化工进展 ›› 2022, Vol. 41 ›› Issue (S1): 150-159.DOI: 10.16085/j.issn.1000-6613.2021-2660
废旧聚丙烯/活性炭微波共裂解制取可燃裂解气与轻质裂解油
刘楠1( ), 胡一铭1, 杨颖1, 李红晋2, 高竹青1(
), 胡一铭1, 杨颖1, 李红晋2, 高竹青1( ), 郝秀丽1
), 郝秀丽1
- 1.太原科技大学化学与生物工程学院,山西 太原 030024
2.太原科技大学机械学院,山西 太原 030024
-
收稿日期:2021-12-31修回日期:2022-03-04出版日期:2022-10-20发布日期:2022-11-10 -
通讯作者:高竹青 -
作者简介:刘楠(1995—),女,硕士研究生,研究方向为有机固废物的资源化利用。E-mail:614934750@qq.com。 -
基金资助:国家自然科学基金青年基金(21701120);山西省科技厅重点研发计划(高新领域)(201803D121099);太原科技大学博士启动基金项目(20182020)
Microwave assisted co-pyrolysis of waste polypropylene /activated carbon to produce combustible pyrolysis gas and light pyrolysis oil
LIU Nan1( ), HU Yiming1, YANG Ying1, LI Hongjin2, GAO Zhuqing1(
), HU Yiming1, YANG Ying1, LI Hongjin2, GAO Zhuqing1( ), HAO Xiuli1
), HAO Xiuli1
- 1.College of Chemical and Biological Engineering, Taiyuan University of Science and Technology, Taiyuan 030024, Shanxi, China
2.School of Mechanical Engineering, Taiyuan University of Science and Technology, Taiyuan 030024, Shanxi, China
