化工进展 ›› 2023, Vol. 42 ›› Issue (2): 783-793.DOI: 10.16085/j.issn.1000-6613.2022-0630
焦炉煤气常量含碳气氛对加氢脱硫催化剂活性、选择性和积炭的影响
李乃珍1( ), 孙瑞洁1, 秦志峰1,2,3(
), 孙瑞洁1, 秦志峰1,2,3( ), 苗茂谦1, 吴琼笑4, 常丽萍1, 孙鹏程2, 曾剑2, 刘毅3
), 苗茂谦1, 吴琼笑4, 常丽萍1, 孙鹏程2, 曾剑2, 刘毅3
- 1.太原理工大学省部共建煤基能源清洁高效利用国家重点实验室,山西 太原 030024
2.山西省环境规划院 博士后科研工作站,山西 太原 030002
3.清华大学环境科学与工程博士后流动站,北京 100084
4.山西道生鑫宇清洁能源有限公司,山西 忻州 036300
-
收稿日期:2022-04-12修回日期:2022-06-19出版日期:2023-02-25发布日期:2023-03-13 -
通讯作者:秦志峰 -
作者简介:李乃珍(1995—),女,硕士研究生,研究方向为气体净化与工业催化。E-mail:lnzlldmmmmd@163.com。 -
基金资助:2016年度山西省科技重大专项(MJH2016-03);东莞市能源投资集团有限公司攻关项目(RH2000004464)
Effects of constant carbon atmosphere on the activity, selectivity and coking of catalysts in hydrodesulfurization of coke oven gas
LI Naizhen1( ), SUN Ruijie1, QIN Zhifeng1,2,3(
), SUN Ruijie1, QIN Zhifeng1,2,3( ), MIAO Maoqian1, WU Qiongxiao4, CHANG Liping1, SUN Pengcheng2, ZENG Jian2, LIU Yi3
), MIAO Maoqian1, WU Qiongxiao4, CHANG Liping1, SUN Pengcheng2, ZENG Jian2, LIU Yi3
- 1.Provincial and Ministry Jointly Build the State Key Laboratory of Clean and Efficient Utilization of Coal-based Energy, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
2.Postdoctoral Research Station, Environmental Planning Institute of Shanxi Province, Taiyuan 030002, Shanxi, China
3.Environmental Science and Engineering Postdoctoral Mobile Station, Tsinghua University, Beijing 100084, China
4.Daosheng Xinyu Clean Energy Company Limited, Xinzhou 036300, Shanxi, China
-
Received:2022-04-12Revised:2022-06-19Online:2023-02-25Published:2023-03-13 -
Contact:QIN Zhifeng
摘要:
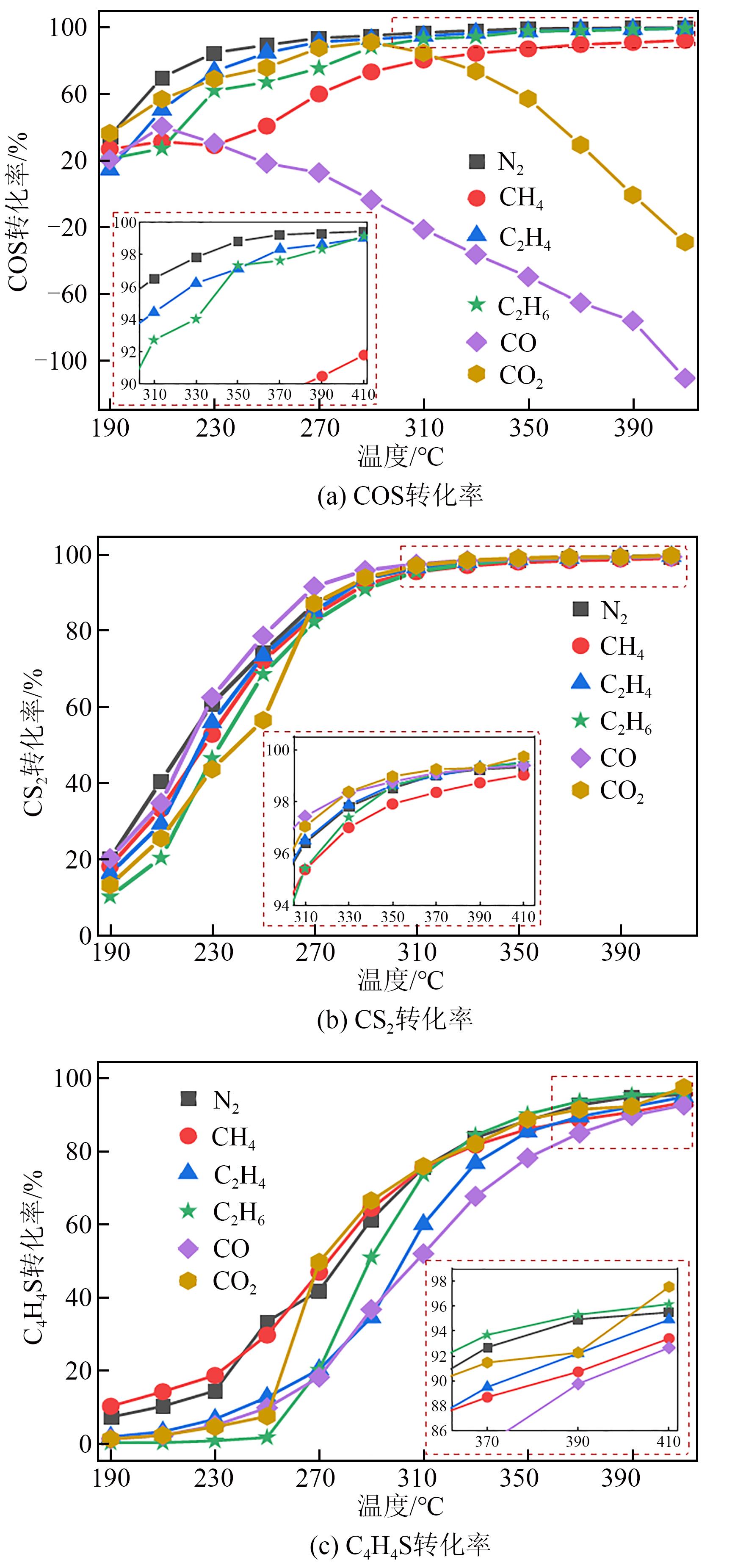
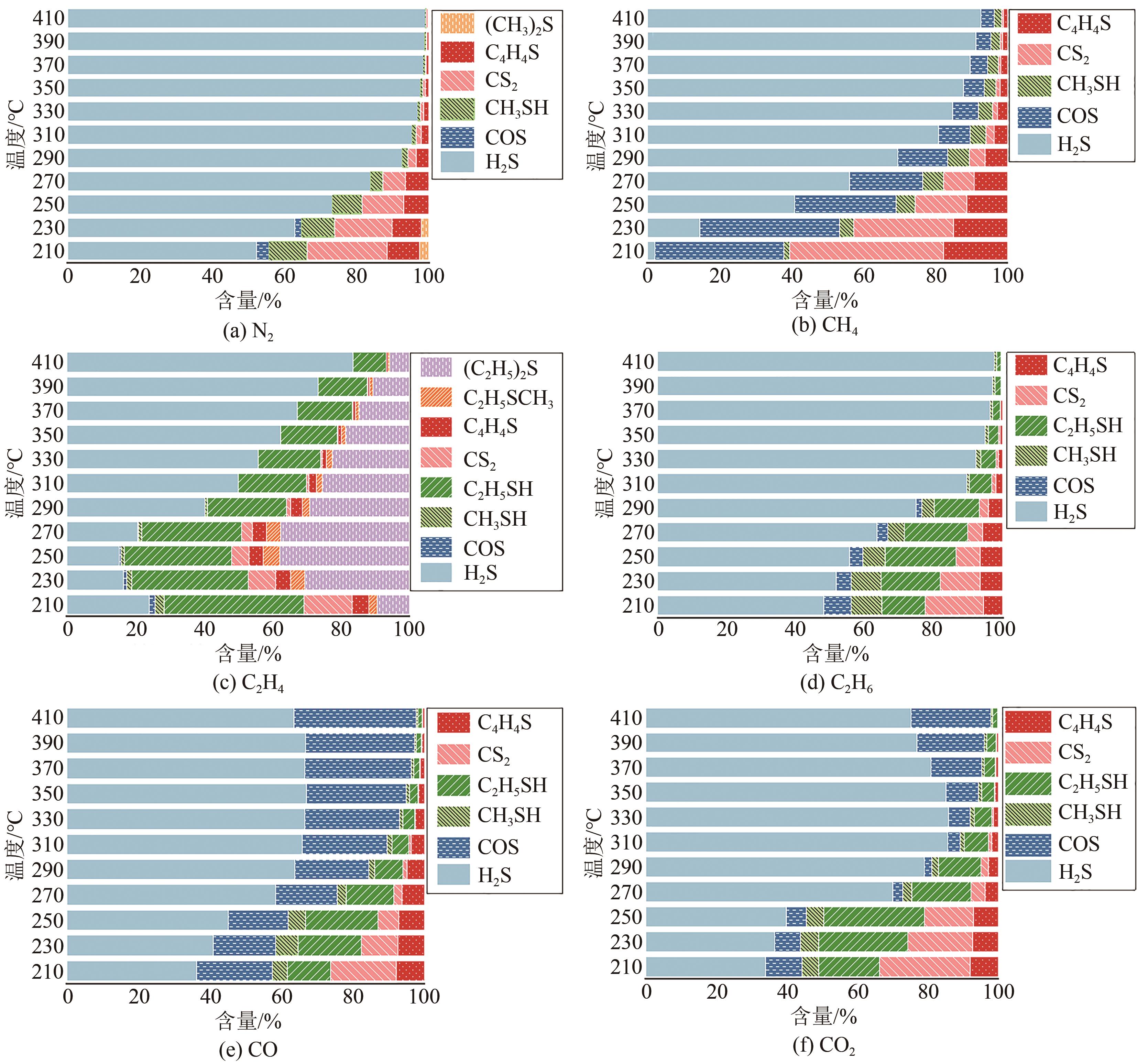
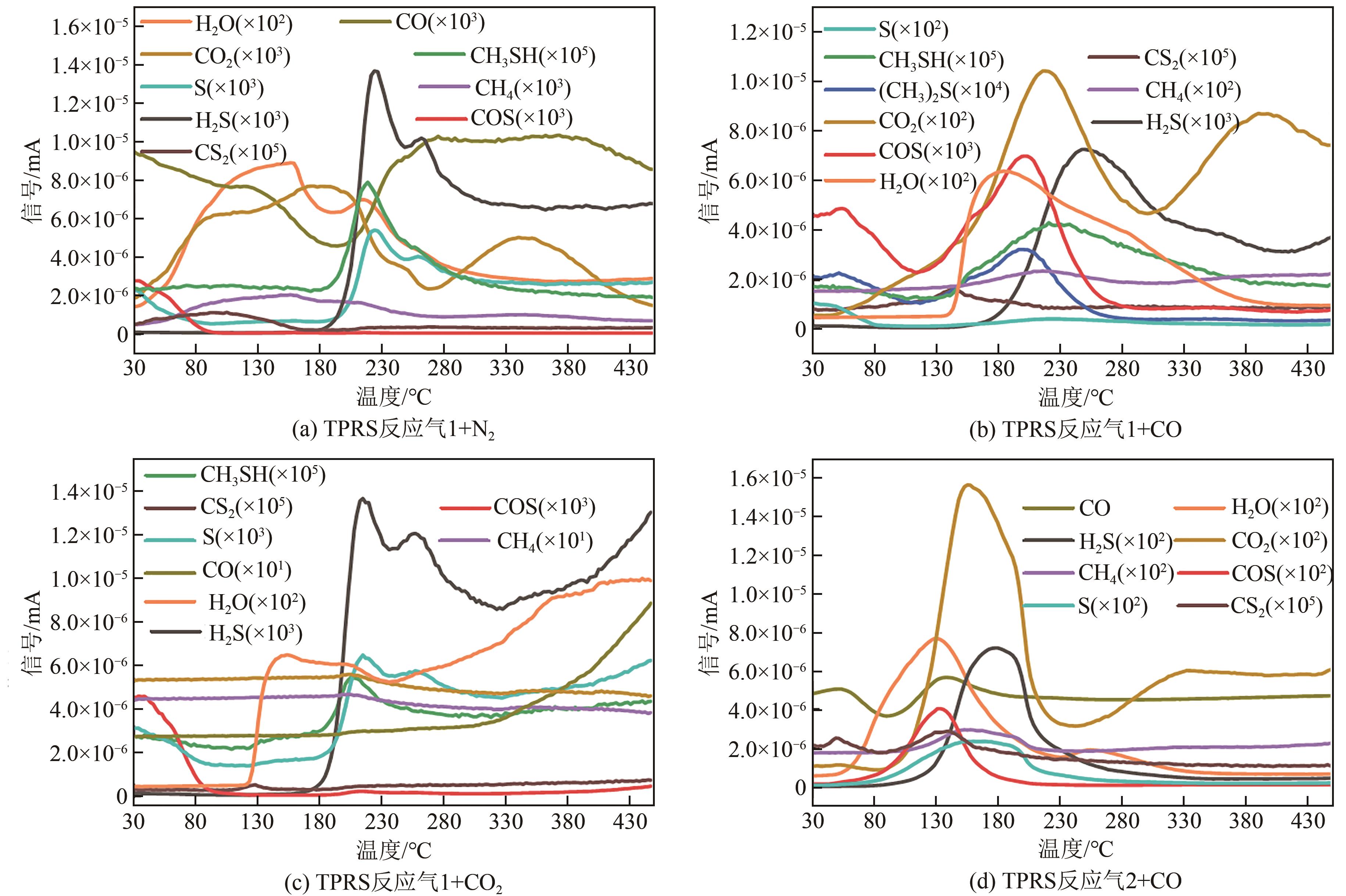
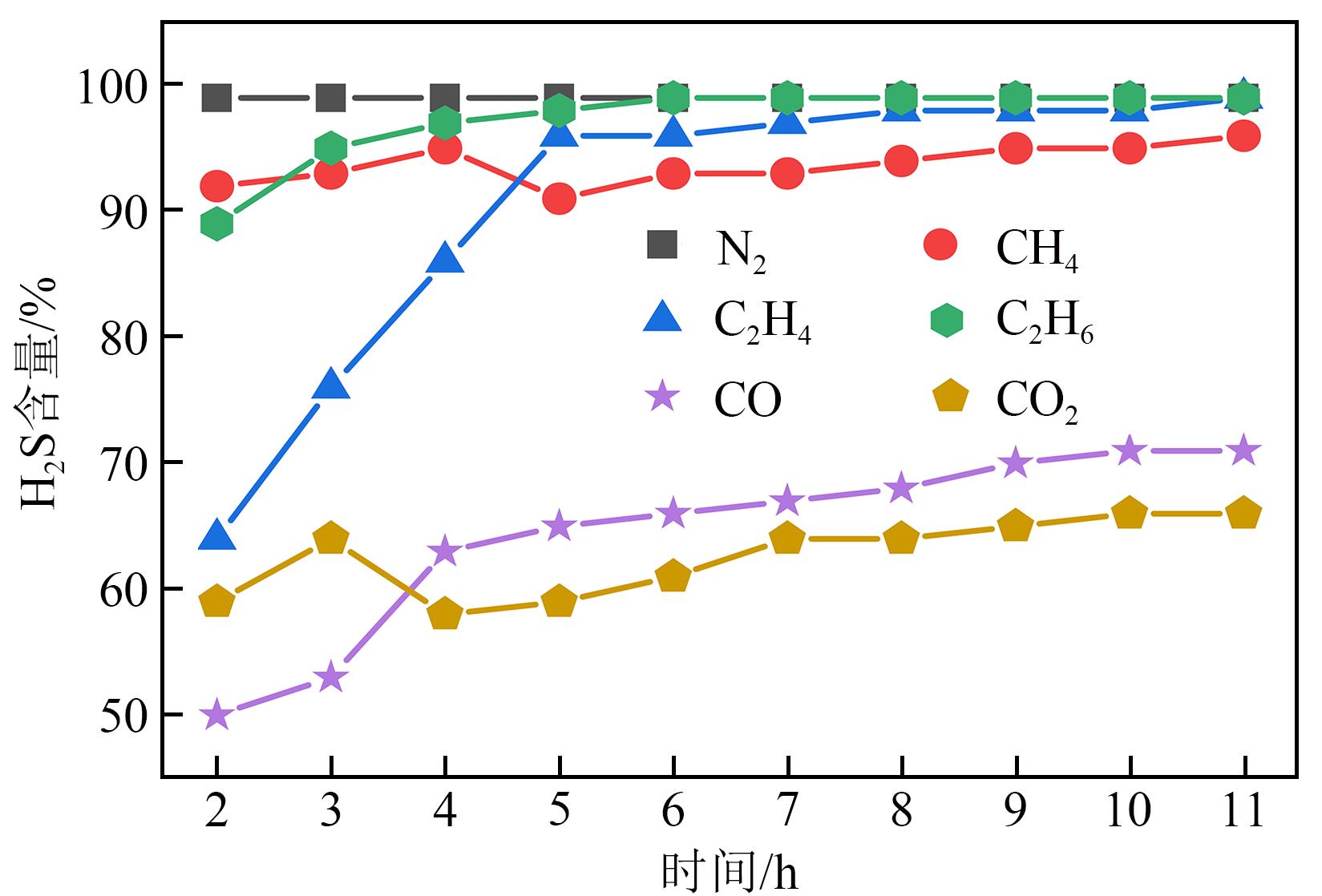
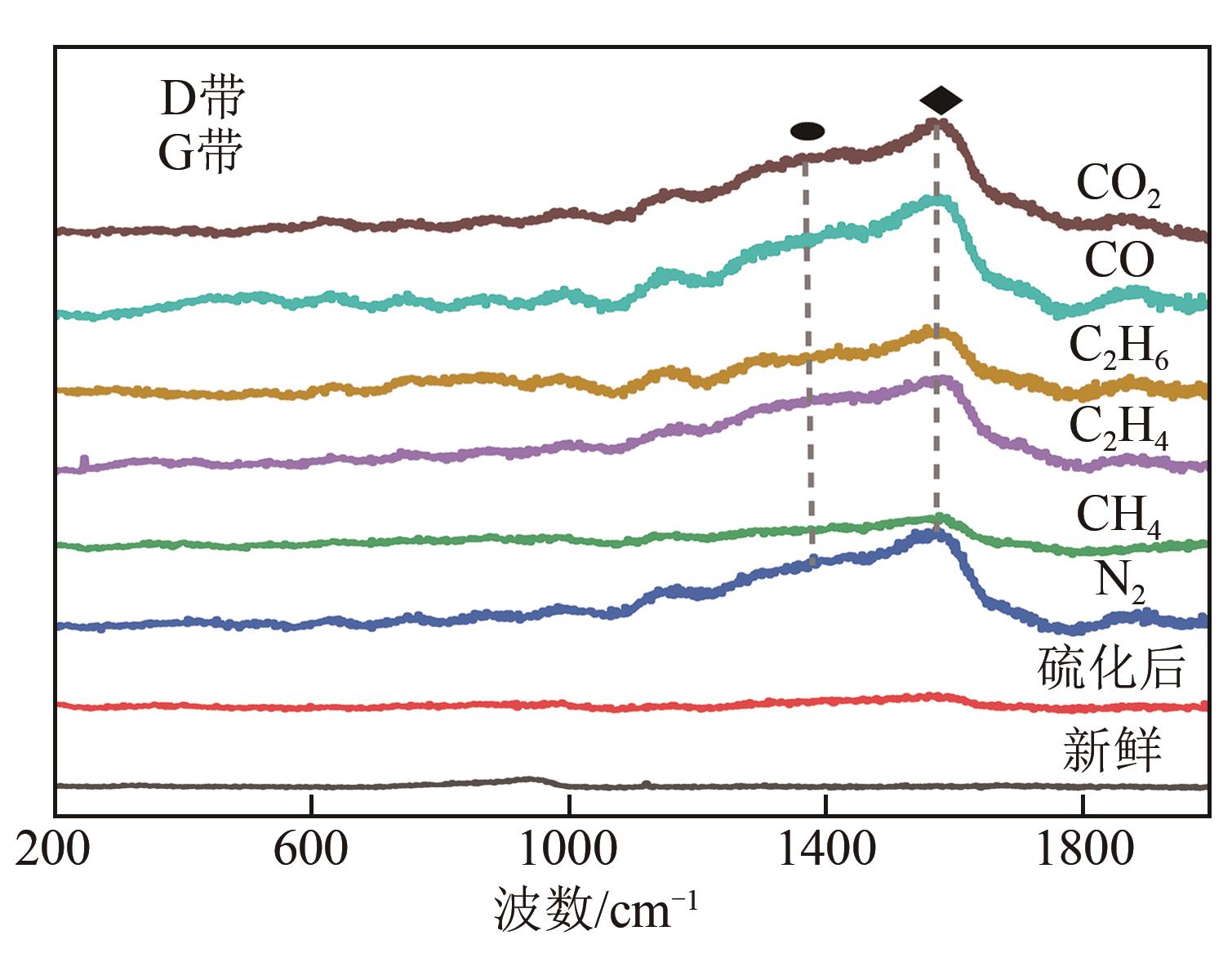
利用微型固定床反应装置对工业Fe-Mo预加氢脱硫催化剂进行加氢脱硫(HDS)评价,研究焦炉煤气中不同常量含碳原料组分(CH4、C2H4、C2H6、CO、CO2)对催化剂加氢活性、选择性以及积炭的影响,并采用红外碳硫分析仪、N2吸附-脱附、Raman以及TPRS-MS对催化剂进行表征。结果表明:在N2气氛下,COS、CS2和C4H4S加氢转化由易到难顺序为:COS>CS2>C4H4S,但COS加氢转化受含碳气氛影响最明显,致使焦炉煤气加氢脱硫中COS难以完全脱除;不同气氛对硫化物加氢选择性都会产生影响,其中C2H4气氛对选择性影响最明显,而对H2S收率影响最明显的是CO2和CO;不同含碳气氛在410℃下稳定反应11h后催化剂均发生积炭,主要以石墨化炭为主,其中C2H4气氛下积炭最严重,气氛对积炭影响顺序为:C2H4>CO2>CO>CH4 >N2>C2H6。
中图分类号:
引用本文
李乃珍, 孙瑞洁, 秦志峰, 苗茂谦, 吴琼笑, 常丽萍, 孙鹏程, 曾剑, 刘毅. 焦炉煤气常量含碳气氛对加氢脱硫催化剂活性、选择性和积炭的影响[J]. 化工进展, 2023, 42(2): 783-793.
LI Naizhen, SUN Ruijie, QIN Zhifeng, MIAO Maoqian, WU Qiongxiao, CHANG Liping, SUN Pengcheng, ZENG Jian, LIU Yi. Effects of constant carbon atmosphere on the activity, selectivity and coking of catalysts in hydrodesulfurization of coke oven gas[J]. Chemical Industry and Engineering Progress, 2023, 42(2): 783-793.
| 气体名称 | 纯度(体积分数)或含量 | 平衡气体 |
|---|---|---|
| 甲烷 | 99.99% | — |
| 乙烯 | 99.9% | — |
| 乙烷 | 99.9% | — |
| 一氧化碳 | 99.99% | — |
| 二氧化碳 | 99.99% | — |
| 预硫化气体 | H2S约3% | H2 |
| 含硫反应气体 | COS约119.48mg/m3、CS2约91.28mg/m3、C4H4S约12.39mg/m3 | H2 |
| TPRS反应气1 | COS约2080mg/m3 | H2 |
