化工进展 ›› 2022, Vol. 41 ›› Issue (7): 3784-3793.DOI: 10.16085/j.issn.1000-6613.2021-1819
球磨改性热解炭吸附磺胺甲 唑
唑
黄平安1( ), 徐俊2, 杨宇轩1, 潘宇涵1, 王新文2, 黄群星1(
), 徐俊2, 杨宇轩1, 潘宇涵1, 王新文2, 黄群星1( )
)
- 1.浙江大学热能工程研究所,能源清洁利用国家重点实验室,浙江 杭州 310027
2.中策橡胶集团有限公司,浙江 杭州 310018
-
收稿日期:2021-08-25修回日期:2021-11-09出版日期:2022-07-25发布日期:2022-07-23 -
通讯作者:黄群星 -
作者简介:黄平安(1996—),男,硕士研究生,研究方向为废轮胎资源化利用。E-mail:3150104444@zju.edu.cn 。 -
基金资助:浙江省重点研发计划(2020C03084)
Ball milled modified pyrolysis carbon adsorb sulfamethoxazole
HUANG Ping’an1( ), XU Jun2, YANG Yuxuan1, PAN Yuhan1, WANG Xinwen2, HUANG Qunxing1(
), XU Jun2, YANG Yuxuan1, PAN Yuhan1, WANG Xinwen2, HUANG Qunxing1( )
)
- 1.State Key Laboratory of Clean Energy Utilization, Institute for Thermal Power Engineering, Zhejiang University, Hangzhou 310027, Zhejiang, China
2.Zhongce Rubber Group Company Limited, Hangzhou 310018, Zhejiang, China
-
