化工进展 ›› 2024, Vol. 43 ›› Issue (11): 6428-6442.DOI: 10.16085/j.issn.1000-6613.2023-1822
• 资源与环境化工 • 上一篇
钯基催化剂在电催化加氢脱氯技术的应用与挑战
- 中国石油大学(华东)化学化工学院重质油国家重点实验室,山东 青岛 266580
-
收稿日期:2023-10-16修回日期:2024-01-25出版日期:2024-11-15发布日期:2024-12-07 -
通讯作者:柳云骐 -
作者简介:李俊熙(1995—),男,博士研究生,研究方向为环境污染治理技术与材料。E-mail:B20030038@s.upc.edu.cn。 -
基金资助:国家自然科学基金(22178388)
Application and challenges of palladium-based catalysts in electrocatalytic hydrodechlorination
- State Key Laboratory of Heavy Oil Processing, College of Chemistry and Chemical Engineering, China University of Petroleum (East China), Qingdao 266580, Shandong, China
-
Received:2023-10-16Revised:2024-01-25Online:2024-11-15Published:2024-12-07 -
Contact:LIU Yunqi
摘要:
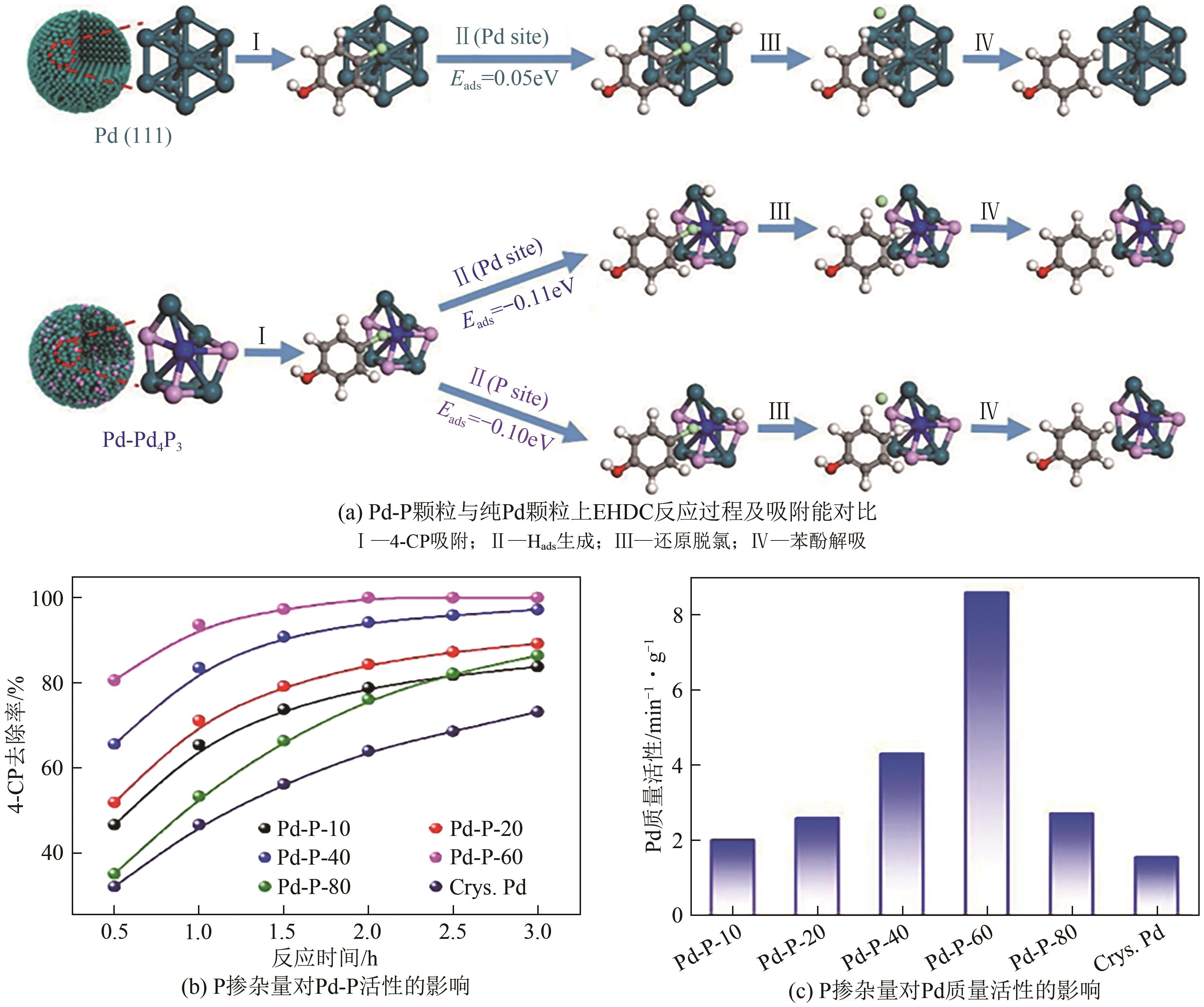
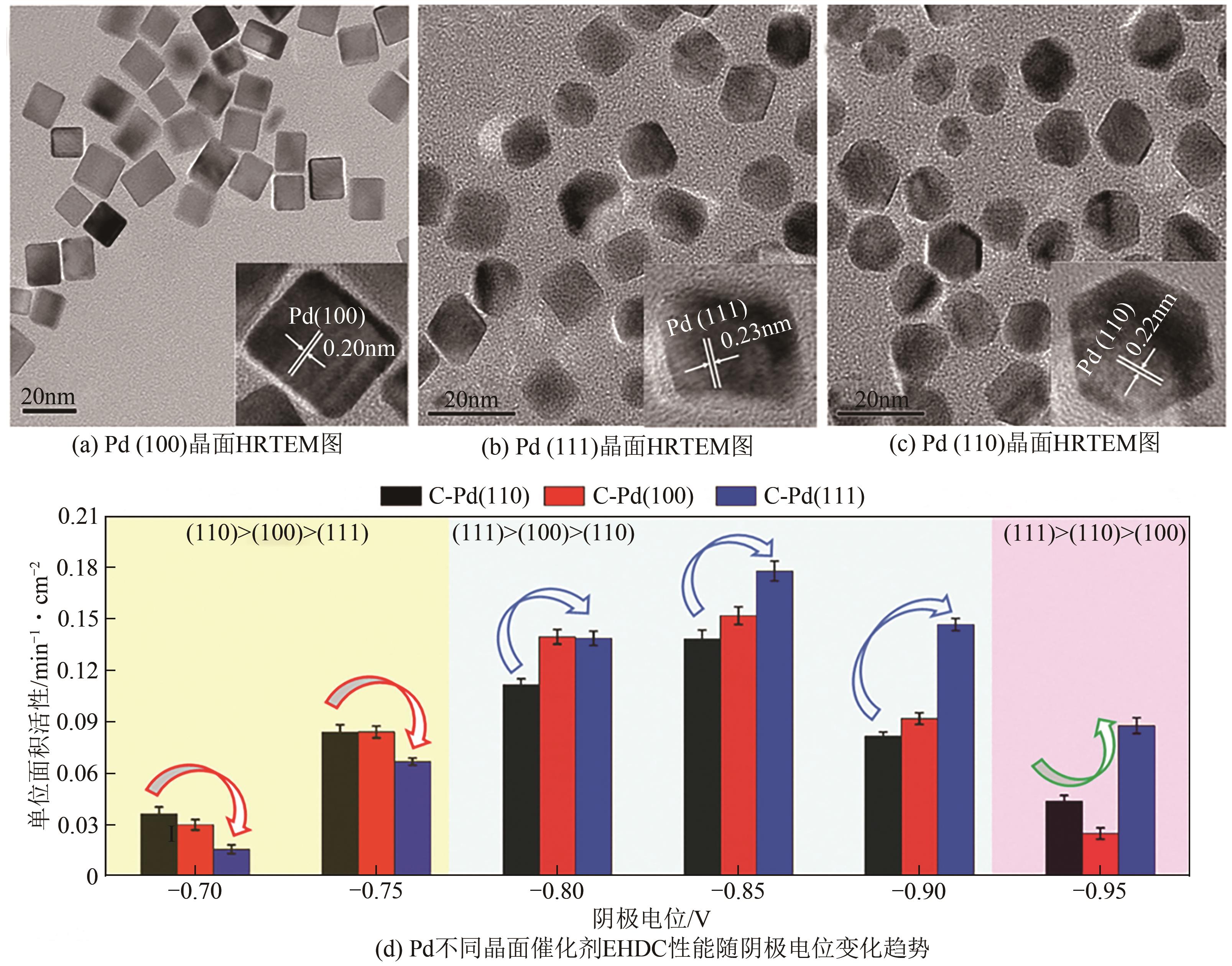
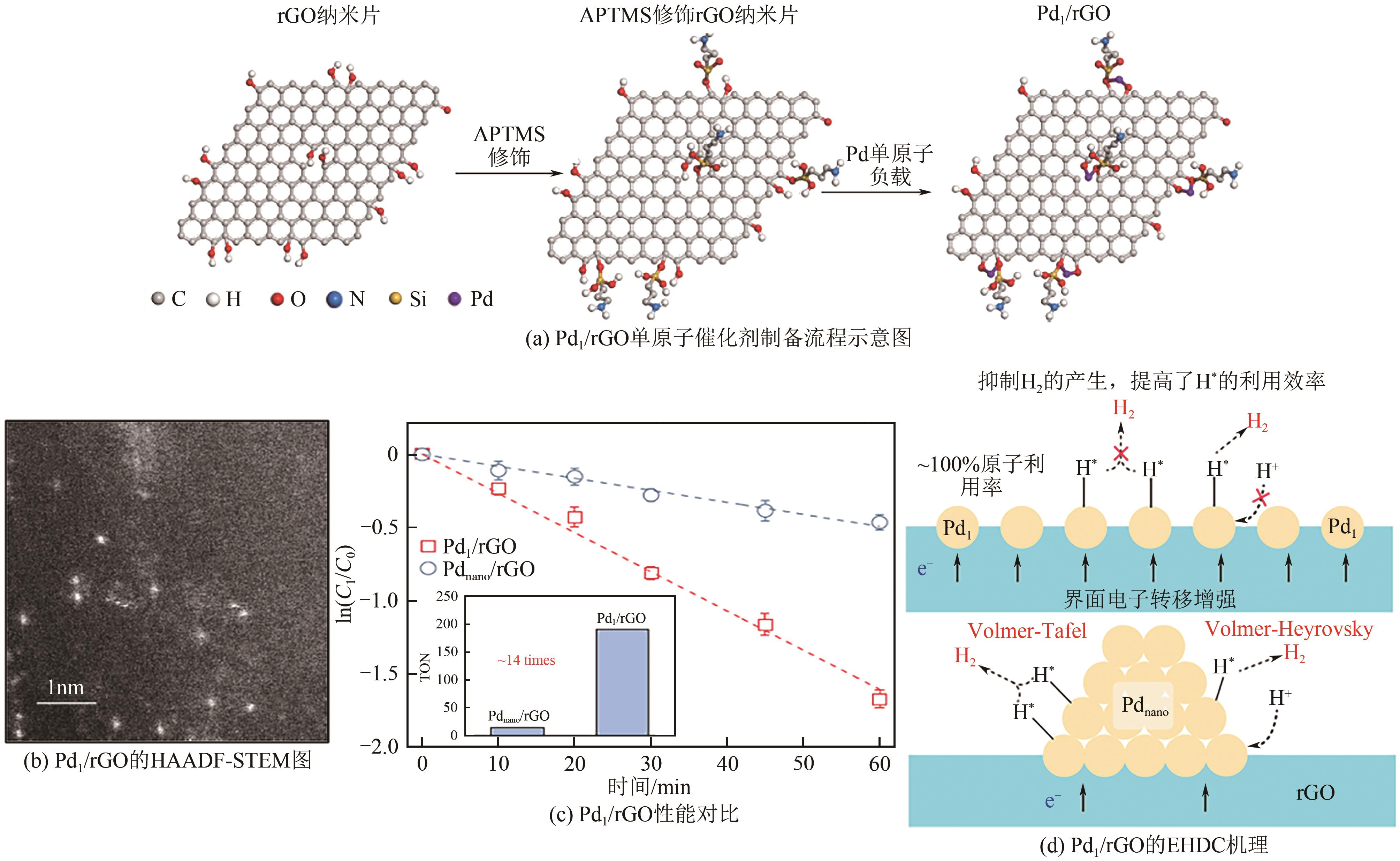
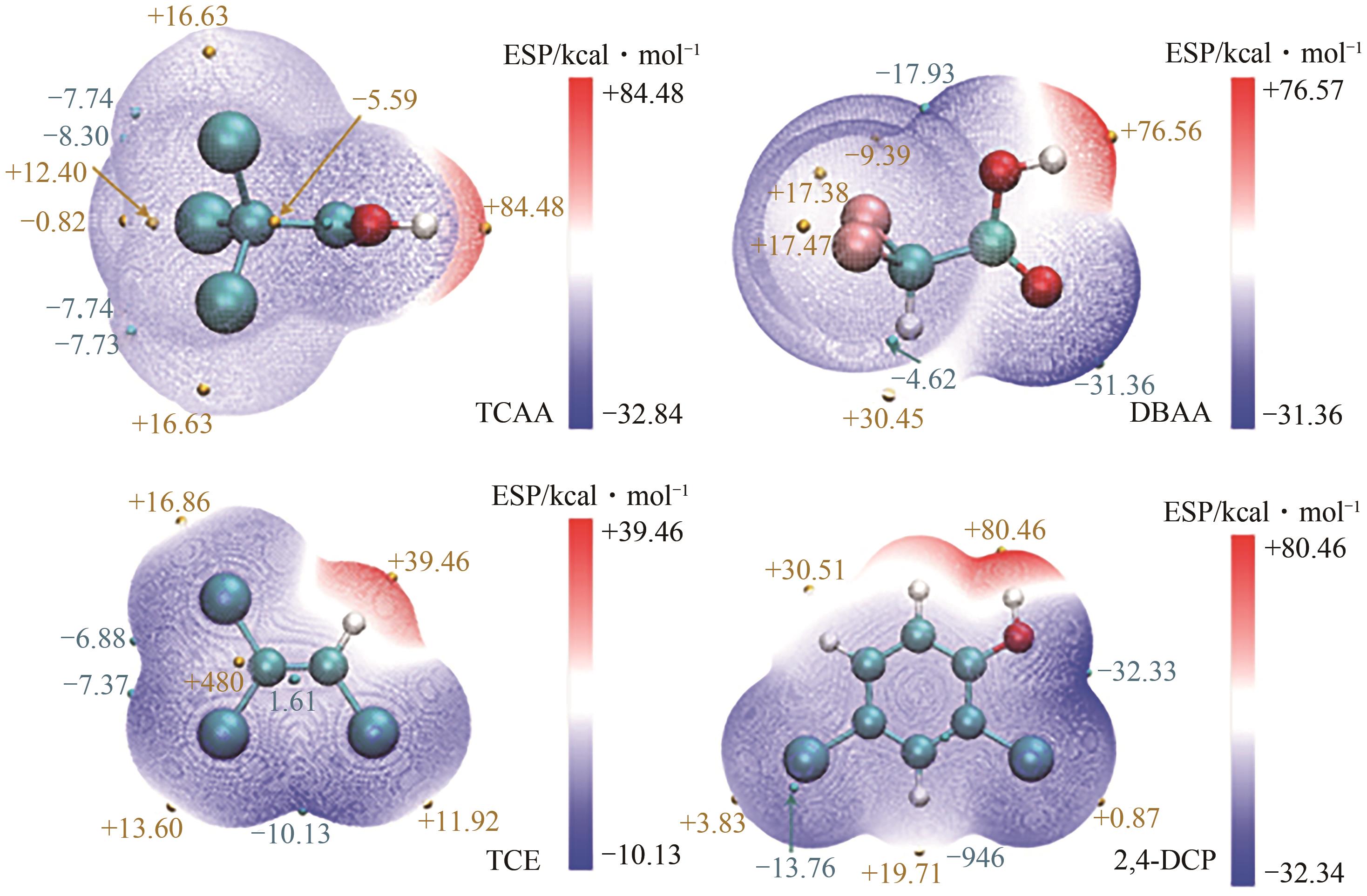
电催化加氢脱氯(EHDC)技术是一种新型的高效、安全、绿色的水处理技术。钯(Pd)催化剂作为较有效的EHDC催化剂由于其易失活和价格昂贵的原因限制其工业应用。本文分析了调控Pd的形态结构(分散度、颗粒大小、晶面)、尺度效应(单原子催化剂、双原子催化剂)和电子结构调控对提升Pd的EHDC性能的研究进展,提出提升Pd对污染物的有效吸附能够提升EHDC性能。探讨了其他因素(工作电位、共存离子、pH等)对其活性的影响。对EHDC技术未来应用进行了展望,以简单方式制备Pd单原子催化剂,实现100%的原子利用率,显著降低Pd的成本,耦合可再生能源技术降低能源成本,推动工业化应用是未来的一个研究方向。
中图分类号:
引用本文
李俊熙, 柳云骐. 钯基催化剂在电催化加氢脱氯技术的应用与挑战[J]. 化工进展, 2024, 43(11): 6428-6442.
