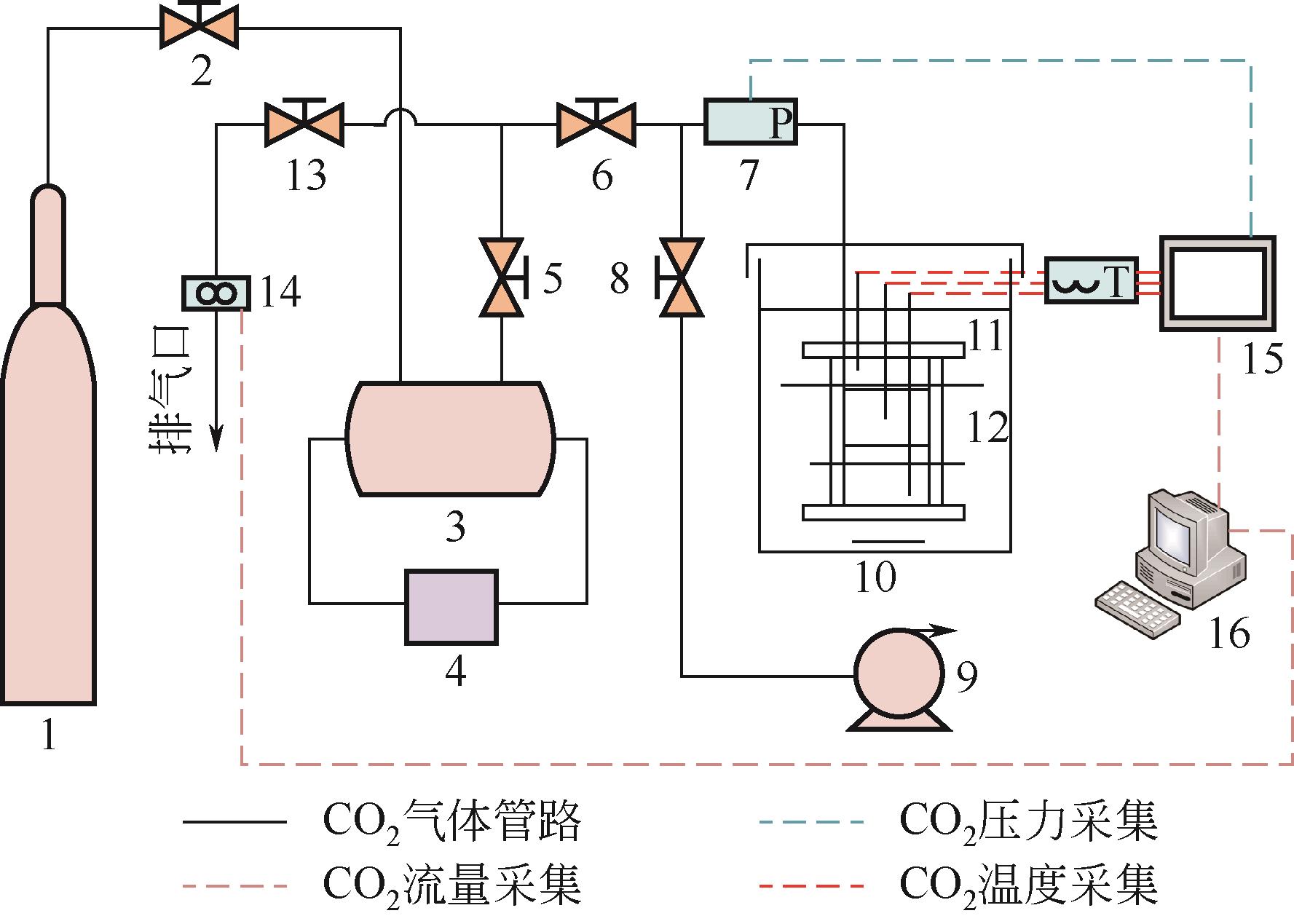
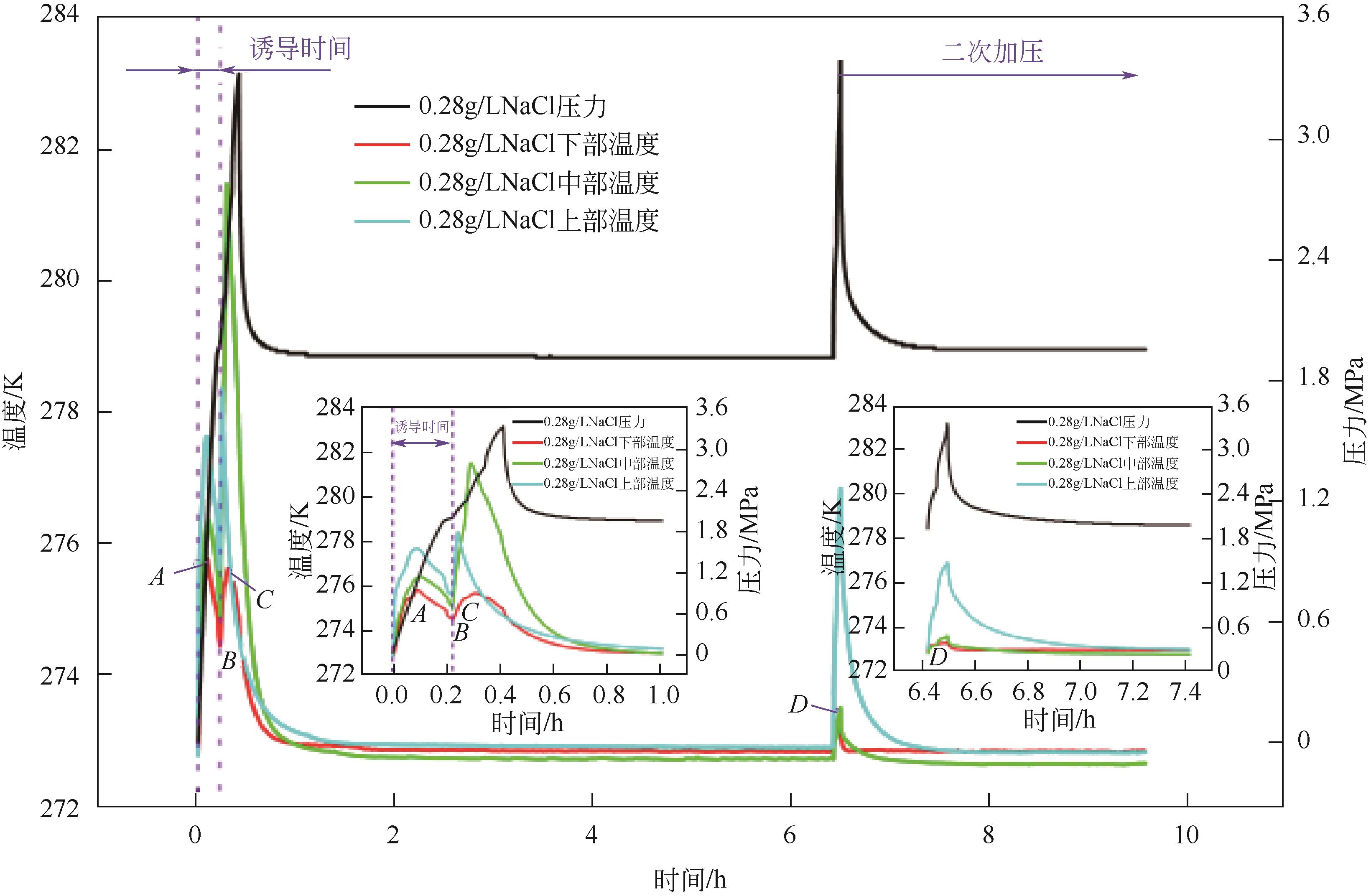
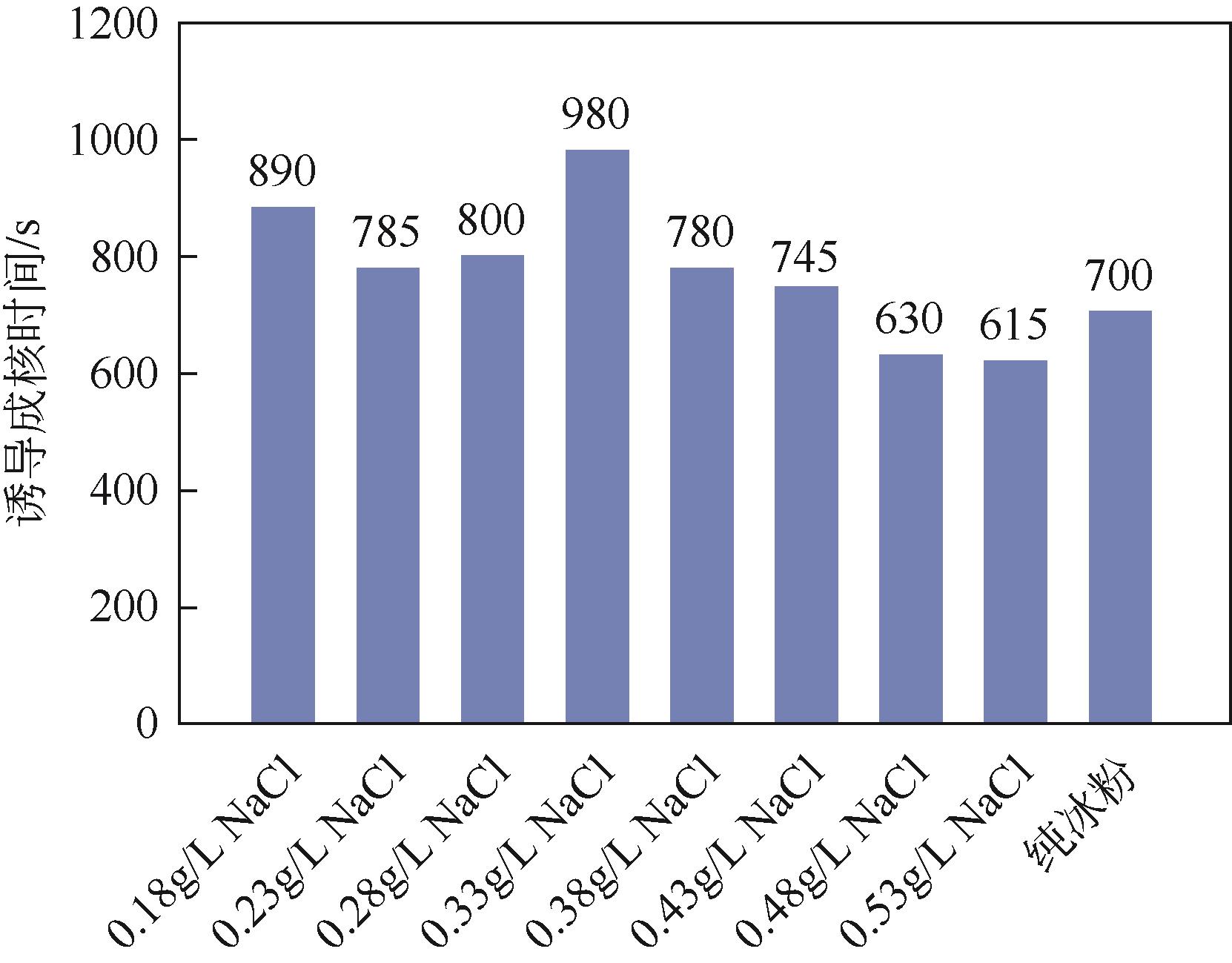
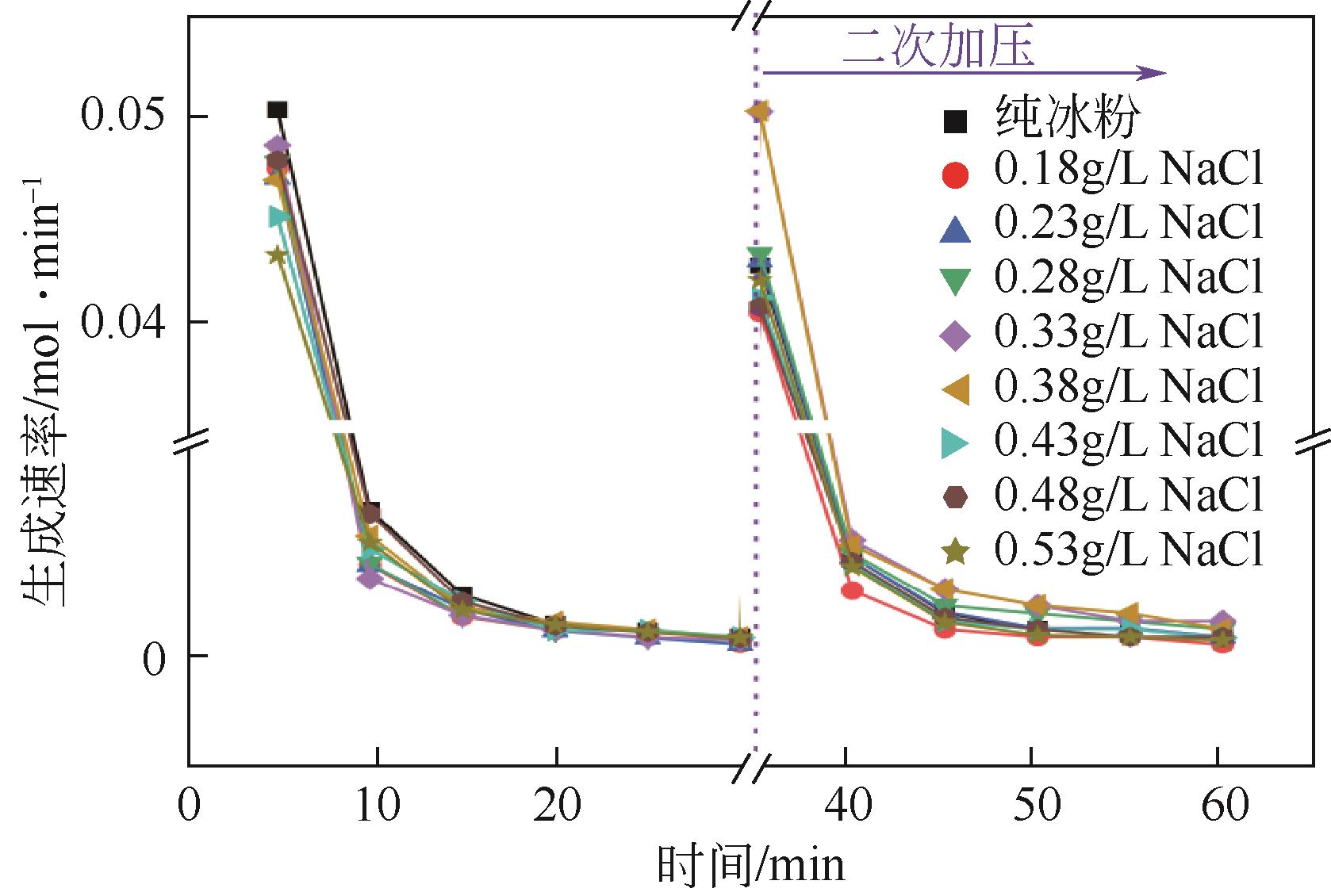
| 1 |
BOZZANO Giulia, MANENTI Flavio. Efficient methanol synthesis: Perspectives, technologies and optimization strategies[J]. Progress in Energy and Combustion Science, 2016, 56: 71-105.