Received:2021-08-25Revised:2021-11-09Online:2022-07-25Published:2022-07-23 -
Contact:HUANG Qunxing
摘要:
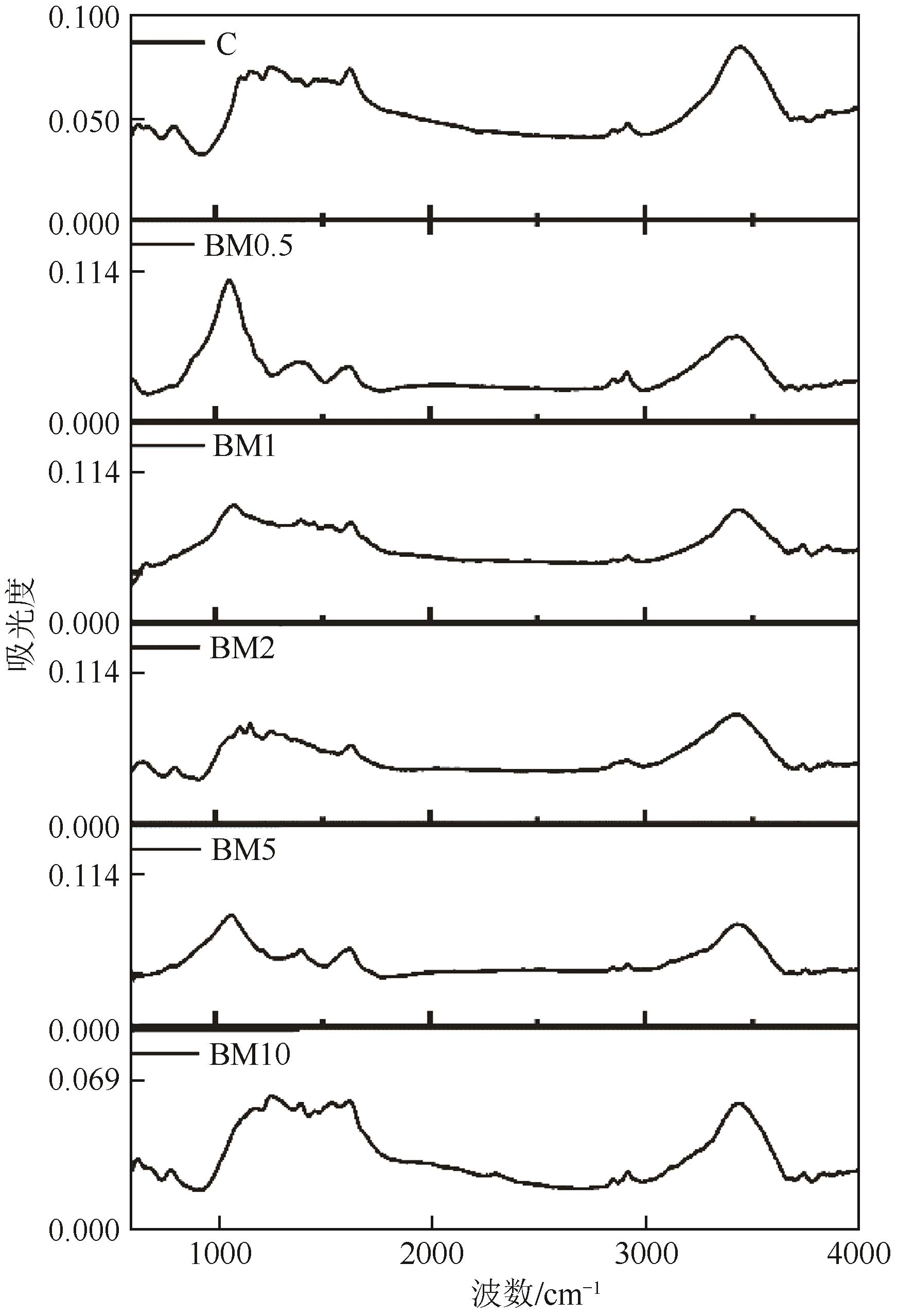
针对热解炭颗粒大、表面活性弱、吸附能力差的问题,本文提出了一种机械球磨表面改性方法,探讨了不同球磨改性参数下热解炭对磺胺甲 唑(SMZ)的吸附效果。以废橡胶连续热解炭为原料,采用不锈钢球磨制得具有不同表面性质的球磨炭,分析了球磨前后热解炭的结构、表面性质及表面形貌,并对比了球磨改性前后的SMZ吸附性能。结果表明,球磨改性过程可以有效改善废轮胎热解炭结构及表面性质,球磨处理2h的热解炭对SMZ的吸附效果最好,吸附量达到59.37mg/g,吸附动力学符合伪二级吸附模型。
唑(SMZ)的吸附效果。以废橡胶连续热解炭为原料,采用不锈钢球磨制得具有不同表面性质的球磨炭,分析了球磨前后热解炭的结构、表面性质及表面形貌,并对比了球磨改性前后的SMZ吸附性能。结果表明,球磨改性过程可以有效改善废轮胎热解炭结构及表面性质,球磨处理2h的热解炭对SMZ的吸附效果最好,吸附量达到59.37mg/g,吸附动力学符合伪二级吸附模型。
中图分类号:
引用本文
黄平安, 徐俊, 杨宇轩, 潘宇涵, 王新文, 黄群星. 球磨改性热解炭吸附磺胺甲 唑[J]. 化工进展, 2022, 41(7): 3784-3793.
唑[J]. 化工进展, 2022, 41(7): 3784-3793.
HUANG Ping’an, XU Jun, YANG Yuxuan, PAN Yuhan, WANG Xinwen, HUANG Qunxing. Ball milled modified pyrolysis carbon adsorb sulfamethoxazole[J]. Chemical Industry and Engineering Progress, 2022, 41(7): 3784-3793.
