化工进展 ›› 2022, Vol. 41 ›› Issue (5): 2778-2787.DOI: 10.16085/j.issn.1000-6613.2021-1254
钇/羟基磷灰石的制备及对含磷废水的净化
黄敏1,2( ), 王彬1,2(
), 王彬1,2( ), 周明罗1,2, 谌书1,2, 张瀚文1,2, 杨远坤1,2, 傅开彬1,2, 梁宏3
), 周明罗1,2, 谌书1,2, 张瀚文1,2, 杨远坤1,2, 傅开彬1,2, 梁宏3
- 1.西南科技大学环境与资源学院,四川 绵阳 621010
2.低成本废水处理技术四川省国际科技合作基地,四川 绵阳 621010
3.西南石油大学化学化工学院,四川 成都 610500
-
收稿日期:2021-06-15修回日期:2021-09-06出版日期:2022-05-05发布日期:2022-05-24 -
通讯作者:王彬 -
作者简介:黄敏(1997—),女,硕士研究生,研究方向为水污染控制技术与工程应用。E-mail:hm19970815@163.com 。 -
基金资助:四川省环境治理与生态保护重大科技专项(2018SZDZX0020)
Preparation of yttrium/hydroxyapatite and purification of wastewater containing phosphorus
HUANG Min1,2( ), WANG Bin1,2(
), WANG Bin1,2( ), ZHOU Mingluo1,2, CHEN Shu1,2, ZHANG Hanwen1,2, YANG Yuankun1,2, FU Kaibin1,2, LIANG Hong3
), ZHOU Mingluo1,2, CHEN Shu1,2, ZHANG Hanwen1,2, YANG Yuankun1,2, FU Kaibin1,2, LIANG Hong3
