化工进展 ›› 2022, Vol. 41 ›› Issue (12): 6489-6499.DOI: 10.16085/j.issn.1000-6613.2022-0445
酸改性猪粪生物炭的制备及其对直接红23染料的吸附性能
江汝清1,2( ), 余广炜1(
), 余广炜1( ), 王玉1,2, 黎长江1, 邢贞娇1
), 王玉1,2, 黎长江1, 邢贞娇1
- 1.中国科学院城市环境研究所城市污染物转化重点实验室,福建 厦门 361021
2.中国科学院大学,北京 100049
-
收稿日期:2022-03-22修回日期:2022-04-18出版日期:2022-12-20发布日期:2022-12-29 -
通讯作者:余广炜 -
作者简介:江汝清(1996—),女,硕士,研究方向为固废处置。E-mail:rqjiang@iue.ac.cn。 -
基金资助:中国科学院A类战略性先导科技专项(XDA23020504);国家重点研发计划(2020YFC1908904);福建省自然科学基金(2019J01135)
Preparation of acid-modified pig manure biochar and its adsorption performance on Direct Red 23
JIANG Ruqing1,2( ), YU Guangwei1(
), YU Guangwei1( ), WANG Yu1,2, LI Changjiang1, XING Zhenjiao1
), WANG Yu1,2, LI Changjiang1, XING Zhenjiao1
- 1.Key Laboratory of Urban Pollutant Conversion, Institute of Urban Environment, Chinese Academy of Sciences, Xiamen 361021, Fujian, China
2.University of Chinese Academy of Sciences, Beijing 100049, China
-
Received:2022-03-22Revised:2022-04-18Online:2022-12-20Published:2022-12-29 -
Contact:YU Guangwei
摘要:
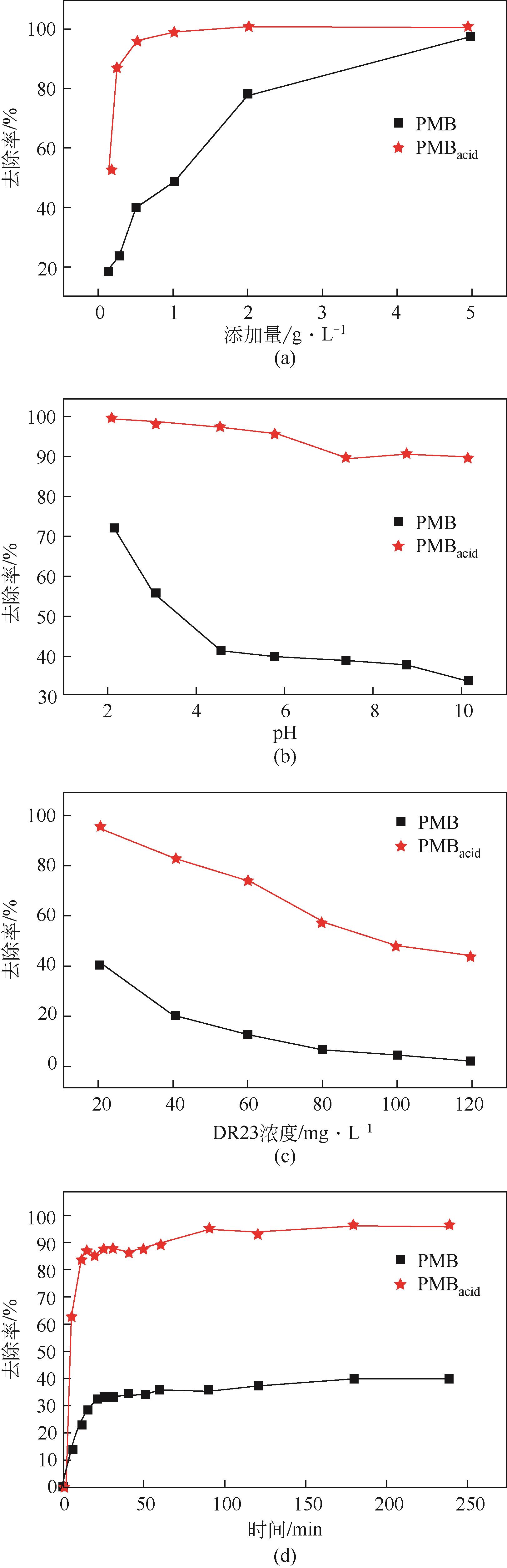
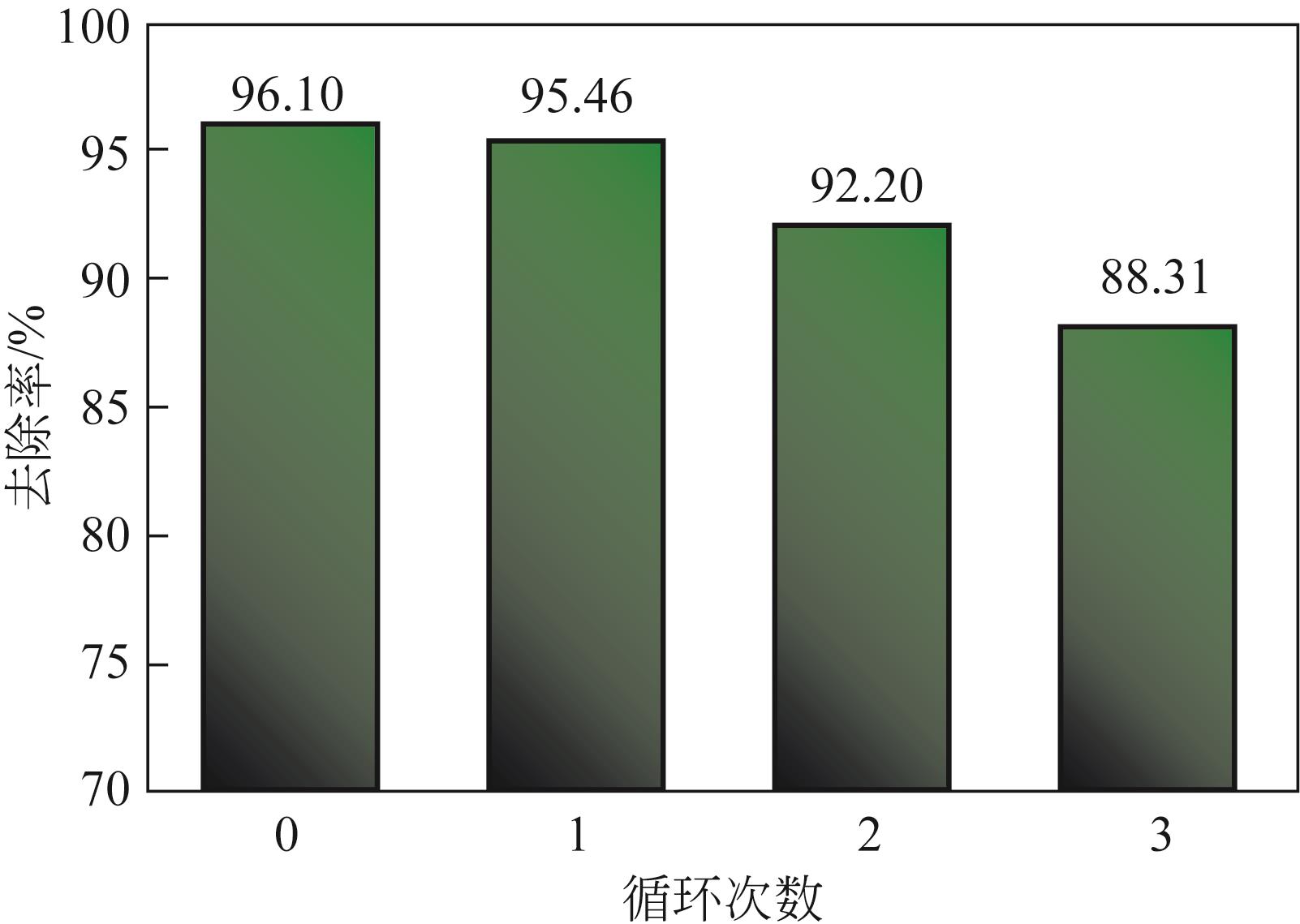
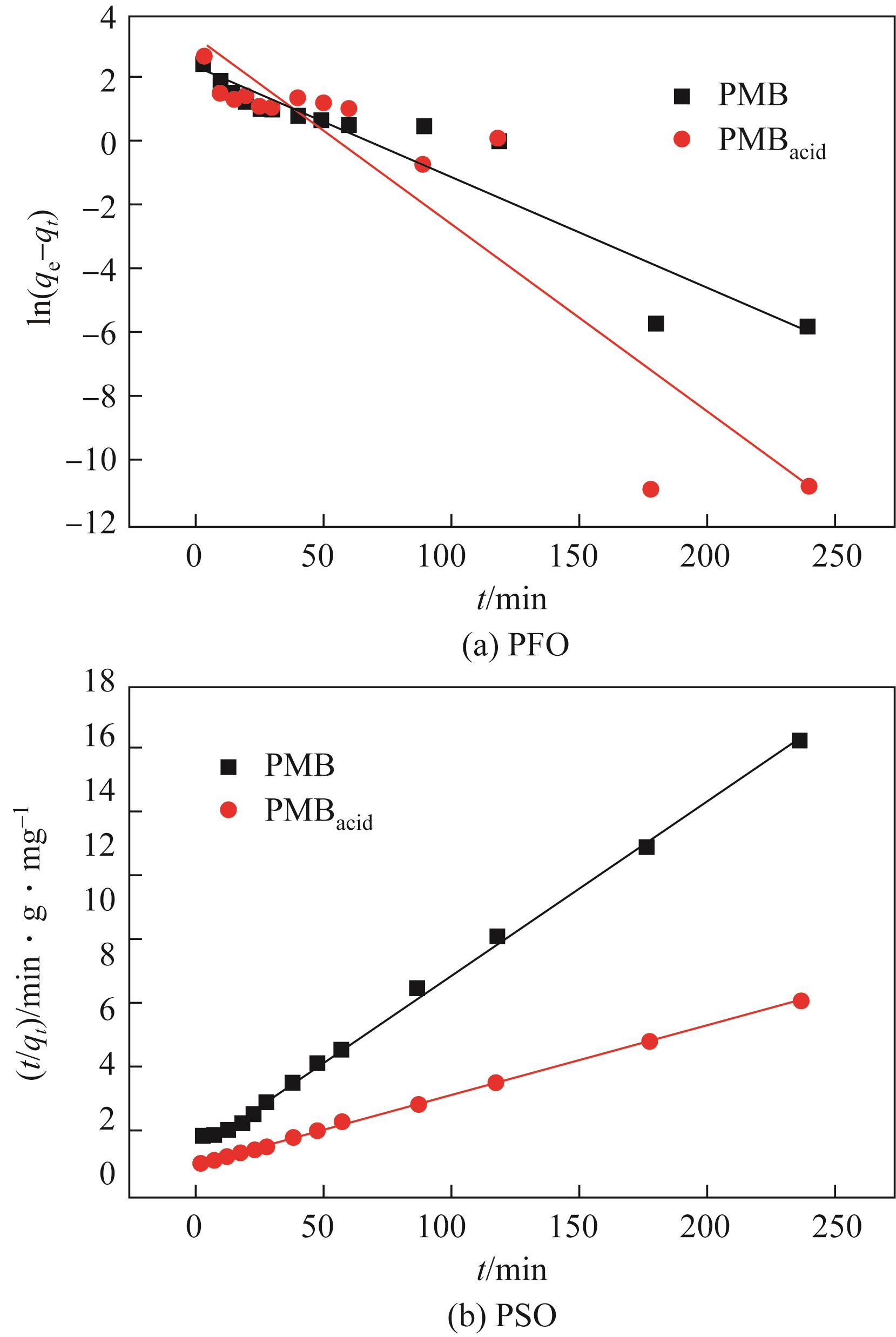
以直接红23染料(DR23)溶液模拟印染废水,对比分析了酸改性前后猪粪生物炭对DR23的吸附特性与机理。通过静态吸附实验考察了DR23溶液的pH、初始浓度、吸附时间、吸附温度、吸附剂添加量等条件对吸附效果的影响,并确定了该吸附过程的吸附动力学和吸附等温线。研究发现,相比于未改性生物炭(PMB),酸改性后生物炭(PMBacid)结构变得疏松多孔,表面官能团丰富,表现出更优的脱色性能,对DR23的吸附去除率最高可达96.10%,最大饱和吸附量为111.51mg/g,且在经过3次循环再生后,PMBacid对DR23的去除率仍可达到88.31%;此外,pH对PMBacid的脱色吸附性能影响较小。PMBacid对DR23的吸附是一个受反应速率和扩散控制的复杂过程,符合于伪二级动力学模型和Langmuir等温吸附模型;PMBacid对DR23的吸附机理取决于吸附剂的物理化学性质,其孔结构及各官能团通过不同的机制参与了生物炭对DR23的吸附过程。
中图分类号:
引用本文
江汝清, 余广炜, 王玉, 黎长江, 邢贞娇. 酸改性猪粪生物炭的制备及其对直接红23染料的吸附性能[J]. 化工进展, 2022, 41(12): 6489-6499.
JIANG Ruqing, YU Guangwei, WANG Yu, LI Changjiang, XING Zhenjiao. Preparation of acid-modified pig manure biochar and its adsorption performance on Direct Red 23[J]. Chemical Industry and Engineering Progress, 2022, 41(12): 6489-6499.