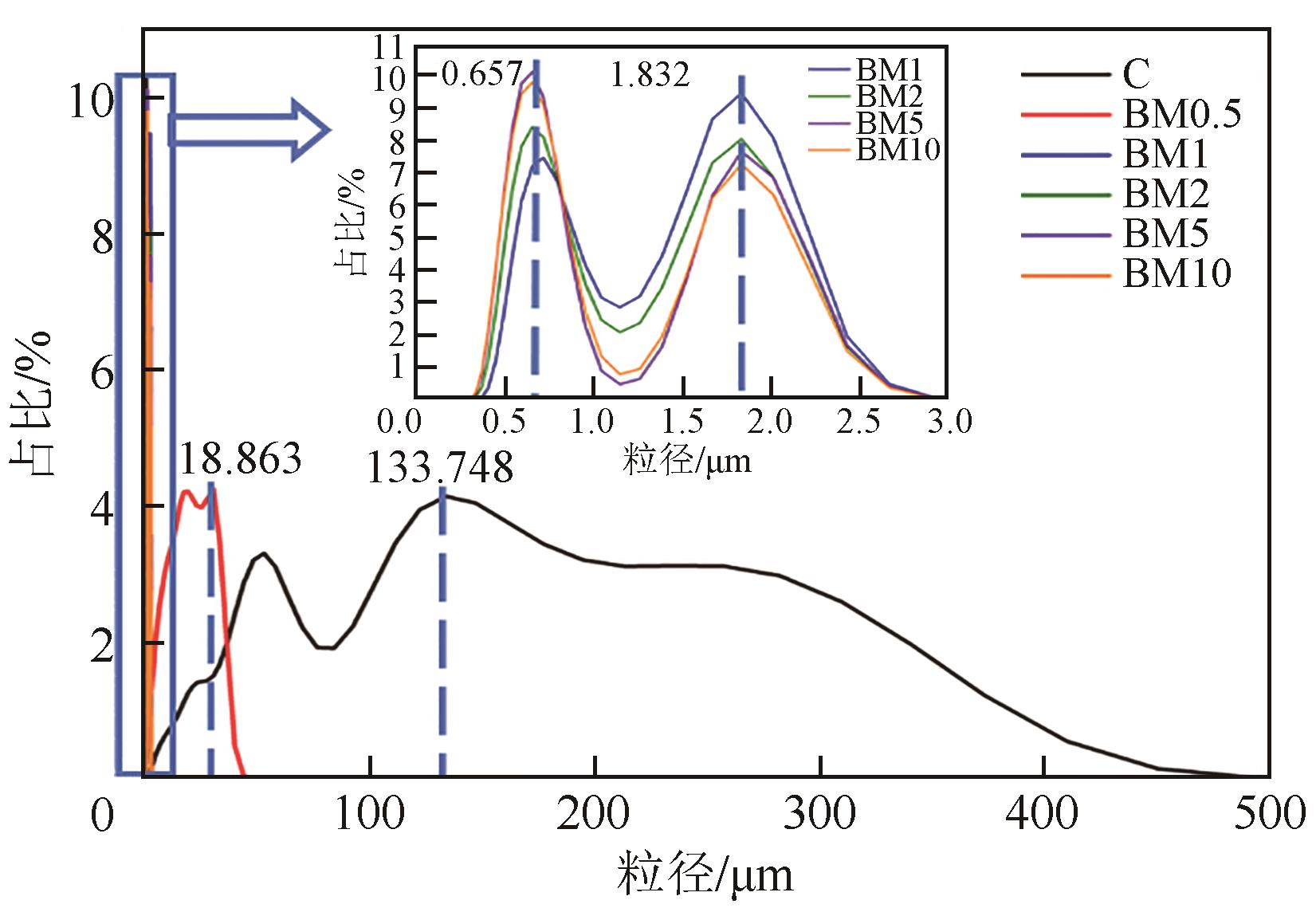
| 样品 | 最小粒径/μm | 最大粒径/μm | 平均粒径/μm | d90/μm |
|---|---|---|---|---|
| C | 0.08 | 541.89 | 120.30 | 278.50 |
| BM0.5 | 0.08 | 43.67 | 14.07 | 30.24 |
| BM1 | 0.34 | 2.92 | 1.31 | 2.15 |
| BM2 | 0.31 | 2.92 | 1.19 | 2.11 |
| BM5 | 0.28 | 2.92 | 1.12 | 2.11 |
| BM10 | 0.28 | 2.92 | 1.09 | 2.08 |
表1 球磨前后热解炭的粒度参数
| 样品 | 最小粒径/μm | 最大粒径/μm | 平均粒径/μm | d90/μm |
|---|---|---|---|---|
| C | 0.08 | 541.89 | 120.30 | 278.50 |
| BM0.5 | 0.08 | 43.67 | 14.07 | 30.24 |
| BM1 | 0.34 | 2.92 | 1.31 | 2.15 |
| BM2 | 0.31 | 2.92 | 1.19 | 2.11 |
| BM5 | 0.28 | 2.92 | 1.12 | 2.11 |
| BM10 | 0.28 | 2.92 | 1.09 | 2.08 |
| 样品 | SBET/m2?g-1 | Smicro/m2?g-1 | RSmicro/% | PS/nm | Vtotal/cm3?g-1 | Vmicro/cm3?g-1 | RVmicro/% | DP/μm |
|---|---|---|---|---|---|---|---|---|
| C | 88.31 | 8.03 | 9.09 | 28.57 | 0.63 | 0.0025 | 0.39 | 120.30 |
| BM0.5 | 112.87 | 17.06 | 15.11 | 13.61 | 0.38 | 0.0073 | 1.90 | 14.07 |
| BM1 | 113.52 | 20.14 | 17.74 | 11.57 | 0.33 | 0.0092 | 2.80 | 1.31 |
| BM2 | 223.73 | 101.44 | 45.34 | 5.56 | 0.31 | 0.0433 | 13.91 | 1.19 |
| BM5 | 203.03 | 94.75 | 46.67 | 5.54 | 0.28 | 0.0402 | 14.31 | 1.12 |
| BM10 | 113.97 | 44.27 | 38.84 | 7.71 | 0.22 | 0.0190 | 8.64 | 1.09 |
表2 球磨前后热解炭的吸附能力、结构参数、平均粒度
| 样品 | SBET/m2?g-1 | Smicro/m2?g-1 | RSmicro/% | PS/nm | Vtotal/cm3?g-1 | Vmicro/cm3?g-1 | RVmicro/% | DP/μm |
|---|---|---|---|---|---|---|---|---|
| C | 88.31 | 8.03 | 9.09 | 28.57 | 0.63 | 0.0025 | 0.39 | 120.30 |
| BM0.5 | 112.87 | 17.06 | 15.11 | 13.61 | 0.38 | 0.0073 | 1.90 | 14.07 |
| BM1 | 113.52 | 20.14 | 17.74 | 11.57 | 0.33 | 0.0092 | 2.80 | 1.31 |
| BM2 | 223.73 | 101.44 | 45.34 | 5.56 | 0.31 | 0.0433 | 13.91 | 1.19 |
| BM5 | 203.03 | 94.75 | 46.67 | 5.54 | 0.28 | 0.0402 | 14.31 | 1.12 |
| BM10 | 113.97 | 44.27 | 38.84 | 7.71 | 0.22 | 0.0190 | 8.64 | 1.09 |
| 样品 | 元素/% | |||
|---|---|---|---|---|
| C | O | S | Si | |
| C | 76.88 | 20.56 | 2.07 | 0.49 |
| BM0.5 | 76.14 | 21.69 | 1.83 | 0.35 |
| BM1 | 76.51 | 21.86 | 1.22 | 0.42 |
| BM2 | 73.19 | 23.80 | 2.40 | 0.62 |
| BM5 | 70.20 | 26.25 | 2.85 | 0.70 |