- 1.School of Environment and Resources, Southwest University of Science and Technology, Mianyang 621010, Sichuan, China
2.Sichuan Provincial Sci-Tech Cooperation Base of Low-cost Wastewater Treatment Technology, Mianyang 621010, Sichuan, China
3.School of Chemistry and Chemical Engineering, Southwest Petroleum University, Chengdu 610500, Sichuan, China
-
Received:2021-06-15Revised:2021-09-06Online:2022-05-05Published:2022-05-24 -
Contact:WANG Bin
摘要:
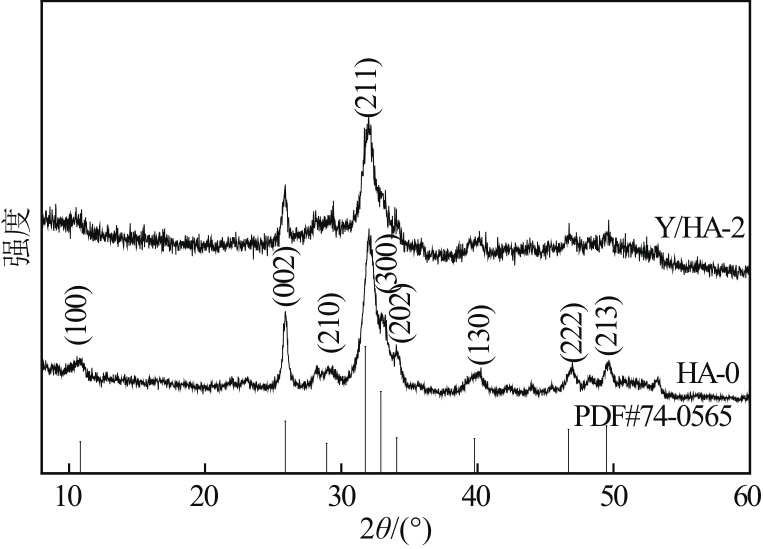
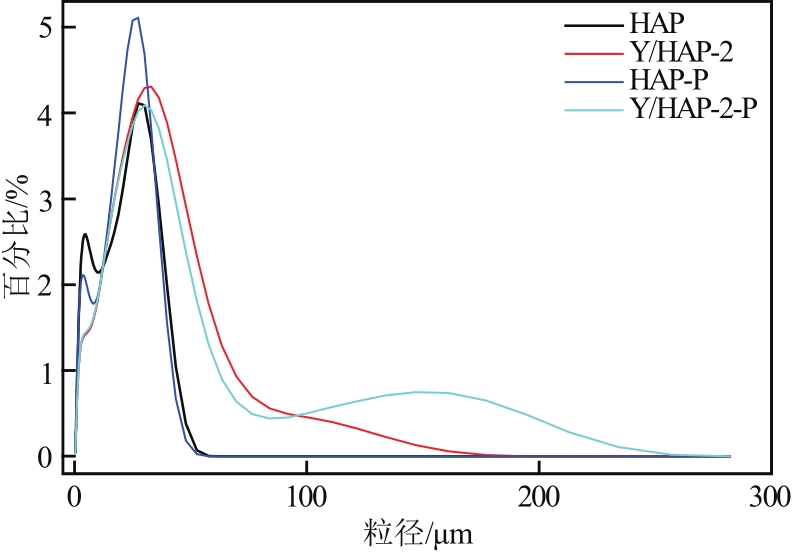
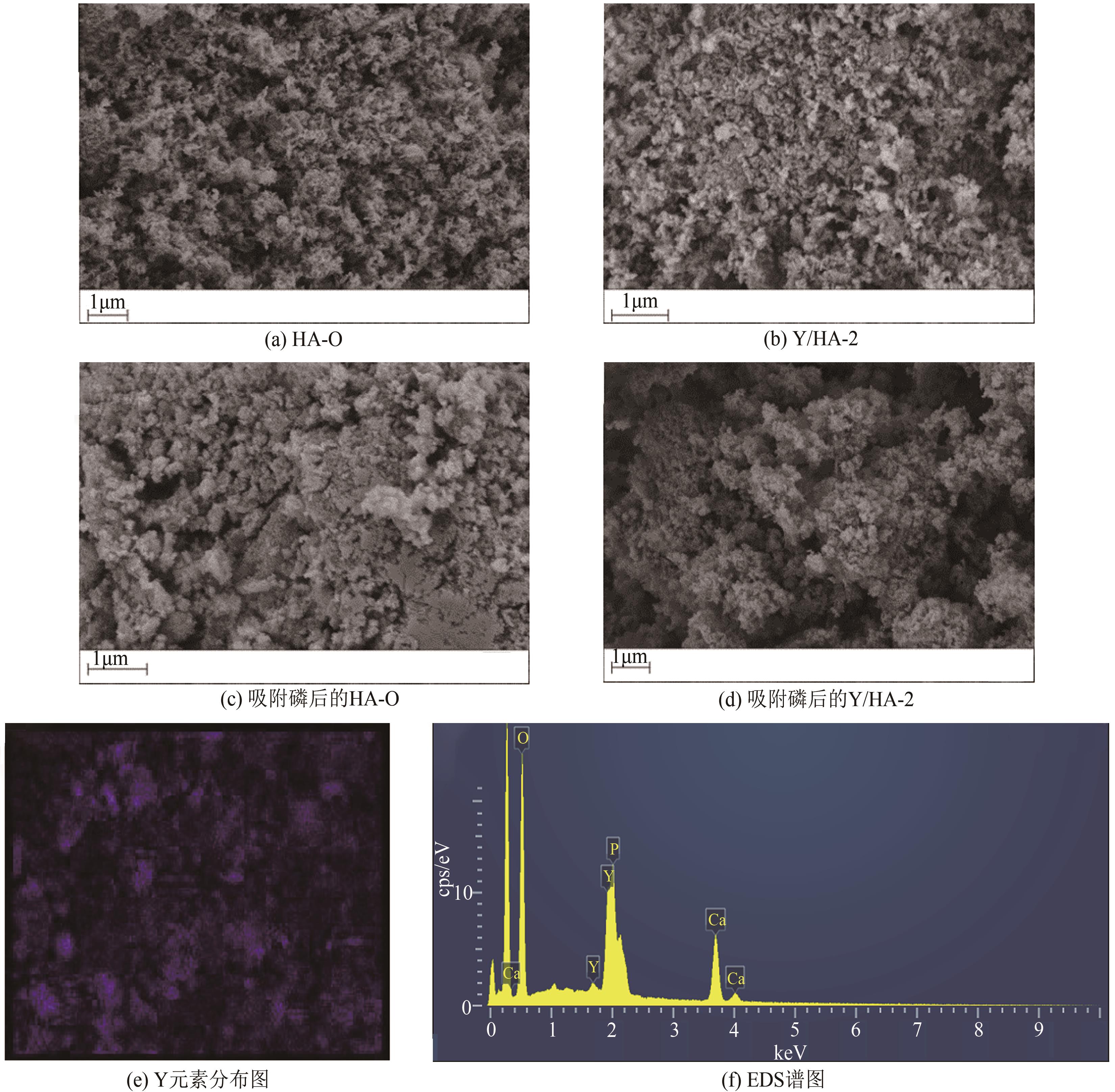
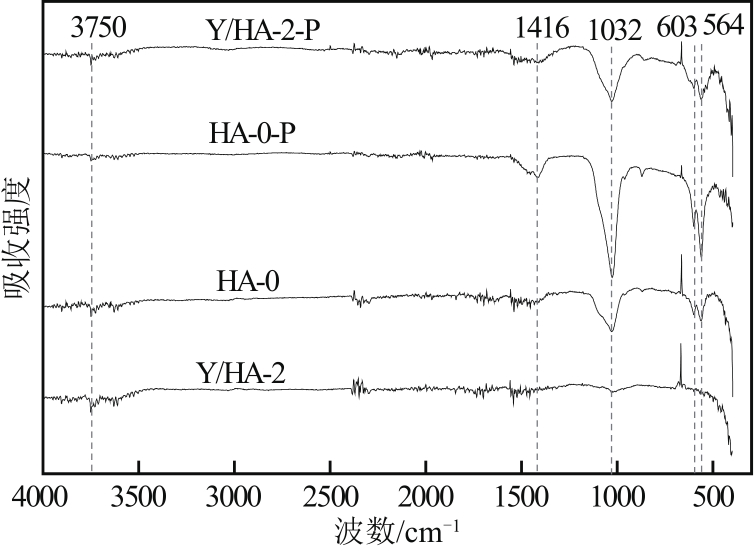
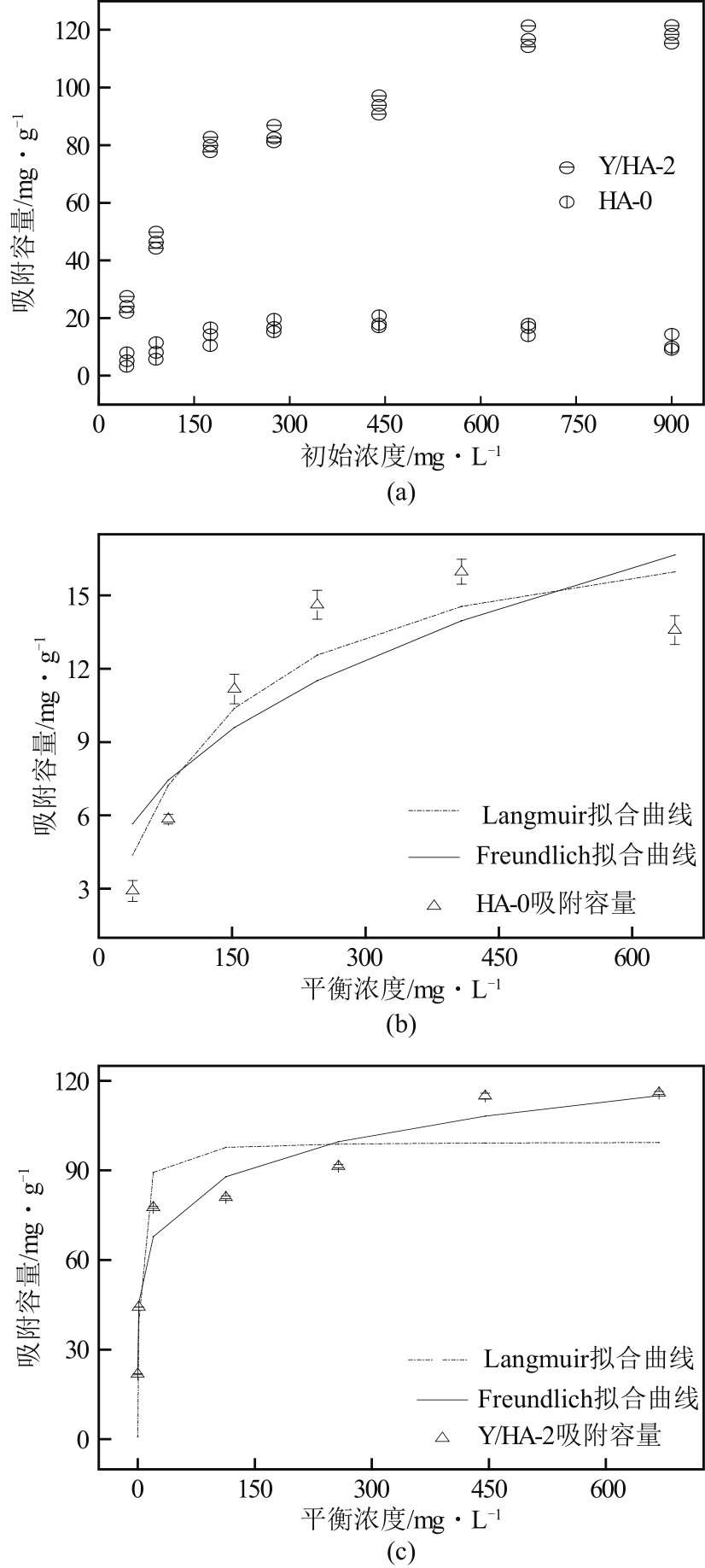
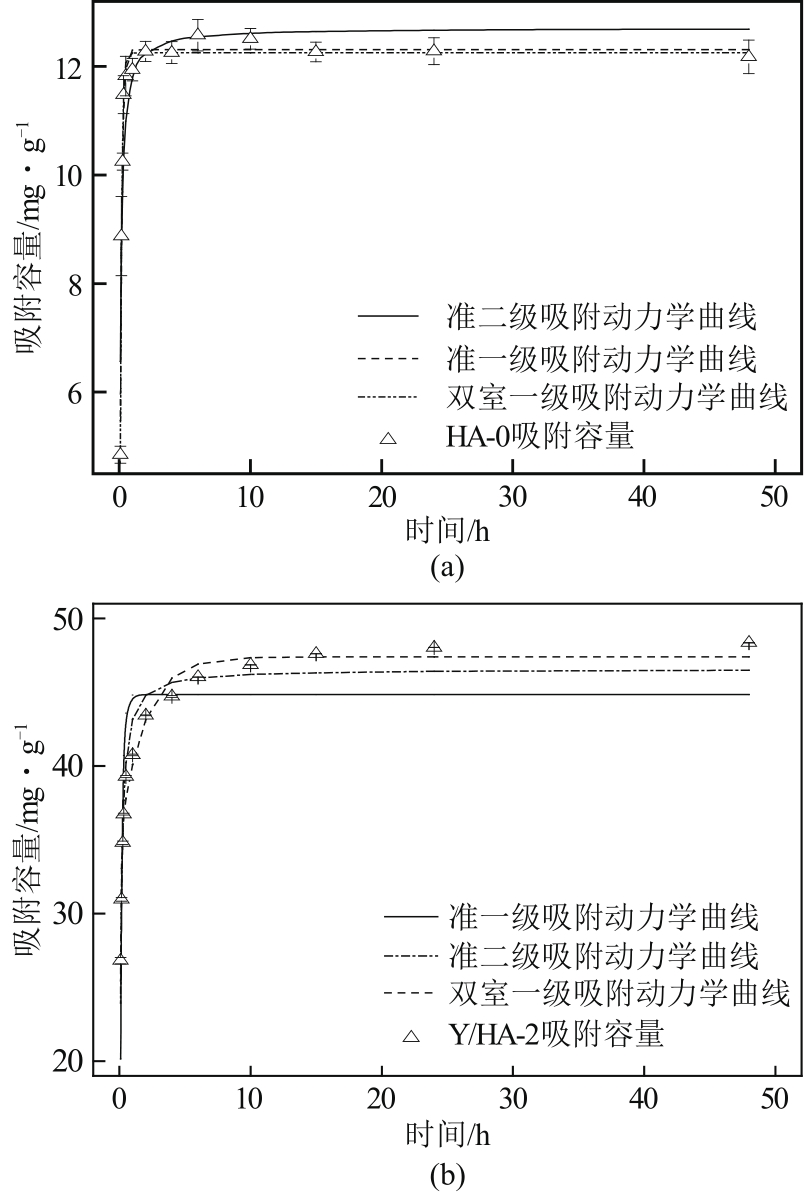
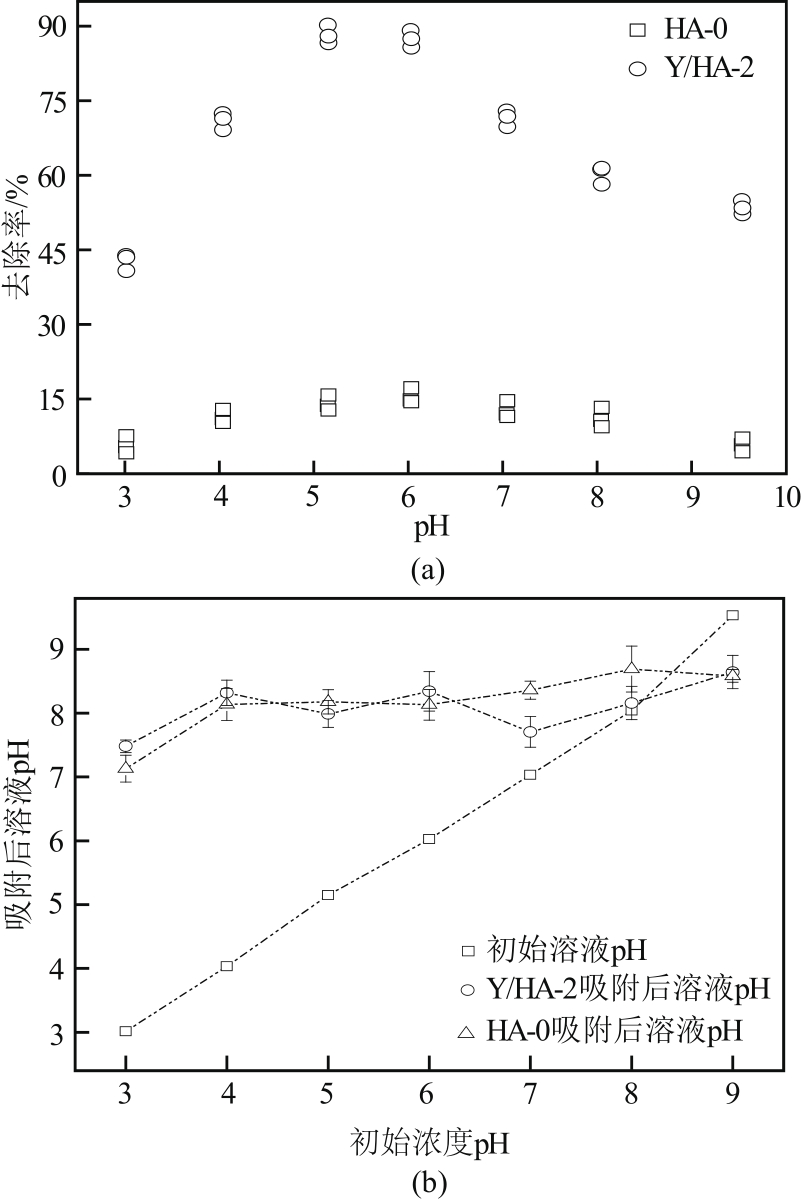
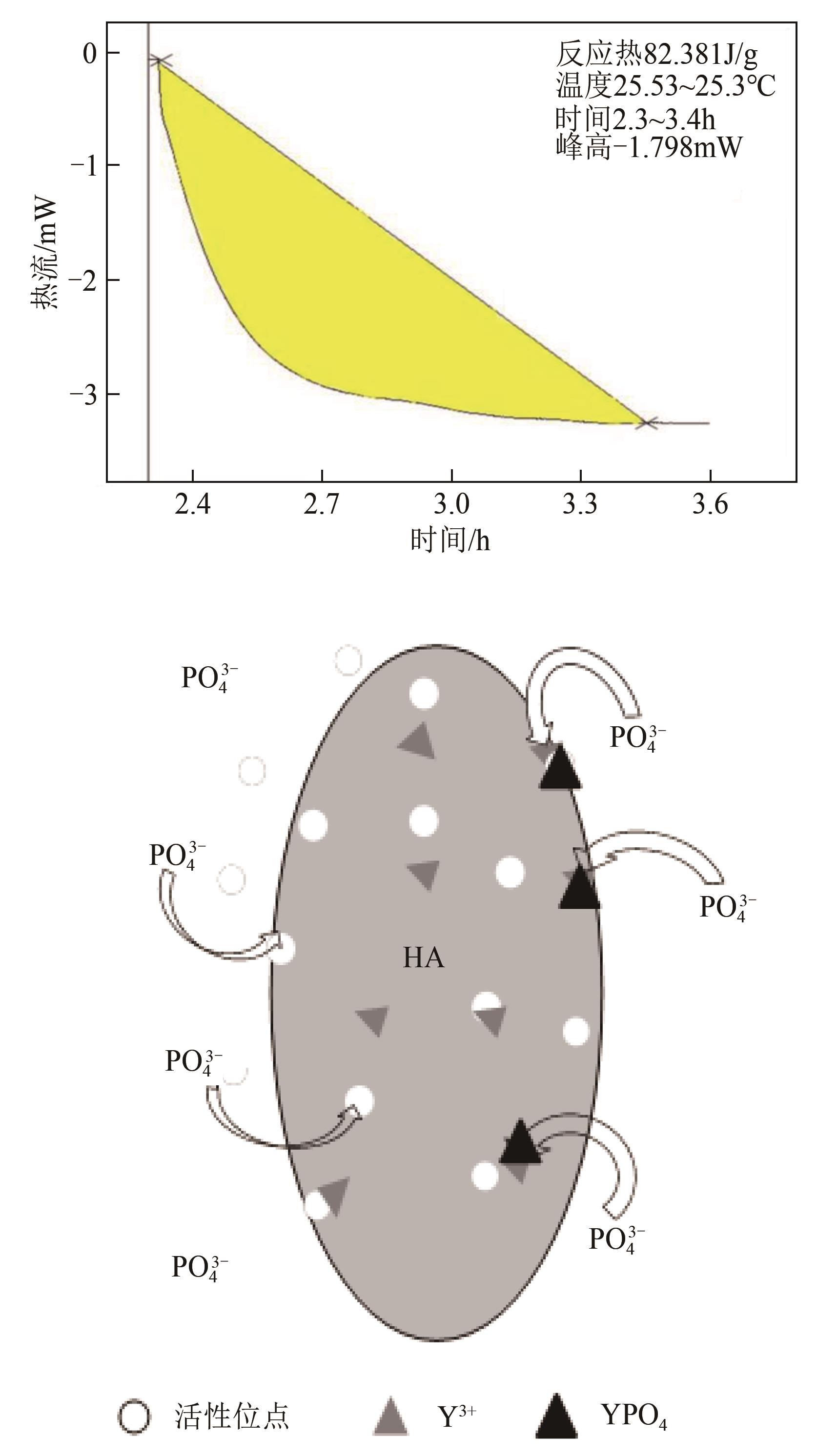
以氢氧化钙和磷酸二氢钠为原料制备羟基磷灰石,通过化学沉淀法制备钇/羟基磷灰石复合材料,利用SEM-EDS(扫描电子显微镜)、XRD(X射线衍射)、FTIR(傅里叶变换红外光谱)等表征其晶体结构与形貌,并采用静态吸附实验考察吸附剂的负载比例、pH、吸附时间和初始浓度对磷酸盐的吸附性能的影响。结果显示:羟基磷灰石为分散均匀的稻米状颗粒,XRD图谱显示为典型的羟基磷灰石,钇/羟基磷灰石颗粒发生团聚且粒径变大,特征峰峰强减弱,但未出现新的强特征峰。吸附研究表明:钙与钇的摩尔比为2∶1时吸附效率最高,复合材料的吸附容量随初始浓度的增加而增大,最大吸附量为116.38mg/g,吸附过程符合准二级动力学模型、双室一级动力学模型和Freundlich等温吸附模型,吸附过程由化学吸附主导,随pH在3~9的范围增加,复合材料的吸附效率先增后减,在pH为5~6时达到最大值。
中图分类号:
引用本文
黄敏, 王彬, 周明罗, 谌书, 张瀚文, 杨远坤, 傅开彬, 梁宏. 钇/羟基磷灰石的制备及对含磷废水的净化[J]. 化工进展, 2022, 41(5): 2778-2787.