| TPRS反应气2 | H2S约500mg/m3 | H2 |
| 硫化物标气 | H2S约143.32mg/m3、COS约155.40mg/m3、CH3SH约99.35mg/m3、C2H5SH约98.54mg/m3、CH3SCH3约102.23mg/m3、CS2约157.02mg/m3、C4H4S约147.57mg/m3、CH3S2CH3约148.57mg/m3、C2H5SCH3约146.79mg/m3、(C2H5)S约146.45mg/m3 | N2 |
表1 实验所用气体组分及纯度
| 气体名称 | 纯度(体积分数)或含量 | 平衡气体 |
|---|---|---|
| 甲烷 | 99.99% | — |
| 乙烯 | 99.9% | — |
| 乙烷 | 99.9% | — |
| 一氧化碳 | 99.99% | — |
| 二氧化碳 | 99.99% | — |
| 预硫化气体 | H2S约3% | H2 |
| 含硫反应气体 | COS约119.48mg/m3、CS2约91.28mg/m3、C4H4S约12.39mg/m3 | H2 |
| TPRS反应气1 | COS约2080mg/m3 | H2 |
| TPRS反应气2 | H2S约500mg/m3 | H2 |
| 硫化物标气 | H2S约143.32mg/m3、COS约155.40mg/m3、CH3SH约99.35mg/m3、C2H5SH约98.54mg/m3、CH3SCH3约102.23mg/m3、CS2约157.02mg/m3、C4H4S约147.57mg/m3、CH3S2CH3约148.57mg/m3、C2H5SCH3约146.79mg/m3、(C2H5)S约146.45mg/m3 | N2 |
| 序号 | 反应方程式 | ΔG/kJ·mol-1 | lgK | ||
|---|---|---|---|---|---|
| 250℃ | 350℃ | 250℃ | 350℃ | ||
| (1) | COS+H2=CO+H2S | -2.444 | -3.277 | 1.021 | 1.149 |
| (2) | COS+4H2=H2S+CH4+H2O | -20.480 | -13.451 | 8.556 | 4.718 |
| (3) | COS+H2O=CO2+H2S | -10.755 | -12.811 | 4.493 | 4.493 |
| (4) | 2COS+4H2=CH4+CS2+2H2O | -13.972 | -8.843 | 5.837 | 3.102 |
| (5) | CS2+4H2=2H2S+CH4 | -34.279 | -29.596 | 14.322 | 10.381 |
| (6) | C4H4S+4H2=H2S+i-C4H10 | -25.936 | -17.916 | 10.836 | 6.284 |
| (7) | CO2+CS2=2COS | -3.044 | -3.335 | 1.272 | 1.170 |
| (8) | CO2+H2=CH4+2H2O | -17.016 | -12.178 | 7.109 | 4.271 |
| (9) | H2S+CO=COS+H2+CH4 | -14.350 | -6.113 | 5.996 | -1.999 |
| (10) | H2+CO=CO2+CH4 | -26.348 | -19.708 | 11.008 | 6.912 |
| (11) | COS+3H2=CH3SH+H2O | -2.893 | 4.073 | 1.209 | -1.429 |
| (12) | CS2+3H2=CH3SH+H2S | -16.692 | -12.072 | 6.974 | 4.234 |
| (13) | 2CH3SH=(CH3)2S+H2S | -0.146 | 0.284 | 0.061 | -0.100 |
| (14) | CH3SH+H2=CH4+H2S | -17.587 | -17.524 | 7.348 | 6.147 |
| (15) | (CH3)2S+2H2=2CH4+H2S | -35.028 | -35.332 | 14.634 | 12.393 |
| (16) | C2H4+H2S=C2H5SH | -2.850 | 0.202 | 1.191 | -0.071 |
| (17) | 2C2H5SH=(C2H5)2S+H2S | -1.122 | -0.968 | 0.469 | 0.340 |
| (18) | C2H5SH+C2H4=(C2H5)2S | -3.972 | -0.767 | 1.660 | 0.269 |
| (19) | C2H4+CH3SH=CH3SC2H5 | -3.373 | -0.021 | 1.409 | 0.007 |
| (20) | C2H5SH+H2=C2H6+H2S | -14.574 | -14.504 | 6.089 | 5.087 |
| (21) | CO+3H2=CH4+H2O | -18.036 | -10.174 | 7.535 | 3.569 |
| (22) | CO+H2O=CO2+H2 | -8.312 | -9.534 | 3.473 | 3.344 |
表2 反应方程及热力学参数汇总
| 序号 | 反应方程式 | ΔG/kJ·mol-1 | lgK | ||
|---|---|---|---|---|---|
| 250℃ | 350℃ | 250℃ | 350℃ | ||
| (1) | COS+H2=CO+H2S | -2.444 | -3.277 | 1.021 | 1.149 |
| (2) | COS+4H2=H2S+CH4+H2O | -20.480 | -13.451 | 8.556 | 4.718 |
| (3) | COS+H2O=CO2+H2S | -10.755 | -12.811 | 4.493 | 4.493 |
| (4) | 2COS+4H2=CH4+CS2+2H2O | -13.972 | -8.843 | 5.837 | 3.102 |
| (5) | CS2+4H2=2H2S+CH4 | -34.279 | -29.596 | 14.322 | 10.381 |
| (6) | C4H4S+4H2=H2S+i-C4H10 | -25.936 | -17.916 | 10.836 | 6.284 |
| (7) | CO2+CS2=2COS | -3.044 | -3.335 | 1.272 | 1.170 |
| (8) | CO2+H2=CH4+2H2O | -17.016 | -12.178 | 7.109 | 4.271 |