| BM10 | 68.46 | 28.19 | 2.67 | 0.68 |
表3 球磨前后热解炭的EDS数据(质量分数)
| 样品 | 元素/% | |||
|---|---|---|---|---|
| C | O | S | Si | |
| C | 76.88 | 20.56 | 2.07 | 0.49 |
| BM0.5 | 76.14 | 21.69 | 1.83 | 0.35 |
| BM1 | 76.51 | 21.86 | 1.22 | 0.42 |
| BM2 | 73.19 | 23.80 | 2.40 | 0.62 |
| BM5 | 70.20 | 26.25 | 2.85 | 0.70 |
| BM10 | 68.46 | 28.19 | 2.67 | 0.68 |
| 样品 | Langmuir等温线模型 | Freundlich等温线模型 | |||||
|---|---|---|---|---|---|---|---|
| qm/mg·g-1 | KL | R2 | KF | n | R2 | ||
| C | 1.939 | 0.255 | 0.984 | 0.590 | 3.214 | 0.909 | |
| BM0.5 | 26.767 | 0.278 | 0.989 | 7.463 | 2.821 | 0.947 | |
| BM1 | 27.981 | 0.324 | 0.992 | 8.192 | 2.879 | 0.923 | |
| BM2 | 55.424 | 0.942 | 0.990 | 21.238 | 3.036 | 0.830 | |
| BM5 | 50.118 | 0.816 | 0.990 | 18.974 | 3.099 | 0.867 | |
| BM10 | 33.897 | 0.293 | 0.993 | 9.452 | 2.774 | 0.938 | |
表4 两种等温线模型的拟合参数
| 样品 | Langmuir等温线模型 | Freundlich等温线模型 | |||||
|---|---|---|---|---|---|---|---|
| qm/mg·g-1 | KL | R2 | KF | n | R2 | ||
| C | 1.939 | 0.255 | 0.984 | 0.590 | 3.214 | 0.909 | |
| BM0.5 | 26.767 | 0.278 | 0.989 | 7.463 | 2.821 | 0.947 | |
| BM1 | 27.981 | 0.324 | 0.992 | 8.192 | 2.879 | 0.923 | |
| BM2 | 55.424 | 0.942 | 0.990 | 21.238 | 3.036 | 0.830 | |
| BM5 | 50.118 | 0.816 | 0.990 | 18.974 | 3.099 | 0.867 | |
| BM10 | 33.897 | 0.293 | 0.993 | 9.452 | 2.774 | 0.938 | |
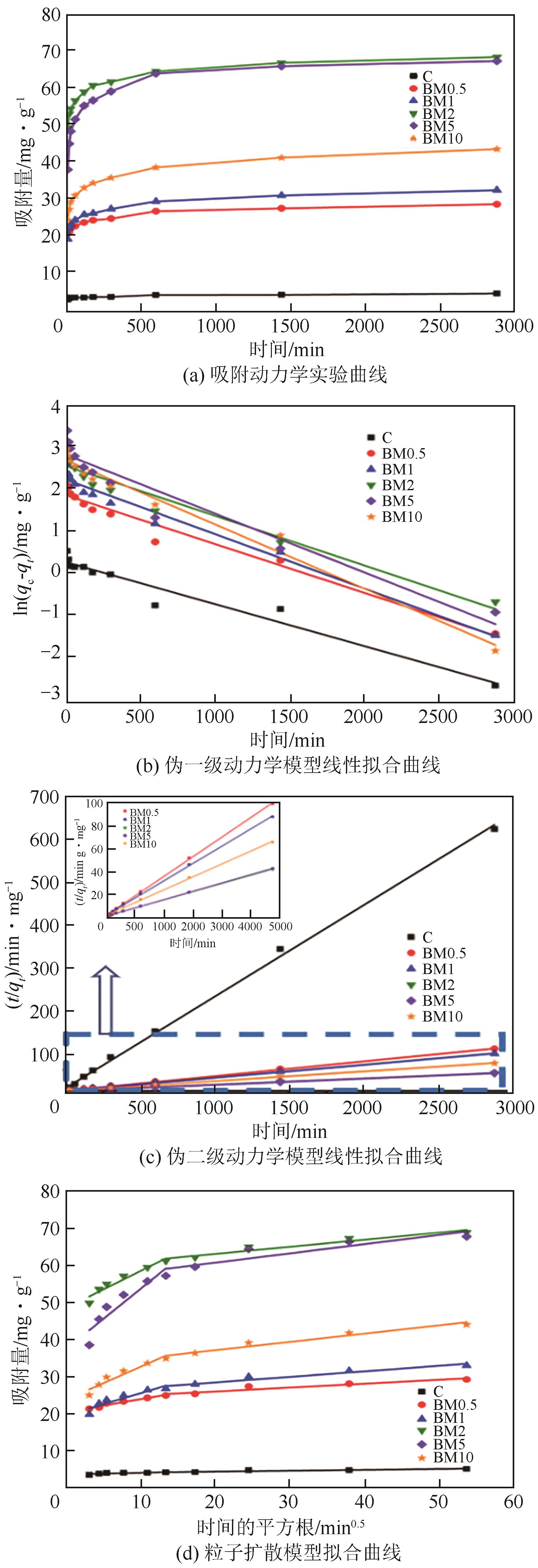
| 样品 | 伪一级动力学模型 | 伪二级动力学模型 | |||||
|---|---|---|---|---|---|---|---|
| k1/mg | qe,cal/mg | R2 | k2/g | qe,cal/mg | R2 | ||
| C | 0.00099 | 1.31 | 0.946 | 0.0062 | 4.63 | 0.999 | |
| BM0.5 | 0.0012 | 6.42 | 0.965 | 0.0015 | 28.80 | 0.999 | |
| BM1 | 0.0013 | 9.29 | 0.971 | 0.0010 | 32.63 | 0.999 | |
| BM2 | 0.0012 | 12.82 | 0.944 | 0.0008 | 68.68 | 0.999 | |
| BM5 | 0.0014 | 16.81 | 0.919 | 0.0006 | 67.80 | 0.999 | |
| BM10 | 0.0015 | 14.77 | 0.976 | 0.0006 | 43.74 | 0.999 | |
表5 两种动力学模型的拟合参数
| 样品 | 伪一级动力学模型 | 伪二级动力学模型 | |||||
|---|---|---|---|---|---|---|---|
| k1/mg | qe,cal/mg | R2 | k2/g | qe,cal/mg | R2 | ||
| C | 0.00099 | 1.31 | 0.946 | 0.0062 | 4.63 | 0.999 | |
| BM0.5 | 0.0012 | 6.42 | 0.965 | 0.0015 | 28.80 | 0.999 | |