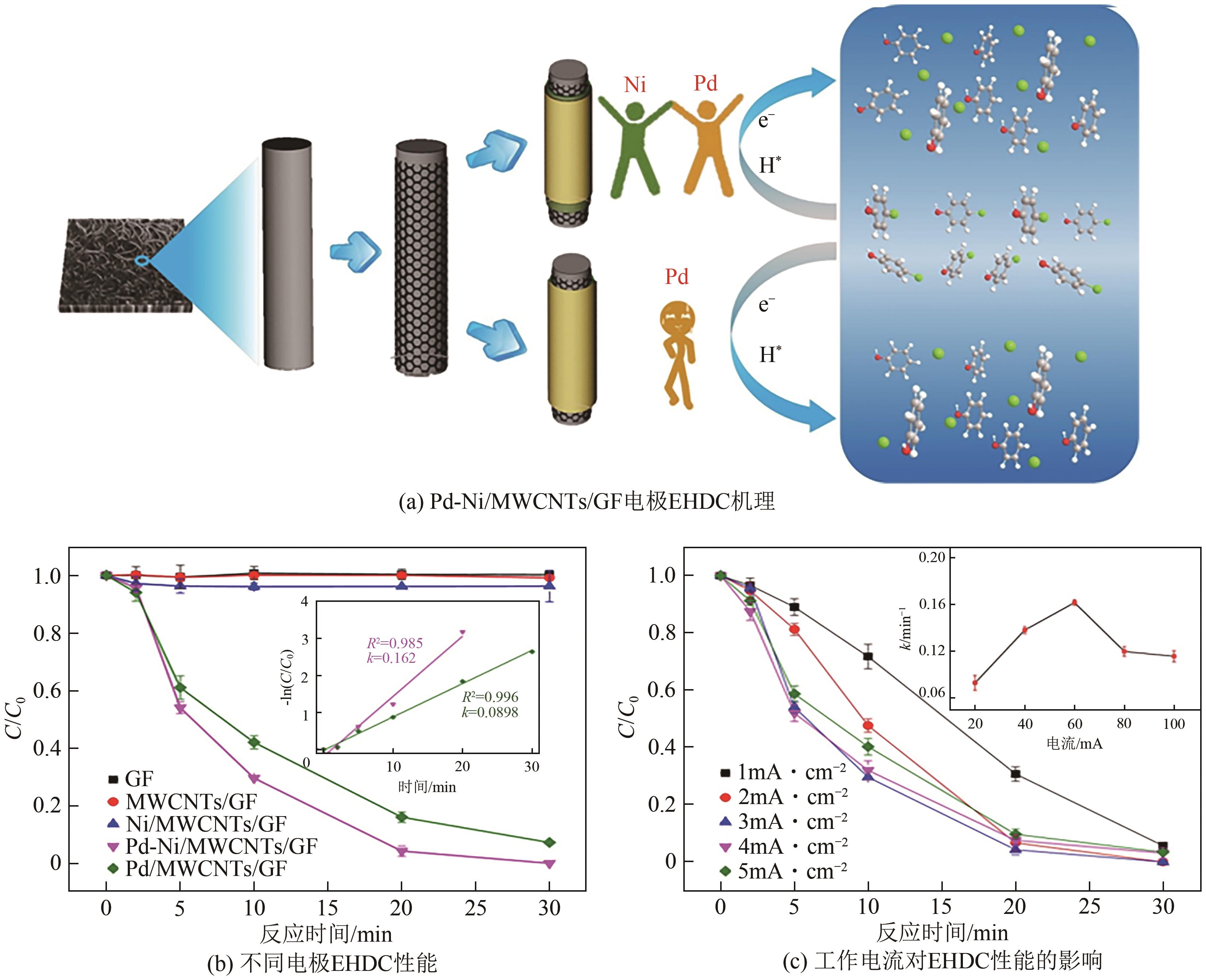
LI Junxi, LIU Yunqi. Application and challenges of palladium-based catalysts in electrocatalytic hydrodechlorination[J]. Chemical Industry and Engineering Progress, 2024, 43(11): 6428-6442.
催化剂 (催化剂量) | 污染物 (添加量) | 测试条件 | 转化率/% | 电流效率/% | 参考文献 |
|---|---|---|---|---|---|
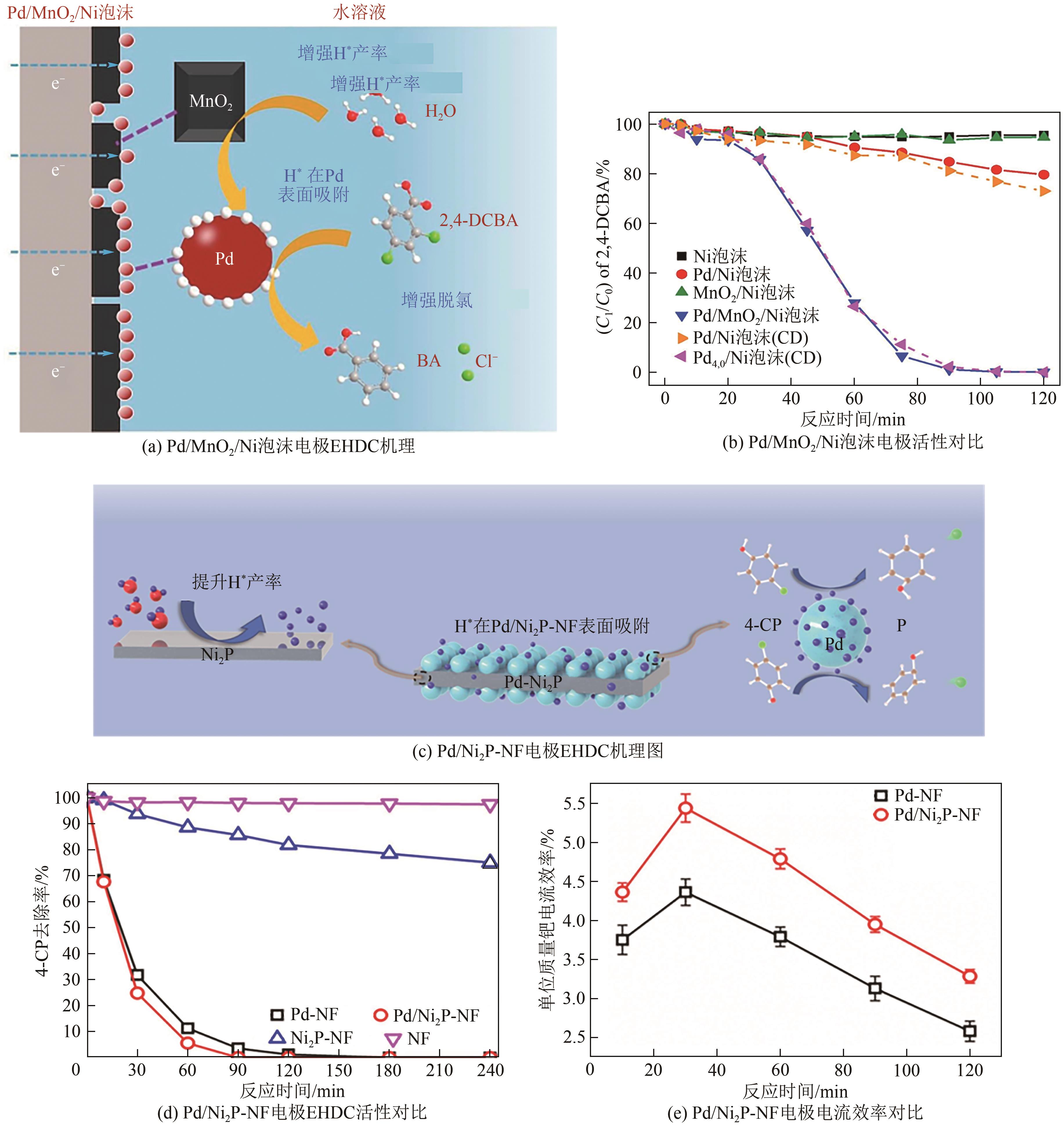
| Pd/MnO2/Ni泡沫 (0.44mg/cm2) | 2,4-二氯苯甲酸 (0.2mmol/L) | 10mA 10mmol/L Na2SO4;T=303K;pH=4.0 | 100(120min) | 70 | [ |
| TiC-Pd/Ni泡沫 (0.44mg/cm2) | 2,4-二氯苯甲酸 (0.2mmol/L) | -0.85V vs. Ag/AgCl 10mmol/L Na2SO4;T=(298±0.3K);pH=4 | 99.8(90min) | — | [ |
Pd/AC (8mg) | 2,4-二氯苯甲酸 (0.156mmol/L) | 10mA 10mmol/L Na2SO4;T=313K;pH=4.0 | 98(180min) | 6 | [ |
| Pd-Co3O4/Ni泡沫 (1.88mg/cm2) | 2,4-二氯苯氧基乙酸 (50mg/L) | 1.5mA/cm2 17mmol/L Na2SO4T=(298.15±1)K | 94.2(120min) | 12.1 | [ |
| Pd/TiN-Ni泡沫 (0.44mg/cm2) | 2,4-二氯苯氧基乙酸 (0.23mmol/L) | 1.667mA/cm2 10mmol/L Na2SO4; T=298.15K | 100(120min) | — | [ |
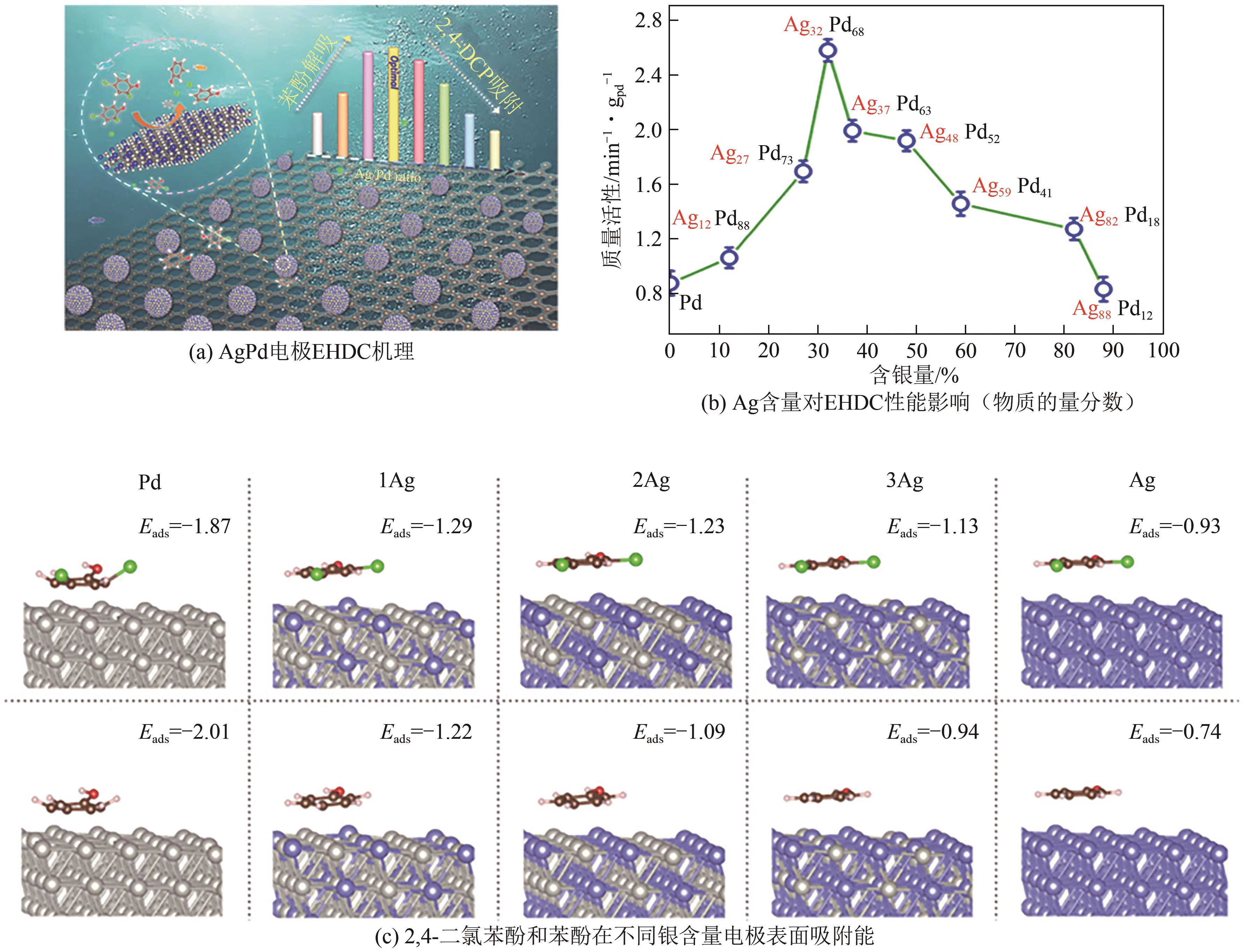
| 碳载Ag32Pd68合金 (4.79mg) | 2,4-二氯苯酚 (0.31 mmol/L) | -0.70V vs. Ag/AgCl 50mmol/L Na2SO4; T=298.15K;pH=6.8 | <90(240min) | <40 | [ |
| Pd-TiO2 | 2,4-二氯苯酚 (0.31mmol/L) | -0.85V vs. Ag/AgCl 50mmol/L Na2SO4; T=298.15K;pH=6.8 | <80(180min) | 25.8 | [ |
Pd/TiN [(3.20±0.4)mg] | 2,4-二氯苯酚 (0.31mmol/L) | -0.80V vs. Ag/AgCl 50mmol/L Na2SO4; T=298.15K;pH=6.8 | 96.4(240min) | <40 | [ |
Pd/MnO2/Ni泡沫 (0.205mg/cm2) | 2,4-二氯苯酚 (0.31mmol/L) | -0.85V vs. Ag/AgCl 50mmol/L Na2SO4; T=298.15K;pH=7.0 | 100(150min) | 26.3 | [ |
| Pd/Ni2P-Ni泡沫 (0.41mg/cm2) | 对氯苯酚 (100mg/L) | -0.85V vs. Ag/AgCl 50mmol/L Na2SO4; T=298.15K;pH=7.0 | 100(120min) | 28.3 | [ |
| Pd/PPy-MWCNTs/Ti (3.72mg/cm2) | 对氯苯酚 (0.78mmol/L) | 1.0mA/cm2 0.1mol/L Na2SO4T=298.15K;pH=2.08 | 99.82(120min) | 13.8 | [ |
| Pd-P-60 NPs (0.8mg/cm2) | 对氯苯酚 (50mg/L) | -0.80V vs. SCE 25 mmol/L K2SO4; T=298.15K;pH=6.8 | 100(120min) | <20 | [ |
| 碳载Pd7Au3合金 | 对氯苯酚 (50mg/L) | -1.10V vs. SCE 50mmol/L K2SO4; T=298.15K;pH=6.8 | 98.35(240min) | <14 | [ |
表1 Pd基催化剂EHDC性能对比
催化剂 (催化剂量) | 污染物 (添加量) | 测试条件 | 转化率/% | 电流效率/% | 参考文献 |
|---|---|---|---|---|---|
| Pd/MnO2/Ni泡沫 (0.44mg/cm2) | 2,4-二氯苯甲酸 (0.2mmol/L) | 10mA 10mmol/L Na2SO4;T=303K;pH=4.0 | 100(120min) | 70 | [ |
| TiC-Pd/Ni泡沫 (0.44mg/cm2) | 2,4-二氯苯甲酸 (0.2mmol/L) | -0.85V vs. Ag/AgCl 10mmol/L Na2SO4;T=(298±0.3K);pH=4 | 99.8(90min) | — | [ |
Pd/AC (8mg) | 2,4-二氯苯甲酸 (0.156mmol/L) | 10mA 10mmol/L Na2SO4;T=313K;pH=4.0 | 98(180min) | 6 | [ |
| Pd-Co3O4/Ni泡沫 (1.88mg/cm2) | 2,4-二氯苯氧基乙酸 (50mg/L) | 1.5mA/cm2 17mmol/L Na2SO4T=(298.15±1)K | 94.2(120min) | 12.1 | [ |
| Pd/TiN-Ni泡沫 (0.44mg/cm2) | 2,4-二氯苯氧基乙酸 (0.23mmol/L) | 1.667mA/cm2 10mmol/L Na2SO4; T=298.15K | 100(120min) | — | [ |
| 碳载Ag32Pd68合金 (4.79mg) | 2,4-二氯苯酚 (0.31 mmol/L) | -0.70V vs. Ag/AgCl 50mmol/L Na2SO4; T=298.15K;pH=6.8 | <90(240min) | <40 | [ |
| Pd-TiO2 | 2,4-二氯苯酚 (0.31mmol/L) | -0.85V vs. Ag/AgCl 50mmol/L Na2SO4; T=298.15K;pH=6.8 | <80(180min) | 25.8 | [ |
Pd/TiN [(3.20±0.4)mg] | 2,4-二氯苯酚 (0.31mmol/L) | -0.80V vs. Ag/AgCl 50mmol/L Na2SO4; T=298.15K;pH=6.8 | 96.4(240min) | <40 | [ |
Pd/MnO2/Ni泡沫 (0.205mg/cm2) | 2,4-二氯苯酚 (0.31mmol/L) | -0.85V vs. Ag/AgCl 50mmol/L Na2SO4; T=298.15K;pH=7.0 | 100(150min) | 26.3 | [ |
| Pd/Ni2P-Ni泡沫 (0.41mg/cm2) | 对氯苯酚 (100mg/L) | -0.85V vs. Ag/AgCl 50mmol/L Na2SO4; T=298.15K;pH=7.0 | 100(120min) | 28.3 | [ |
| Pd/PPy-MWCNTs/Ti (3.72mg/cm2) | 对氯苯酚 (0.78mmol/L) | 1.0mA/cm2 0.1mol/L Na2SO4T=298.15K;pH=2.08 | 99.82(120min) | 13.8 | [ |
| Pd-P-60 NPs (0.8mg/cm2) | 对氯苯酚 (50mg/L) | -0.80V vs. SCE 25 mmol/L K2SO4; T=298.15K;pH=6.8 | 100(120min) | <20 | [ |
| 碳载Pd7Au3合金 | 对氯苯酚 (50mg/L) | -1.10V vs. SCE 50mmol/L K2SO4; T=298.15K;pH=6.8 | 98.35(240min) | <14 | [ |
| 1 | RENAGULI Aikebaier, FERNANDO Sujan, HOPKE Philip K, et al. Nontargeted screening of halogenated organic compounds in fish fillet tissues from the great lakes[J]. Environmental Science & Technology, 2020, 54(23): 15035-15045. |