| 参数 | 数值 |
|---|---|
| 工业分析(干基,质量分数) | |
| 灰分/% | 19.22 |
| 挥发分/% | 66.98 |
| 固定碳/% | 13.80 |
| 元素分析(干基,质量分数) | |
| C/% | 38.11 |
| H/% | 6.03 |
| N/% | 4.21 |
| S/% | 0.79 |
| pH | 7.05 |
| 电导率(EC) | 3.06 |
| 阳离子交换量(CEC)/cmol·kg-1 | 92.04 |
| 孔隙结构 | |
| BET比表面积/m2·g-1 | 1.0315 |
| 总孔体积/cm3·g-1 | 0.0075 |
| 平均孔直径/nm | 26.4431 |
表1 猪粪的基本性质
| 参数 | 数值 |
|---|---|
| 工业分析(干基,质量分数) | |
| 灰分/% | 19.22 |
| 挥发分/% | 66.98 |
| 固定碳/% | 13.80 |
| 元素分析(干基,质量分数) | |
| C/% | 38.11 |
| H/% | 6.03 |
| N/% | 4.21 |
| S/% | 0.79 |
| pH | 7.05 |
| 电导率(EC) | 3.06 |
| 阳离子交换量(CEC)/cmol·kg-1 | 92.04 |
| 孔隙结构 | |
| BET比表面积/m2·g-1 | 1.0315 |
| 总孔体积/cm3·g-1 | 0.0075 |
| 平均孔直径/nm | 26.4431 |
| 有机染料 | 分子式 | 分子量 | 离子性 | 分子结构 |
|---|---|---|---|---|
| DR23 | C35H25N7Na2O10S2 | 813.72 | 阴离子 |  |
表2 DR23的理化性质
| 有机染料 | 分子式 | 分子量 | 离子性 | 分子结构 |
|---|---|---|---|---|
| DR23 | C35H25N7Na2O10S2 | 813.72 | 阴离子 |  |
| 生物炭 | 温度/℃ | Freundlich方程式(3) | Langmuir方程式(4) | ||||
|---|---|---|---|---|---|---|---|
| Kf | 1/n | R2 | b | qm | R2 | ||
| PMB | 25 | 14.68 | 0.01302 | -0.22 | 0.64 | 15.89 | 0.97 |
| 35 | 21.21 | 0.01433 | -0.24 | 0.25 | 18.32 | 0.97 | |
| 45 | 21.73 | 0.00366 | -0.24 | 0.36 | 20.68 | 0.96 | |
| PMBacid | 25 | 41.48 | 0.22907 | 0.94 | 0.31 | 105.82 | 0.99 |
| 35 | 49.51 | 0.19545 | 0.97 | 0.41 | 108.17 | 0.99 | |
| 45 | 58.12 | 0.16298 | 0.97 | 0.47 | 111.51 | 0.99 | |
表3 不同温度下DR23吸附等温线拟合参数
| 生物炭 | 温度/℃ | Freundlich方程式(3) | Langmuir方程式(4) | ||||
|---|---|---|---|---|---|---|---|
| Kf | 1/n | R2 | b | qm | R2 | ||
| PMB | 25 | 14.68 | 0.01302 | -0.22 | 0.64 | 15.89 | 0.97 |
| 35 | 21.21 | 0.01433 | -0.24 | 0.25 | 18.32 | 0.97 | |
| 45 | 21.73 | 0.00366 | -0.24 | 0.36 | 20.68 | 0.96 | |
| PMBacid | 25 | 41.48 | 0.22907 | 0.94 | 0.31 | 105.82 | 0.99 |
| 35 | 49.51 | 0.19545 | 0.97 | 0.41 | 108.17 | 0.99 | |
| 45 | 58.12 | 0.16298 | 0.97 | 0.47 | 111.51 | 0.99 | |
| 染料 | 吸附剂 | 吸附能力/mg·g-1 | 参考文献 |
|---|---|---|---|
| DR23 | PMB | 20.68 | 本研究 |
| DR23 | PMBacid | 111.51 | 本研究 |
| DR23 | 猪粪生物炭 | 17.82 | [ |
| DR23 | 污泥生物炭 | 111.98 | [ |
| 直接红80 | 大蒜秸秆 | 107.53 | [ |
| 直接红80 | 杏仁壳 | 20.5 | [ |
| 刚果红 | 斜发沸石 | 16.90 | [ |
| 刚果红 | 铁改性斜发沸石 | 36.70 | [ |
表4 几种吸附剂的吸附能力对比
| 染料 | 吸附剂 | 吸附能力/mg·g-1 | 参考文献 |
|---|---|---|---|
| DR23 | PMB | 20.68 | 本研究 |
| DR23 | PMBacid | 111.51 | 本研究 |
| DR23 | 猪粪生物炭 | 17.82 | [ |
| DR23 | 污泥生物炭 | 111.98 | [ |
| 直接红80 | 大蒜秸秆 | 107.53 | [ |
| 直接红80 | 杏仁壳 | 20.5 | [ |
| 刚果红 | 斜发沸石 | 16.90 | [ |
| 刚果红 | 铁改性斜发沸石 | 36.70 | [ |
| 生物炭 | qe,exp/mg·g-1 | 伪一级动力学方程式(5) | 伪二级动力学方程式(6) | ||||
|---|---|---|---|---|---|---|---|
| k1/min-1 | qcal/mg·g-1 | R2 | k2/g·mg-1·min-1 | qcal/mg·g-1 | R2 | ||
| PMB | 15.01 | 0.03 | 10.49 | 0.8765 | 6.80×10-3 | 15.54 | 0.9983 |
| PMBacid | 38.18 | 0.06 | 26.04 | 0.8460 | 7.95×10-3 | 38.62 | 0.9996 |
表5 生物炭吸附DR23的伪一级、伪二级动力学参数
| 生物炭 | qe,exp/mg·g-1 | 伪一级动力学方程式(5) | 伪二级动力学方程式(6) | ||||
|---|---|---|---|---|---|---|---|
| k1/min-1 | qcal/mg·g-1 | R2 | k2/g·mg-1·min-1 | qcal/mg·g-1 | R2 | ||