| BM1 | 0.0013 | 9.29 | 0.971 | 0.0010 | 32.63 | 0.999 | |
| BM2 | 0.0012 | 12.82 | 0.944 | 0.0008 | 68.68 | 0.999 | |
| BM5 | 0.0014 | 16.81 | 0.919 | 0.0006 | 67.80 | 0.999 | |
| BM10 | 0.0015 | 14.77 | 0.976 | 0.0006 | 43.74 | 0.999 | |
| 样品 | kd1/mg | kd2/mg |
|---|---|---|
| C | 0.05 | 0.02 |
| BM0.5 | 0.35 | 0.11 |
| BM1 | 0.60 | 0.15 |
| BM2 | 1.01 | 0.19 |
| BM5 | 1.63 | 0.25 |
| BM10 | 0.89 | 0.22 |
表6 粒子扩散模型的拟合参数
| 样品 | kd1/mg | kd2/mg |
|---|---|---|
| C | 0.05 | 0.02 |
| BM0.5 | 0.35 | 0.11 |
| BM1 | 0.60 | 0.15 |
| BM2 | 1.01 | 0.19 |
| BM5 | 1.63 | 0.25 |
| BM10 | 0.89 | 0.22 |
| 1 | SIENKIEWICZ M, KUCINSKA-LIPKA J, JANIK H, et al. Progress in used tyres management in the European Union: a review[J]. Waste Management, 2012, 32(10): 1742-1751. |
| 2 | MANCHÓN-VIZUETE E, MACÍAS-GARCÍA A, NADAL GISBERT A, et al. Adsorption of mercury by carbonaceous adsorbents prepared from rubber of tyre wastes[J]. Journal of Hazardous Materials, 2005, 119(1/2/3): 231-238. |
| 3 | MARTÍNEZ J D, PUY N, MURILLO R, et al. Waste tyre pyrolysis—A review[J]. Renewable and Sustainable Energy Reviews, 2013, 23: 179-213. |
| 4 | ACOSTA R, NABARLATZ D, SÁNCHEZ-SÁNCHEZ A, et al. Adsorption of Bisphenol A on KOH-activated tyre pyrolysis char[J]. Journal of Environmental Chemical Engineering, 2018, 6(1): 823-833. |
| 5 | 杨殿才, 潘宇涵, 黄群星, 等. 废轮胎热解炭低温催化焦油重整制备富氢气体的研究[J]. 化工学报, 2020, 71(2): 642-650. |
| YANG Diancai, PAN Yuhan, HUANG Qunxing, et al. Study on catalytic reforming of tar at low temperature to produce hydrogen-rich gas by tire pyrolysis char[J]. CIESC Journal, 2020, 71(2): 642-650. | |
| 6 | 刘英俊, 乔慧君, 杜爱华. 废轮胎热裂解研究进展[J]. 世界橡胶工业, 2015, 42(1): 41-46. |
| LIU Yingjun, QIAO Huijun, DU Aihua. Research progress on the pyrolysis of scrap tire[J]. World Rubber Industry, 2015, 42(1): 41-46. | |
| 7 | 周作艳. 废旧轮胎热解炭黑的改性及应用研究[D]. 青岛: 青岛科技大学, 2017. |
| ZHOU Zuoyan. The modification and applycation of waste tire's pyrolytic carbon black[D]. Qingdao, China: Qingdao University of Science & Technology, 2017. | |