| (9) | H2S+CO=COS+H2+CH4 | -14.350 | -6.113 | 5.996 | -1.999 |
| (10) | H2+CO=CO2+CH4 | -26.348 | -19.708 | 11.008 | 6.912 |
| (11) | COS+3H2=CH3SH+H2O | -2.893 | 4.073 | 1.209 | -1.429 |
| (12) | CS2+3H2=CH3SH+H2S | -16.692 | -12.072 | 6.974 | 4.234 |
| (13) | 2CH3SH=(CH3)2S+H2S | -0.146 | 0.284 | 0.061 | -0.100 |
| (14) | CH3SH+H2=CH4+H2S | -17.587 | -17.524 | 7.348 | 6.147 |
| (15) | (CH3)2S+2H2=2CH4+H2S | -35.028 | -35.332 | 14.634 | 12.393 |
| (16) | C2H4+H2S=C2H5SH | -2.850 | 0.202 | 1.191 | -0.071 |
| (17) | 2C2H5SH=(C2H5)2S+H2S | -1.122 | -0.968 | 0.469 | 0.340 |
| (18) | C2H5SH+C2H4=(C2H5)2S | -3.972 | -0.767 | 1.660 | 0.269 |
| (19) | C2H4+CH3SH=CH3SC2H5 | -3.373 | -0.021 | 1.409 | 0.007 |
| (20) | C2H5SH+H2=C2H6+H2S | -14.574 | -14.504 | 6.089 | 5.087 |
| (21) | CO+3H2=CH4+H2O | -18.036 | -10.174 | 7.535 | 3.569 |
| (22) | CO+H2O=CO2+H2 | -8.312 | -9.534 | 3.473 | 3.344 |
| 样品 | 孔体积/cm3·g-1 | 比表面积/m2·g-1 | 平均孔径/nm | 碳质量分数/% | 硫质量分数/% |
|---|---|---|---|---|---|
| 新鲜催化剂 | 0.47 | 210.4 | 4.4 | 0.12 | 0.12 |
| 硫化后催化剂 | 0.45 | 190.8 | 4.4 | 0.29 | 3.62 |
| N2反应后 | 0.43 | 185.6 | 4.5 | 0.38 | 2.48 |
| CH4反应后 | 0.41 | 181.9 | 4.5 | 0.44 | 2.64 |
| C2H4反应后 | 0.41 | 178.1 | 4.6 | 0.77 | 2.56 |
| C2H6反应后 | 0.41 | 181.4 | 4.5 | 0.29 | 2.72 |
| CO反应后 | 0.42 | 182.5 | 4.5 | 0.47 | 2.56 |
| CO2反应后 | 0.42 | 184.4 | 4.5 | 0.54 | 2.58 |
表3 不同常量含碳气氛反应后催化剂物理性能
| 样品 | 孔体积/cm3·g-1 | 比表面积/m2·g-1 | 平均孔径/nm | 碳质量分数/% | 硫质量分数/% |
|---|---|---|---|---|---|
| 新鲜催化剂 | 0.47 | 210.4 | 4.4 | 0.12 | 0.12 |
| 硫化后催化剂 | 0.45 | 190.8 | 4.4 | 0.29 | 3.62 |
| N2反应后 | 0.43 | 185.6 | 4.5 | 0.38 | 2.48 |
| CH4反应后 | 0.41 | 181.9 | 4.5 | 0.44 | 2.64 |
| C2H4反应后 | 0.41 | 178.1 | 4.6 | 0.77 | 2.56 |
| C2H6反应后 | 0.41 | 181.4 | 4.5 | 0.29 | 2.72 |
| CO反应后 | 0.42 | 182.5 | 4.5 | 0.47 | 2.56 |
| CO2反应后 | 0.42 | 184.4 | 4.5 | 0.54 | 2.58 |
| 1 | PORTHA J F, URIBE-SOTO W, COMMENGE J M, et al. Techno-economic and carbon footprint analyses of a coke oven gas reuse process for methanol production[J]. Processes, 2021, 9(6): 1042. |
| 2 | 李慧敏. 焦炉煤气制天然气的重要意义[J]. 山西化工, 2019, 39(1): 103-104. |
| LI Huimin. The important significance of coke oven gas for natural gas production[J]. Shanxi Chemical Industry, 2019, 39(1): 103-104. | |
| 3 | 杜雄伟. 焦炉煤气制天然气工艺技术探讨[J]. 天然气化工(C1化学与化工), 2014, 39(4): 74-76. |
| DU Xiongwei. A discussion on processes for conversion of coke oven gas to natural gas[J]. Natural Gas Chemical Industry, 2014, 39(4): 74-76. | |
| 4 | 吴越峰, 吴双月. 利用焦炉气生产合成氨工艺路线探讨[C]//全国炼焦行业利用焦炉煤气生产甲醇及应用研讨会. 北京, 2006: 135-150. |
| WU Yuefeng, WU Shuangyue. Discussion on the process route of using coke oven gas to produce synthetic ammonia[C]//National Coking Industry Using Coke Oven Gas to Produce Methanol and Application Seminar. Beijing, 2006: 135-150. | |
