Chang, et al. The invention relates to a synthesis method of guanine: CN112522351A[P]. 2021-03-19.
|
| 11 |
邵伟清, 毛宏伟. 2, 4, 5-三氨基-6-羟基嘧啶硫酸盐的合成[J]. 中国医药工业杂志, 2002, 33(12): 579.
|
|
SHAO Weiqing, MAO Hongwei. Synthesis of 2, 4, 5-triamino-6-hydroxypyrimidine sulfate[J]. Chinese Journal of Pharmaceuticals, 2002, 33(12): 579.
|
| 12 |
黄永明, 吕银祥, 刘文达, 等. 正交设计法催化氢化制备2,4,5-三氨基-6-羟基嘧啶[J]. 复旦学报(自然科学版), 2000, 39(4): 459-462.
|
|
HUANG Yongming, LU Yinxiang, LIU Wenda, et al. Preparation of 6-hydro-2,4,5-triaminopyrimidine by using orthogonal design test[J]. Journal of Fudan University, 2000, 39(4): 459-462.
|
| 13 |
沈晓勇. 一种 2,4,5-三氨基-6-羟基嘧啶硫酸盐的制备方法: CN115286584A[P]. 2022-11-04.
|
|
SHEN Xiaoyong. The invention relates to a preparation method of 2, 4, 5-triamino-6-hydroxypyrimidine sulfate: CN115286584A[P]. 2022-11-04.
|
| 14 |
魏作君, 贺晓东, 林志毅. 一种制备 2,4,5-三氨基-6-羟基嘧啶硫酸盐的方法: CN102399194A[P]. 2012-04-04.
|
|
WEI Zuojun, HE Xiaodong, LIN Zhiyi. A method for preparing 2,4,5-triamino-6-hydroxypyrimidine sulfate: CN102399194A[P]. 2012-04-04.
|
| 15 |
STEFFEN K D. Process for the preparation of pure guanine: EP415028[P]. 1991-03-06.
|
| 16 |
THEIS Christoph, BRUHN Stefan. Process for preparing guanine under superatmospheric pressure: US6214993[P]. 2001-04-10.
|
| 17 |
THEIS Christoph, BRUHN Stephan. Process for the preparation of guanine: US6242599[P]. 2001-06-05.
|
| 18 |
THALHAMMER Franz, GRAEFE Jurgen. Production of purines via reductive formylation: US5688947[P]. 1997-11-18.
|
| 19 |
陈文华, 唐健国. 鸟嘌呤的合成工艺改进[J]. 广州化工, 2011, 39(24): 59-60.
|
|
CHEN Wenhua, TANG Jianguo. Improvement on synthesis of guanine[J]. Guangzhou Chemical Industry, 2011, 39(24): 59-60.
|
| 20 |
张从芬, 王凤各, 游志英, 等. 甲酸钠法合成鸟嘌呤的研究[J]. 中国当代医药, 2013, 20(28): 9-10, 13.
|
|
ZHANG Congfen, WANG Fengge, YOU Zhiying, et al. Study on synthesis and analysis of guanine by sodium formate[J]. China Modern Medicine, 2013, 20(28): 9-10, 13.
|
| 21 |
楚玮明, 孔立, 徐汉青, 等. 一种制备鸟嘌呤的新方法: CN106117207A[P]. 2016-11-16.
|
|
CHU Weiming, KONG Li, XU Hanqing, et al. A new method for preparing guanine: CN106117207A[P]. 2016-11-16.
|
| 22 |
赵传武, 杨金路, 汪天洋, 等. 喹啉多环系列化合物的一锅法合成[J]. 精细化工, 2023, 40(2): 456-464.
|
|
ZHAO Chuanwu, YANG Jinlu, WANG Tianyang, et al. One-pot synthesis of quinoline polycyclic compounds[J]. Fine Chemicals, 2023, 40(2): 456-464.
|
 ), 刘飞1, 张天永1,2(
), 刘飞1, 张天永1,2( ), 姜爽1,2(
), 姜爽1,2( )
)
 ), LIU Fei1, ZHANG Tianyong1,2(
), LIU Fei1, ZHANG Tianyong1,2( ), JIANG Shuang1,2(
), JIANG Shuang1,2( )
)