| 8 | GUPTA V K, NAYAK A, AGARWAL S, et al. Potential of activated carbon from waste rubber tire for the adsorption of phenolics: effect of pre-treatment conditions[J]. Journal of Colloid and Interface Science, 2014, 417: 420-430. |
| 9 | TRUBETSKAYA A, KLING J, ERSHAG O, et al. Removal of phenol and chlorine from wastewater using steam activated biomass soot and tire carbon black[J]. Journal of Hazardous Materials, 2019, 365: 846-856. |
| 10 | CONDE-RIVERA L R, SUAREZ-ESCOBAR A F, MARIN-PEREZ J J, et al. TiO2 supported on activated carbon from tire waste for ibuprofen removal[J]. Materials Letters, 2021, 291: 129590. |
| 11 | ACOSTA R, FIERRO V, MARTINEZ DE YUSO A, et al. Tetracycline adsorption onto activated carbons produced by KOH activation of tyre pyrolysis char[J]. Chemosphere, 2016, 149: 168-176. |
| 12 | XING T, SUNARSO J, YANG W R, et al. Ball milling: a green mechanochemical approach for synthesis of nitrogen doped carbon nanoparticles[J]. Nanoscale, 2013, 5(17): 7970. |
| 13 | NAGHDI M, TAHERAN M, BRAR S K, et al. A green method for production of nanobiochar by ball milling-optimization and characterization[J]. Journal of Cleaner Production, 2017, 164: 1394-1405. |
| 14 | LYU H H, GAO B, HE F, et al. Experimental and modeling investigations of ball-milled biochar for the removal of aqueous methylene blue[J]. Chemical Engineering Journal, 2018, 335: 110-119. |
| 15 | XU X Y, XU Z B, HUANG J S, et al. Sorption of reactive red by biochars ball milled in different atmospheres: co-effect of surface morphology and functional groups[J]. Chemical Engineering Journal, 2021, 413: 127468. |
| 16 | HUANG J S, ZIMMERMAN A R, CHEN H, et al. Ball milled biochar effectively removes sulfamethoxazole and sulfapyridine antibiotics from water and wastewater[J]. Environmental Pollution, 2020, 258: 113809. |
| 17 | ZHANG D W, HE Q Q, HU X L, et al. Enhanced adsorption for the removal of tetracycline hydrochloride (TC) using ball-milled biochar derived from crayfish shell[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 615: 126254. |