| 5 | LIU Xin, YUAN Zengwei. Life cycle environmental performance of by-product coke production in China[J]. Journal of Cleaner Production, 2016, 112: 1292-1301. |
| 6 | VAN ACHT S C J, LAYCOCK C J, CARR S J W, et al. Optimization of VPSA-EHP/C process for high-pressure hydrogen recovery from coke oven gas using CO selective adsorbent[J]. International Journal of Hydrogen Energy, 2021, 46(1): 709-725. |
| 7 | 张华西, 安楚玉, 张礼树, 等. 焦炉煤气加氢脱硫催化剂的制备及应用[J]. 天然气化工(C1化学与化工), 2019, 44(2): 46-49. |
| ZHANG Huaxi, AN Chuyu, ZHANG Lishu, et al. Preparation and application of catalysts for hydrogenation desulfurization of coke-oven gas[J]. Natural Gas Chemical Industry, 2019, 44(2): 46-49. | |
| 8 | 康凤楠. 镁基储氢材料用于焦炉煤气加氢脱硫影响因素的研究——储氢材料与噻吩的反应[D]. 青岛: 山东科技大学, 2012. |
| KANG Fengnan. Study on influencing factors of magnesium-based hydrogen storage materials used in hydrodesulfurization of coke oven gas reaction of hydrogenstorage materials with thiophene[D]. Qingdao: Shandong University of Science and Technology, 2012. | |
| 9 | DE OLIVEIRA CARNEIRO L, DE VASCONCELOS S F, DE FARIAS NETO G W, et al. Improving H2S removal in the coke oven gas purification process[J]. Separation and Purification Technology, 2021, 257: 117862. |
| 10 | 周晓奇. 焦炉煤气深度净化工艺及其催化剂的研究开发[J]. 中国科技成果, 2011, 12(7): 24-25, 28. |
| ZHOU Xiaoqi. Research and development of advanced purification process of coke oven gas and its catalyst[J]. China Science and Technology Achievements, 2011, 12 (7): 24-25, 28. | |
| 11 | 刘慧. 焦炉煤气干法脱硫工艺的改进[J]. 燃料与化工, 2017, 48(6): 49-52. |
| LIU Hui. Improvement on dry desulfurization of coke oven gas[J]. Fuel & Chemical Processes, 2017, 48(6): 49-52. | |
| 12 | 裴学国, 亓栋, 丁卉, 等. T202型催化剂在焦炉气二级加氢脱硫工艺中的应用[J]. 化肥设计, 2011, 49(5): 48-51. |
| PEI Xueguo, QI Dong, DING Hui, et al. Application for T202 type catalyst in hydrogenation desulphurization process of coking-oven gas in 2 stages[J]. Chemical Fertilizer Design, 2011, 49(5): 48-51. | |
| 13 | 杨宝刚, 黄成忠, 朱军利. JT-8型加氢催化剂在焦炉煤气制甲醇装置上的工业应用[J]. 工业催化, 2014, 22(8): 628-631. |
| YANG Baogang, HUANG Chengzhong, ZHU Junli. Commercial application of JT-8 hydrogenation catalyst in the plant of methanol synthesized from coke oven gas[J]. Industrial Catalysis, 2014, 22(8): 628-631. | |
| 14 | 朱军利, 张林生, 盛明泽, 等. JT-8型焦炉煤气加氢催化剂失活样品剖析[J]. 工业催化, 2020, 28(9): 45-50. |
| ZHU Junli, ZHANG Linsheng, SHENG Mingze, et al. Analysis of deactivated hydrogenation catalyst JT-8[J]. Industrial Catalysis, 2020, 28(9): 45-50. | |
| 15 | 郭玉峰, 蒋晓娟. 焦炉气净化中的有机硫加氢工艺应用技术[J]. 山西化工, 2017, 37(3): 52-55. |
| GUO Yufeng, JIANG Xiaojuan. Application technology of organic sulfur hydrogenation process in purification of coke oven gas[J]. Shanxi Chemical Industry, 2017, 37(3): 52-55. | |
| 16 | 张书森. 铁钼预加氢化学清洗再生方法[J]. 中国化工贸易, 2015, 7(31): 297. |
| ZHANG Shusen. Fe-Mo pre-hydrogenation chemical cleaning and regeneration method[J]. China Chemical Trade, 2015, 7(31): 297. | |