| PMB | 15.01 | 0.03 | 10.49 | 0.8765 | 6.80×10-3 | 15.54 | 0.9983 |
| PMBacid | 38.18 | 0.06 | 26.04 | 0.8460 | 7.95×10-3 | 38.62 | 0.9996 |
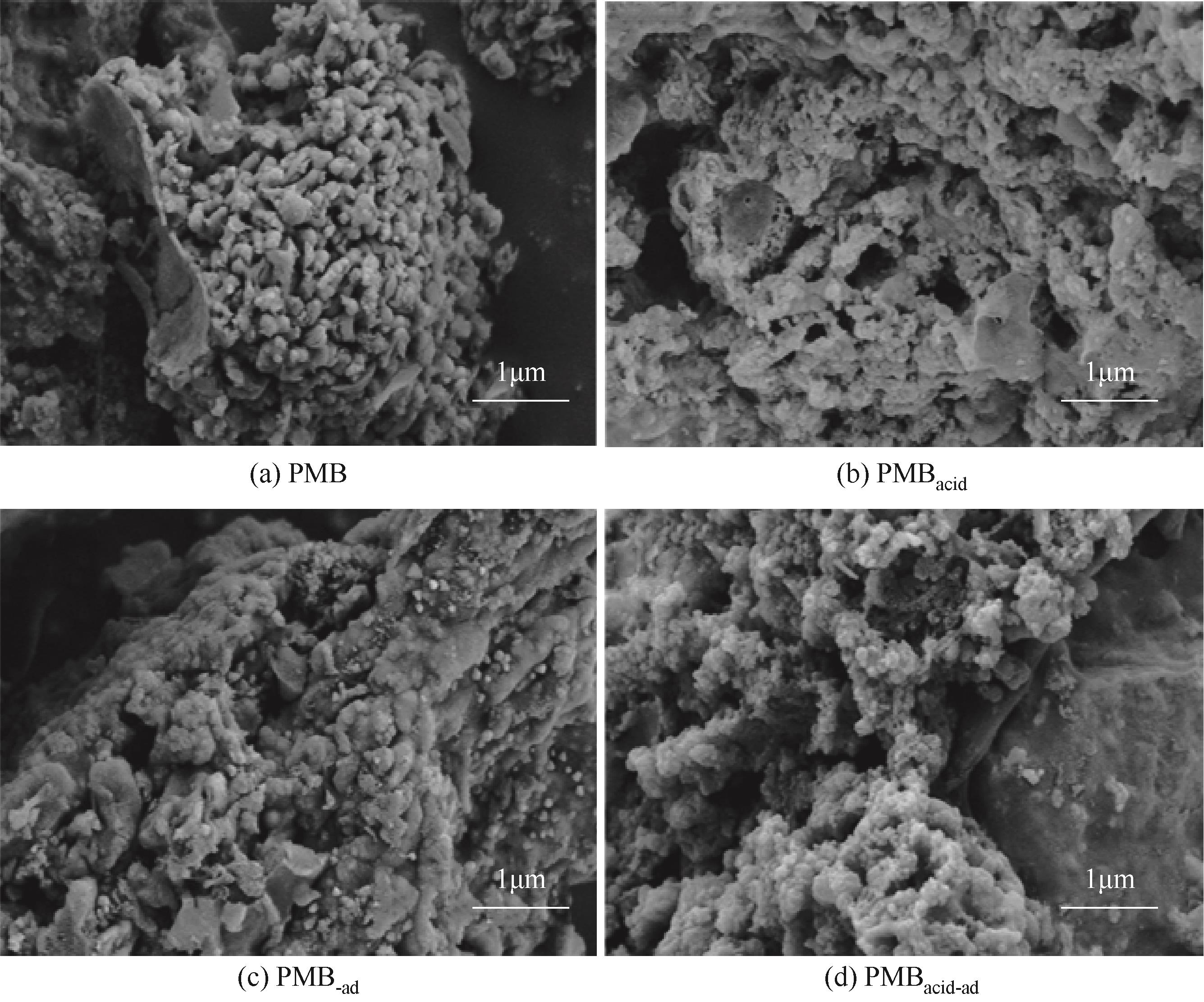
| 生物炭 | 比表面积/m2·g-1 | 总孔体积/cm3·g-1 | 平均孔径/nm |
|---|---|---|---|
| PMB | 2.1775 | 0.0109 | 14.8857 |
| PMB-ad | 1.7825 | 0.0135 | 12.4721 |
| PMBacid | 85.8710 | 0.0850 | 4.0232 |
| PMBacid-ad | 84.6621 | 0.0952 | 3.9592 |
表6 生物炭吸附DR23前后的孔径结构
| 生物炭 | 比表面积/m2·g-1 | 总孔体积/cm3·g-1 | 平均孔径/nm |
|---|---|---|---|
| PMB | 2.1775 | 0.0109 | 14.8857 |
| PMB-ad | 1.7825 | 0.0135 | 12.4721 |
| PMBacid | 85.8710 | 0.0850 | 4.0232 |
| PMBacid-ad | 84.6621 | 0.0952 | 3.9592 |
| 1 | 秦翠兰, 王磊元, 刘飞, 等. 畜禽粪便生物质资源利用的现状与展望[J]. 农机化研究, 2015, 37(6): 234-238. |
| QIN Cuilan, WANG Leiyuan, LIU Fei, et al. Status and prospects of livestock manure utilization of biomass resources[J]. Journal of Agricultural Mechanization Research, 2015, 37(6): 234-238. | |
| 2 | 王广, 曾加其, 王丹凤. 畜禽粪污综合利用模式探讨[J]. 畜禽业, 2021, 32(1): 28. |
| WANG Guang, ZENG Jiaqi, WANG Danfeng. Comprehensive utilization model of livestock and poultry manure [J]. Livestock and Poultry Industry, 2021, 32(1): 28. | |
| 3 | CANTRELL K B, HUNT P G, UCHIMIYA M, et al. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar[J]. Bioresource Technology, 2012, 107: 419-428. |
| 4 | 张祖康. 污水处理场废渣的减量和资源化[J]. 石油化工环境保护, 2000(3): 1-5. |
| ZHANG Z K. Decreasing and utilizing waste sludge of wastewater treatment plant[J]. Environment Protection in Petrochemical Industry, 2000(3): 1-5. | |
| 5 | 蔺丽丽, 蒋文举, 金燕, 等. 微波法制备污泥活性炭研究[J]. 环境工程学报, 2007, 1(4): 119-122. |
| LIN Lili, JIANG Wenju, JIN Yan, et al. Study on activated carbon made from sewage sludge by microwave[J]. Chinese Journal of Environmental Engineering, 2007, 1(4): 119-122. | |
| 6 | GONZÁLEZ-GUTIÉRREZ L V, GONZÁLEZ-ALATORRE G, ESCAMILLA-SILVA E M. Proposed pathways for the reduction of a reactive azo dye in an anaerobic fixed bed reactor[J]. World Journal of Microbiology and Biotechnology, 2009, 25(3): 415-426. |
| 7 | ARDEJANI F D, BADII K, LIMAEE N Y, et al. Adsorption of Direct Red 80 dye from aqueous solution onto almond shells: effect of pH, initial concentration and shell type[J]. Journal of Hazardous Materials, 2008, 151(2/3): 730-737. |
| 8 | KALLEL F, BOUAZIZ F, CHAARI F, et al. Interactive effect of garlic straw on the sorption and desorption of Direct Red 80 from aqueous solution[J]. Process Safety and Environmental Protection, 2016, 102: 30-43. |
| 9 | AHMAD A L, PUASA S W, ZULKALI M M D. Micellar-enhanced ultrafiltration for removal of reactive dyes from an aqueous solution[J]. Desalination, 2006, 191(1/2/3): 153-161. |
| 10 | ALVENTOSA-DELARA E, BARREDO-DAMAS S, ALCAINA-MIRANDA M I, et al. Ultrafiltration technology with a ceramic membrane for reactive dye removal: optimization of membrane performance[J]. Journal of Hazardous Materials, 2012, 209/210: 492-500. |