|
| 2 |
DUAN Huiming, WANG Siqi, HE Chenglin, et al. Application of a novel grey Bernoulli model to predict the global consumption of renewable energy[J]. Energy Reports, 2021, 7: 7200-7211.
|
| 3 |
NGUYEN N N, LA V T, HUYNH C D, et al. Technical and economic perspectives of hydrate-based carbon dioxide capture[J]. Applied Energy, 2022, 307: 118237.
|
| 4 |
樊栓狮, 尤莎莉, 郎雪梅, 等. 笼型水合物膜分离和捕获二氧化碳研究进展[J]. 化工进展, 2020, 39(4): 1211-1218.
|
|
FAN Shuanshi, YOU Shali, LANG Xuemei, et al. Separation and capture carbon dioxide by clathrate-hydrate membranes: A review[J]. Chemical Industry and Engineering Progress, 2020, 39(4): 1211-1218.
|
| 5 |
王磊, 方桂英, 阳庆元. 金属-有机骨架材料CO2捕获性能的大规模计算筛选[J]. 化工学报, 2019, 70(3): 1135-1143.
|
|
WANG Lei, FANG Guiying, YANG Qingyuan. Performance of metal-organic frameworks for CO2 capture from large-scale computational screening[J]. CIESC Journal, 2019, 70(3): 1135-1143.