| 17 | 陈程, 曹晓娜, 徐广通, 等. 渣油加氢失活催化剂的积炭规律[J]. 石油学报(石油加工), 2016, 32(6): 1221-1227. |
| CHEN Cheng, CAO Xiaona, XU Guangtong, et al. Pattern of coke deposition on the spent residue hydrotreating catalysts[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2016, 32(6): 1221-1227. | |
| 18 | 陈云宝, 刘建明. 抑制焦炉煤气精脱硫工序积碳对策探讨[C]//中国炼焦行业协会2012年中国焦化行业科技大会. 北京, 2012: 387-394. |
| CHENG Yunbao, LIU Jianming. Discussion on countermeasures for inhibiting carbon deposit in coke oven gas fine desulfurization process[C]// Proceedings of China Coking Industry Association’s 2012 China Coking Industry Science and Technology Conference. Beijing, 2012: 387-394. | |
| 19 | GUISNET M, MAGNOUX P. Coking and deactivation of zeolites[J]. Applied Catalysis, 1989, 54(1): 1-27. |
| 20 | 汪佩华, 秦志峰, 吴琼笑, 等. 磷添加方式对NiMo/Al2O3催化剂加氢脱硫性能的影响[J]. 化工进展, 2021, 40(2): 890-900. |
| WANG Peihua, QIN Zhifeng, WU Qiongxiao, et al. Effect of phosphorus adding manners on the performance of NiMo/Al2O3 catalyst in hydrodesulfurization[J]. Chemical Industry and Engineering Progress, 2021, 40(2): 890-900. | |
| 21 | 王芳芳, 赵海, 张德祥, 等. 铁锰系脱硫剂对煤气中羰基硫的脱硫机理初探[J]. 煤炭学报, 2008, 33(2): 197-200. |
| WANG Fangfang, ZHAO Hai, ZHANG Dexiang, et al. The desulfurization mechanism of iron-manganese compound oxide desulfurizer for removal of COS from coal gas[J]. Journal of China Coal Society, 2008, 33(2): 197-200. | |
| 22 | 张海鹰, 王旭珍, 赵宗彬, 等. 载Mo和Ni-Mo酚醛树脂基活性炭加氢催化转化羰基硫的研究[J]. 燃料化学学报, 2009, 37(5): 618-623. |
| ZHANG Haiying, WANG Xuzhen, ZHAO Zongbin, et al. Hydrodesulfurization of carbonyl sulfide over the catalysts Mo or Ni-Mo supported on phenolic resin-based activated carbon[J]. Journal of Fuel Chemistry and Technology, 2009, 37(5): 618-623. | |
| 23 | QU Yixin, XU Heming, ZHAO Jianfeng, et al. Conversion and reaction kinetics of coke oven gas over a commercial Fe-Mo/Al2O3 catalyst[J]. Journal of Central South University, 2016, 23(2): 293-302. |
| 24 | GUTIÉRREZ O Y, KAUFMANN C, HRABAR A, et al. Synthesis of methyl mercaptan from carbonyl sulfide over sulfide K2MoO4/SiO2 [J]. Journal of Catalysis, 2011, 280(2): 264-273. |
| 25 | BENSON J W, SCHRADER G L, ANGELICI R J. Studies of the mechanism of thiophene hydrodesulfurization: 2H NMR and mass spectral analysis of 1,3-butadiene produced in the deuterodesulfurization (DDS) of thiophene over PbMo6S8 catalyst[J]. Journal of Molecular Catalysis A: Chemical, 1995, 96(3): 283-299. |
| 26 | YANG Hongyuan, LIU Xin, LIU Qing, et al. WO x modified Ni catalyst supported on mesoporous silica with extra large mesopores for CO methanation[J]. Energy Technology, 2020, 8(6): 2000097. |
| 27 | LI Jiangwei, SONG Qi, LI Jiangbing, et al. La-enhanced Ni nanoparticles highly dispersed on SiC for low-temperature CO methanation performance[J]. Rare Metals, 2021, 40(7): 1753-1761. |
