化工进展 ›› 2024, Vol. 43 ›› Issue (10): 5369-5380.DOI: 10.16085/j.issn.1000-6613.2023-1527
• 化工过程与装备 • 上一篇
CO2化学转化碳酸二甲酯/乙二醇的能量集成和碳流分析
- 1.大连交通大学环境与化学工程学院,辽宁 大连 116028
2.中国科学院过程工程研究所,北京 100190
-
收稿日期:2023-09-01修回日期:2023-11-22出版日期:2024-10-15发布日期:2024-10-29 -
通讯作者:李英 -
作者简介:纵华健(2000—),男,硕士研究生,研究方向为化工过程模拟。E-mail:2422379654@qq.com。 -
基金资助:国家自然科学基金(U22A20416);大连理工大学精细化工国家重点实验室开放课题基金(KF2116)
Energy integration and carbon flow analysis of process of CO2 chemical transformation to dimethyl carbonate and ethylene glycol
ZONG Huajian1( ), LI Ying1(
), LI Ying1( ), ZHANG Xiangping2
), ZHANG Xiangping2
- 1.School of Environmental and Chemical Engineering, Dalian Jiaotong University, Dalian 116028, Liaoning, China
2.Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China
-
Received:2023-09-01Revised:2023-11-22Online:2024-10-15Published:2024-10-29 -
Contact:LI Ying
摘要:
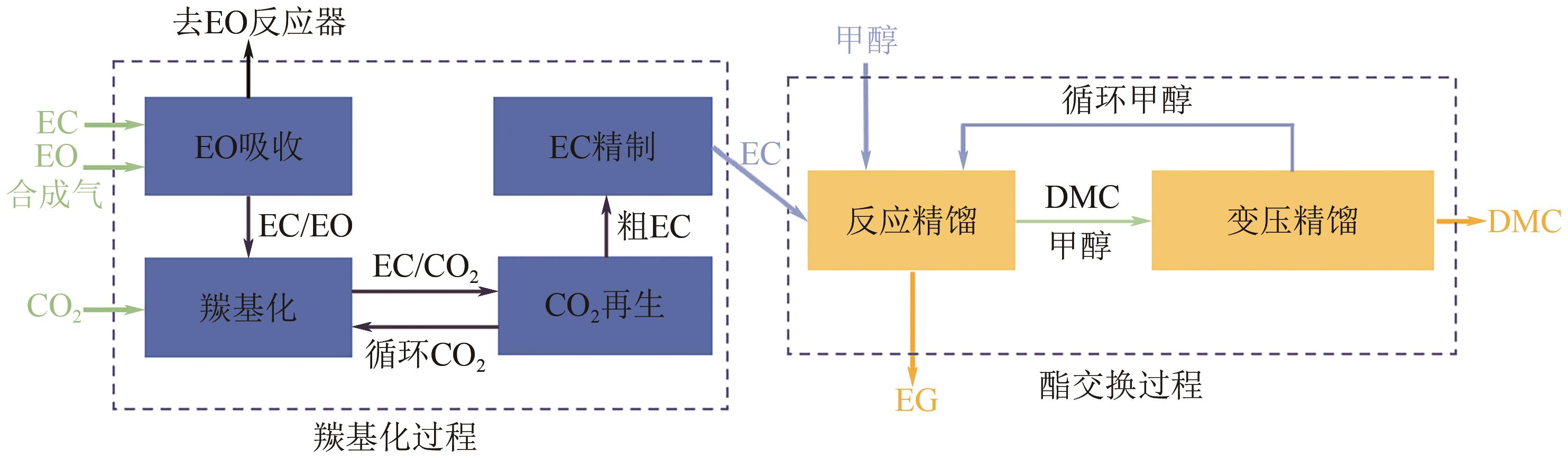
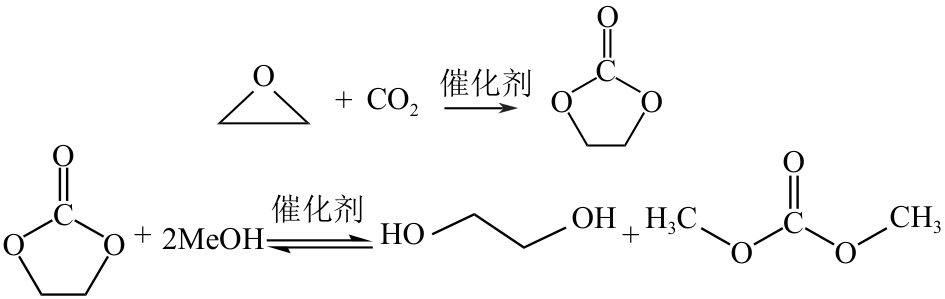
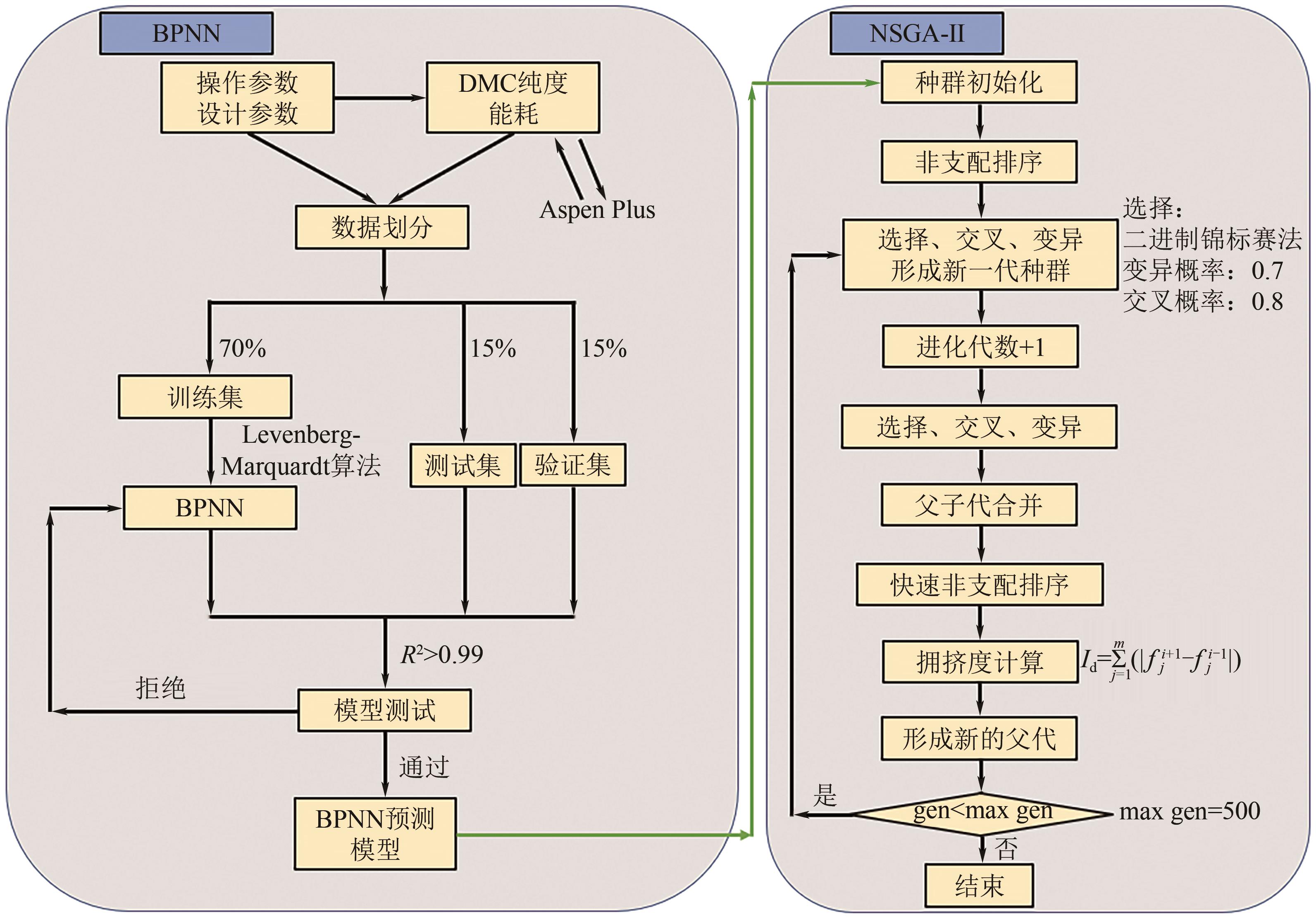
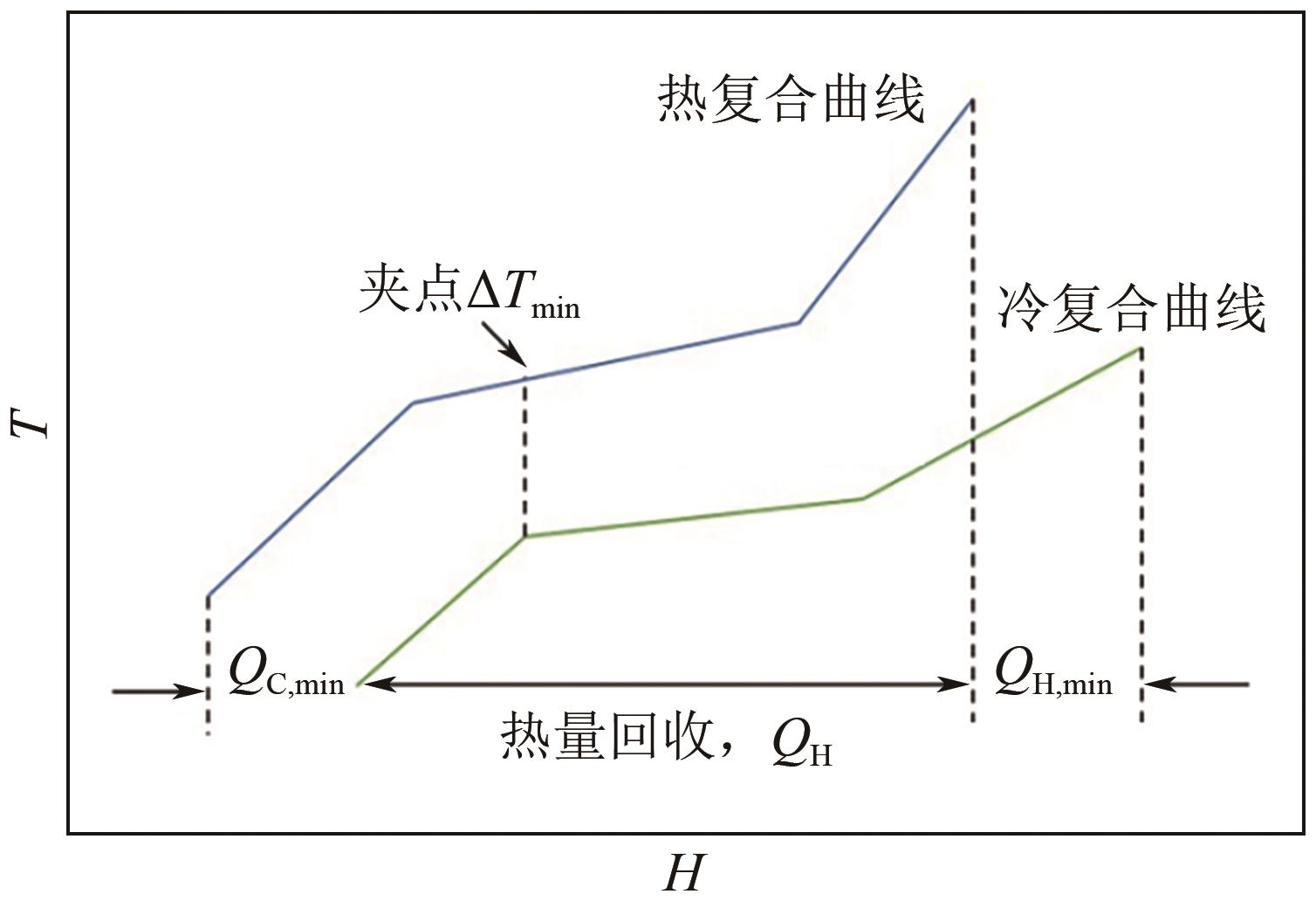
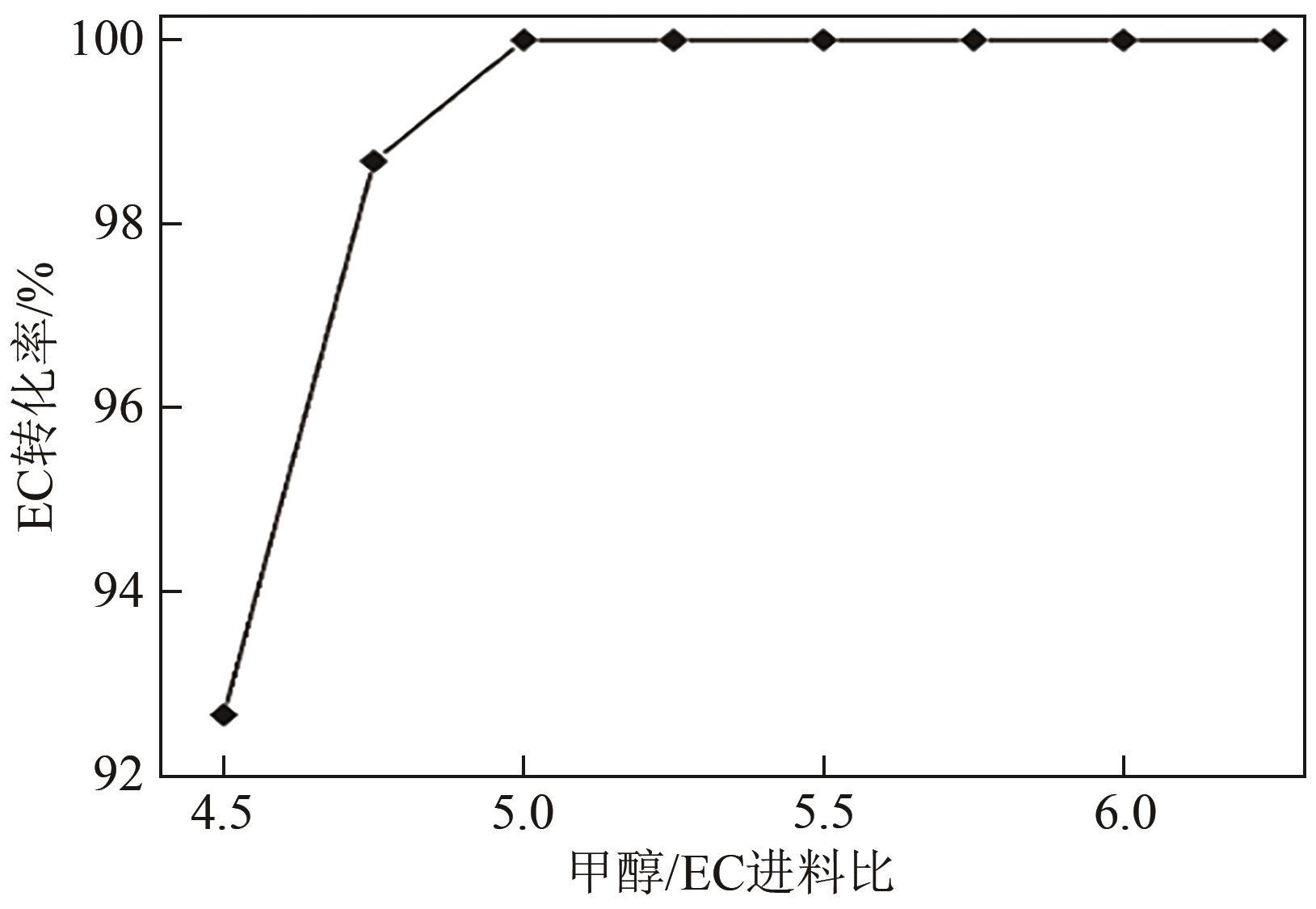
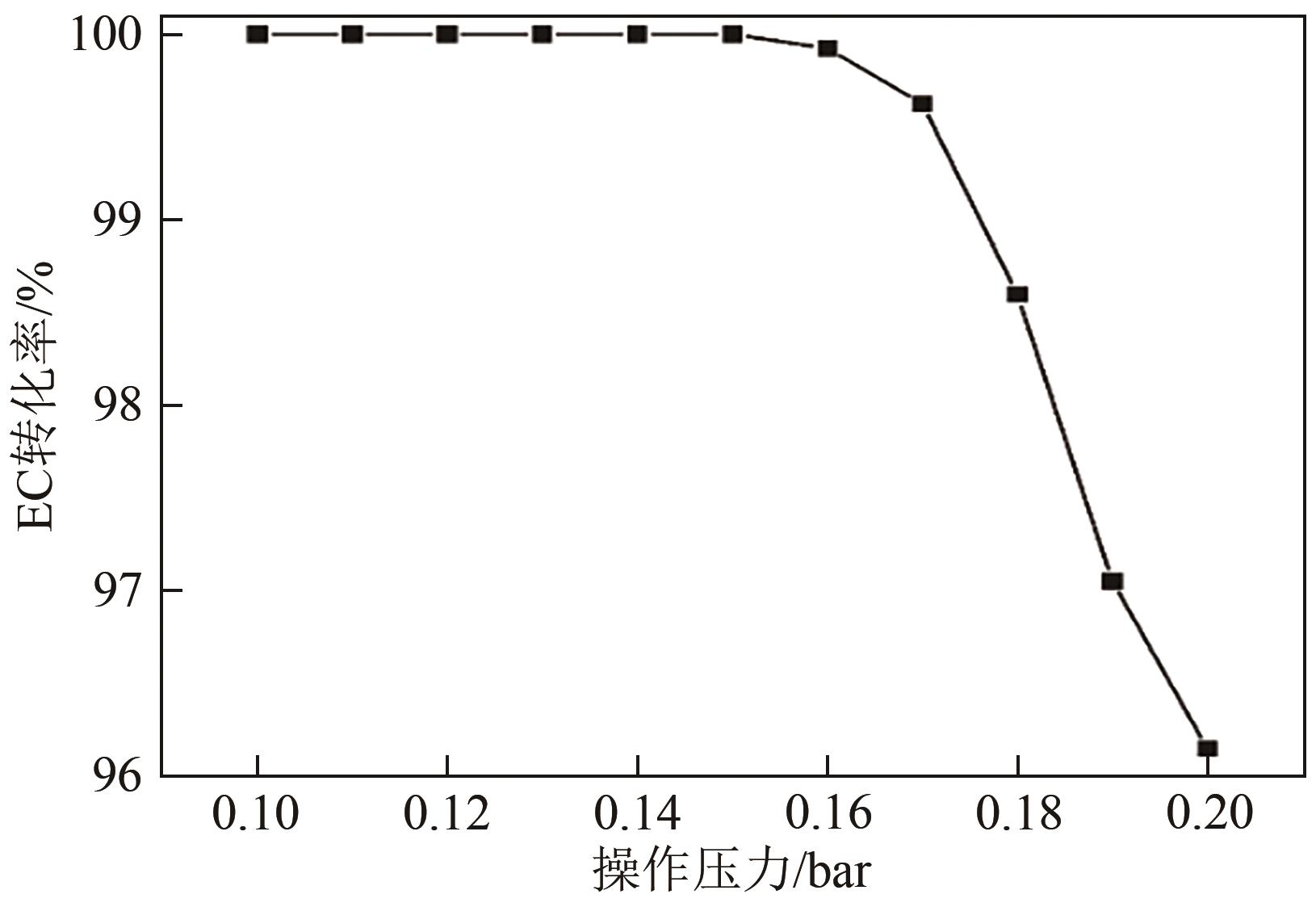
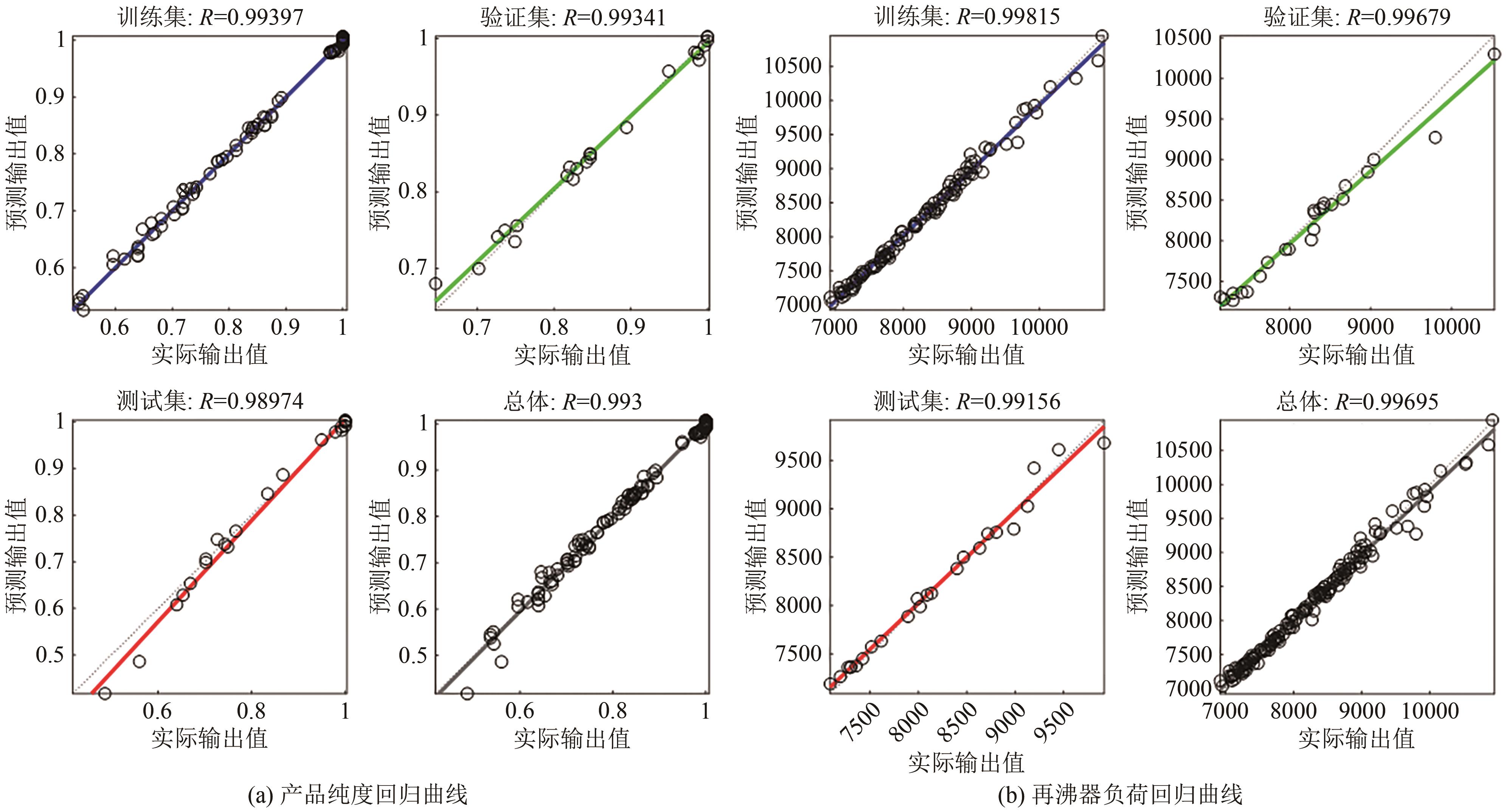
CO2化学转化可获得高值化学品,实现CO2资源化利用,是解决“碳中和”问题的理想方法之一。两步酯交换法生产碳酸二甲酯(DMC)、副产乙二醇,是实现CO2高值化利用的有效途径。针对该过程面临的CO2活化难、生产成本高的技术难题,本文采用碳酸乙烯酯一步吸收环氧乙烷、离子液体催化剂、反应精馏实现酯交换以及变压精馏分离碳酸二甲酯和甲醇共沸物等过程强化方法实现CO2两步转化。本文首先利用Aspen Plus完成全流程模拟,再采用BP神经网络和第二代非支配排序多目标遗传算法(NSGA-Ⅱ)优化酯交换过程参数,基于夹点技术对酯交换过程进行能量集成并对全过程进行严格的碳流分析。能量集成结果表明,酯交换过程热公用工程用量降低40.34%;碳流分析结果表明,全过程总碳原子利用率达到99.81%,考虑能源消耗的间接碳排放,碳原子利用效率为86.90%,净CO2排放量0.314kg CO2/kg DMC。与文献报道的工艺相比,本文工艺流程所得DMC产品纯度较高(99.9995%)、能耗更小(1.10kW·h/kg DMC),可为CO2化学转化碳酸二甲酯和乙二醇提供技术指导。
中图分类号:
引用本文
纵华健, 李英, 张香平. CO2化学转化碳酸二甲酯/乙二醇的能量集成和碳流分析[J]. 化工进展, 2024, 43(10): 5369-5380.
ZONG Huajian, LI Ying, ZHANG Xiangping. Energy integration and carbon flow analysis of process of CO2 chemical transformation to dimethyl carbonate and ethylene glycol[J]. Chemical Industry and Engineering Progress, 2024, 43(10): 5369-5380.
| 组分i | 组分j | aij | aji | bij | bji | cij | cji |
|---|---|---|---|---|---|---|---|
| 甲醇 | DMC | -2.0637 | 2.6639 | 642.56 | -1104.8 | 0 | 0 |
| 甲醇 | EC | -0.5409 | 15.892 | 4589.3 | -1278.9 | -2.2771 | -2.0488 |
| 甲醇 | EG | -32.587 | 2.2712 | 10000 | -599.11 | 0 | 0 |
| DMC | EC | 2.5273 | -6.7598 | 167.34 | 442.34 | -0.7321 | 1.0398 |
| DMC | EG | 0 | 0 | -236.13 | -146.88 | 0 | 0 |
| EC | EG | 0 | 0 | -275.66 | -27.96 | 0 | 0 |
表1 甲醇-DMC-EG-EC共沸物UNIQUAC模型交互参数[22,25]
| 组分i | 组分j | aij | aji | bij | bji | cij | cji |
|---|---|---|---|---|---|---|---|
| 甲醇 | DMC | -2.0637 | 2.6639 | 642.56 | -1104.8 | 0 | 0 |
