| 4 |
LIU Y, BISSON T M, YANG H Q, et al. Recent developments in novel sorbents for flue gas clean up[J]. Fuel Processing Technology, 2010, 91(10): 1175-1197.
|
| 5 |
GAO X, LIU S J, ZHANG Y, et al. Physicochemical properties of metal-doped activated carbons and relationship with their performance in the removal of SO2 and NO[J]. Journal of Hazardous Materials, 2011, 188(1/2/3): 58-66.
|
| 6 |
BAI B C, LEE C W, LEE Y S, et al. Metal impregnate on activated carbon fiber for SO2 gas removal: a ssessment of pore structure, Cu supporter, breakthrough, and bed utilization[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2016, 509: 73-79.
|
| 7 |
ZHANG K, HE Y, WANG Z H, et al. Multi-stage semi-coke activation for the removal of SO2 and NO[J]. Fuel,2017,210: 738-747.
|
| 8 |
CHENG G, ZHANG C X. Desulfurization and denitrification technologies of coal-fired flue gas[J]. Polish Journal of Environmental Studies, 2018, 27(2): 481-489.
|
| 9 |
LIU S J, YU X N, LIN G X, et al. Insights into the effect of adsorption-desorption cycles on SO2 removal over an activated carbon[J]. Aerosol and Air Quality Research, 2019, 19(2): 411-421.
|
| 10 |
LI B, MA C Y. Study on the mechanism of SO2 removal by activated carbon[J]. Energy Procedia, 2018, 153: 471-477.
|
| 11 |
KARATEPE N, ORBAK İ, YAVUZ R, et al. Sulfur dioxide adsorption by activated carbons having different textural and chemical properties[J]. Fuel, 2008, 87(15/16): 3207-3215.
|
| 12 |
RAYMUNDO-PIÑERO E, CAZORLA-AMORÓS D, SALINAS-MARTINEZ DE LECEA C, et al. Factors controling the SO2 removal by porous carbons: relevance of the SO2 oxidation step[J]. Carbon, 2000, 38(3): 335-344.
|
| 13 |
WANG A N, FAN R Q, PI X X, et al. Nitrogen-doped microporous carbons derived from pyridine ligand-based metal-organic complexes as high-performance SO2 adsorption sorbents[J]. ACS Applied Materials & Interfaces, 2018, 10(43): 37407-37416.
|
| 14 |
LI Y Q, YU Y F, LIU L, et al. Synthesis of N-doped carbon spheres using extended stöber method for SO2 adsorption[J]. Nano, 2017, 12(1): 1750004.
|
| 15 |
QIE Z P, SUN F, GAO J H, et al. Enhanced SO2 fluidized adsorption dynamic by hierarchically porous activated coke[J]. Journal of the Energy Institute, 2020, 93(2): 802-810.
|
| 16 |
ZHANG X, ZHENG H H, LI G Y, et al. Ammoniated and activated microporous biochar for enhancement of SO2 adsorption[J]. Journal of Analytical and Applied Pyrolysis, 2021, 156: 105119.
|
| 17 |
ALVAREZ-MERINO M A, CARRASCO-MARÍN F, MORENO-CASTILLA C. Adsorption of SO2 from flowing air by alkaline-oxide-containing activated carbons[J]. Applied Catalysis B: Environmental, 1997, 13(3/4): 229-240.
|
| 18 |
GUO J, LUA A C. Adsorption of sulfur dioxide onto activated carbons prepared from oil-palm shells impregnated with potassium hydroxide[J]. Journal of Chemical Technology & Biotechnology, 2000, 75(11): 971-976.
|
| 19 |
LIU D D, SU R, HAO Z K, et al. Catalytic effect of NaCl on the improvement of the physicochemical structure of coal-based activated carbons for SO2 adsorption[J]. Processes, 2019, 7(6): 338.
|
| 20 |
FORTIER H, ZELENIETZ C, DAHN T R, et al. SO2 adsorption capacity of K2CO3-impregnated activated carbon as a function of K2CO3 content loaded by soaking and incipient wetness[J]. Applied Surface Science, 2007, 253(6): 3201-3207.
|
| 21 |
LI J H, CHANG H Z, MA L, et al. Low-temperature selective catalytic reduction of NO x with NH3 over metal oxide and zeolite catalysts: a review[J]. Catalysis Today, 2011, 175(1): 147-156.