|
| 6 |
SUN Q B, KANG Y T. Review on CO2 hydrate formation/dissociation and its cold energy application[J]. Renewable and Sustainable Energy Reviews, 2016, 62: 478-494.
|
| 7 |
DASHTI H, ZHEHAO Y L, LOU X. Recent advances in gas hydrate-based CO2 capture[J]. Journal of Natural Gas Science and Engineering, 2015, 23: 195-207.
|
| 8 |
ZHANG Lunxiang, YANG Lei, WANG Jiaqi, et al. Enhanced CH4 recovery and CO2 storage via thermal stimulation in the CH4/CO2 replacement of methane hydrate[J]. Chemical Engineering Journal, 2017, 308: 40-49.
|
| 9 |
XU Chungang, YU Yisong, XIE Wenjun, et al. Study on developing a novel continuous separation device and carbon dioxide separation by process of hydrate combined with chemical absorption[J]. Applied Energy, 2019, 255: 113791.
|
| 10 |
CHUNG W, ROH K, LEE J H. Design and evaluation of CO2 capture plants for the steelmaking industry by means of amine scrubbing and membrane separation[J]. International Journal of Greenhouse Gas Control, 2018, 74: 259-270.
|
| 11 |
WANG Tian, ZHANG Lunxiang, SUN Lingjie, et al. Methane recovery and carbon dioxide storage from gas hydrates in fine marine sediments by using CH4/CO2 replacement[J]. Chemical Engineering Journal, 2021, 425: 131562.
|
| 12 |
WANG Yanhong, LANG Xuemei, FAN Shuanshi. Hydrate capture CO2 from shifted synthesis gas, flue gas and sour natural gas or biogas[J]. Journal of Energy Chemistry, 2013, 22(1): 39-47.
|
| 13 |
SUN Ronghui, YANG Mingjun, SONG Yongchen. Effect of NaCl concentration on depressurization-induced methane hydrate dissociation near ice-freezing point: Associated with metastable phases[J]. Journal of Natural Gas Science and Engineering, 2021, 96: 104304.
|
| 14 |
Junghoon MOK, CHOI Wonjung, KIM Sungwoo, et al. NaCl-induced enhancement of thermodynamic and kinetic CO2 selectivity in CO2+N2 hydrate formation and its significance for CO2 sequestration[J]. Chemical Engineering Journal, 2023, 451: 138633.
|
| 15 |
SMIT Berend. Molecular simulations of fluid phase equilibria[J]. Fluid Phase Equilibria, 1996, 116(1/2): 249-256.
|
| 16 |
NASEH Moeinoddin, FALAMAKI Cavus, MOHEBBI Vahid. Equilibrium conditions of CO2+C3H8 hydrates in pure and saline water solutions of NaCl[J]. Journal of Natural Gas Science and Engineering, 2022, 106: 104734.
|
| 17 |
WANG Jianlong, SUN Jinsheng, WANG Ren, et al. Mechanisms of synergistic inhibition of NaCl and glycine mixtures on methane hydrate formation: Experimental and molecular dynamic simulation[J]. Gas Science and Engineering, 2023: 204880.
|
| 18 |
HOLZAMMER Christine, FINCKENSTEIN Agnes, WILL Stefan, et al. How sodium chloride salt inhibits the formation of CO2 gas hydrates[J]. The Joural of Physical Chemistry B, 2022, 120(9): 2452-2459.
|
| 19 |
CHOI Wonjung, LEE Yohan, Junghoon MOK, et al. Thermodynamic and kinetic influences of NaCl on HFC-125a hydrates and their significance in gas hydrate-based desalination[J]. Chemical Engineering Journal, 2019, 358: 598-605.
|
| 20 |
SUN Xian, LIU Dejun, CHANG Dongchao, et al. Analysis of natural gas hydrate formation in sodium dodecyl sulfate and quartz sand complex system under saline environment[J]. Petroleum Science and Technology, 2018, 36(14): 1073-1079.
|
| 21 |
XU Jiafang, DU Shuai, HAO Yongchao, et al. Molecular simulation study of methane hydrate formation mechanism in NaCl solutions with different concentrations[J]. Chemical Physics, 2021, 551: 111323.
|
| 22 |
张金锋. 甲烷水合物在不同体系中的生成动力学研究[D]. 杭州: 浙江工业大学, 2003.