| 甲醇 | EC | -0.5409 | 15.892 | 4589.3 | -1278.9 | -2.2771 | -2.0488 |
| 甲醇 | EG | -32.587 | 2.2712 | 10000 | -599.11 | 0 | 0 |
| DMC | EC | 2.5273 | -6.7598 | 167.34 | 442.34 | -0.7321 | 1.0398 |
| DMC | EG | 0 | 0 | -236.13 | -146.88 | 0 | 0 |
| EC | EG | 0 | 0 | -275.66 | -27.96 | 0 | 0 |
| 上下限 | 反应精馏塔 | 高压塔 | 低压塔 | |||
|---|---|---|---|---|---|---|
| 压力/bar | 回流比 | 压力/bar | 回流比 | 压力/bar | 回流比 | |
| 上限 | 0.1 | 0.2 | 10 | 0.2 | 1 | 3 |
| 下限 | 0.3 | 1.0 | 20 | 1.2 | 5 | 9 |
表2 操作参数的上下限
| 上下限 | 反应精馏塔 | 高压塔 | 低压塔 | |||
|---|---|---|---|---|---|---|
| 压力/bar | 回流比 | 压力/bar | 回流比 | 压力/bar | 回流比 | |
| 上限 | 0.1 | 0.2 | 10 | 0.2 | 1 | 3 |
| 下限 | 0.3 | 1.0 | 20 | 1.2 | 5 | 9 |
| 流股 | 温度 /℃ | 压力 /bar | 摩尔流量 /kmol·h-1 | 质量流量/kg·h-1 | ||||
|---|---|---|---|---|---|---|---|---|
| EO | CO2 | CH4 | C2H4 | EC | ||||
| S1-1 | 25 | 20 | 45 | 0 | 0 | 0 | 0 | 3962.8 |
| S1-2 | 50 | 20 | 169.5 | 220.3 | 126.1 | 1603.7 | 1729.8 | 0 |
| S1-3 | 39.5 | 20 | 160 | 6.26 | 119.7 | 1584 | 1639.2 | 0.06 |
| S1-4 | 58.1 | 20.2 | 54.47 | 214 | 6.38 | 19.76 | 90.67 | 3962.7 |
| S1-5 | -111.4 | 5 | 4.61 | 0.05 | 6.38 | 19.76 | 90.67 | 0 |
| S1-6 | 171 | 5 | 49.86 | 213.9 | 0 | 0 | 0 | 3962.8 |
| S1-7 | 120 | 20 | 5.03 | 0 | 221.4 | 0 | 0 | 0 |
| S1-8 | 120 | 20 | 54.72 | 0.15 | 212.3 | 0 | 0 | 4393.5 |
| S1-9 | 105 | 0.98 | 4.69 | 0.09 | 204.65 | 0 | 0 | 3.15 |
| S1-10 | 105 | 0.98 | 50.03 | 0.06 | 7.66 | 0 | 0 | 4390.4 |
| S1-11 | -99.5 | 0.5 | 0.17 | 0.06 | 7.66 | 0 | 0 | 0 |
| S1-12 | 219.5 | 0.5 | 49.855 | 0 | 0 | 0 | 0 | 4390.4 |
表3 羰基化过程工艺物流数据
| 流股 | 温度 /℃ | 压力 /bar | 摩尔流量 /kmol·h-1 | 质量流量/kg·h-1 | ||||
|---|---|---|---|---|---|---|---|---|
| EO | CO2 | CH4 | C2H4 | EC | ||||
| S1-1 | 25 | 20 | 45 | 0 | 0 | 0 | 0 | 3962.8 |
| S1-2 | 50 | 20 | 169.5 | 220.3 | 126.1 | 1603.7 | 1729.8 | 0 |
| S1-3 | 39.5 | 20 | 160 | 6.26 | 119.7 | 1584 | 1639.2 | 0.06 |
| S1-4 | 58.1 | 20.2 | 54.47 | 214 | 6.38 | 19.76 | 90.67 | 3962.7 |
| S1-5 | -111.4 | 5 | 4.61 | 0.05 | 6.38 | 19.76 | 90.67 | 0 |
| S1-6 | 171 | 5 | 49.86 | 213.9 | 0 | 0 | 0 | 3962.8 |
| S1-7 | 120 | 20 | 5.03 | 0 | 221.4 | 0 | 0 | 0 |
| S1-8 | 120 | 20 | 54.72 | 0.15 | 212.3 | 0 | 0 | 4393.5 |
| S1-9 | 105 | 0.98 | 4.69 | 0.09 | 204.65 | 0 | 0 | 3.15 |
| S1-10 | 105 | 0.98 | 50.03 | 0.06 | 7.66 | 0 | 0 | 4390.4 |
| S1-11 | -99.5 | 0.5 | 0.17 | 0.06 | 7.66 | 0 | 0 | 0 |
| S1-12 | 219.5 | 0.5 | 49.855 | 0 | 0 | 0 | 0 | 4390.4 |
| 流股 | 温度 /℃ | 压力 /bar | 摩尔流量 /kmol·h-1 | 质量流量/kg·h-1 | |||
|---|---|---|---|---|---|---|---|
| 甲醇 | EC | DMC | EG | ||||
| S2-1 | 25 | 0.4 | 49.855 | 0 | 4390.4 | 0 | 0 |
| S2-2 | 25 | 0.1 | 99.71 | 3194.9 | 0 | 0 | 0 |