| 2 | ILUNGA Ali K, MAMBA Bhekie B, NKAMBULE Thabo T I. Catalytic hydrodehalogenation of halogenated disinfection byproducts for clean drinking water production: A review[J]. Journal of Water Process Engineering, 2021, 44: 102402. |
| 3 | HALSE Anne Karine, SCHLABACH Martin, SCHUSTER Jasmin K, et al. Endosulfan, pentachlorobenzene and short-chain chlorinated paraffins in background soils from Western Europe[J]. Environmental Pollution, 2015, 196: 21-28. |
| 4 | MCGOLDRICK Daryl J, MURPHY Elizabeth W. Concentration and distribution of contaminants in lake trout and walleye from the Laurentian Great Lakes (2008—2012)[J]. Environmental Pollution, 2016, 217: 85-96. |
| 5 | RANI Lata, THAPA Komal, KANOJIA Neha, et al. An extensive review on the consequences of chemical pesticides on human health and environment[J]. Journal of Cleaner Production, 2021, 283: 124657. |
| 6 | DENG Jia, HU Xinming, GAO Enlai, et al. Electrochemical reductive remediation of trichloroethylene contaminated groundwater using biomimetic iron-nitrogen-doped carbon[J]. Journal of Hazardous Materials, 2021, 419: 126458. |
| 7 | SHEN Yi, TONG Yiwen, XU Jinli, et al. Ni-based layered metal-organic frameworks with palladium for electrochemical dechlorination[J]. Applied Catalysis B: Environmental, 2020, 264: 118505. |
| 8 | LOU Yaoyin, HAPIOT Philippe, FLONER Didier, et al. Efficient dechlorination of α-halocarbonyl and α-haloallyl pollutants by electroreduction on bismuth[J]. Environmental Science & Technology, 2020, 54(1): 559-567. |
| 9 | LI Junxi, PENG Yiyin, ZHANG Wendong, et al. Hierarchical Pd/MnO2 nanosheet array supported on Ni foam: An advanced electrode for electrocatalytic hydrodechlorination reaction[J]. Applied Surface Science, 2020, 509: 145369. |
| 10 | JIANG Guangming, LI Xiangjun, SHEN Yu, et al. Mechanistic insight into the electrocatalytic hydrodechlorination reaction on palladium by a facet effect study[J]. Journal of Catalysis, 2020, 391: 414-423. |
| 11 | MAO Ran, HUANG Chao, ZHAO Xu, et al. Dechlorination of triclosan by enhanced atomic hydrogen-mediated electrochemical reduction: Kinetics, mechanism, and toxicity assessment[J]. Applied Catalysis B: Environmental, 2019, 241: 120-129. |
| 12 | JIANG Guangming, LAN Mengna, ZHANG Zhiyong, et al. Identification of active hydrogen species on palladium nanoparticles for an enhanced electrocatalytic hydrodechlorination of 2, 4-dichlorophenol in water[J]. Environmental Science & Technology, 2017, 51(13): 7599-7605. |
| 13 | ZHOU Jiasheng, LOU Zimo, XU Jiang, et al. Enhanced electrocatalytic dechlorination by dispersed and moveable activated carbon supported palladium catalyst[J]. Chemical Engineering Journal, 2019, 358: 1176-1185. |
| 14 | LI Junjing, WANG Huan, QI Ziyan, et al. Kinetics and mechanisms of electrocatalytic hydrodechlorination of diclofenac on Pd-Ni/PPy-rGO/Ni electrodes[J]. Applied Catalysis B: Environmental, 2020, 268: 118696. |
| 15 | LIU Yong, LIU Lan, SHAN Jun, et al. Electrodeposition of palladium and reduced graphene oxide nanocomposites on foam-nickel electrode for electrocatalytic hydrodechlorination of 4-chlorophenol[J]. Journal of Hazardous Materials, 2015, 290: 1-8. |
| 16 | SHU Song, WANG Peng, ZHANG Wendong, et al. Pd nanoparticles on defective polymer carbon nitride: Enhanced activity and origin for electrocatalytic hydrodechlorination reaction[J]. Chinese Chemical Letters, 2020, 31(10): 2762-2768. |
| 17 | SUN Chen, LOU Zimo, LIU Yu, et al. Influence of environmental factors on the electrocatalytic dechlorination of 2,4-dichlorophenoxyacetic acid on nTiN doped Pd/Ni foam electrode[J]. Chemical Engineering Journal, 2015, 281: 183-191. |
| 18 | LIU Rui, ZHAO Huachao, ZHAO Xiaoyu, et al. Defect sites in ultrathin Pd nanowires facilitate the highly efficient electrochemical hydrodechlorination of pollutants by H*ads [J]. Environmental Science & Technology, 2018, 52(17): 9992-10002. |