| 18 | XIANG W, WAN Y S, ZHANG X Y, et al. Adsorption of tetracycline hydrochloride onto ball-milled biochar: governing factors and mechanisms[J]. Chemosphere, 2020, 255: 127057. |
| 19 | ZHUANG Z C, WANG L, TANG J C. Efficient removal of volatile organic compound by ball-milled biochars from different preparing conditions[J]. Journal of Hazardous Materials, 2021, 406: 124676. |
| 20 | YANG Y X, LIN B C, SUN C, et al. Facile synthesis of tailored mesopore-enriched hierarchical porous carbon from food waste for rapid removal of aromatic VOCs[J]. Science of the Total Environment, 2021, 773: 145453. |
| 21 | YANG Y X, SUN C, LIN B C, et al. Surface modified and activated waste bone char for rapid and efficient VOCs adsorption[J]. Chemosphere, 2020, 256: 127054. |
| 22 | WANG A Y, SUN K, WU L P, et al. Co-carbonization of biomass and oily sludge to prepare sulfamethoxazole super-adsorbent materials[J]. Science of the Total Environment, 2020, 698: 134238. |
| 23 | KUMAR M, XIONG X N, WAN Z H, et al. Ball milling as a mechanochemical technology for fabrication of novel biochar nanomaterials[J]. Bioresource Technology, 2020, 312: 123613. |
| 24 | NASRULLAH A, KHAN A S, BHAT A H, et al. Effect of short time ball milling on physicochemical and adsorption performance of activated carbon prepared from mangosteen peel waste[J]. Renewable Energy, 2021, 168: 723-733. |
| 25 | ABU-ZIED B M, SCHWIEGER W, ASIRI A M. Effect of ball milling on the structural and textural features of MCM-41 mesoporous material[J]. Microporous and Mesoporous Materials, 2015, 218: 153-159. |
| 26 | XIANG W, ZHANG X Y, CHEN K Q, et al. Enhanced adsorption performance and governing mechanisms of ball-milled biochar for the removal of volatile organic compounds (VOCs)[J]. Chemical Engineering Journal, 2020, 385: 123842. |
| 27 | ABOULKAS A, HAMMANI H, ACHABY M EL, et al. Valorization of algal waste via pyrolysis in a fixed-bed reactor: production and characterization of bio-oil and bio-char[J]. Bioresource Technology, 2017, 243: 400-408. |
| 28 | MORAL-RODRÍGUEZ A I, LEYVA-RAMOS R, OCAMPO-PÉREZ R, et al. Removal of ronidazole and sulfamethoxazole from water solutions by adsorption on granular activated carbon: equilibrium and intraparticle diffusion mechanisms[J]. Adsorption, 2016, 22(1): 89-103. |