| S2-3 | 19.2 | 1 | 258.04 | 6131.3 | 0 | 6007.1 | 0 |
| S2-4 | 19.9 | 16.4 | 258.04 | 6131.3 | 0 | 6007.1 | 0 |
| S2-5 | 160.6 | 16.3 | 258.04 | 6131.3 | 0 | 6007.1 | 0 |
| S2-6 | 132.2 | 0.1 | 49.86 | 0.16 | 0.18 | 0 | 3094.3 |
| S2-7 | 157.2 | 16.3 | 198.69 | 6062.4 | 0 | 854.5 | 0 |
| S2-8 | 206.1 | 16.3 | 59.35 | 68.8 | 0 | 5152.6 | 0 |
| S2-9 | 112.4 | 2.2 | 59.35 | 68.8 | 0 | 5152.6 | 0 |
| S2-10 | 97.3 | 2.2 | 9.50 | 68.8 | 0 | 662.0 | 0 |
| S2-11 | 116.8 | 2.2 | 49.853 | 0.008 | 0 | 4490.69 | 0 |
表4 酯交换过程工艺物流数据
| 流股 | 温度 /℃ | 压力 /bar | 摩尔流量 /kmol·h-1 | 质量流量/kg·h-1 | |||
|---|---|---|---|---|---|---|---|
| 甲醇 | EC | DMC | EG | ||||
| S2-1 | 25 | 0.4 | 49.855 | 0 | 4390.4 | 0 | 0 |
| S2-2 | 25 | 0.1 | 99.71 | 3194.9 | 0 | 0 | 0 |
| S2-3 | 19.2 | 1 | 258.04 | 6131.3 | 0 | 6007.1 | 0 |
| S2-4 | 19.9 | 16.4 | 258.04 | 6131.3 | 0 | 6007.1 | 0 |
| S2-5 | 160.6 | 16.3 | 258.04 | 6131.3 | 0 | 6007.1 | 0 |
| S2-6 | 132.2 | 0.1 | 49.86 | 0.16 | 0.18 | 0 | 3094.3 |
| S2-7 | 157.2 | 16.3 | 198.69 | 6062.4 | 0 | 854.5 | 0 |
| S2-8 | 206.1 | 16.3 | 59.35 | 68.8 | 0 | 5152.6 | 0 |
| S2-9 | 112.4 | 2.2 | 59.35 | 68.8 | 0 | 5152.6 | 0 |
| S2-10 | 97.3 | 2.2 | 9.50 | 68.8 | 0 | 662.0 | 0 |
| S2-11 | 116.8 | 2.2 | 49.853 | 0.008 | 0 | 4490.69 | 0 |
| 项目 | 能量集成前 | 能量集成后 | 对比/% |
|---|---|---|---|
| 热公用工程/kW | 7418.4 | 4425.9 | -40.34 |
| 冷公用工程/kW | 7292.7 | 4297.2 | -41.07 |
| 总换热面积/m2 | 268.74 | 322.08 | 19.85 |
| 总投资成本/103USD | 177.07 | 226.05 | 27.66 |
| 加热操作费/103USD·a-1 | 536.89 | 329.77 | -38.58 |
| 冷却操作费/103USD·a-1 | 562.47 | 542.51 | -3.54 |
| 年度总成本/103USD·a-1 | 1146.10 | 927.29 | -19.09 |
表5 能量集成前后模拟结果比较
| 项目 | 能量集成前 | 能量集成后 | 对比/% |
|---|---|---|---|
| 热公用工程/kW | 7418.4 | 4425.9 | -40.34 |
| 冷公用工程/kW | 7292.7 | 4297.2 | -41.07 |
| 总换热面积/m2 | 268.74 | 322.08 | 19.85 |
| 总投资成本/103USD | 177.07 | 226.05 | 27.66 |
| 加热操作费/103USD·a-1 | 536.89 | 329.77 | -38.58 |
| 冷却操作费/103USD·a-1 | 562.47 | 542.51 | -3.54 |
| 年度总成本/103USD·a-1 | 1146.10 | 927.29 | -19.09 |
| 工艺 | DMC纯度/% | EG纯度/% | 热量消耗/kW·h·(kg DMC)-1 | 参考文献 | |
|---|---|---|---|---|---|
| 集成前 | 集成后 | ||||
| EC路线 | 99.16 (99.2) | 99.99 (99.9) | 2.54 | — | [ |
| 尿素路线 | 99.74 (99.7) | — | 16.49 | — | [ |
| BAYER法 | 100.0 (100.0) | — | 2.93 | — | [ |
| 酯交换-变压精馏 | 100.0 | 99.82 | 2.44 | 1.25 | [ |
| 酯交换-变压精馏 | 99.99 | 99.80 | 11.08 | — | [ |
| 酯交换-苯酚萃取精馏 | 99.80 | 99.80 | 4.34 | — | [ |
| 酯交换-苯胺萃取精馏 | 99.50 | 99.50 | 2.78 | — | [ |
| 酯交换-苯胺萃取精馏 | 99.50 | 99.50 | — | 1.85 | [ |
| 本文 | 99.999 | 99.986 | 1.76 | 1.10 | 模拟 |
表6 本文结果与文献中工艺产品纯度和生产能耗的比较
| 工艺 | DMC纯度/% | EG纯度/% | 热量消耗/kW·h·(kg DMC)-1 | 参考文献 | |