| 11 | KIM T H, PARK C, YANG J, et al. Comparison of disperse and reactive dye removals by chemical coagulation and Fenton oxidation[J]. Journal of Hazardous Materials, 2004, 112(1/2): 95-103. |
| 12 | SZPYRKOWICZ L, JUZZOLINO C, KAUL S N. A comparative study on oxidation of disperse dyes by electrochemical process, ozone, hypochlorite and Fenton reagent[J]. Water Research, 2001, 35(9): 2129-2136. |
| 13 | NOROOZI B, SORIAL G A. Applicable models for multi-component adsorption of dyes: a review[J]. Journal of Environmental Sciences, 2013, 25(3): 419-429. |
| 14 | SADEGH H, ALI G A M, GUPTA V K, et al. The role of nanomaterials as effective adsorbents and their applications in wastewater treatment[J]. Journal of Nanostructure in Chemistry, 2017, 7(1): 1-14. |
| 15 | SUZAIMI N D, GOH P S, MALEK N A N N, et al. Enhancing the performance of porous rice husk silica through branched polyethyleneimine grafting for phosphate adsorption[J]. Arabian Journal of Chemistry, 2020, 13(8): 6682-6695. |
| 16 | AHMADI Z, RAMEZANI H, AZIZI S N, et al. Synthesis of zeolite NaY supported Mn-doped ZnS quantum dots and investigation of their photodegradation ability towards organic dyes[J]. Environmental Science and Pollution Research International, 2020, 27(9): 9707-9717. |
| 17 | ALAKHRAS F, ALHAJRI E, HAOUNATI R, et al. A comparative study of photocatalytic degradation of Rhodamine B using natural-based zeolite composites[J]. Surfaces and Interfaces, 2020, 20: 100611. |
| 18 | BOUDECHICHE N, FARES M, OUYAHIA S, et al. Comparative study on removal of two basic dyes in aqueous medium by adsorption using activated carbon from Ziziphus lotus stones[J]. Microchemical Journal, 2019, 146: 1010-1018. |
| 19 | OTERO M, ROZADA F, CALVO L F, et al. Kinetic and equilibrium modelling of the methylene blue removal from solution by adsorbent materials produced from sewage sludges[J]. Biochemical Engineering Journal, 2003, 15(1): 59-68. |
| 20 | SMITH K M, FOWLER G D, PULLKET S, et al. Sewage sludge-based adsorbents: a review of their production, properties and use in water treatment applications[J]. Water Research, 2009, 43(10): 2569-2594. |
| 21 | 龚真萍, 赵红, 郑永杰, 等. 生物炭材料的制备及其在印染废水处理中的应用[J]. 染整技术, 2021, 43(12): 1-4. |
| GONG Zhenping, ZHAO Hong, ZHENG Yongjie, et al. Preparation of biochar material and its application in dyeing and printing wastewater treatment[J]. Textile Dyeing and Finishing Journal, 2021, 43(12): 1-4. | |
| 22 | 朱国婷, 邢献军, 汪家权, 等. 酸预处理活性炭对废水染料的吸附研究[J]. 环境科学与技术, 2016, 39(S2): 160-165. |
| ZHU Guoting, XING Xianjun, WANG Jiaquan, et al. Study on the adsorption of dyes in wastewater on activated carbon pre-treated with acid[J]. Environmental Science & Technology, 2016, 39(S2): 160-165. | |
| 23 | 张志芳, 邵红, 孔祥西, 等. 花生壳活性炭的制备及其对染料废水的脱色性能研究[J]. 沈阳化工大学学报, 2014, 28(2): 130-136. |
| ZHANG Zhifang, SHAO Hong, KONG Xiangxi, et al. Decoloring capability of activated carbon from peanut shell to dye wastewater[J]. Journal of Shenyang University of Chemical Technology, 2014, 28(2): 130-136. | |