|
| 22 |
YUAN J, JIANG X, ZOU M J, et al. Copper ore-modified activated coke: highly efficient and regenerable catalysts for the removal of SO2 [J]. Industrial & Engineering Chemistry Research, 2018, 57(46): 15731-15739.
|
| 23 |
SILAS K, GHANI W A W A K, CHOONG T S Y, et al. Breakthrough studies of Co3O4 supported activated carbon monolith for simultaneous SO2/NO x removal from flue gas[J]. Fuel Processing Technology, 2018, 180: 155-165.
|
| 24 |
DAVINI P. Influence of surface properties and iron addition on the SO2 adsorption capacity of activated carbons[J]. Carbon, 2002, 40(5): 729-734.
|
| 1 |
REZAEI F, ROWNAGHI A A, MONJEZI S, et al. SO x /NO x removal from flue gas streams by solid adsorbents: a review of current challenges and future directions[J]. Energy & Fuels, 2015, 29(9): 5467-5486.
|
| 2 |
MATHIEU Y, TZANIS L, SOULARD M, et al. Adsorption of SO x by oxide materials: a review[J]. Fuel Processing Technology, 2013, 114: 81-100.
|
| 25 |
MA J R, LIU Z Y, LIU S J, et al. A regenerable Fe/AC desulfurizer for SO2 adsorption at low temperatures[J]. Applied Catalysis B: Environmental, 2003, 45(4): 301-309.
|
| 26 |
CARABINEIRO S A C, RAMOS A M, VITAL J, et al. Adsorption of SO2 using vanadium and vanadium-copper supported on activated carbon[J]. Catalysis Today, 2003, 78(1/2/3/4): 203-210.
|
| 3 |
ABDULRASHEED A A, JALIL A A, TRIWAHYONO S, et al. Surface modification of activated carbon for adsorption of SO2 and NO x : a review of existing and emerging technologies[J]. Renewable and Sustainable Energy Reviews, 2018, 94: 1067-1085.
|
| 27 |
TSENG H H, WEY M Y. Study of SO2 adsorption and thermal regeneration over activated carbon-supported copper oxide catalysts[J]. Carbon, 2004, 42(11): 2269-2278.
|
| 28 |
YAO L, YANG L, JIANG W J, et al. Removal of SO2 from flue gas on a copper-modified activated coke prepared by a novel one-step carbonization activation blending method[J]. Industrial & Engineering Chemistry Research, 2019, 58(34): 15693-15700.
|
| 29 |
MARCU I C, SANDULESCU I. Study of sulfur dioxide adsorption on Y zeolite[J]. Journal of the Serbian Chemical Society, 2004, 69(7): 563-569.
|
| 30 |
ROUF S A, EIĆ M. Adsorption of SO2 from wet mixtures on hydrophobic zeolites[J]. Adsorption, 1998, 4(1): 25-33.
|
| 31 |
TANTET J, EIĆ M, DESAI R. Breakthrough study of the adsorption and separation of sulfur dioxide from wet gas using hydrophobic zeolites[J]. Gas Separation & Purification, 1995, 9(3): 213-220.
|
| 32 |
NASLUZOV V A, SHOR A M, NÖRTEMANN F, et al. Density functional study of SO2 adsorption in HY zeolites[J]. Journal of Molecular Structure, 1999, 466(1/2/3): 235-244.
|
| 33 |
KIRSCHHOCK C E A, SULTANA A, GODARD E, et al. Adsorption chemistry of sulfur dioxide in hydrated Na-Y zeolite[J]. Angewandte Chemie International Edition, 2004, 43(28): 3722-3724.
|
| 34 |
SAKURAI Y, TAKAHASHI Y, MAKINO T. Laboratory measurement of absorption and oxidation of sulphur dioxide by zeolite[J]. Journal of Geochemical Exploration, 1998, 64(1/2/3): 315-319.
|
| 35 |
LANIECKI M, ZIOLEK M, KARGE H G. Effect of water on the formation of bisulfite ions upon sulfur dioxide adsorption onto faujasite-type zeolites[J]. The Journal of Physical Chemistry, 1987, 91(1): 4-6.
|
| 36 |
SRINIVASAN A, GRUTZECK M W. The adsorption of SO2 by zeolites synthesized from fly ash[J]. Environmental Science & Technology, 1999, 33(9): 1464-1469.
|
| 37 |
PEDROLO D R S, DE MENEZES QUINES L K, DE SOUZA G, et al. Synthesis of zeolites from Brazilian coal ash and its application in SO2 adsorption[J]. Journal of Environmental Chemical Engineering, 2017, 5(5): 4788-4794.