|---|---|---|---|---|---|
| 集成前 | 集成后 | ||||
| EC路线 | 99.16 (99.2) | 99.99 (99.9) | 2.54 | — | [ |
| 尿素路线 | 99.74 (99.7) | — | 16.49 | — | [ |
| BAYER法 | 100.0 (100.0) | — | 2.93 | — | [ |
| 酯交换-变压精馏 | 100.0 | 99.82 | 2.44 | 1.25 | [ |
| 酯交换-变压精馏 | 99.99 | 99.80 | 11.08 | — | [ |
| 酯交换-苯酚萃取精馏 | 99.80 | 99.80 | 4.34 | — | [ |
| 酯交换-苯胺萃取精馏 | 99.50 | 99.50 | 2.78 | — | [ |
| 酯交换-苯胺萃取精馏 | 99.50 | 99.50 | — | 1.85 | [ |
| 本文 | 99.999 | 99.986 | 1.76 | 1.10 | 模拟 |
| 工艺 | 净CO2排放/kg CO2·(kg DMC)-1 | 参考文献 |
|---|---|---|
| EC路线 | 0.45 | [ |
| 尿素路线 | 1.28 | [ |
| 膜生物反应器 | 0.52 | [ |
| 渗透汽化 | 0.67 | [ |
| 脱水反应精馏 | 0.71 | [ |
| CO2与MeOH直接合成 | 0.34 | [ |
| 本文 | 0.31 | 模拟 |
表7 净CO2排放对比
| 工艺 | 净CO2排放/kg CO2·(kg DMC)-1 | 参考文献 |
|---|---|---|
| EC路线 | 0.45 | [ |
| 尿素路线 | 1.28 | [ |
| 膜生物反应器 | 0.52 | [ |
| 渗透汽化 | 0.67 | [ |
| 脱水反应精馏 | 0.71 | [ |
| CO2与MeOH直接合成 | 0.34 | [ |
| 本文 | 0.31 | 模拟 |
| 1 | 张凡, 王树众, 李艳辉, 等. 中国制造业碳排放问题分析与减排对策建议[J]. 化工进展, 2022, 41(3): 1645-1653. |
| ZHANG Fan, WANG Shuzhong, LI Yanhui, et al. Analysis of CO2 emission and countermeasures and suggestions for emission reduction in Chinese manufacturing[J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1645-1653. | |
| 2 | 巩金龙. CO2化学转化研究进展概述[J]. 化工学报, 2017, 68(4): 1282-1285. |
| GONG Jinlong. A brief overview on recent progress on chemical conversion of CO2 [J]. CIESC Journal, 2017, 68(4): 1282-1285. | |
| 3 | Hrvoje MIKULČIĆ, RIDJAN SKOV Iva, DOMINKOVIĆ Dominik Franjo, et al. Flexible Carbon Capture and Utilization technologies in future energy systems and the utilization pathways of captured CO2 [J]. Renewable and Sustainable Energy Reviews, 2019, 114: 109338. |
| 4 | 姚向阳, 张彦, 高嵩, 等. 面向可持续发展的二氧化碳化学研究进展[J]. 华中师范大学学报(自然科学版), 2019, 53(6): 834-846. |
| YAO Xiangyang, ZHANG Yan, GAO Song, et al. Progress on carbon dioxide chemistry towards sustainable development[J]. Journal of Central China Normal University (Natural Sciences), 2019, 53(6): 834-846. | |
| 5 | YANG Na, WANG Rui. Sustainable technologies for the reclamation of greenhouse gas CO2 [J]. Journal of Cleaner Production, 2015, 103: 784-792. |
| 6 | FIORANI G, PEROSA A, SELVA M. Dimethyl carbonate: A versatile reagent for a sustainable valorization of renewables[J]. Green Chemistry, 2018, 20(2): 288-322. |
| 7 | XU Baohua, WANG Jinquan, SUN Jian, et al. Fixation of CO2 into cyclic carbonates catalyzed by ionic liquids: A multi-scale approach[J]. Green Chemistry, 2015, 17(1): 108-122. |
| 8 | TUNDO P, MUSOLINO M, ARICÒ F. The reactions of dimethyl carbonate and its derivatives[J]. Green Chemistry, 2018, 20(1): 28-85. |