| 24 | 孙良媛, 刘涛, 张乐. 中国规模化畜禽养殖的现状及其对生态环境的影响[J]. 华南农业大学学报(社会科学版), 2016, 15(2): 23-30. |
| SUN Liangyuan, LIU Tao, ZHANG Le. The pollution of scale livestock and poultry breeding and its influence on eco-environment[J]. Journal of South China Agricultural University (Social Science Edition), 2016, 15(2): 23-30. | |
| 25 | 武淑霞, 刘宏斌, 黄宏坤, 等. 我国畜禽养殖粪污产生量及其资源化分析[J]. 中国工程科学, 2018, 20(5): 103-111. |
| WU Shuxia, LIU Hongbin, HUANG Hongkun, et al. Analysis on the amount and utilization of manure in livestock and poultry breeding in China[J]. Engineering Science, 2018, 20(5): 103-111. | |
| 26 | CHLOPIN W, BALANDIN A. Über Die adsorption des bariumchlorids durch das kolloidale mangansuperoxydhydrat in wäßrigen lösungen[J]. Zeitschrift Für Anorganische Und Allgemeine Chemie, 1925, 149(1): 157-166. |
| 27 | LANGMUIR I. The constitution and fundamental properties of solids and liquids[J]. Journal of the Franklin Institute, 1917, 183(1): 102-105. |
| 28 | AKSU Z. Biosorption of reactive dyes by dried activated sludge: equilibrium and kinetic modelling[J]. Biochemical Engineering Journal, 2001, 7(1): 79-84. |
| 29 | HO Y S, MCKAY G. Pseudo-second order model for sorption processes[J]. Process Biochemistry, 1999, 34(5): 451-465. |
| 30 | NAUTIYAL P, SUBRAMANIAN K A, DASTIDAR M G. Adsorptive removal of dye using biochar derived from residual algae after in situ transesterification: alternate use of waste of biodiesel industry[J]. Journal of Environmental Management, 2016, 182: 187-197. |
| 31 | AKGÜL M. Enhancement of the anionic dye adsorption capacity of clinoptilolite by Fe3+-grafting[J]. Journal of Hazardous Materials, 2014, 267: 1-8. |
| 32 | LIU N, ZHU M L, WANG H, et al. Adsorption characteristics of Direct Red 23 from aqueous solution by biochar[J]. Journal of Molecular Liquids, 2016, 223: 335-342. |
| 33 | JIANG R Q, YU G W, NDAGIJIMANA P, et al. Effective adsorption of direct Red 23 by sludge biochar-based adsorbent: adsorption kinetics, thermodynamics and mechanisms study[J]. Water Science and Technology, 2021, 83(10): 2424-2436. |
| 34 | REN X L, LAI X H, ZHU K J, et al. Removal of acid turquoise blue 2G from aqueous solution by adsorbent derived from sludge and straw: kinetic, isotherm and thermodynamic study[J]. Desalination and Water Treatment, 2016, 57(1): 440-448. |
| 35 | SUN Y B, DING C C, CHENG W C, et al. Simultaneous adsorption and reduction of U( Ⅵ ) on reduced graphene oxide-supported nanoscale zerovalent iron[J]. Journal of Hazardous Materials, 2014, 280: 399-408. |
| 36 | VADIVELAN V, KUMAR K V. Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk[J]. Journal of Colloid and Interface Science, 2005, 286(1): 90-100. |
| 37 | 韩闯. 污泥生物炭水热制备及其对染料脱色研究[D]. 上海: 东华大学, 2017. |
| HAN Chuang. Preparation of sludge biochar and its decolorization of dye[D]. Shanghai: Donghua University, 2017. | |