|
| 38 |
GOLLAKOTA S V, CHRISWELL C D. Study of an adsorption process using silicalite for sulfur dioxide removal from combustion gases[J]. Industrial & Engineering Chemistry Research, 1988, 27(1): 139-143.
|
| 39 |
BU N J, WU J, MAO R, et al. Characterization of activated carbon-13X zeolite composite and its adsorption mechanism on SO2 [J]. Journal of Nanoscience and Nanotechnology, 2016, 16(8): 8839-8845.
|
| 40 |
IVANOVA E, KOUMANOVA B. Adsorption of sulfur dioxide on natural clinoptilolite chemically modified with salt solutions[J]. Journal of Hazardous Materials, 2009, 167(1/2/3): 306-312.
|
| 41 |
TERAOKA Y, MOTOI Y, YAMASAKI H, et al. Adsorption of sulfur dioxide on Y-type zeolites[J]. Studies in Surface Science and Catalysis, 1997, 105: 1787-1793.
|
| 42 |
SHIN C S, NIIYAMA H. Oxidative sorption of SO2 by Cu/zeolite[J]. Journal of the Japan Petroleum Institute, 1988, 31(2): 147-153.
|
| 43 |
DATHE H, SEDLMAIR C, JENTYS A, et al. Adsorption of SO2 on different metal impregnated zeolites[J]. Studies in Surface Science and Catalysis, 2004, 154: 3003-3009.
|
| 44 |
MATHIEU Y, SOULARD M, PATARIN J, et al. Mesoporous materials for the removal of SO2 from gas streams[J]. Fuel Processing Technology, 2012, 99: 35-42.
|
| 45 |
DEBERRY D W, SLADEK K J. Rates of reaction of SO2 with metal oxides[J]. The Canadian Journal of Chemical Engineering, 1971, 49(6): 781-785.
|
| 46 |
LOWELL P S, SCHWITZGEBEL K, PARSONS T B, et al. Selection of metal oxides for removing SO2 from flue gas[J]. Industrial & Engineering Chemistry Process Design and Development, 1971, 10(3): 384-390.
|
| 47 |
WAQIF M, SAAD A M, BENSITEL M, et al. Comparative study of SO2 adsorption on metal oxides[J]. Journal of the Chemical Society, Faraday Transactions, 1992, 88(19): 2931.
|
| 48 |
PHAM X M, PHAM D L, HANH N T, et al. An initial evaluation on the adsorption of SO2 and NO2 over porous Fe3O4 nanoparticles synthesized by facile scalable method[J]. Journal of Chemistry, 2019, 2019: 1-7.
|
| 49 |
LUO Y G, LI D J. Experimental study of nanometer TiO2 for use as an adsorbent for SO2 removal[J]. Developments in Chemical Engineering and Mineral Processing, 2002, 10(3/4): 443-457.
|
| 50 |
RODRÍGUEZ J. Environmental catalysis and the chemistry of SO2 on oxide surfaces: fundamental principles for the cleavage of S—O bonds[J]. Ciencia, 2011, 9(2): 139-154.
|
| 51 |
BALTRUSAITIS J, JAYAWEERA P M, GRASSIAN V H. Sulfur dioxide adsorption on TiO2 nanoparticles: influence of particle size, coadsorbates, sample pretreatment, and light on surface speciation and surface coverage[J]. The Journal of Physical Chemistry C, 2011, 115(2): 492-500.
|
| 52 |
ZHANG X Y, ZHUANG G S, CHEN J M, et al. Heterogeneous reactions of sulfur dioxide on typical mineral particles[J]. The Journal of Physical Chemistry B, 2006, 110(25): 12588-12596.
|
| 53 |
FU H B, WANG X, WU H B, et al. Heterogeneous uptake and oxidation of SO2 on iron oxides[J]. The Journal of Physical Chemistry C, 2007, 111(16): 6077-6085.
|
| 54 |
WANG H L, HAO R, GAO M P, et al. High temperature adsorption of SO2 on mixed oxides derived from CaAl hydrotalcite-like compounds[J]. Processes, 2021, 9(2): 325.
|
| 55 |
MA C B, YI H H, TANG X L, et al. Improving simultaneous removal efficiency of SO2 and NO x from flue gas by surface modification of MgO with organic component[J]. Journal of Cleaner Production, 2019, 230: 508-517.