-
Received:2021-12-31Revised:2022-03-04Online:2022-10-20Published:2022-11-10 -
Contact:GAO Zhuqing
摘要:
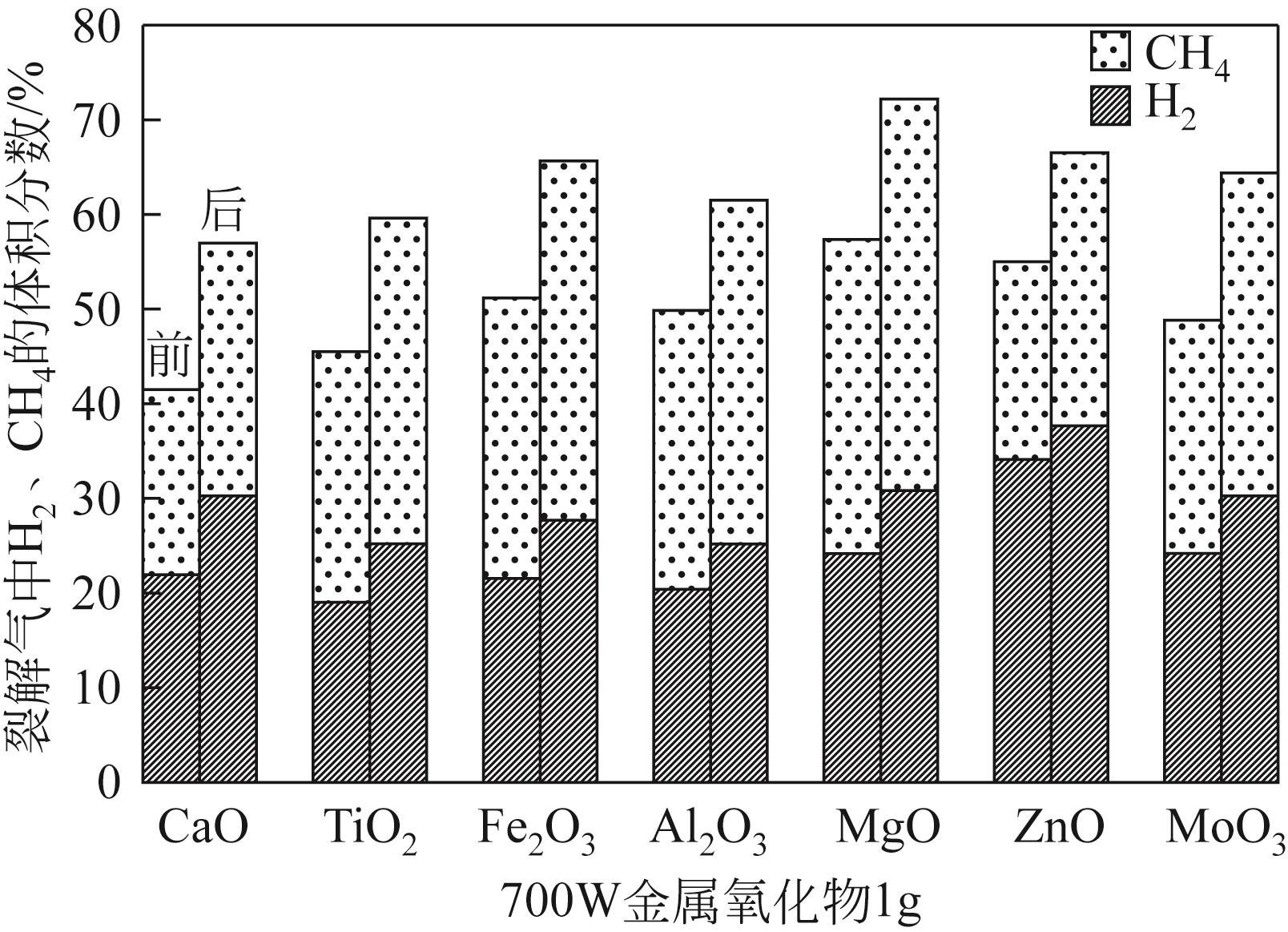
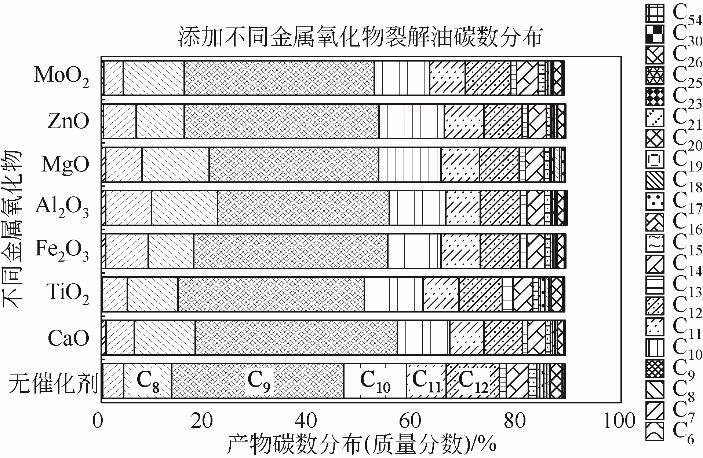
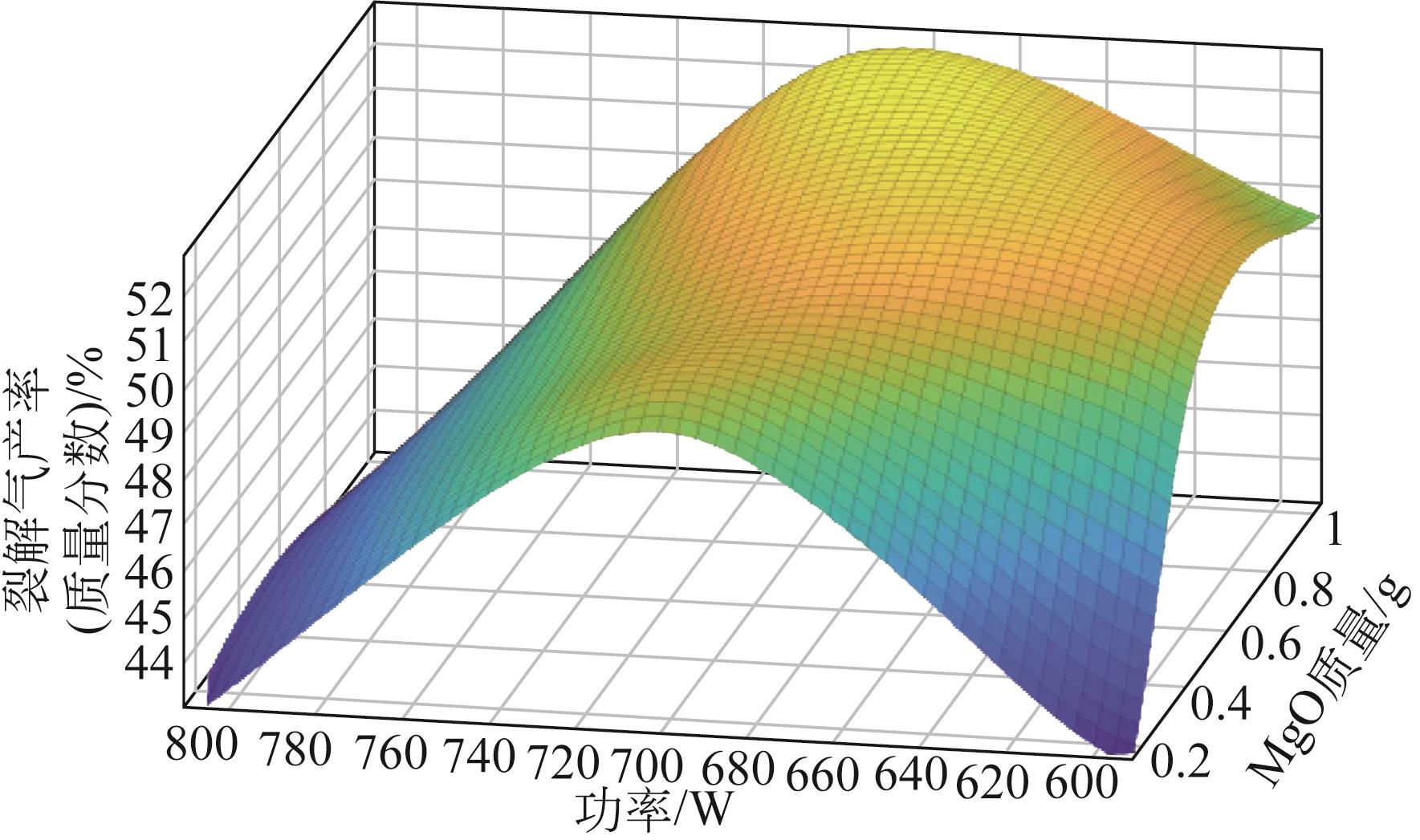
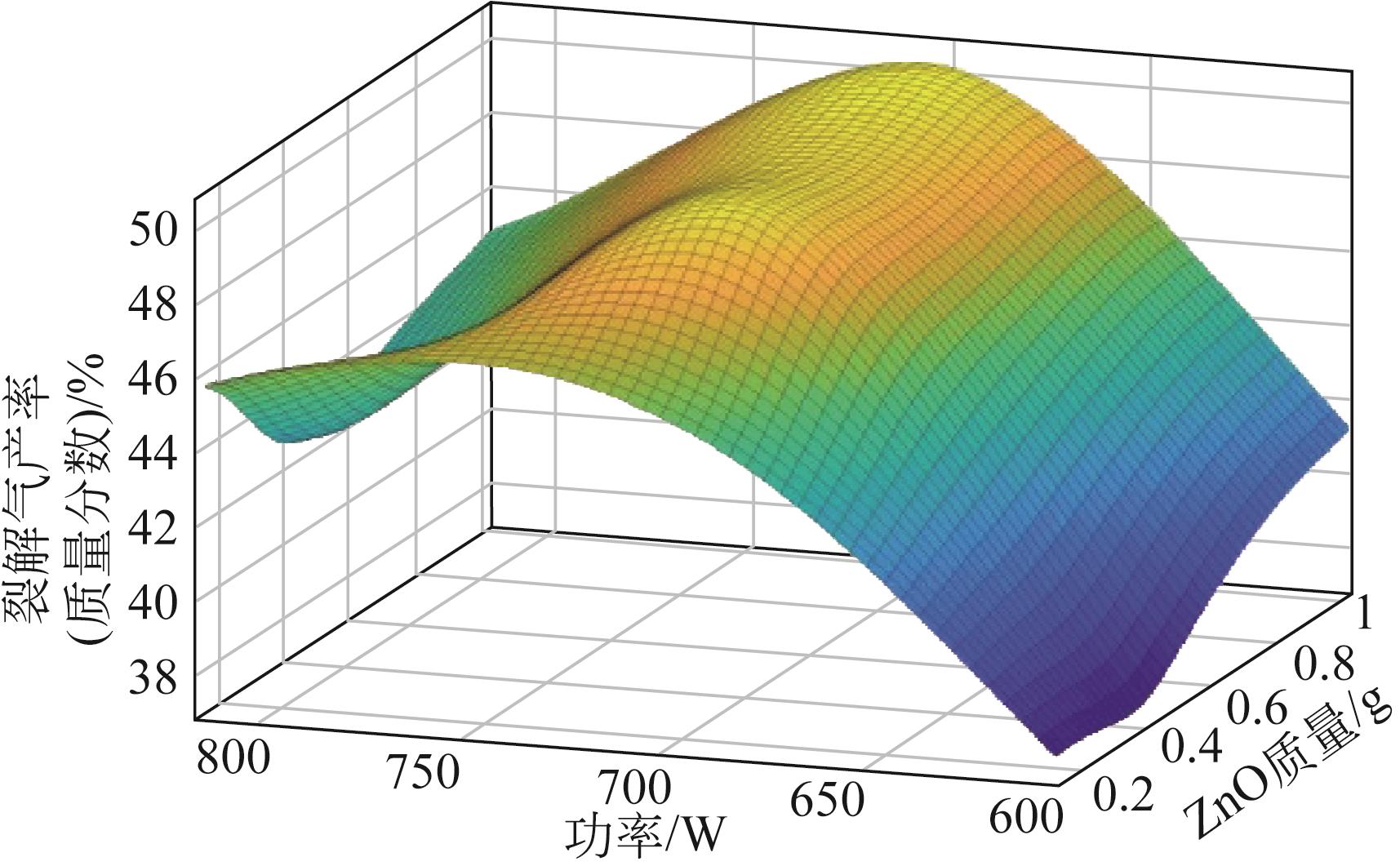
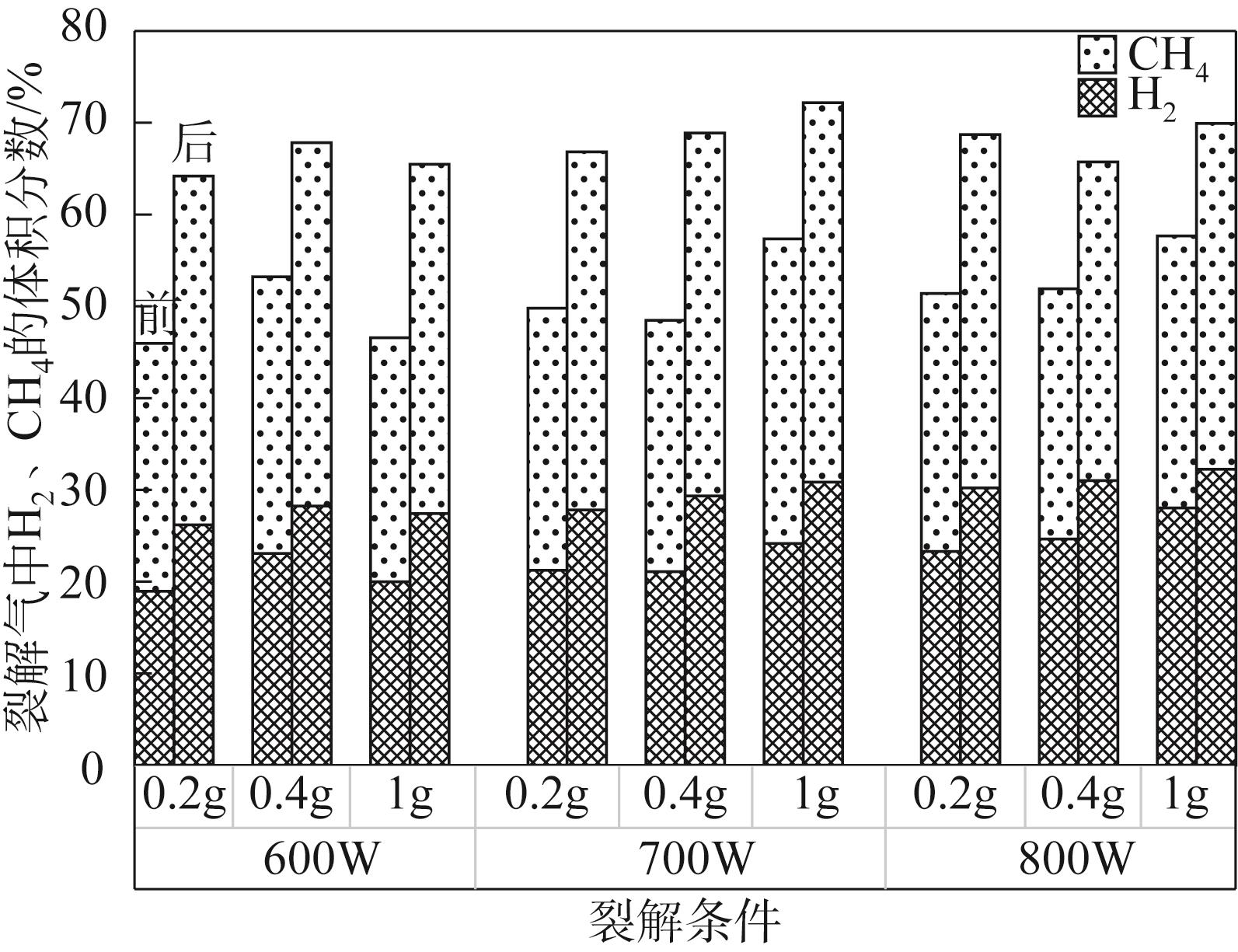
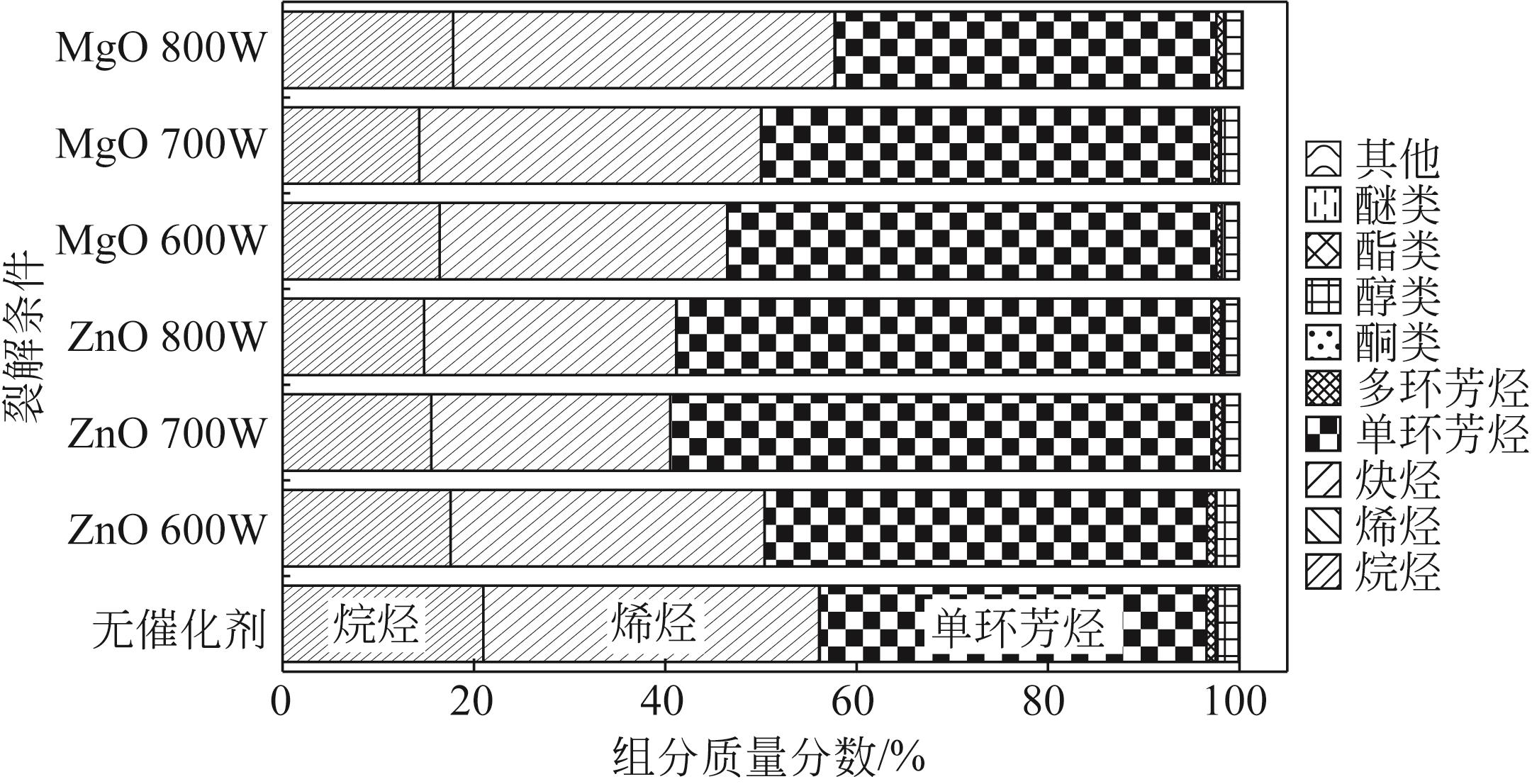
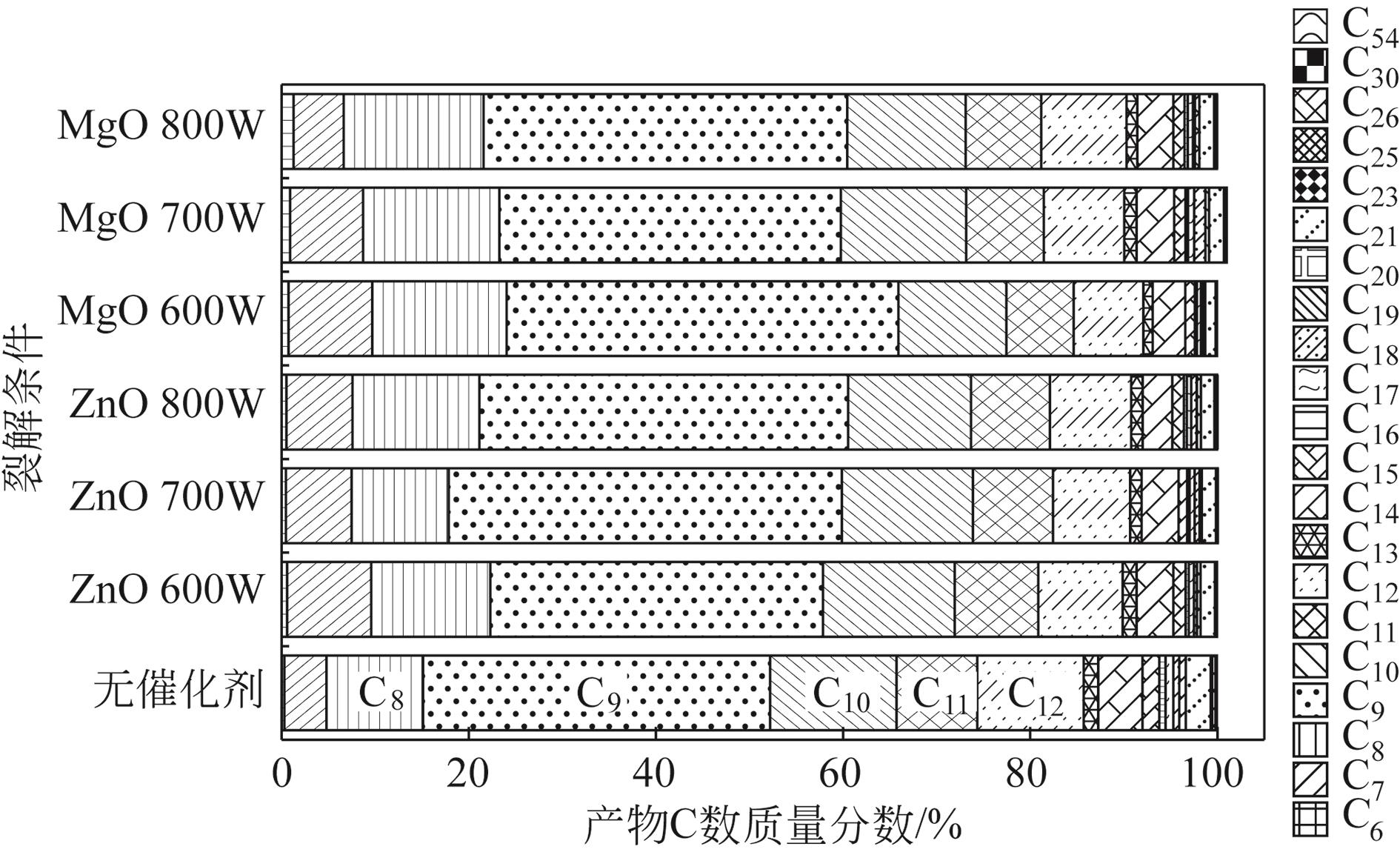