| 19 | WU Yifan, GAN Ling, ZHANG Shupeng, et al. Carbon-nanotube-doped Pd-Ni bimetallic three-dimensional electrode for electrocatalytic hydrodechlorination of 4-chlorophenol: Enhanced activity and stability[J]. Journal of Hazardous Materials, 2018, 356: 17-25. |
| 20 | WU Yifan, GAN Ling, ZHANG Shupeng, et al. Enhanced electrocatalytic dechlorination of para-chloronitrobenzene based on Ni/Pd foam electrode[J]. Chemical Engineering Journal, 2017, 316: 146-153. |
| 21 | LOU Zimo, XU Jiang, ZHOU Jiasheng, et al. Insight into atomic H* generation, H2 evolution, and cathode potential of MnO2 induced Pd/Ni foam cathode for electrocatalytic hydrodechlorination[J]. Chemical Engineering Journal, 2019, 374: 211-220. |
| 22 | YU Weiting, JIANG He, FANG Jinhui, et al. Designing an electron-deficient Pd/NiCo2O4 bifunctional electrocatalyst with an enhanced hydrodechlorination activity to reduce the consumption of Pd[J]. Environmental Science & Technology, 2021, 55(14): 10087-10096. |
| 23 | LI Junxi, CHEN Yanju, BAI Ruiyu, et al. Construction of Pd/Ni2P-Ni foam nanosheet array electrode by in situ phosphatization-electrodeposition strategy for synergistic electrocatalytic hydrodechlorination[J]. Chemical Engineering Journal, 2022, 435: 134932. |
| 24 | LOU Zimo, ZHOU Jiasheng, SUN Mei, et al. MnO2 enhances electrocatalytic hydrodechlorination by Pd/Ni foam electrodes and reduces Pd needs[J]. Chemical Engineering Journal, 2018, 352: 549-557. |
| 25 | CHEN Ge, WANG Zhenyao, XIA Dingguo. Electrochemically codeposited palladium/molybdenum oxide electrode for electrocatalytic reductive dechlorination of 4-chlorophenol[J]. Electrochemistry Communications, 2004, 6(3): 268-272. |
| 26 | LIAO Hanbin, WEI Chao, WANG Jingxian, et al. A multisite strategy for enhancing the hydrogen evolution reaction on a nano-Pd surface in alkaline media[J]. Advanced Energy Materials, 2017, 7(21): 1701129. |
| 27 | LUO Mingchuan, GUO Shaojun. Strain-controlled electrocatalysis on multimetallic nanomaterials[J]. Nature Reviews Materials, 2017, 2(11): 17059. |
| 28 | RATCLIFF Erin L, Clayton SHALLCROSS R, ARMSTRONG Neal R. Introduction: Electronic materials[J]. Chemical Reviews, 2016, 116(21): 12821-12822. |
| 29 | GAO Fei, ZHANG Yangping, SONG Pingping, et al. Self-template construction of Sub-24nmPd Ag hollow nanodendrites as highly efficient electrocatalysts for ethylene glycol oxidation[J]. Journal of Power Sources, 2019, 418: 186-192. |
| 30 | LI Angzhen, ZHAO Xu, HOU Yining, et al. The electrocatalytic dechlorination of chloroacetic acids at electrodeposited Pd/Fe-modified carbon paper electrode[J]. Applied Catalysis B: Environmental, 2012, 111: 628-635. |
| 31 | CHEN Yali, XIONG Lu, SONG Xiangning, et al. Electrocatalytic hydrodehalogenation of atrazine in aqueous solution by Cu@Pd/Ti catalyst[J]. Chemosphere, 2015, 125: 57-63. |
| 32 | ESCOBEDO Ericson, KIM Jihun, Dasom OH, et al. Electrocatalytic dehalogenation of aqueous pollutants by dealloyed nanoporous Pd/Ti cathode[J]. Catalysis Today, 2021, 361: 63-68. |
| 33 | PENG Yiyin, CUI Meiyang, ZHANG Zhiyong, et al. Bimetallic composition-promoted electrocatalytic hydrodechlorination reaction on silver-palladium alloy nanoparticles[J]. ACS Catalysis, 2019, 9(12): 10803-10811. |
| 34 | CHEN Yanju, FENG Chao, WANG Wenhong, et al. Electronic structure engineering of bimetallic Pd-Au alloy nanocatalysts for improving electrocatalytic hydrodechlorination performance[J]. Separation and Purification Technology, 2022, 289: 120731. |
| 35 | CHEN Yanju, LIU Zhi, LIU Shoujie, et al. In-situ doping-induced crystal form transition of amorphous Pd-P catalyst for robust electrocatalytic hydrodechlorination[J]. Applied Catalysis B: Environmental, 2021, 284: 119713. |
| 36 | WANG Kaifeng, SHU Song, CHEN Min, et al. Pd-TiO2 Schottky heterojunction catalyst boost the electrocatalytic hydrodechlorination reaction[J]. Chemical Engineering Journal, 2020, 381: 122673. |