|
| 56 |
ZHAO L, BI S N, PEI J S, et al. Adsorption performance of SO2 over ZnAl2O4 nanospheres[J]. Journal of Industrial and Engineering Chemistry, 2016, 41: 151-157.
|
| 57 |
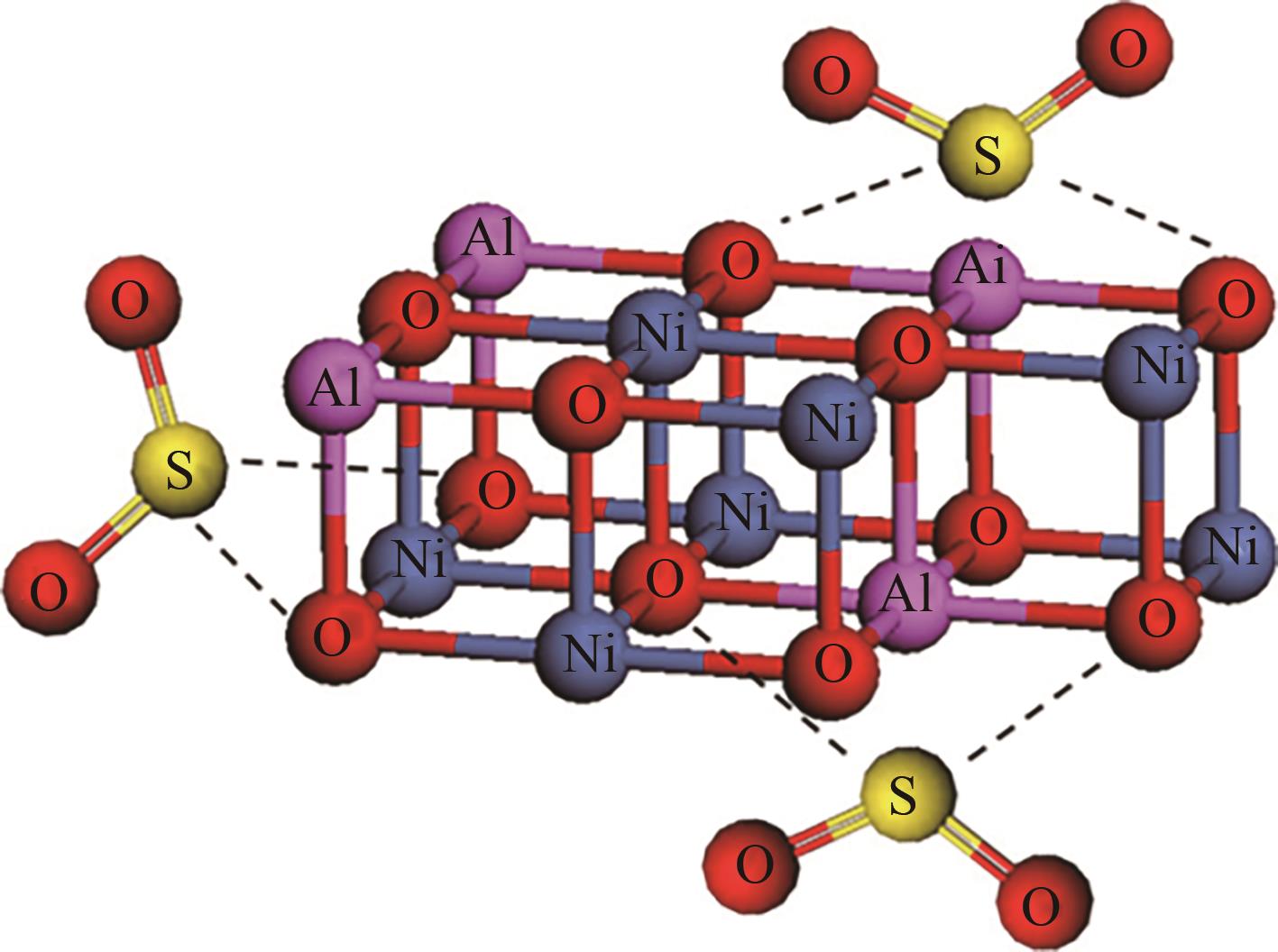
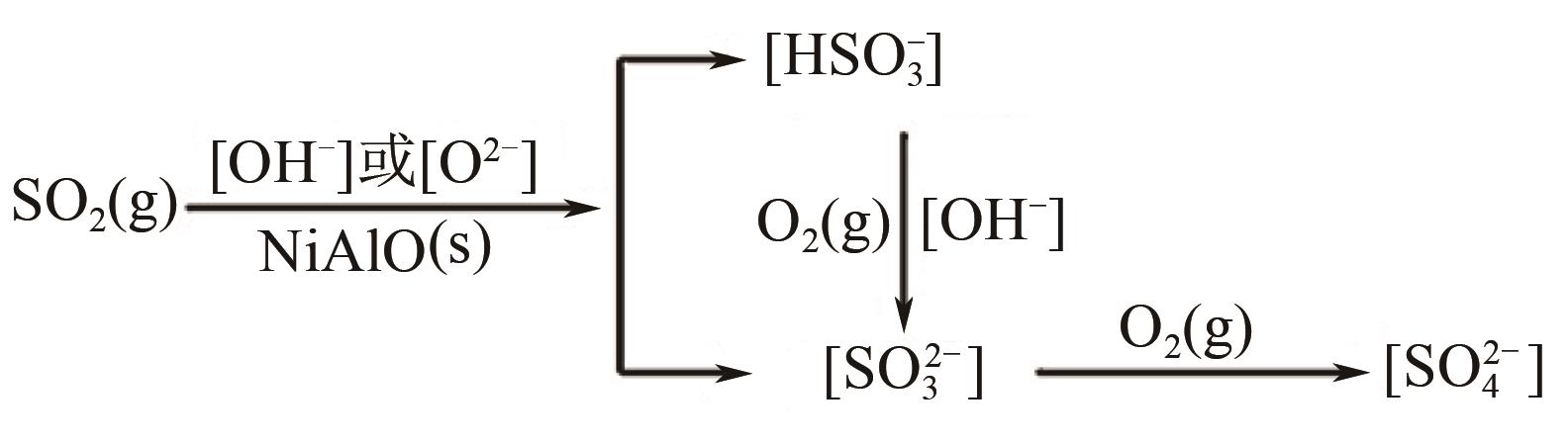
ZHAO L, LI X Y, HAO C, et al. SO2 adsorption and transformation on calcined NiAl hydrotalcite-like compounds surfaces: an in situ FTIR and DFT study[J]. Applied Catalysis B: Environmental, 2012, 117/118: 339-345.
|
| 58 |
ZHAO L, LI X, QUAN X, et al. Effects of surface features on sulfur dioxide adsorption on calcined NiAl hydrotalcite-like compounds[J]. Environmental Science & Technology, 2011, 45(12): 5373-5379.
|
| 59 |
ZHAO L, LI X Y, ZHAO J. Correlation of structural and chemical characteristics with catalytic performance of hydrotalcite-based CuNiAl mixed oxides for SO2 abatement[J]. Chemical Engineering Journal, 2013, 223: 164-171.
|
| 60 |
WAQIF M, SAUR O, LAVALLEY J C, et al. Nature and mechanism of formation of sulfate species on copper/alumina sorbent-catalysts for sulfur dioxide removal[J]. The Journal of Physical Chemistry, 1991, 95(10): 4051-4058.