为实现对废旧塑料的资源化利用,本文采用微波裂解法,以废弃聚丙烯(PP)为裂解原料、颗粒状活性炭为吸波材料通过微波共裂解制取可燃裂解气与轻质裂解油。实验研究了不添加催化剂时微波功率对裂解所得裂解气、裂解油和固体碳的影响,以及添加不同种类金属氧化物作为辅助催化剂时对裂解产物的影响,并详细研究了MgO、ZnO的添加量和功率对产物的影响。研究发现,不添加催化剂时裂解气的产率可达40%,其中H2、CH4约占气体总体积的40%,裂解油的产率为40%左右,固体碳的产率为15%左右。裂解油中烷烃、烯烃和单环芳烃三者的总含量可达90%以上,裂解油的相对密度介于0.7~0.8之间,属于轻质裂解油;添加不同金属氧化物后部分金属氧化物可加深PP的裂化程度,其中MgO可显著提高CH4的含量,ZnO可显著提高H2的含量,且金属氧化物可进一步提高裂解油中单环芳烃的含量;结合响应面分析PP的最佳裂解条件为:加入MgO后功率范围在660~720W,催化剂量在0.6~1g;加入ZnO后功率范围在680~740W,催化剂量在0.4~1g。
中图分类号:
引用本文
刘楠, 胡一铭, 杨颖, 李红晋, 高竹青, 郝秀丽. 废旧聚丙烯/活性炭微波共裂解制取可燃裂解气与轻质裂解油[J]. 化工进展, 2022, 41(S1): 150-159.