|
|
ZHANG Jinfeng. Research on the kinetics of methane hydrate formation in various systems[D]. Hangzhou: Zhejiang University of Technology, 2003.
|
| 23 |
ZHANG Baoyong, WU Qiang, GAO Xia, et al. Memory effect on hydrate formation and influential factors of its sustainability in new hydrate-based coal mine methane separation method[J]. International Journal of Environment and Pollution, 2013, 53(3/4): 201.
|
| 24 |
SMITH J M, VAN NESS H C, ABBOTT M M, et al. Introduction to chemical engineering thermodynamics[M]. Eighth edition. New York, NY: McGraw-Hill Education, 2018.
|
| 25 |
SUN Duo, ENGLEZOS Peter. Storage of CO2 in a partially water saturated porous medium at gas hydrate formation conditions[J]. International Journal of Greenhouse Gas Control, 2014, 25: 1-8.
|
| 26 |
NATARAJAN V, BISHNOI P R, KALOGERAKIS N. Induction phenomena in gas hydrate nucleation[J]. Chemical Engineering Science, 1994, 49(13): 2075-2087.
|
| 27 |
DENDY S E. Clathrate hydrates of natural gases[M]. Boca Raton, FL: CRC Press, 2008.
|
| 28 |
张强, 吴强, 张保勇, 等. NaCl-SDS复合溶液中多组分瓦斯水合物成核动力学机理[J]. 煤炭学报, 2015, 40(10): 2430-2436.
|
|
ZHANG Qiang, WU Qiang, ZHANG Baoyong, et al. Nucleation kinetics mechanism of multi-component mine gas hydrate in NaCl-SDS mixed solutions[J]. Journal of China Coal Society, 2015, 40(10): 2430-2436.
|
| 29 |
BAI Dongsheng, WU Ziyan, LIN Cijie, et al. The effect of aqueous NaCl solution on methane hydrate nucleation and growth[J]. Fluid Phase Equilibria, 2019, 487: 76-82.
|
| 30 |
WANG Xiaohui, WANG Yunfei, XIE Yan, et al. Study on the decomposition conditions of gas hydrate in quartz sand-brine mixture systems[J]. The Journal of Chemical Thermodynamics, 2019, 131: 247-253.
|
| 31 |
曹学文, 杨凯然, 夏闻竹, 等. CO2水合物分解实验及分解速率模型[J]. 天然气工业, 2021, 41(7): 152-159.
|
|
CAO Xuewen, YANG Kairan, XIA Wenzhu, et al. Dissociation experiment and dissociation rate model of CO2 hydrate[J]. Natural Gas Industry, 2021, 41(7): 152-159.
|
| 32 |
陈雪萍, 张鹏, 吴青柏. “自保护”态CO2水合物分解动力及影响因素[J]. 天然气地球科学, 2020, 31(2): 184-193.
|
|
CHEN Xueping, ZHANG Peng, WU Qingbai. Decomposition dynamics and influencing factors of CO2 hydrate in self-preservation state[J]. Natural Gas Geoscience, 2020, 31(2): 184-193.
|
| 33 |
FALENTY A, KUHS W F, GLOCKZIN M, et al. “self-preservation” of CH4 hydrates for gas transport technology: Pressure-temperature dependence and ice microstructures[J]. Energy & Fuels, 2014, 28(10): 6275-6283.
|
| 34 |
YAGASAKI Takuma, MATSUMOTO Masakazu, TANAKA Hideki. Effects of thermodynamic inhibitors on the dissociation of methane hydrate: A molecular dynamics study[J]. Physical Chemistry Chemical Physics: PCCP, 2015, 17(48): 32347-32357.
|
| 35 |
QURESHI M F, KHANDELWAL H, USADI A, et al. CO2 hydrate stability in oceanic sediments under brine conditions[J]. Energy, 2022, 256: 124625.
|
 ), 刘生浩1,2,3, 滕亚栋1,2,3, 王立瑾1,2,3, 焦雯泽1,2,3
), 刘生浩1,2,3, 滕亚栋1,2,3, 王立瑾1,2,3, 焦雯泽1,2,3
 ), LIU Shenghao1,2,3, TENG Yadong1,2,3, WANG Lijin1,2,3, JIAO Wenze1,2,3
), LIU Shenghao1,2,3, TENG Yadong1,2,3, WANG Lijin1,2,3, JIAO Wenze1,2,3