| 9 | SONG Qingwen, ZHOU Zhihua, HE Liangnian. Efficient, selective and sustainable catalysis of carbon dioxide[J]. Green Chemistry, 2017, 19(16): 3707-3728. |
| 10 | ZHANG Suojiang, SUN Jian, ZHANG Xiaochun, et al. Ionic liquid-based green processes for energy production[J]. Chemical Society Reviews, 2014, 43(22): 7838-7869. |
| 11 | PETER Goodrich, NIMAL Gunaratne H Q, JOHAN Jacquemin, et al. Sustainable cyclic carbonate production, utilizing carbon dioxide and azolate ionic liquids[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(7): 5635-5641. |
| 12 | YANG Chaokun, LIU Mengshuai, ZHANG Jiaxu, et al. Facile synthesis of DBU-based ionic liquids cooperated with ZnI2 as catalysts for efficient cycloaddition of CO2 to epoxides under mild and solvent-free conditions[J]. Molecular Catalysis, 2018, 450: 39-45. |
| 13 | LIU Mengshuai, LI Xin, LIANG Lin, et al. Protonated triethanolamine as multi-hydrogen bond donors catalyst for efficient cycloaddition of CO2 to epoxides under mild and cocatalyst-free conditions[J]. Journal of CO2 Utilization, 2016, 16: 384-390. |
| 14 | WANG Lin, ZHANG Guangyou, KODAMA Koichi, et al. An efficient metal- and solvent-free organocatalytic system for chemical fixation of CO2 into cyclic carbonates under mild conditions[J]. Green Chemistry, 2016, 18(5): 1229-1233. |
| 15 | 郭艳东, 佟佳欢, 刘晓敏, 等. 负载型离子液体的研究进展及发展趋势[J]. 中国科学(化学), 2016, 46(12): 1305-1316. |
| GUO Yandong, TONG Jiahuan, LIU Xiaomin, et al. Recent advances and development of supported ionic liquids[J]. SCIENTIA SINICA Chimica, 2016, 46(12): 1305-1316. | |
| 16 | CHENG Weiguo, CHEN Xi, SUN Jian, et al. SBA-15 supported triazolium-based ionic liquids as highly efficient and recyclable catalysts for fixation of CO2 with epoxides[J]. Catalysis Today, 2013, 200: 117-124. |
| 17 | 贺玥玥, 顾晓华, 潘子鹤, 等. KF/MgO催化醇解制备碳酸二甲酯的超声强化研究[J]. 石油化工, 2019, 48(10): 996-1000. |
| HE Yueyue, GU Xiaohua, PAN Zihe, et al. Enhancement of ultrasonic on preparation of dimethyl carbonate through alcoholysis catalyzed by KF/MgO[J]. Petrochemical Technology, 2019, 48(10): 996-1000. | |
| 18 | WANG San-Jang, YU Cheng-Ching, HUANG Hsiao-Ping. Plant-wide design and control of DMC synthesis process via reactive distillation and thermally coupled extractive distillation[J]. Computers & Chemical Engineering, 2010, 34(3): 361-373. |
| 19 | 董营, 肖颖, 黄耀东, 等. 萃取精馏分离碳酸二甲酯-乙醇二元共沸物[J]. 化工进展, 2013, 32(4): 750-756, 768. |
| DONG Ying, XIAO Ying, HUANG Yaodong, et al. Separation of binary azeotrope ethanol-dimethyl carbonate by extractive distillation[J]. Chemical Industry and Engineering Progress, 2013, 32(4): 750-756, 768. | |