LIU Nan, HU Yiming, YANG Ying, LI Hongjin, GAO Zhuqing, HAO Xiuli. Microwave assisted co-pyrolysis of waste polypropylene /activated carbon to produce combustible pyrolysis gas and light pyrolysis oil[J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 150-159.
功率 /W | 裂解气质量 /g | 裂解气产率 (质量分数)/% | 裂解油质量 /g | 裂解油产率 (质量分数)/% | 固体碳质量 /g | 固体碳产率 (质量分数)/% |
|---|---|---|---|---|---|---|
| 500 | — | — | — | — | — | — |
| 600 | 8.95 | 44.75 | 7.92 | 39.60 | 3.13 | 15.65 |
| 700 | 8.35 | 41.75 | 8.84 | 44.20 | 2.81 | 14.05 |
| 800 | 10.05 | 52.25 | 6.98 | 34.90 | 2.97 | 14.85 |
表1 功率对裂解产物的影响(PP 20g,活性炭20g)
功率 /W | 裂解气质量 /g | 裂解气产率 (质量分数)/% | 裂解油质量 /g | 裂解油产率 (质量分数)/% | 固体碳质量 /g | 固体碳产率 (质量分数)/% |
|---|---|---|---|---|---|---|
| 500 | — | — | — | — | — | — |
| 600 | 8.95 | 44.75 | 7.92 | 39.60 | 3.13 | 15.65 |
| 700 | 8.35 | 41.75 | 8.84 | 44.20 | 2.81 | 14.05 |
| 800 | 10.05 | 52.25 | 6.98 | 34.90 | 2.97 | 14.85 |
| 功率/W | |||
|---|---|---|---|
| 500 | — | — | — |
| 600 | 16.77 | 24.80 | 41.57 |
| 700 | 18.13 | 24.09 | 42.22 |
| 800 | 23.04 | 27.09 | 50.13 |
表2 功率对裂解气组分的影响(PP20g,活性炭20g)
| 功率/W | |||
|---|---|---|---|
| 500 | — | — | — |
| 600 | 16.77 | 24.80 | 41.57 |
| 700 | 18.13 | 24.09 | 42.22 |
| 800 | 23.04 | 27.09 | 50.13 |
| 金属氧化物 | 裂解气质量/g | 裂解气产率 (质量分数)/ % | 裂解油质量/g | 裂解油产率 (质量分数)/ % | 固体碳质量 /g | 固体碳产率 (质量分数)/% |
|---|---|---|---|---|---|---|
| 无催化剂 | 8.35 | 41.75 | 8.84 | 44.20 | 2.81 | 14.05 |
| CaO | 8.60 | 43.00 | 9.05 | 45.25 | 2.35 | 11.75 |
| TiO2 | 7.49 | 37.45 | 9.22 | 46.10 | 3.29 | 16.45 |
| Fe2O3 | 9.75 | 48.75 | 8.18 | 40.90 | 2.07 | 10.35 |
| Al2O3 | 8.97 | 44.85 | 8.88 | 44.40 | 2.15 | 10.75 |
| MgO | 10.49 | 52.45 | 7.92 | 39.60 | 1.59 | 7.90 |
| ZnO | 10.05 | 50.25 | 7.73 | 38.65 | 2.22 | 11.10 |
| MoO3 | 9.20 | 46.00 | 8.21 | 41.05 | 2.59 | 12.95 |
表3 不同金属氧化物(1g)对产物的影响(PP 20g,活性炭20g)
| 金属氧化物 | 裂解气质量/g | 裂解气产率 (质量分数)/ % | 裂解油质量/g | 裂解油产率 (质量分数)/ % | 固体碳质量 /g | 固体碳产率 (质量分数)/% |
|---|---|---|---|---|---|---|
| 无催化剂 | 8.35 | 41.75 | 8.84 | 44.20 | 2.81 | 14.05 |
| CaO | 8.60 | 43.00 | 9.05 | 45.25 | 2.35 | 11.75 |
| TiO2 | 7.49 | 37.45 | 9.22 | 46.10 | 3.29 | 16.45 |
| Fe2O3 | 9.75 | 48.75 | 8.18 | 40.90 | 2.07 | 10.35 |
| Al2O3 | 8.97 | 44.85 | 8.88 | 44.40 | 2.15 | 10.75 |
| MgO | 10.49 | 52.45 | 7.92 | 39.60 | 1.59 | 7.90 |
| ZnO | 10.05 | 50.25 | 7.73 | 38.65 | 2.22 | 11.10 |
| MoO3 | 9.20 | 46.00 | 8.21 | 41.05 | 2.59 | 12.95 |
| 催化剂 | 相对密度 |
|---|---|
| 无 | 0.806 |
| CaO | 0.723 |
| Al2O3 | 0.750 |
| TiO2 | 0.790 |
| MoO3 | 0.776 |
| ZnO | 0.720 |
| Fe2O3 | 0.760 |
| MgO | 0.753 |
表4 不同裂解条件下裂解油相对密度
| 催化剂 | 相对密度 |
|---|---|
| 无 | 0.806 |
| CaO | 0.723 |
| Al2O3 | 0.750 |
| TiO2 | 0.790 |
| MoO3 | 0.776 |
| ZnO | 0.720 |
| Fe2O3 | 0.760 |
| MgO | 0.753 |
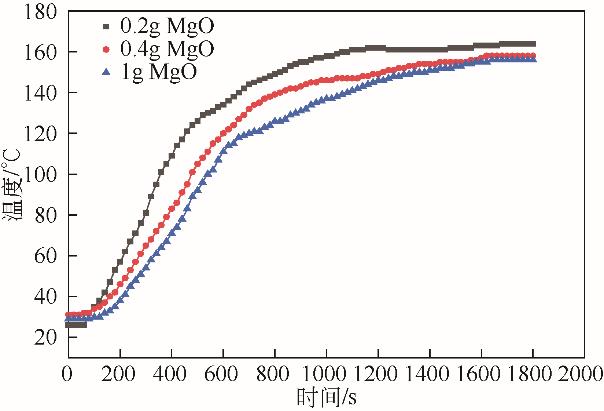
| 功率/W | MgO质量 /g | PP∶MgO (质量比) | 裂解气质量 /g | 裂解气产率 (质量分数)/% | 裂解油质量 /g | 裂解油产率 (质量分数)/% | 固体碳质量 /g | 固体碳产率 (质量分数)/% |
|---|---|---|---|---|---|---|---|---|
| 600 | 0.2 | 100∶1 | 8.64 | 43.20 | 7.57 | 37.85 | 3.79 | 18.95 |
| 600 | 0.4 | 50∶1 | 9.86 | 49.30 | 7.03 | 35.13 | 3.11 | 15.55 |
| 600 | 1.0 | 20∶1 | 9.94 | 49.70 | 7.56 | 37.80 | 2.50 | 12.50 |
| 700 | 0.2 | 100∶1 | 10.01 | 50.05 | 7.50 | 37.50 | 2.49 | 12.45 |
| 700 | 0.4 | 50∶1 | 10.07 | 50.35 | 7.69 | 38.45 | 2.24 | 11.20 |
| 700 | 1.0 | 20∶1 | 10.49 | 52.45 | 7.92 | 39.60 | 1.59 | 7.90 |
| 800 | 0.2 | 100∶1 | 9.11 | 43.55 | 9.13 | 45.65 | 1.76 | 8.80 |
| 800 | 0.4 | 50∶1 | 8.83 | 44.15 | 9.02 | 45.10 | 2.15 | 10.75 |