|
| 61 |
XIE G Y, LIU Z Y, ZHU Z P, et al. Reductive regeneration of sulfated CuO/Al2O3 catalyst-sorbent in ammonia[J]. Applied Catalysis B: Environmental, 2003, 45(3): 213-221.
|
| 62 |
JEONG S M, KIM S D. Enhancement of the SO2 sorption capacity of CuO/γ-Al2O3 sorbent by an alkali-salt promoter[J]. Industrial & Engineering Chemistry Research, 1997, 36(12): 5425-5431.
|
| 63 |
DATHE H, JENTYS A, HAIDER P, et al. On the trapping of SO x on CaO-Al2O3-based novel high capacity sorbents[J]. Physical Chemistry Chemical Physics, 2006, 8(13): 1601-1613.
|
| 64 |
KYLHAMMAR L, CARLSSON P A, INGELSTEN H H, et al. Regenerable ceria-based SO x traps for sulfur removal in lean exhausts[J]. Applied Catalysis B: Environmental, 2008, 84(1/2): 268-276.
|
| 65 |
SINHA A, SUZUKI K, TAKAHARA M, et al. Mesostructured manganese oxide/gold nanoparticle composites for extensive air purification[J]. Angewandte Chemie International Edition, 2007, 46(16): 2891-2894.
|
| 66 |
DIAF A, GARCIA J L, BECKMAN E J. Thermally reversible polymeric sorbents for acid gases: CO2, SO2, and NO x [J]. Journal of Applied Polymer Science, 1994, 53(7): 857-875.