HUANG Min, WANG Bin, ZHOU Mingluo, CHEN Shu, ZHANG Hanwen, YANG Yuankun, FU Kaibin, LIANG Hong. Preparation of yttrium/hydroxyapatite and purification of wastewater containing phosphorus[J]. Chemical Industry and Engineering Progress, 2022, 41(5): 2778-2787.
| 元素 | 质量分数/% | 原子分数/% | 氧化物 | 氧化物质量分数/% |
|---|---|---|---|---|
| O | 32.45 | 60.20 | CaO | 36.56 |
| Ca | 26.13 | 19.35 | Y2O3 | 39.13 |
| Y | 30.81 | 10.29 | P2O5 | 24.30 |
| P | 10.61 | 10.16 |
表1 Y/Ca10(PO4)6(OH)2表面主要元素含量
| 元素 | 质量分数/% | 原子分数/% | 氧化物 | 氧化物质量分数/% |
|---|---|---|---|---|
| O | 32.45 | 60.20 | CaO | 36.56 |
| Ca | 26.13 | 19.35 | Y2O3 | 39.13 |
| Y | 30.81 | 10.29 | P2O5 | 24.30 |
| P | 10.61 | 10.16 |
| 材料 | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| qm | KL | R2 | KF | 1/n | R2 | ||
| HA-0 | 20.07 | 0.0071 | 0.869 | 1.334 | 0.395 | 0.741 | |
| 17.68 | 0.0084 | 0.853 | 1.468 | 0.364 | 0.688 | ||
| 19.71 | 0.0078 | 0.915 | 1.449 | 0.383 | 0.780 | ||
| Y/HA-2 | 99.40 | 0.427 | 0.854 | 43.14 | 0.149 | 0.963 | |
| 99.92 | 0.438 | 0.847 | 43.03 | 0.151 | 0.965 | ||
| 99.69 | 0.452 | 0.848 | 43.19 | 0.151 | 0.962 | ||
表2 材料的吸附等温线拟合参数
| 材料 | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| qm | KL | R2 | KF | 1/n | R2 | ||
| HA-0 | 20.07 | 0.0071 | 0.869 | 1.334 | 0.395 | 0.741 | |
| 17.68 | 0.0084 | 0.853 | 1.468 | 0.364 | 0.688 | ||
| 19.71 | 0.0078 | 0.915 | 1.449 | 0.383 | 0.780 | ||
| Y/HA-2 | 99.40 | 0.427 | 0.854 | 43.14 | 0.149 | 0.963 | |
| 99.92 | 0.438 | 0.847 | 43.03 | 0.151 | 0.965 | ||
| 99.69 | 0.452 | 0.848 | 43.19 | 0.151 | 0.962 | ||
| 材料 | qe(exp) | 准一级吸附动力学 | 准二级吸附动力学 | 双室一级动力学 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qe(cal) | k1a | R2 | qe(cal) | k2a | R2 | qe(cal) | R2 | |||||
| HA-0 | 12.27 | 12.28 | 6.84 | 0.989 | 12.68 | 0.972 | 0.989 | 12.25 | 0.992 | |||
| 12.42 | 12.48 | 6.89 | 0.980 | 12.88 | 0.975 | 0.876 | 12.42 | 0.989 | ||||
| 11.84 | 12.16 | 7.47 | 0.953 | 12.56 | 1.084 | 0.871 | 12.08 | 0.966 | ||||
| Y/HA-2 | 48.35 | 44.82 | 7.16 | 0.793 | 46.55 | 0.272 | 0.954 | 47.40 | 0.977 | |||
| 48.35 | 44.83 | 7.21 | 0.788 | 46.56 | 0.275 | 0.952 | 47.37 | 0.975 | ||||
| 48.34 | 44.84 | 7.10 | 0.801 | 46.58 | 0.269 | 0.956 | 47.40 | 0.966 | ||||
表3 材料的吸附动力学模型参数
| 材料 | qe(exp) | 准一级吸附动力学 | 准二级吸附动力学 | 双室一级动力学 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qe(cal) | k1a | R2 | qe(cal) | k2a | R2 | qe(cal) | R2 | |||||
| HA-0 | 12.27 | 12.28 | 6.84 | 0.989 | 12.68 | 0.972 | 0.989 | 12.25 | 0.992 | |||
| 12.42 | 12.48 | 6.89 | 0.980 | 12.88 | 0.975 | 0.876 | 12.42 | 0.989 | ||||
| 11.84 | 12.16 | 7.47 | 0.953 | 12.56 | 1.084 | 0.871 | 12.08 | 0.966 | ||||
| Y/HA-2 | 48.35 | 44.82 | 7.16 | 0.793 | 46.55 | 0.272 | 0.954 | 47.40 | 0.977 | |||
| 48.35 | 44.83 | 7.21 | 0.788 | 46.56 | 0.275 | 0.952 | 47.37 | 0.975 | ||||
| 48.34 | 44.84 | 7.10 | 0.801 | 46.58 | 0.269 | 0.956 | 47.40 | 0.966 | ||||
| 材料 | k1 | k2 | f1 | f2 | k1/k2 |
|---|---|---|---|---|---|
| HA-0 | 12.061 | 9.643 | 0.625 | 0.375 | 1.251 |
| 12.140 | 10.827 | 0.632 | 0.368 | 1.121 | |
| 12.037 | 10.751 | 0.631 | 0.369 | 1.199 | |
| Y/HA-2 | 15.006 | 0.531 | 0.734 | 0.266 | 27.944 |
| 15.508 | 0.562 | 0.731 | 0.269 | 27.594 | |
| 14.661 | 0.529 | 0.737 | 0.263 | 27.714 |
表4 双室一级动力学模型拟合结果
| 材料 | k1 | k2 | f1 | f2 | k1/k2 |
|---|---|---|---|---|---|
| HA-0 | 12.061 | 9.643 | 0.625 | 0.375 | 1.251 |
| 12.140 | 10.827 | 0.632 | 0.368 | 1.121 | |
| 12.037 | 10.751 | 0.631 | 0.369 | 1.199 | |
| Y/HA-2 | 15.006 | 0.531 | 0.734 | 0.266 | 27.944 |
| 15.508 | 0.562 | 0.731 | 0.269 | 27.594 | |
| 14.661 | 0.529 | 0.737 | 0.263 | 27.714 |