| 800 | 1.0 | 20∶1 | 8.70 | 43.50 | 9.13 | 45.65 | 2.17 | 10.85 |
表5 MgO添加量及功率对产物的影响(PP 20g,活性炭20g)
| 功率/W | MgO质量 /g | PP∶MgO (质量比) | 裂解气质量 /g | 裂解气产率 (质量分数)/% | 裂解油质量 /g | 裂解油产率 (质量分数)/% | 固体碳质量 /g | 固体碳产率 (质量分数)/% |
|---|---|---|---|---|---|---|---|---|
| 600 | 0.2 | 100∶1 | 8.64 | 43.20 | 7.57 | 37.85 | 3.79 | 18.95 |
| 600 | 0.4 | 50∶1 | 9.86 | 49.30 | 7.03 | 35.13 | 3.11 | 15.55 |
| 600 | 1.0 | 20∶1 | 9.94 | 49.70 | 7.56 | 37.80 | 2.50 | 12.50 |
| 700 | 0.2 | 100∶1 | 10.01 | 50.05 | 7.50 | 37.50 | 2.49 | 12.45 |
| 700 | 0.4 | 50∶1 | 10.07 | 50.35 | 7.69 | 38.45 | 2.24 | 11.20 |
| 700 | 1.0 | 20∶1 | 10.49 | 52.45 | 7.92 | 39.60 | 1.59 | 7.90 |
| 800 | 0.2 | 100∶1 | 9.11 | 43.55 | 9.13 | 45.65 | 1.76 | 8.80 |
| 800 | 0.4 | 50∶1 | 8.83 | 44.15 | 9.02 | 45.10 | 2.15 | 10.75 |
| 800 | 1.0 | 20∶1 | 8.70 | 43.50 | 9.13 | 45.65 | 2.17 | 10.85 |
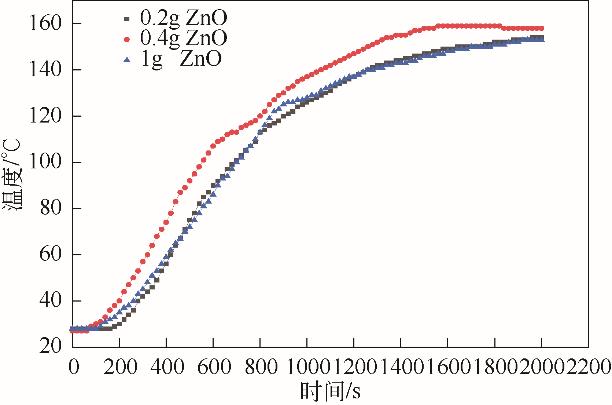
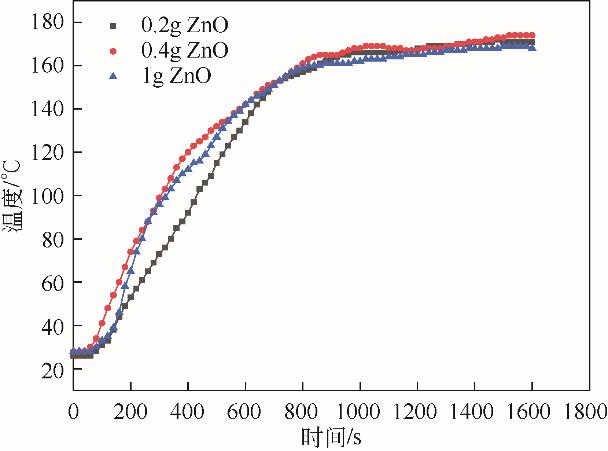
| 功率/W | ZnO质量 /g | PP∶ZnO (质量比) | 裂解气质量 /g | 裂解气产率 (质量分数)/% | 裂解油质量 /g | 裂解油产率(质量分数)/% | 固体碳质量 /g | 固体碳产率(质量分数)/% |
|---|---|---|---|---|---|---|---|---|
| 600 | 0.2 | 100∶1 | 7.52 | 37.60 | 8.28 | 41.40 | 4.20 | 21.00 |
| 600 | 0.4 | 50∶1 | 7.49 | 37.45 | 9.22 | 46.10 | 3.29 | 16.45 |
| 600 | 1.0 | 20∶1 | 8.23 | 41.15 | 8.71 | 43.55 | 3.06 | 15.30 |
| 700 | 0.2 | 100∶1 | 9.34 | 46.70 | 8.52 | 42.60 | 2.14 | 10.70 |
| 700 | 0.4 | 50∶1 | 9.70 | 48.50 | 7.80 | 39.00 | 2.50 | 12.50 |
| 700 | 1.0 | 20∶1 | 10.05 | 50.25 | 7.73 | 38.65 | 2.22 | 11.10 |
| 800 | 0.2 | 100∶1 | 9.18 | 45.90 | 8.82 | 44.10 | 2.00 | 10.00 |
| 800 | 0.4 | 50∶1 | 8.66 | 43.30 | 9.22 | 46.10 | 2.12 | 10.60 |
| 800 | 1.0 | 20∶1 | 9.10 | 45.50 | 8.26 | 41.30 | 2.64 | 13.20 |
表6 ZnO添加量及功率对产物的影响(PP 20g,活性炭20g)
| 功率/W | ZnO质量 /g | PP∶ZnO (质量比) | 裂解气质量 /g | 裂解气产率 (质量分数)/% | 裂解油质量 /g | 裂解油产率(质量分数)/% | 固体碳质量 /g | 固体碳产率(质量分数)/% |
|---|---|---|---|---|---|---|---|---|
| 600 | 0.2 | 100∶1 | 7.52 | 37.60 | 8.28 | 41.40 | 4.20 | 21.00 |
| 600 | 0.4 | 50∶1 | 7.49 | 37.45 | 9.22 | 46.10 | 3.29 | 16.45 |
| 600 | 1.0 | 20∶1 | 8.23 | 41.15 | 8.71 | 43.55 | 3.06 | 15.30 |
| 700 | 0.2 | 100∶1 | 9.34 | 46.70 | 8.52 | 42.60 | 2.14 | 10.70 |
| 700 | 0.4 | 50∶1 | 9.70 | 48.50 | 7.80 | 39.00 | 2.50 | 12.50 |
| 700 | 1.0 | 20∶1 | 10.05 | 50.25 | 7.73 | 38.65 | 2.22 | 11.10 |
| 800 | 0.2 | 100∶1 | 9.18 | 45.90 | 8.82 | 44.10 | 2.00 | 10.00 |
| 800 | 0.4 | 50∶1 | 8.66 | 43.30 | 9.22 | 46.10 | 2.12 | 10.60 |
| 800 | 1.0 | 20∶1 | 9.10 | 45.50 | 8.26 | 41.30 | 2.64 | 13.20 |
| 功率r/W | 催化剂质量/g | ZnO相对密度 | MgO相对密度 |
|---|---|---|---|
| 600 | 0.2 | 0.776 | 0.750 |
| 600 | 0.4 | 0.780 | 0.750 |
| 600 | 1.0 | 0.776 | 0.740 |
| 700 | 0.2 | 0.750 | 0.792 |
| 700 | 0.4 | 0.760 | 0.793 |
| 700 | 1.0 | 0.720 | 0.753 |
800 800 800 | 0.2 0.4 1.0 | 0.780 0.777 0.779 | 0.771 0.772 0.760 |
表7 不同裂解条件下裂解油参数
| 功率r/W | 催化剂质量/g | ZnO相对密度 | MgO相对密度 |
|---|---|---|---|
| 600 | 0.2 | 0.776 | 0.750 |
| 600 | 0.4 | 0.780 | 0.750 |
| 600 | 1.0 | 0.776 | 0.740 |
| 700 | 0.2 | 0.750 | 0.792 |
| 700 | 0.4 | 0.760 | 0.793 |
| 700 | 1.0 | 0.720 | 0.753 |
800 800 800 | 0.2 0.4 1.0 | 0.780 0.777 0.779 | 0.771 0.772 0.760 |
| 1 | LI Kangqiang, CHEN Guo, LI Xiteng, et al. High-temperature dielectric properties and pyrolysis reduction characteristics of different biomass-pyrolusite mixtures in microwave field[J]. Bioresource Technology, 2019, 294: 122217. |