| 28 | SAMOKHVALOV A, TATARCHUK B J. Characterization of active sites, determination of mechanisms of H2S, COS and CS2 sorption and regeneration of ZnO low-temperature sorbents: Past, current and perspectives[J]. Physical Chemistry Chemical Physics: PCCP, 2011, 13(8): 3197-3209. |
| 29 | 周强. 煤的热解行为及硫的脱除[D]. 大连: 大连理工大学, 2004. |
| ZHOU Qiang. Behavior of coal and sulfur removal during pyrolysis[D]. Dalian: Dalian University of Technology, 2004. | |
| 30 | 沈芳, 上官炬, 梁生兆. 气氛对中温羰基硫水解催化剂的影响[C]//第十三届全国催化学术会议. 兰州, 2006. |
| SHEN Fang, SHANGGUAN Ju, LIANG Shengzhao. Influence of atmosphere on mesothermal carbonyl sulfide hydrolysis catalyst[C]// Proceedings of the 13th National Conference on Catalysis. Lanzhou: 2006. | |
| 31 | WHELAN M E, LENNARTZ S T, GIMENO T E, et al. Reviews and syntheses: Carbonyl sulfide as a multi-scale tracer for carbon and water cycles[J]. Biogeosciences, 2018, 15(12): 3625-3657. |
| 32 | GARCIA-MONTOTO V, VERDIER S, MAROUN Z, et al. Understanding the removal of V, Ni and S in crude oil atmospheric residue hydrodemetallization and hydrodesulfurization[J]. Fuel Processing Technology, 2020, 201: 106341. |
| 33 | 刘志凯. 焦炉煤气加氢脱硫FeMo催化剂的研究[D]. 武汉: 武汉理工大学, 2010. |
| LIU Zhikai. Study on FeMo hydrodesulfurization catalyst for the coke oven gas[D]. Wuhan: Wuhan University of Technology, 2010. | |
| 34 | LÓPEZ-BENÍTEZ A, BERHAULT G, SILVA-RODRIGO R, et al. Evaluation of the interest of NiMo catalysts supported on MgO-TiO2 for hydrodesulfurization applications[J]. Catalysis Letters, 2019, 149(10): 2656-2670. |
| 35 | 王杰, 刘永杰, 田云清, 等. 采用天然气制备生产甲硫醇和/或二甲基硫醚用中间原料的工艺: CN107010606A[P]. 2017-08-04. |
| WANG Jie, LIU Yongjie, TIAN Yunqing, et al. Technique for preparing middle raw material for producing methyl mercaptan and/or dimethyl sulfide by natural gas: CN107010606A[P]. 2017-08-04. | |
| 36 | 崔玉, 仲倩, 杨小凤, 等. 碳碳双键亲电加成反应的启发式教学[J]. 化工高等教育, 2012, 29(4): 81-83. |
| CUI Yu, ZHONG Qian, YANG Xiaofeng, et al. The heuristic teaching about electrophilic addition reaction of the carbon-carbon double bonds[J]. Higher Education in Chemical Engineering, 2012, 29(4): 81-83. | |
| 37 | 吴景梅, 邰燕芳, 朱银邦, 等. 关于烯烃加成反应历程的探讨[J]. 西昌学院学报(自然科学版), 2017, 31(4): 111-114. |
| WU Jingmei, TAI Yanfang, ZHU Yinbang, et al. Discussion on the addition reaction process of alkene[J]. Journal of Xichang University(Natural Science Edition), 2017, 31(4): 111-114. | |
| 38 | 舒畅, 袁照华, 王日杰, 等. 乙硫醇合成催化剂Co-Mo/γ-Al2O3的研究[C]//第九届全国工业催化技术及应用年会. 厦门, 2012. |
| SHU Chang, YUAN Zhaohua, WANG Rijie, et al. Study on Co-Mo/γ-Al2O3 as Catalyst for Ethanethiol Synthesis[C]//Proceedings of the 9th National Annual Conference on Industrial Catalysis Technology and Application. Xiamen, 2012. | |
| 39 | 何磊. 硫化氢下游产品开发与应用[J]. 化工中间体, 2003(S2): 10-14. |
| HE Lei. Development and application of downstream products of hydrogen sulfide[J]. Chemical Intermediate, 2003(S2): 10-14. | |
| 40 | 徐琼, 尹笃林. 硫化氢加成直接合成硫醇的催化剂研究进展[J]. 精细化工中间体, 2005, 35(1): 17-19. |