| 38 | KANG J, ZHANG H Y, DUAN X G, et al. Magnetic Ni-Co alloy encapsulated N-doped carbon nanotubes for catalytic membrane degradation of emerging contaminants[J]. Chemical Engineering Journal, 2019, 362: 251-261. |
| 39 | 王泽庆, 朱耀辉, 仲茜溪, 等. 南疆棉花秸秆生物炭对水中亚甲基蓝的吸附特性[J]. 广东化工, 2020, 47(5): 22-26. |
| WANG Z Q, ZHU Y H, ZHONG X X, et al. Adsorption characteristics of aqueous methylene blue by biochar pyrolyzed from cotton straw in south Xinjiang[J]. Guangdong Chemical Industry, 2020, 47(5): 22-26. | |
| 40 | YAO Y, ZHANG Y, GAO B, et al. Removal of sulfamethoxazole (SMX) and sulfapyridine (SPY) from aqueous solutions by biochars derived from anaerobically digested bagasse[J]. Environmental Science and Pollution Research International, 2018, 25(26): 25659-25667. |
| 41 | LUO F, CHEN Z L, MEGHARAJ M, et al. Simultaneous removal of trichloroethylene and hexavalent chromium by green synthesized agarose-Fe nanoparticles hydrogel[J]. Chemical Engineering Journal, 2016, 294: 290-297. |
| 42 | WENG X L, LIN S, ZHONG Y H, et al. Chitosan stabilized bimetallic Fe/Ni nanoparticles used to remove mixed contaminants-amoxicillin and Cd (II) from aqueous solutions[J]. Chemical Engineering Journal, 2013, 229: 27-34. |
| 43 | GONG J, LIU J, JIANG Z W, et al. A facile approach to prepare porous cup-stacked carbon nanotube with high performance in adsorption of methylene blue[J]. Journal of Colloid and Interface Science, 2015, 445: 195-204. |
| 44 | SONG X D, XUE X Y, CHEN D Z, et al. Application of biochar from sewage sludge to plant cultivation: influence of pyrolysis temperature and biochar-to-soil ratio on yield and heavy metal accumulation[J]. Chemosphere, 2014, 109: 213-220. |
| 45 | 陈思. 污泥-稻壳共热解及生物炭吸附特性研究[D]. 武汉: 武汉大学, 2019. |
| CHEN Si. Study on the co-pyrolysis of sludge-rice hull and the adsorption properties of biochar[D]. Wuhan: Wuhan University, 2019. |
| [1] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [2] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [3] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [4] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [5] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [6] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [7] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [8] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [9] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| [10] | 王浩然, 殷全玉, 方明, 侯建林, 李军, 何斌, 张明月. 近临界水处理废弃烟梗工艺优化[J]. 化工进展, 2023, 42(9): 5019-5027. |
| [11] | 姜晶, 陈霄宇, 张瑞妍, 盛光遥. 载锰生物炭制备及其在环境修复中应用研究进展[J]. 化工进展, 2023, 42(8): 4385-4397. |
| [12] | 张振, 李丹, 陈辰, 吴菁岚, 应汉杰, 乔浩. 吸附树脂对唾液酸的分离纯化[J]. 化工进展, 2023, 42(8): 4153-4158. |
| [13] | 王知彩, 刘伟伟, 周璁, 潘春秀, 闫洪雷, 李占库, 颜井冲, 任世彪, 雷智平, 水恒福. 基于煤基腐殖酸的高效减水剂合成与性能表征[J]. 化工进展, 2023, 42(7): 3634-3642. |
| [14] | 于静文, 宋璐娜, 刘砚超, 吕瑞东, 武蒙蒙, 冯宇, 李忠, 米杰. 一种吲哚基超交联聚合物In-HCP对水中碘的吸附作用[J]. 化工进展, 2023, 42(7): 3674-3683. |
| [15] | 李艳玲, 卓振, 池亮, 陈曦, 孙堂磊, 刘鹏, 雷廷宙. 氮掺杂生物炭的制备与应用研究进展[J]. 化工进展, 2023, 42(7): 3720-3735. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||