| 37 | CHEN Min, SHU Song, LI Junxi, et al. Activating palladium nanoparticles via a Mott-Schottky heterojunction in electrocatalytic hydrodechlorination reaction[J]. Journal of Hazardous Materials, 2020, 389: 121876. |
| 38 | WANG Peng, SHI Xuelin, FU Chunhong, et al. Strong pyrrolic-N-Pd interactions boost the electrocatalytic hydrodechlorination reaction on palladium nanoparticles[J]. Nanoscale, 2020, 12(2): 843-850. |
| 39 | LOU Zimo, YU Chaochao, WEN Xiaofei, et al. Construction of Pd nanoparticles/two-dimensional Co-MOF nanosheets heterojunction for enhanced electrocatalytic hydrodechlorination[J]. Applied Catalysis B: Environmental, 2022, 317: 121730. |
| 40 | FU Wenyang, SHU Song, LI Junxi, et al. Identifying the rate-determining step of the electrocatalytic hydrodechlorination reaction on palladium nanoparticles[J]. Nanoscale, 2019, 11(34): 15892-15899. |
| 41 | FENG Jiyu, RAMACHANDRAN Ranjith K, SOLANO Eduardo, et al. Tuning size and coverage of Pd nanoparticles using atomic layer deposition[J]. Applied Surface Science, 2021, 539: 148238. |
| 42 | YUAN Qiuyi, DOAN Hieu A, GRABOW Lars C, et al. Finite size effects in submonolayer catalysts investigated by CO electrosorption on PtsML/Pd(100)[J]. Journal of the American Chemical Society, 2017, 139(39): 13676-13679. |
| 43 | ZHANG Zhiqiang, LU Jinsuo, ZHANG Bing, et al. Insight into the size effect of Pd nanoparticles on the catalytic reduction of nitrite in water over Pd/C catalysts[J]. Environmental Science: Nano, 2020, 7(7): 2117-2129. |
| 44 | HE Zhiqiao, JIAN Qiwei, TANG Juntao, et al. Improvement of electrochemical reductive dechlorination of 2,4-dichlorophenoxyacetic acid using palladium catalysts prepared by a pulsed electrodeposition method[J]. Electrochimica Acta, 2016, 222: 488-498. |
| 45 | SHU Xiaoyu, YANG Qi, YAO Fubing, et al. Electrocatalytic hydrodechlorination of 4-chlorophenol on Pd supported multi-walled carbon nanotubes particle electrodes[J]. Chemical Engineering Journal, 2019, 358: 903-911. |
| 46 | MAO Mingyue, WU Jie, WANG Yi, et al. Active site and adsorption behavior engineering of subsize PdNi nanoparticles for boosting electrocatalytic hydrodechlorination of 4-chlorophenol[J]. Applied Surface Science, 2022, 600: 153988. |
| 47 | LEE Hyunjoo. Utilization of shape-controlled nanoparticles as catalysts with enhanced activity and selectivity[J]. RSC Advances, 2014, 4(77): 41017-41027. |
| 48 | WU Zhijie, PAN Tao, CHAI Yan, et al. Synthesis of palladium phosphides for aqueous phase hydrodechlorination: Kinetic study and deactivation resistance[J]. Journal of Catalysis, 2018, 366: 80-90. |
| 49 | QIN Shiyi, LEI Chao, WANG Xuxu, et al. Electrocatalytic activation of organic chlorides via direct and indirect electron transfer using atomic vacancy control of palladium-based catalyst[J]. Cell Reports Physical Science, 2022, 3(1): 100713. |
| 50 | LU Suwei, WENG Bo, CHEN Aizhu, et al. Facet engineering of Pd nanocrystals for enhancing photocatalytic hydrogenation: Modulation of the Schottky barrier height and enrichment of surface reactants[J]. ACS Applied Materials & Interfaces, 2021, 13(11): 13044-13054. |
| 51 | LOU Yaoyin, XIAO Chi, FANG Jiayi, et al. High activity of step sites on Pd nanocatalysts in electrocatalytic dechlorination[J]. Physical Chemistry Chemical Physics, 2022, 24(6): 3896-3904. |
| 52 | HE Zhen, DUAN Qiaohui, WANG Chengming, et al. Atom-stepped surface-regulated Pd nanowires for boosting alcohol oxidation activity[J]. Journal of Colloid and Interface Science, 2023, 646: 529-537. |
| 53 | WANG Minmin, ZHANG Hui, LIU Yunqi, et al. Research progress of precise structural regulation of single atom catalyst for accelerating electrocatalytic oxygen reduction reaction[J]. Journal of Energy Chemistry, 2022, 72: 56-72. |