| XU Qiong, YIN Dulin. Developments of catalyts in the synthesis of mercaptans by the of alkene with hydrogen sulfide[J]. Fine Chemical Intermediates, 2005, 35(1): 17-19. | |
| 41 | 张宏, 李望, 赵和平, 等. 以废气中的硫化氢开发含硫化学品的研究进展[J]. 化工进展, 2017, 36(10): 3832-3849. |
| ZHANG Hong, LI Wang, ZHAO Heping, et al. Latest development of the sulfur-containing chemicals from hydrogen sulfide in waste gas[J]. Chemical Industry and Engineering Progress, 2017, 36(10): 3832-3849. | |
| 42 | XU Hao, FAN Xing, LI Guosheng, et al. Preparation of Co-Mo/γ-Al2O3 catalyst and the catalytic hydrogenation effects on coal-related model compounds[J]. Journal of the Energy Institute, 2021, 96: 52-60. |
| 43 | SAID S, MIKHAIL S, RIAD M. Recent progress in preparations and applications of meso-porous alumina[J]. Materials Science for Energy Technologies, 2019, 2(2): 288-297. |
| 44 | 李陶琦, 邹若松, 陆晋喜. 乙硫醚合成工艺研究[J]. 陕西化工, 1993, 22(4): 35-36. |
| LI Taoqi, ZOU Ruosong, LU Jinxi. Study on synthesis technology of diethyl sulfide[J]. Shaanxi Chemical Industry, 1993, 22(4): 35-36. | |
| 45 | 李立武, 刘文汇, 张中宁, 等. 天然气中H2S含量及其硫同位素组成质谱直接分析法[C]//第十一届全国有机地球化学学术会议. 昆明, 2007: 509-510. |
| LI Liwu, LIU Wenhui, ZHANG Zhongning, et al. Direct analysis of H2S content and sulfur isotope composition in natural gas by mass spectrometry[C]// Proceedings of the 11th National Conference on OrganicGeochemistry. Kunming, 2007: 509-510. | |
| 46 | ALISHA G D, TRISUNARYANTI W, SYOUFIAN A. Hydrocracking of waste palm cooking oil into hydrocarbon compounds over Mo catalyst impregnated on SBA-15[J]. Silicon, 2022, 14(5): 2309-2315. |
| 47 | MOQADAM R G, TAVASOLI A, SALIMI M. What is the effect of promoter loading on alkalized bimetallic Co-Mo catalyst for higher alcohols synthesis from syngas?[J]. Catalysis in Industry, 2019, 11(3): 208-215. |
| 48 | 徐军科, 羌宁. 反应器材质对Ni-Co双金属催化剂上沼气重整制氢性能与积炭的影响[J]. 天然气化工(C1化学与化工), 2021, 46(4): 52-57, 69. |
| XU Junke, QIANG Ning. Effect of reactor material on performance and carbon deposition of biogas reforming for hydrogen production over Ni-Co bimetallic catalyst[J]. Natural Gas Chemical Industry, 2021, 46(4): 52-57, 69. | |
| 49 | SHAMSI A, BALTRUS J P, SPIVEY J J. Characterization of coke deposited on Pt/alumina catalyst during reforming of liquid hydrocarbons[J]. Applied Catalysis A: General, 2005, 293: 145-152. |
| 50 | LI Qing, SUI Zhijun, ZHOU Xinggui, et al. Coke formation on Pt-Sn/Al2O3 catalyst in propane dehydrogenation: coke characterization and kinetic study[J]. Topics in Catalysis, 2011, 54(13): 888-896. |
| 51 | CASTAÑO P, ELORDI G, OLAZAR M, et al. Insights into the coke deposited on HZSM-5, Hβ and HY zeolites during the cracking of polyethylene[J]. Applied Catalysis B: Environmental, 2011, 104(1/2): 91-100. |
| 52 | 王雪, 孙昱东, 张强, 等. 催化剂上积炭结构和组成的分析研究方法[J]. 分析测试技术与仪器, 2013, 19(1): 6-11. |