|
| 67 |
DIAF A, BECKMAN E J. Thermally reversible polymeric sorbents for acid gases, Ⅳ. Affinity tuning for the selective dry sorption of NO x [J]. Reactive Polymers, 1995, 25(1): 89-96.
|
| 68 |
KHATRI R A, CHUANG S S C, SOONG Y, et al. Thermal and chemical stability of regenerable solid amine sorbent for CO2 capture[J]. Energy & Fuels, 2006, 20(4): 1514-1520.
|
| 69 |
BELMABKHOUT Y, SAYARI A. Isothermal versus non-isothermal adsorption-desorption cycling of triamine-grafted pore-expanded MCM-41 mesoporous silica for CO2 capture from flue gas[J]. Energy & Fuels, 2010, 24(9): 5273-5280.
|
| 70 |
LEE H J, LEE K I, KIM M, et al. Diamine-anchored polystyrene resins for reversible SO2 adsorption[J]. ACS Sustainable Chemistry & Engineering, 2016, 4(4): 2012-2019.
|
| 71 |
WEI L, GAO Z, WANG Y. Integrated two-stage adsorption for selective removal of CO2 and SO2 by amine-functionalized SBA-15[J]. Asia-Pacific Journal of Chemical Engineering, 2017, 12(4): 660-670.
|
| 72 |
YU X, HAO J L, XI Z C, et al. Investigation of low concentration SO2 adsorption performance on different amine-modified Merrifield resins[J]. Atmospheric Pollution Research, 2019, 10(2): 404-411.
|
| 73 |
SHAO J G, ZHANG J J, ZHANG X, et al. Enhance SO2 adsorption performance of biochar modified by CO2 activation and amine impregnation[J]. Fuel, 2018, 224: 138-146.
|
| 74 |
XU X C, SONG C S, MILLER B G, et al. Adsorption separation of carbon dioxide from flue gas of natural gas-fired boiler by a novel nanoporous "molecular basket” adsorbent[J]. Fuel Processing Technology, 2005, 86(14/15): 1457-1472.
|
| 75 |
UYANGA I J, IDEM R O. Studies of SO2- and O2- induced degradation of aqueous MEA during CO2 capture from power plant flue gas streams[J]. Industrial & Engineering Chemistry Research, 2007, 46(8): 2558-2566.
|
| 76 |
DATHE H, JENTYS A, LERCHER J A. Adsorption of SO2 on Ba impregnated metal organic framework materials[M]//Molecular sieves: from basic research to industrial applications, proceedings of the 3rd international zeolite symposium. Amsterdam: Elsevier, 2005: 995-1002.
|
| 77 |
FERNANDEZ C A, THALLAPALLY P K, MOTKURI R K, et al. Gas-induced expansion and contraction of a fluorinated metal-organic framework[J]. Crystal Growth & Design, 2010, 10(3): 1037-1039.
|
| 78 |
BRITT D, TRANCHEMONTAGNE D, YAGHI O M. Metal-organic frameworks with high capacity and selectivity for harmful gases[J]. PNAS, 2008, 105(33): 11623-11627.
|
| 79 |
TAN K, ZULUAGA S, WANG H, et al. Interaction of acid gases SO2 and NO2 with coordinatively unsaturated metal organic frameworks: M-MOF-74 (M=Zn, Mg, Ni, Co)[J]. Chemistry of Materials, 2017, 29(10): 4227-4235.
|
| 80 |
HUNGERFORD J, BHATTACHARYYA S, TUMULURI U, et al. DMOF-1 as a representative MOF for SO2 adsorption in both humid and dry conditions[J]. The Journal of Physical Chemistry C, 2018, 122(41): 23493-23500.
|
| 81 |
GLOMB S, WOSCHKO D, MAKHLOUFI G, et al. Metal-organic frameworks with internal urea-functionalized dicarboxylate linkers for SO2 and NH3 adsorption[J]. ACS Applied Materials & Interfaces, 2017, 9(42): 37419-37434.
|
| 82 |
ZHANG Y, CHEN Z H, LIU X, et al. Efficient SO2 removal using a microporous metal-organic framework with molecular sieving effect[J]. Industrial & Engineering Chemistry Research, 2020, 59(2): 874-882.
|
 ), LI Chuankun, YANG Zhe(
), LI Chuankun, YANG Zhe( ), GOU Chengdong, GAO Xinjiang
), GOU Chengdong, GAO Xinjiang