| 2 | RATNASARI D K, NAHIL M A, WILLIAMS P T. Catalytic pyrolysis of waste plastics using staged catalysis for production of gasoline range hydrocarbon oils[J]. Journal of Analytical and Applied Pyrolysis, 2017, 124: 631-637. |
| 3 | CAO Changqing, BIAN Ce, WANG Gaoyun, et al. Co-gasification of plastic wastes and soda lignin in supercritical water[J]. Chemical Engineering Journal, 2020, 388: 124277. |
| 4 | JAMBECK J R, GEYER R, WILCOX C, et al. Plastic waste inputs from land into the ocean[J]. Science, 2015, 347(6223): 768-771. |
| 5 | KASAR P, SHARMA D K, AHMARUZZAMAN M. Thermal and catalytic decomposition of waste plastics and its co-processing with petroleum residue through pyrolysis process[J]. Journal of Cleaner Production, 2020, 265: 121639. |
| 6 | KLINGER J L, WESTOVER T L, EMERSON R M, et al. Effect of biomass type, heating rate, and sample size on microwave-enhanced fast pyrolysis product yields and qualities[J]. Applied Energy, 2018, 228: 535-545. |
| 7 | 郭良. 生物质微波裂解实验研究[D]. 上海: 华东理工大学, 2018. |
| GUO Liang. Experimental study on biomass microwave cracking[D]. Shanghai: East China University of Science and Technology, 2018. | |
| 8 | DAI Leilei, HE Chao, WANG Yunpu, et al. Comparative study on microwave and conventional hydrothermal pretreatment of bamboo sawdust: Hydrochar properties and its pyrolysis behaviors[J]. Energy Conversion and Management, 2017, 146: 1-7. |
| 9 | JING Xiaodong, WEN Hao, GONG Xuzhong, et al. Heating strategies for the system of PP and spherical activated carbon during microwave cracking for obtaining value-added products[J]. Fuel Processing Technology, 2020, 199: 106265. |
| 10 | 高腾飞, 肖天存, 闫巍, 等. 固体废弃物微波技术处理及其资源化[J]. 工业催化, 2016, 24(7): 1-10. |
| GAO Tengfei, XIAO Tiancun, YAN Wei, et al. Treatment and recovery of solid waste by microwave techniques[J]. Industrial Catalysis, 2016, 24(7): 1-10. | |
| 11 | SURIAPPARAO D V, VINU R, SHUKLA A, et al. Effective deoxygenation for the production of liquid biofuels via microwave assisted co-pyrolysis of agro residues and waste plastics combined with catalytic upgradation[J]. Bioresource Technology, 2020, 302: 122775. |
| 12 | UNDRI A, ROSI L, FREDIANI M, et al. Efficient disposal of waste polyolefins through microwave assisted pyrolysis[J]. Fuel, 2014, 116: 662-671. |
| 13 | LOPEZ G, ARTETXE M, AMUTIO M, et al. Thermochemical routes for the valorization of waste polyolefinic plastics to produce fuels and chemicals. A review[J]. Renewable and Sustainable Energy Reviews, 2017, 73: 346-368. |
| 14 | MULLEN C A, DORADO C, BOATENG A A. Catalytic co-pyrolysis of switchgrass and polyethylene over HZSM-5: catalyst deactivation and coke formation[J]. Journal of Analytical and Applied Pyrolysis, 2018, 129: 195-203. |
| 15 | RUSSELL A D, ANTREOU E I, LAM S S, et al. Microwave-assisted pyrolysis of HDPE using an activated carbon bed[J]. RSC Advances, 2012, 2(17): 6756. |
| 16 | SAIFUDDIN N, PRIATHARSINI P, HAKIM S B. Microwave-assisted co-pyrolysis of bamboo biomass with plastic waste for hydrogen-rich syngas production[J]. American Journal of Applied Sciences, 2016, 13(5): 511-521. |
| 17 | ZHANG Donghong, LIN Xiaona, ZHANG Qingfa, et al. Catalytic pyrolysis of wood-plastic composite waste over activated carbon catalyst for aromatics production: Effect of preparation process of activated carbon[J]. Energy, 2020, 212: 118983. |
| 18 | LI Chao, SUN Yifan, DONG Dehua, et al. Co-pyrolysis of cellulose/lignin and sawdust: Influence of secondary condensation of the volatiles on characteristics of biochar[J]. Energy, 2021, 226: 120442. |