| 1 | 秦延文, 马迎群, 温泉, 等. 沱江流域总磷污染负荷、成因及控制对策研究[J]. 环境科学与管理, 2020, 45(2): 20-25. |
| QIN Yanwen, MA Yingqun, WEN Quan, et al. Pollution load, causes and control strategy of total phosphorus pollution in Tuojiang River Basin[J]. Environmental Science and Management, 2020, 45(2): 20-25. | |
| 2 | 秦延文, 马迎群. 沱江上游(德阳段)磷污染特征及影响因素分析[J]. 磷肥与复肥, 2020, 35(8): 15-18. |
| QIN Yanwen, MA Yingqun. Characteristics and effecting factors of phosphorus pollution in upstream of Tuojiang River(Deyang section)[J]. Phosphate & Compound Fertilizer, 2020, 35(8): 15-18. | |
| 3 | 赵伟华, 李健伟, 王梅香, 等. 前置A2NSBR系统硝化和反硝化除磷的特性[J]. 中国环境科学, 2019, 39(11): 4660-4665. |
| ZHAO Weihua, LI Jianwei, WANG Meixiang, et al. Nitrification and denitrifying phosphorus removal performance in the pre-A2 NSBR system[J]. China Environmental Science, 2019, 39(11): 4660-4665. | |
| 4 | 赵伟华, 王梅香, 李健伟, 等. A2O工艺和A2O+BCO工艺的脱氮除磷性能比较[J]. 中国环境科学, 2019, 39(3): 994-999. |
| ZHAO Weihua, WANG Meixiang, LI Jianwei, et al. Nitrogen and phosphorus removal performance comparison between A2O and A2O+BCO system[J]. China Environmental Science, 2019, 39(3): 994-999. | |
| 5 | LI Shiyang, HUANG Xiangfeng, LIU Jia, et al. PVA/PEI crosslinked electrospun nanofibers with embedded La(OH)3 nanorod for selective adsorption of high flux low concentration phosphorus[J]. Journal of Hazardous Materials, 2020, 384: 121457. |
| 6 | WANG Bing, MA Yuena, LEE Xinqing, et al. Environmental-friendly coal gangue-biochar composites reclaiming phosphate from water as a slow-release fertilizer[J]. Science of the Total Environment, 2021, 758: 143664. |
| 7 | YU Xiaolong, NAKAMURA Y, OTSUKA M, et al. Development of a novel phosphorus recovery system using incinerated sewage sludge ash (ISSA) and phosphorus-selective adsorbent[J]. Waste Management, 2021, 120: 41-49. |
| 8 | GULYÁS A, GENÇ S, CAN Z S, et al. Phosphate recovery from sewage sludge supernatants using magnetic nanoparticles[J]. Journal of Water Process Engineering, 2021, 40: 101843. |
| 9 | XU Rui, Tao LYU, ZHANG Meiyi, et al. Molecular-level investigations of effective biogenic phosphorus adsorption by a lanthanum/aluminum-hydroxide composite[J]. Science of the Total Environment, 2020, 725: 138424. |
| 10 | ZHAO Qian, LIU Chengfang, SONG Hongquan, et al. Mechanism of phosphate adsorption on superparamagnetic microparticles modified with transitional elements: Experimental observation and computational modelling[J]. Chemosphere, 2020, 258: 127327. |
| 11 | HERNÁNDEZ-COCOLETZI H, SALINAS R A, ÁGUILA-ALMANZA E, et al. Natural hydroxyapatite from fishbone waste for the rapid adsorption of heavy metals of aqueous effluent[J]. Environmental Technology & Innovation, 2020, 20: 101109. |
| 12 | ROUFF A A, MA Ning, KUSTKA A B. Adsorption of arsenic with struvite and hydroxylapatite in phosphate-bearing solutions[J]. Chemosphere, 2016, 146: 574-581. |
| 13 | KOLMAS J, KRUKOWSKI S, LASKUS A, et al. Synthetic hydroxyapatite in pharmaceutical applications[J]. Ceramics International, 2016, 42(2): 2472-2487. |
| 14 | KONG Y M, KIM S, KIM H E, et al. Reinforcement of hydroxyapatite bioceramic by addition of ZrO2 coated with Al2O3 [J]. Journal of the American Ceramic Society, 1999, 82(11): 2963-2968. |
| 15 | WHITE A A, BEST S M, KINLOCH I A. Hydroxyapatite-carbon nanotube composites for biomedical applications: a review[J]. International Journal of Applied Ceramic Technology, 2007, 4(1): 1-13. |
| 16 | BARABÁS R, DEEMTER D, KATONA G, et al. Comparative study on physicochemical and mechanical characterization of newnanocarbon-based hydroxyapatite nanocomposites[J]. Turkish Journal of Chemistry, 2019, 43(3): 809-824. |