| 54 | PAN Yuan, LI Min, MI Wanliang, et al. Single-atomic Mn sites coupled with Fe3C nanoparticles encapsulated in carbon matrixes derived from bimetallic Mn/Fe polyphthalocyanine conjugated polymer networks for accelerating electrocatalytic oxygen reduction[J]. Nano Research, 2022, 15(9): 7976-7985. |
| 55 | CHEN Zupeng, VOROBYEVA Evgeniya, MITCHELL Sharon, et al. A heterogeneous single-atom palladium catalyst surpassing homogeneous systems for Suzuki coupling[J]. Nature Nanotechnology, 2018, 13(8): 702-707. |
| 56 | PAN Yuan, ZHANG Chao, LIN Yan, et al. Electrocatalyst engineering and structure-activity relationship in hydrogen evolution reaction: From nanostructures to single atoms[J]. Science China Materials, 2020, 63(6): 921-948. |
| 57 | LI Min, ZHU Houyu, YUAN Qing, et al. Proximity electronic effect of Ni/Co diatomic sites for synergistic promotion of electrocatalytic oxygen reduction and hydrogen evolution[J]. Advanced Functional Materials, 2023, 33(4): 2210867. |
| 58 | HUANG Dahong, KIM David J, RIGBY Kali, et al. Elucidating the role of single-atom Pd for electrocatalytic hydrodechlorination[J]. Environmental Science & Technology, 2021, 55(19): 13306-13316. |
| 59 | MIN Yuan, ZHOU Xiao, CHEN Jiejie, et al. Integrating single-cobalt-site and electric field of boron nitride in dechlorination electrocatalysts by bioinspired design[J]. Nature Communications, 2021, 12(1): 303. |
| 60 | XU Yinghua, YAO Zeqing, MAO Zhechuan, et al. Single-Ni-atom catalyzes aqueous phase electrochemical reductive dechlorination reaction[J]. Applied Catalysis B: Environmental, 2020, 277: 119057. |
| 61 | CHU Chiheng, HUANG Dahong, GUPTA Srishti, et al. Neighboring Pd single atoms surpass isolated single atoms for selective hydrodehalogenation catalysis[J]. Nature Communications, 2021, 12(1): 5179. |
| 62 | LIU Zhijie, BETTERTON Eric A, ARNOLD Robert G. Electrolytic reduction of low molecular weight chlorinated aliphatic compounds: structural and thermodynamic effects on process kinetics[J]. Environmental Science & Technology, 2000, 34(5): 804-811. |
| 63 | DURANTE Christian, PERAZZOLO Valentina, PERINI Lorenzo, et al. Electrochemical activation of carbon-halogen bonds: Electrocatalysis at silver/copper nanoparticles[J]. Applied Catalysis B: Environmental, 2014, 158: 286-295. |
| 64 | GUO Yun, LI Yang, WANG Zhiwei. Electrocatalytic hydro-dehalogenation of halogenated organic pollutants from wastewater: A critical review[J]. Water Research, 2023, 234: 119810. |
| 65 | LI Zhouyan, LI Xuesong, LI Yang, et al. Efficient removal of micropollutants from low-conductance surface water using an electrochemical Janus ceramic membrane filtration system[J]. Water Research, 2022, 220: 118627. |
| 66 | SCIALDONE Onofrio, GUARISCO Chiara, GALIA Alessandro, et al. Electroreduction of aliphatic chlorides at silver cathodes in water[J]. Journal of Electroanalytical Chemistry, 2010, 641(1/2): 14-22. |
| 67 | GUO Kaiheng, WU Zihao, SHANG Chii, et al. Radical chemistry and structural relationships of PPCP degradation by UV/chlorine treatment in simulated drinking water[J]. Environmental Science & Technology, 2017, 51(18): 10431-10439. |
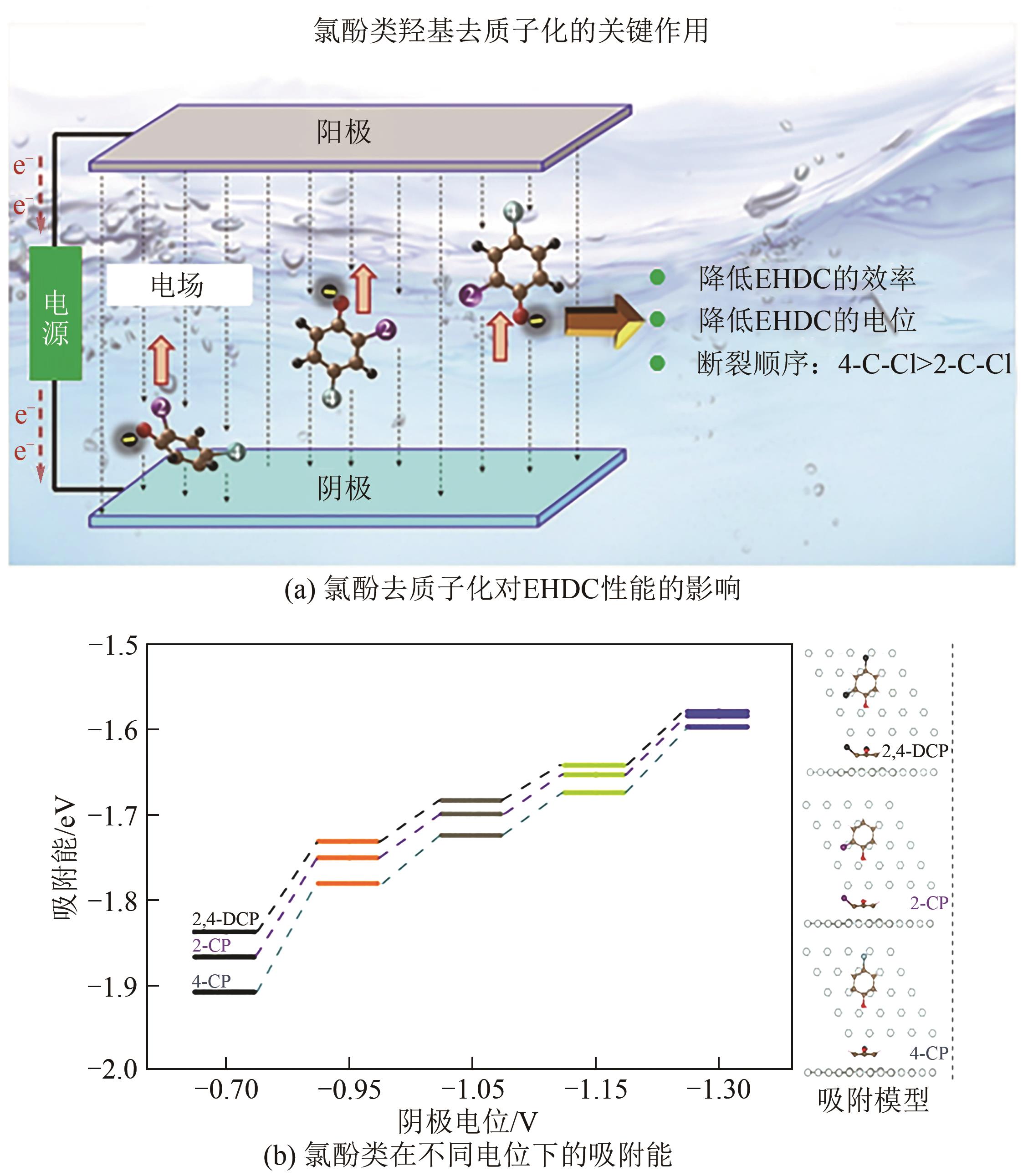
| 68 | SHU Song, FU Wenyang, WANG Peng, et al. Electrocatalytic hydrodechlorination of 2,4-dichlorophenol over palladium nanoparticles: The critical role of hydroxyl group deprotonation[J]. Applied Catalysis A: General, 2019, 583: 117146. |
| 69 | LI Junjing, WANG Huan, WANG Liang, et al. The preparation of Pd/foam-Ni electrode and its electrocatalytic hydrodechlorination for monochlorophenol isomers[J]. Catalysts, 2018, 8(9): 378. |
| 70 | HAN Jun, DEMING Richard L, TAO Fuming. Theoretical study of molecular structures and properties of the complete series of chlorophenols[J]. Journal of Physical Chemistry A, 2004, 108(38): 7736-7743. |
| 71 | LI Junjing, LIU Huiling, CHENG Xiuwen, et al. Stability of palladium-polypyrrole-foam nickel electrode and its electrocatalytic hydrodechlorination for dichlorophenol isomers[J]. Industrial & Engineering Chemistry Research, 2012, 51(48): 15557-15563. |