| 29 | ZHANG Q R, WANG J M, LYU H H, et al. Ball-milled biochar for galaxolide removal: sorption performance and governing mechanisms[J]. Science of the Total Environment, 2019, 659: 1537-1545. |
| 30 | LI R H, ZHANG Y C, DENG H X, et al. Removing tetracycline and Hg(Ⅱ) with ball-milled magnetic nanobiochar and its potential on polluted irrigation water reclamation[J]. Journal of Hazardous Materials, 2020, 384: 121095. |
| 31 | YU F, LI Y, HAN S, et al. Adsorptive removal of antibiotics from aqueous solution using carbon materials[J]. Chemosphere, 2016, 153: 365-385. |
| 32 | 方梦祥, 姚鹏, 岑建孟, 等. 活性炭吸附处理含酚废水的研究进展[J]. 化工进展, 2018, 37(2): 744-751. |
| FANG Mengxiang, YAO Peng, CEN Jianmeng, et al. Adsorption treatment of phenolic wastewater by activated carbon: a review[J]. Chemical Industry and Engineering Progress, 2018, 37(2): 744-751. |
| [1] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [2] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [3] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [4] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [5] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [6] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [7] | 邵博识, 谭宏博. 锯齿波纹板对挥发性有机物低温脱除过程强化模拟分析[J]. 化工进展, 2023, 42(S1): 84-93. |
| [8] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [9] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| [10] | 张丽宏, 金要茹, 程芳琴. 煤气化渣资源化利用[J]. 化工进展, 2023, 42(8): 4447-4457. |
| [11] | 张耀杰, 张传祥, 孙悦, 曾会会, 贾建波, 蒋振东. 煤基石墨烯量子点在超级电容器中的应用[J]. 化工进展, 2023, 42(8): 4340-4350. |
| [12] | 姜晶, 陈霄宇, 张瑞妍, 盛光遥. 载锰生物炭制备及其在环境修复中应用研究进展[J]. 化工进展, 2023, 42(8): 4385-4397. |
| [13] | 张振, 李丹, 陈辰, 吴菁岚, 应汉杰, 乔浩. 吸附树脂对唾液酸的分离纯化[J]. 化工进展, 2023, 42(8): 4153-4158. |
| [14] | 于静文, 宋璐娜, 刘砚超, 吕瑞东, 武蒙蒙, 冯宇, 李忠, 米杰. 一种吲哚基超交联聚合物In-HCP对水中碘的吸附作用[J]. 化工进展, 2023, 42(7): 3674-3683. |
| [15] | 李艳玲, 卓振, 池亮, 陈曦, 孙堂磊, 刘鹏, 雷廷宙. 氮掺杂生物炭的制备与应用研究进展[J]. 化工进展, 2023, 42(7): 3720-3735. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||