| WANG Xue, SUN Yudong, ZHANG Qiang, et al. Analytical methods for structure and composition of coke deposited on catalysts[J]. Analysis and Testing Technology and Instruments, 2013, 19 (1): 6-11. | |
| 53 | 孙锦宜. 工业催化剂的失活与再生[M]. 北京: 化学工业出版社, 2006. |
| SUN Jinyi. Deactivation and regeneration of industrial catalysts[M]. Beijing: Chemical Industry Press, 2006. | |
| 54 | 王梅正, 林民, 朱斌. 钛硅分子筛失活与再生的研究进展[J]. 化工进展, 2007, 26(9): 1258-1262. |
| WANG Meizheng, LIN Min, ZHU Bin. Advances in study of deactivation and regeneration of titanium silicalite[J]. Chemical Industry and Engineering Progress, 2007, 26(9): 1258-1262. | |
| 55 | 张向京, 王燕, 杨立斌, 等. 环己酮氨肟化反应中失活TS-1催化剂上沉积物的物化表征[J]. 燃料化学学报, 2006, 34(2): 234-239. |
| ZHANG Xiangjing, WANG Yan, YANG Libin, et al. Characterization of the deposits on deactivated TS-1 catalyst in cyclohexanone ammoximation process[J]. Journal of Fuel Chemistry and Technology, 2006, 34(2): 234-239. | |
| 56 | 张嘉霖, 孙培永, 张胜红, 等. 乙烯催化转化制备乙二醇反应中TS-1分子筛的失活[J]. 分子催化, 2015, 29(3): 229-237. |
| ZHANG Jialin, SUN Peiyong, ZHANG Shenghong, et al. Deactivation of TS-1 zeolites in the catalytic conversion of ethylene to ethylene glycol[J]. Journal of Molecular Catalysis, 2015, 29(3): 229-237. | |
| 57 | 颜浩, 巩明鑫, 王金玉, 等. 煤焦油催化裂解过程钙-镁催化剂的抗积炭性能[J]. 石油学报(石油加工), 2020, 36(4): 832-838. |
| YAN Hao, GONG Mingxin, WANG Jinyu, et al. Anti-carbon deposition performance of Ca-Mg catalyst in coal tar catalytic cracking process[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2020, 36(4): 832-838. | |
| 58 | RODRIGUEZ A C, SAD M E, CRUCHADE H, et al. Study of catalyst deactivation during 1,3-butanediol dehydration to produce butadiene[J]. Microporous and Mesoporous Materials, 2021, 320: 111066. |
| [1] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [2] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [3] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [4] | 赵景超, 谭明. 表面活性剂对电渗析减量化工业含盐废水的影响[J]. 化工进展, 2023, 42(S1): 529-535. |
| [5] | 李化全, 王明华, 邱贵宝. 硫酸酸解钙钛矿相精矿的行为[J]. 化工进展, 2023, 42(S1): 536-541. |
| [6] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [7] | 王谨航, 何勇, 史伶俐, 龙臻, 梁德青. 气体水合物阻聚剂研究进展[J]. 化工进展, 2023, 42(9): 4587-4602. |
| [8] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [9] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| [10] | 林晓鹏, 肖友华, 管奕琛, 鲁晓东, 宗文杰, 傅深渊. 离子聚合物-金属复合材料(IPMC)柔性电极的研究进展[J]. 化工进展, 2023, 42(9): 4770-4782. |
| [11] | 朱传强, 茹晋波, 孙亭亭, 谢兴旺, 李长明, 高士秋. 固体高分子脱硝剂选择性非催化还原NO x 特性[J]. 化工进展, 2023, 42(9): 4939-4946. |
| [12] | 史天茜, 石永辉, 武新颖, 张益豪, 秦哲, 赵春霞, 路达. Fe2+对厌氧氨氧化EGSB反应器运行性能的影响[J]. 化工进展, 2023, 42(9): 5003-5010. |
| [13] | 张丽宏, 金要茹, 程芳琴. 煤气化渣资源化利用[J]. 化工进展, 2023, 42(8): 4447-4457. |
| [14] | 毛善俊, 王哲, 王勇. 基团辨识加氢:从概念到应用[J]. 化工进展, 2023, 42(8): 3917-3922. |
| [15] | 王报英, 王皝莹, 闫军营, 汪耀明, 徐铜文. 聚合物包覆膜在金属分离回收中的研究进展[J]. 化工进展, 2023, 42(8): 3990-4004. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||