| 72 | CELIK Gokhan, AILAWAR Saurabh A, GUNDUZ Seval, et al. Aqueous-phase hydrodechlorination of trichloroethylene over Pd-based swellable organically modified silica: Catalyst deactivation due to sulfur species[J]. Industrial & Engineering Chemistry Research, 2019, 58(10): 4054-4064. |
| 73 | JIANG Cuishuang, YU Hongbin, WANG Xinhong, et al. Preparation of the palladium/polymeric pyrrole-multi-walled carbon nanotubes film/titanium electrode and its performance for the dechlorination of 4-chlorophenol[J]. International Journal of Electrochemical Science, 2017, 12(6): 5208-5219. |
| 74 | HUANG Binbin, LI Jing, CAO Xingkai, et al. Electrochemical reduction of p-chloronitrobenzene (p-CNB) at silver cathode in dimethylformamide[J]. Electrochimica Acta, 2019, 296: 980-988. |
| 75 | JIANG Guangming, WANG Kaifeng, LI Jieyuan, et al. Electrocatalytic hydrodechlorination of 2,4-dichlorophenol over palladium nanoparticles and its pH-mediated tug-of-war with hydrogen evolution[J]. Chemical Engineering Journal, 2018, 348: 26-34. |
| 76 | SUN Zhirong, WEI Xuefeng, HAN Yanbo, et al. Complete dechlorination of 2,4-dichlorophenol in aqueous solution on palladium/polymeric pyrrole-cetyl trimethyl ammonium bromide/foam-nickel composite electrode[J]. Journal of Hazardous Materials, 2013, 244/245: 287-294. |
| 77 | SUN Zhirong, SHEN Haitao, WEI Xuefeng, et al. Electrocatalytic hydrogenolysis of chlorophenols in aqueous solution on Pd58Ni42 cathode modified with PPy and SDBS[J]. Chemical Engineering Journal, 2014, 241: 433-442. |
| 78 | LOU Zimo, LI Yizhou, ZHOU Jiasheng, et al. TiC doped palladium/nickel foam cathode for electrocatalytic hydrodechlorination of 2,4-DCBA: Enhanced electrical conductivity and reactive activity[J]. Journal of Hazardous Materials, 2019, 362: 148-159. |
| 79 | LIU Qiuxiang, SHEN Yanting, SONG Shuang, et al. Enhanced electrocatalytic hydrodechlorination of 2,4-dichlorophenoxyacetic acid by a Pd-Co3O4/Ni foam electrode[J]. RSC Advances, 2019, 9(21): 12124-12133. |
| 80 | SUN Chen, BAIG Shams ALI, LOU Zimo, et al. Electrocatalytic dechlorination of 2,4-dichlorophenoxyacetic acid using nanosized titanium nitride doped palladium/nickel foam electrodes in aqueous solutions[J]. Applied Catalysis B: Environmental, 2014, 158/159: 38-47. |
| [1] | 林梅洁, 米烁东, 包成. 金属-掺杂氧化铈体系H2/CO电化学反应机理研究进展[J]. 化工进展, 2024, 43(S1): 209-224. |
| [2] | 周渝, 夏太阳, 韦奇, 唐甜, 田磊. 微通道耦合反渗透膜串联处理甲醇制烯烃废水工艺优化[J]. 化工进展, 2024, 43(S1): 43-51. |
| [3] | 马桂璇, 徐子桐, 肖志华, 宁国庆, 魏强, 徐春明. 氧硫双掺杂CNTs水系导电剂辅助构筑高性能石墨/SiO负极[J]. 化工进展, 2024, 43(S1): 443-456. |
| [4] | 朱昊, 刘汉飞, 高源, 黄益平, 费孝诚, 韩卫清. 盐分对电催化降解性能与机理的影响[J]. 化工进展, 2024, 43(S1): 571-580. |
| [5] | 梁宏成, 赵冬妮, 权银, 李敬妮, 胡欣怡. SEI膜形貌与结构对锂离子电池性能的影响[J]. 化工进展, 2024, 43(9): 5049-5062. |
| [6] | 吴剑扬, 王汝娜, 陈耀, 申兰耀, 于永利, 蒋宁, 邱景义, 周恒辉. 锂离子电池高镍正极材料前体的制备工艺[J]. 化工进展, 2024, 43(9): 5079-5085. |
| [7] | 李美萱, 成建凤, 黄国勇, 徐盛明, 郁丰善, 翁雅青, 曹才放, 温嘉玮, 王俊莲, 王春霞, 顾斌涛, 张袁华, 刘斌, 王才平, 潘剑明, 徐泽良, 王翀, 王珂. 高电压镍锰酸锂正极材料的合成与电化学机理[J]. 化工进展, 2024, 43(9): 5086-5094. |
| [8] | 屈芸, 成丽媛, 代国亮, 王刚, 郭羽晴, 孙洁. PAN/MXene同轴纤维电极的制备及性能[J]. 化工进展, 2024, 43(9): 5113-5122. |
| [9] | 安芳芳, 曹少磊, 连增帅, 舒大武, 张岩, 李万新, 韩博. 十二烷基甜菜碱对热活化过硫酸钠降解C.I.活性黑5的影响[J]. 化工进展, 2024, 43(9): 5302-5308. |
| [10] | 王正峰, 谢雨杭, 李伟科, 范永春, 康钟尹, 付乾. 多孔炭修饰的吸附催化一体化电极高效电解碳酸氢盐[J]. 化工进展, 2024, 43(9): 4892-4899. |
| [11] | 宋占龙, 汤涛, 潘蔚, 赵希强, 孙静, 毛岩鹏, 王文龙. 微纳米气泡强化臭氧氧化降解含酚废水[J]. 化工进展, 2024, 43(8): 4614-4623. |
| [12] | 杨光, 姜瑞婷, 张玥, 符子剑, 刘伟. 五氧化二钒/碳纳米复合材料在超级电容器中的应用[J]. 化工进展, 2024, 43(7): 3857-3871. |
| [13] | 罗臻, 王庆吉, 王占生, 杨雪莹, 谢加才, 王浩. 炼化污染场地抽出水强氧化短程处理工艺[J]. 化工进展, 2024, 43(7): 4155-4163. |
| [14] | 朱连燕, 周幸福. 锰掺杂DSA电极及其对印染废水处理的过程优化[J]. 化工进展, 2024, 43(6): 3459-3467. |
| [15] | 刘梦凡, 王华伟, 王亚楠, 张艳茹, 蒋旭彤, 孙英杰. Bio-FeMnCeO x 活化PMS降解四环素效能与机制[J]. 化工进展, 2024, 43(6): 3492-3502. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||