| 20 | 李文秀, 连利燕, 张志刚, 等. 萃取精馏分离碳酸二甲酯和甲醇共沸物[J]. 化学工程, 2012, 40(7): 14-17, 25. |
| LI Wenxiu, LIAN Liyan, ZHANG Zhigang, et al. Separation of dimethyl carbonate-methanol mixture by extractive distillation[J]. Chemical Engineering, 2012, 40(7): 14-17, 25. | |
| 21 | HU Chi-Chih, CHENG Shueh-Hen. Development of alternative methanol/dimethyl carbonate separation systems by extractive distillation—A holistic approach[J]. Chemical Engineering Research and Design, 2017, 127: 189-214. |
| 22 | HSU Kai-Yi, HSIAO Yuan-Chang, I-Lung CHIEN. Design and control of dimethyl carbonate-methanol separation via extractive distillation in the dimethyl carbonate reactive-distillation process[J]. Industrial & Engineering Chemistry Research, 2010, 49(2): 735-749. |
| 23 | SHI Li, WANG San-Jang, WONG David Shan-Hill, et al. Novel process design of synthesizing propylene carbonate for dimethyl carbonate production by indirect alcoholysis of urea[J]. Industrial & Engineering Chemistry Research, 2017, 56(40): 11531-11544. |
| 24 | ZHANG Qingrui, PENG Jiayao, ZHANG Kai. Separation of an azeotropic mixture of dimethyl carbonate and methanol via partial heat integration pressure swing distillation[J]. Asia-Pacific Journal of Chemical Engineering, 2017, 12(1): 50-64. |
| 25 | GU Xincheng, ZHANG Xiaochun, YANG Zifeng, et al. Technical-environmental assessment of CO2 conversion process to dimethyl carbonate/ethylene glycol[J]. Journal of Cleaner Production, 2021, 288: 125598. |
| 26 | Danielle BALLIVET-TKATCHENKO, CHAMBREY Stéphane, KEISKI Riitta, et al. Direct synthesis of dimethyl carbonate with supercritical carbon dioxide: Characterization of a key organotin oxide intermediate[J]. Catalysis Today, 2006, 115(1/2/3/4): 80-87. |
| 27 | Ondřej VOPIČKA, Kryštof PILNÁČEK, FRIESS Karel. Separation of methanol-dimethyl carbonate vapour mixtures with PDMS and PTMSP membranes[J]. Separation and Purification Technology, 2017, 174: 1-11. |
| 28 | LI Qing, KISS Anton A. Novel pervaporation-assisted pressure swing reactive distillation process for intensified synthesis of dimethyl carbonate[J]. Chemical Engineering and Processing: Process Intensification, 2021, 162: 108358. |
| 29 | SOUZA Lorena F S, FERREIRA Priscila R R, DE MEDEIROS José Luiz, et al. Production of DMC from CO2 via indirect route: Technical-economical-environmental assessment and analysis[J]. ACS Sustainable Chemistry & Engineering, 2014, 2(1): 62-69. |
| 30 | KONGPANNA Pichayapan, PAVARAJARN Varong, GANI Rafiqul, et al. Techno-economic evaluation of different CO2-based processes for dimethyl carbonate production[J]. Chemical Engineering Research and Design, 2015, 93: 496-510. |