| 19 | SURIAPPARAO D V, VINU R. Resource recovery from synthetic polymers via microwave pyrolysis using different susceptors[J]. Journal of Analytical and Applied Pyrolysis, 2015, 113: 701-712. |
| 20 | ZHAO D T, WANG X H, MILLER J B, et al. The chemistry and kinetics of polyethylene pyrolysis: a process to produce fuels and chemicals[J]. ChemSusChem, 2020, 13(7): 1764-1774. |
| 21 | 孙凯. 废塑料催化热解制备芳香烃的研究[D]. 杭州: 浙江大学, 2021. |
| SUN Kai. Study on aromatics production from catalytic pyrolysis of waste plastics[D]. Hangzhou: Zhejiang University, 2021. | |
| 22 | DING Kuan, LIU Shasha, HUANG Yong, et al. Catalytic microwave-assisted pyrolysis of plastic waste over NiO and HY for gasoline-range hydrocarbons production[J]. Energy Conversion and Management, 2019, 196: 1316-1325. |
| 23 | ZHOU Nan, DAI Leilei, Yuancai LYU, et al. Catalytic pyrolysis of plastic wastes in a continuous microwave assisted pyrolysis system for fuel production[J]. Chemical Engineering Journal, 2021, 418: 129412. |
| 24 | UNDRI A, ROSI L, FREDIANI M, et al. Upgraded fuel from microwave assisted pyrolysis of waste tire[J]. Fuel, 2014, 115: 600-608. |
| 25 | JING Xiaodong, YAN Guoxun, ZHAO Yuehong, et al. Study on mild cracking of polyolefins to liquid hydrocarbons in a closed batch reactor for subsequent olefin recovery[J]. Polymer Degradation and Stability, 2014, 109: 79-91. |
| 26 | JING Xiaodong, YAN Guoxun, ZHAO Yuehong, et al. Cocracking kinetics of PE/PP and PE/hydrocarbon mixtures (I) PE/PP mixtures[J]. Energy & Fuels, 2014, 28(8): 5396-5405. |
| 27 | LI Yanling, HUANG Sheng, WANG Qian, et al. Hydrogen transfer route and interaction mechanism during co-pyrolysis of Xilinhot lignite and rice husk[J]. Fuel Processing Technology, 2019, 192: 13-20. |
| 28 | HUJURI U, GHOSHAL A K, GUMMA S. Temperature-dependent pyrolytic product evolution profile for polypropylene[J]. Journal of Applied Polymer Science, 2011, 119(4): 2318-2325. |
| [1] | 张耀杰, 张传祥, 孙悦, 曾会会, 贾建波, 蒋振东. 煤基石墨烯量子点在超级电容器中的应用[J]. 化工进展, 2023, 42(8): 4340-4350. |
| [2] | 张丽宏, 金要茹, 程芳琴. 煤气化渣资源化利用[J]. 化工进展, 2023, 42(8): 4447-4457. |
| [3] | 王科菊, 赵成, 胡晓玫, 云军阁, 魏凝涵, 姜雪迎, 邹昀, 陈志航. 金属氧化物低温催化氧化VOCs的研究进展[J]. 化工进展, 2023, 42(5): 2402-2412. |
| [4] | 邢献军, 罗甜, 卜玉蒸, 马培勇. H3PO4活化核桃壳制备活性炭及在Cr(Ⅵ)吸附中的应用[J]. 化工进展, 2023, 42(3): 1527-1539. |
| [5] | 刘雅娟. 浸没式PAC-AMBRs系统中PAC缓解膜污染的研究进展[J]. 化工进展, 2023, 42(1): 457-468. |
| [6] | 孙宪航, 任铸, 张国军, 孙媛, 范开峰, 黄维秋. 超临界CO2作用下甲苯在活性炭中的脱附机理[J]. 化工进展, 2022, 41(S1): 631-636. |
| [7] | 张辛亥, 赵思琛, 朱辉, 张首石, 王凯. 多种碳材料与碳酸钠复合后脱硫性能对比[J]. 化工进展, 2022, 41(S1): 424-435. |
| [8] | 何晨露, 邱晨茜, 方娟, 杨旋, 赖建军, 郑新宇, 吕建华, 陈燕丹, 黄彪. 基于低共熔溶剂体系的氮掺杂超级电容炭[J]. 化工进展, 2022, 41(9): 4946-4953. |
| [9] | 袁权, 李海红, 刘浩杰. HNO3改性活性炭对不同价态离子的电吸附规律[J]. 化工进展, 2022, 41(9): 4986-4994. |
| [10] | 张鹏, 孟凡会, 杨贵楠, 李忠. 金属氧化物在OX-ZEO催化剂中催化CO x 加氢制低碳烯烃研究进展[J]. 化工进展, 2022, 41(8): 4159-4172. |
| [11] | 熊永志, 刘艳艳, 陈晓荭, 卢贝丽, 黄彪, 林冠烽. 甘蔗渣基磷掺杂活性炭的制备及其电化学性能[J]. 化工进展, 2022, 41(8): 4397-4405. |
| [12] | 黄平安, 徐俊, 杨宇轩, 潘宇涵, 王新文, 黄群星. 球磨改性热解炭吸附磺胺甲 唑[J]. 化工进展, 2022, 41(7): 3784-3793. 唑[J]. 化工进展, 2022, 41(7): 3784-3793. |
| [13] | 郑敏, 徐垒, 陈晨, 徐忠宁, 付明来. 稀硝酸催化还原工艺中Pd/AC制备条件优化[J]. 化工进展, 2022, 41(7): 3938-3946. |
| [14] | 武传朋, 李传坤, 杨哲, 苟成冬, 高新江. 固体吸附材料脱除SO2研究进展[J]. 化工进展, 2022, 41(7): 3840-3854. |
| [15] | 蔡思超, 周静, 杜金泽, 李方舟, 李源森, 何林, 李鑫钢, 王成扬. 煤化工酚基精馏釜残资源化利用过程初步分析[J]. 化工进展, 2022, 41(6): 3360-3371. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||