| 31 | YU Bor-Yih, CHEN Mengkai, I-Lung CHIEN. Assessment on CO2 utilization through rigorous simulation: Converting CO2 to dimethyl carbonate[J]. Industrial & Engineering Chemistry Research, 2018, 57(2): 639-652. |
| 32 | 吴青海, 任天瑞. 固载化离子液体催化环氧乙烷和二氧化碳合成碳酸乙烯酯[J]. 过程工程学报, 2012, 12(2): 302-309. |
| WU Qinghai, REN Tianrui. Synthesis of ethylene carbonate via carbon dioxide and ethylene oxide catalyzed by immobilized ionic liquid[J]. The Chinese Journal of Process Engineering, 2012, 12(2): 302-309. | |
| 33 | HE Yueyue, CHENG Huaigang, PAN Zihe, et al. Ultrasonic process intensification during the preparation of dimethyl carbonate based on the alcoholysis of ethylene carbonate and the kinetic behavior of dimethyl carbonate[J]. Reaction Chemistry & Engineering, 2021, 6(11): 2170-2180. |
| 34 | FANG Yunjin, XIAO Wende. Experimental and modeling studies on a homogeneous reactive distillation system for dimethyl carbonate synthesis by transesterification[J]. Separation and Purification Technology, 2004, 34(1/2/3): 255-263. |
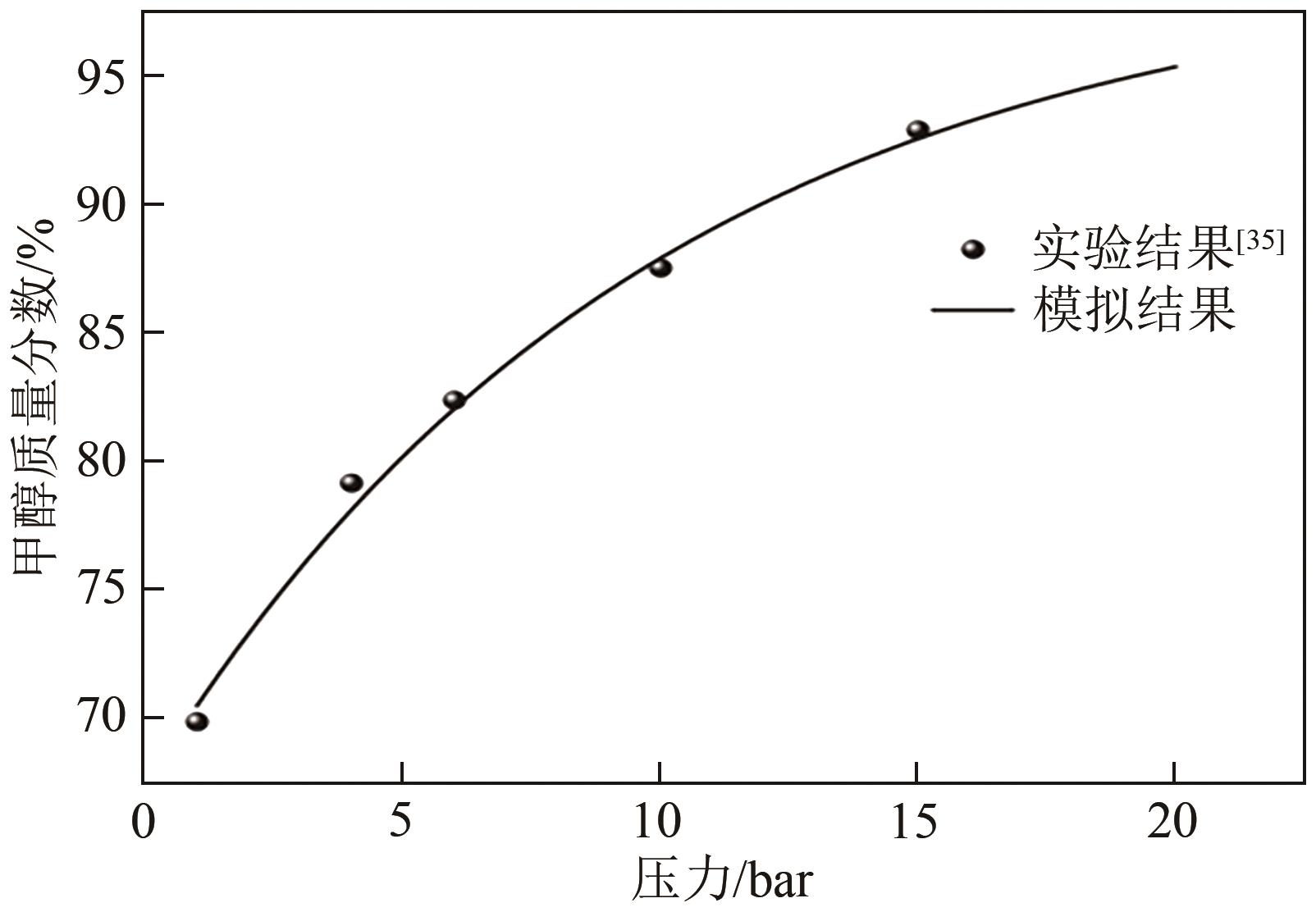
| 35 | 陈嵩嵩, 董丽, 张军平, 等. 酯交换法制备碳酸二甲酯过程模拟与系统㶲分析[J]. 过程工程学报, 2018, 18(6): 1307-1314. |
| CHEN Songsong, DONG Li, ZHANG Junping, et al. Process simulation and system exergy analysis for dimethyl carbonate production with transesterification[J]. The Chinese Journal of Process Engineering, 2018, 18(6): 1307-1314. | |
| 36 | 张莘, 高伟, 齐鸣, 等. 基于多目标优化精馏系统综述[J]. 化工进展, 2019, 38(S1): 1-9. |
| ZHANG Shen, GAO Wei, QI Ming, et al. A review of optimization rectification systems based on multi-objective[J]. Chemical Industry and Engineering Progress, 2019, 38(S1): 1-9. | |
| 37 | 翟建, 刘育良, 李鲁闽, 等. 萃取精馏分离苯/环己烷共沸体系模拟与优化[J]. 化工学报, 2015, 66(9): 3570-3579. |
| ZHAI Jian, LIU Yuliang, LI Lumin, et al. Simulation and optimization of extractive distillation for separation of azeotropic benzene/cyclohexane system[J]. CIESC Journal, 2015, 66(9): 3570-3579. | |
| 38 | 李帅. 基于NSGA-Ⅱ的蒸汽动力系统经济性与环境影响优化[D]. 大连: 大连理工大学, 2019. |
| LI Shuai. Optimization of economic and environmental impact for steam power plant based on NSGA-Ⅱ[D]. Dalian: Dalian University of Technology, 2019. | |
| 39 | 葛玉林. 常减压蒸馏流程模拟与优化及换热网络综合[D]. 大连: 大连理工大学, 2007. |
| GE Yulin. Flow simulation, optimization, and heat exchangers network synthesis of atmosphere-vacuum distillation[D]. Dalian: Dalian University of Technology, 2007. | |
| 40 | GADALLA M, OLUJIĆ Ž, DE RIJKE A, et al. Reducing CO2 emissions of internally heat-integrated distillation columns for separation of close boiling mixtures[J]. Energy, 2005, 31(13): 2409-2417. |
| 41 | LEE Tzong-Shing, LU Wanchen. An evaluation of empirically-based models for predicting energy performance of vapor-compression water chillers[J]. Applied Energy, 2010, 87(11): 3486-3493. |
| 42 | SEIDER W, SEADER J, LEWIN D. Product & process design principles-synthesis, analysis, and evaluation[M]. USA: John Wiley and Sons, Inc., 2006: 565-567. |
| 43 | MRAYED Sabri, SHAMS Mohamed BIN, Mohammed AL-KHAYYAT, et al. Application of pinch analysis to improve the heat integration efficiency in a crude distillation unit[J]. Cleaner Engineering and Technology, 2021, 4: 100168. |
| 44 | KONGPANNA Pichayapan, BABI Deenesh K, PAVARAJARN Varong, et al. Systematic methods and tools for design of sustainable chemical processes for CO2 utilization[J]. Computers & Chemical Engineering, 2016, 87: 125-144. |
| 45 | WU Tsai-Wei, I-Lung CHIEN. CO2 utilization feasibility study: Dimethyl carbonate direct synthesis process with dehydration reactive distillation[J]. Industrial & Engineering Chemistry Research, 2020, 59(3): 1234-1248. |
| 46 | OHNO Hajime, IKHLAYEL Mahdi, TAMURA Masazumi, et al. Direct dimethyl carbonate synthesis from CO2 and methanol catalyzed by CeO2 and assisted by 2-cyanopyridine: A cradle-to-gate greenhouse gas emission study[J]. Green Chemistry, 2021, 23(1): 457-469. |
| [1] | 王亚男, 刘琳琳, 庄钰, 都健. 基于代理模型的环氧乙烷制乙二醇工艺优化同步热集成[J]. 化工进展, 2024, 43(9): 5234-5241. |
| [2] | 韩伟, 韩恒文, 程薇, 汤玮健. 碳中和目标驱动下生物质燃料技术研究进展[J]. 化工进展, 2024, 43(5): 2463-2474. |
| [3] | 李萍, 陈修乐, 张强, 念腾飞, 王育兴, 王盟. 抑烟沥青复掺配比优化及抑烟效果评价[J]. 化工进展, 2024, 43(4): 1923-1933. |
| [4] | 张书铭, 刘化章. 基于BP神经网络模型优化Fe1-x O基氨合成催化剂[J]. 化工进展, 2024, 43(3): 1302-1308. |
| [5] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [6] | 王俊杰, 潘艳秋, 牛亚宾, 俞路. 分子水平催化重整装置模型构建及应用[J]. 化工进展, 2023, 42(7): 3404-3412. |
| [7] | 林海, 王彧斐. 考虑噪声约束的分布式风场布局优化[J]. 化工进展, 2023, 42(7): 3394-3403. |
| [8] | 凌山, 刘聚明, 张前程, 李艳. 模拟移动床分离过程及其优化方法研究进展[J]. 化工进展, 2023, 42(5): 2233-2244. |
| [9] | 代敏, 杨福胜, 张早校, 刘桂莲, 冯霄. 基于多策略集成优化算法的己烷油精馏过程3E多目标优化[J]. 化工进展, 2022, 41(6): 2852-2863. |
| [10] | 韩文韬, 韩振为, 李洪, 高鑫, 李鑫钢. 乙酰丙酸乙酯的反应精馏模型及隔壁塔节能优化设计[J]. 化工进展, 2022, 41(4): 1759-1769. |
| [11] | 陈丹阳, 朱建宇, 吴勤, 王自庆, 张金利. KF/MgO催化甘油和碳酸二甲酯酯交换合成甘油碳酸酯[J]. 化工进展, 2022, 41(4): 2082-2089. |
| [12] | 朱长辉, 朱文超, 罗嘉, 田保河, 孙佳琳, 邹志云. 微波强化酯交换反应制备生物柴油研究进展[J]. 化工进展, 2022, 41(10): 5145-5154. |
| [13] | 李丹, 杨思宇, 钱宇. 耦合溴化锂吸收式制冷与有机朗肯循环的合成气深冷分离工艺[J]. 化工进展, 2022, 41(10): 5236-5246. |
| [14] | 岳倩倩, 高李璟, 肖国民, 魏瑞平, 雷严. 生物柴油连续化生产设备及工艺进展[J]. 化工进展, 2021, 40(S2): 81-88. |
| [15] | 赖佳宁, 高鑫, 从海峰, 李洪, 李鑫钢. 丙酮缩甘油反应精馏工艺全流程模拟与优化[J]. 化工进展, 2021, 40(7): 3584-3590. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||