| 17 | LAHIRI D, SINGH V, KESHRI A K, et al. Carbon nanotube toughened hydroxyapatite by spark plasma sintering: Microstructural evolution and multiscale tribological properties[J]. Carbon, 2010, 48(11): 3103-3120. |
| 18 | BARABÁS R, FARKAS N I, NAGY C L, et al. Adsorption and desorption behavior of natural and synthetic active compounds on hydroxyapatite-based nanocomposites[J]. Ceramics International, 2021, 47(6): 8584-8592. |
| 19 | NÚÑEZ D, CÁCERES R, IDE W, et al. An ecofriendly nanocomposite of bacterial cellulose and hydroxyapatite efficiently removes lead from water[J]. International Journal of Biological Macromolecules, 2020, 165: 2711-2720. |
| 20 | 张连科, 王洋, 王维大, 等. 生物炭负载纳米羟基磷灰石复合材料的制备及对铅离子的吸附特性[J]. 化工进展, 2018, 37(9): 3492-3501. |
| ZHANG Lianke, WANG Yang, WANG Weida, et al. The preparation of biochar-supported nano-hydroxyapatite and its adsorption of Pb2+ [J]. Chemical Industry and Engineering Progress, 2018, 37(9): 3492-3501. | |
| 21 | 王婷庭, 刘敏, 崔桂榕, 等. 五种改性纳米纤维素吸附剂的制备及除磷性能比较[J]. 化工进展, 2017, 36(11): 4279-4285. |
| WANG Tingting, LIU Min, CUI Guirong, et al. Preparation of several modified cellulose nanofiber hybrid adsorbents and performance comparison of phosphate removals[J]. Chemical Industry and Engineering Progress, 2017, 36(11): 4279-4285. | |
| 22 | 罗元, 谢坤, 冯弋洋, 等. 镧改性核桃壳生物炭制备及吸附水体磷酸盐性能[J]. 化工进展, 2021, 40(2): 1121-1129. |
| LUO Yuan, XIE Kun, FENG Yiyang, et al. Preparation of lanthanum modified walnut shell biochar and adsorption of phosphate from aqueous solutions[J]. Chemical Industry and Engineering Progress, 2021, 40(2): 1121-1129. | |
| 23 | 孟庆梅, 孟迪, 张艳丽, 等. 榴莲壳生物炭对磺胺嘧啶的吸附性能[J]. 化工进展, 2020, 39(11): 4651-4659. |
| MENG Qingmei, MENG Di, ZHANG Yanli, et al. Adsorption characteristics of biochar prepared by durian shell on sulfadiazine[J]. Chemical Industry and Engineering Progress, 2020, 39(11): 4651-4659. | |
| 24 | 何豪, 朱宗强, 刘杰, 等. 镁-钙羟基磷灰石吸附剂对水中Pb2+的去除[J]. 环境科学, 2019, 40(9): 4081-4090. |
| HE Hao, ZHU Zongqiang, LIU Jie, et al. Removal of Pb2+ from aqueous solution by magnesium-calcium hydroxyapatite adsorbent[J]. Environmental Science, 2019, 40(9): 4081-4090. | |
| 25 | 王瑜, 孙鹏跃, 种昀楠. 碳羟基磷灰石对模拟刚果红染料废水吸附性能的研究[J]. 化学研究与应用, 2020, 32(4): 599-608. |
| WANG Yu, SUN Pengyue, CHONG Yunnan. Research of the adsorption property of carbon hydroxyapatite to simulated Congo red dye wastewater[J]. Chemical Research and Application, 2020, 32(4): 599-608. | |
| 26 | 傅瑜, 钟旭源. 羟基磷灰石/石墨烯复合物对水中亚甲基蓝的吸附[J]. 广州化工, 2020, 48(6): 84-87. |
| FU Yu, ZHONG Xuyuan. Adsorption of methylene blue from aqueous solution onto hydroxyapatite/graphene composites[J]. Guangzhou Chemical Industry, 2020, 48(6): 84-87. | |
| 27 | 邹雪艳, 赵彦保, 张治军. 纳米羟基磷灰石纳米管的一种简便制备方法及其对溶液中重金属离子的吸附[J]. 无机化学学报, 2020, 36(4): 747-754. |
| ZOU Xueyan, ZHAO Yanbao, ZHANG Zhijun. A facile method to prepare hydroxyapatite nanotubes and immobilization activities against heavy metal ions in solutions[J]. Chinese Journal of Inorganic Chemistry, 2020, 36(4): 747-754. | |
| 28 | 张连科, 王洋, 王维大, 等. 磁性羟基磷灰石/生物炭复合材料的制备及对Pb2+的吸附性能[J]. 环境科学学报, 2018, 38(11): 4360-4370. |
| ZHANG Lianke, WANG Yang, WANG Weida, et al. Preparation of magnetic hydroxyapatite/biochar composite and its adsorption behavior of Pb2+ and recycling performance[J]. Acta Scientiae Circumstantiae, 2018, 38(11): 4360-4370. | |
| 29 | 刘纯玮, 冯莉, 刘忆玲, 等. 制备条件对掺镁羟基磷灰石结构及除氟性能的影响[J]. 硅酸盐学报, 2019, 47(7): 933-941. |
| LIU Chunwei, FENG Li, LIU Yiling, et al. Influence of preparation condition on structure and defluoridation performance of Mg-doped hydroxyapatite[J]. Journal of the Chinese Ceramic Society, 2019, 47(7): 933-941. | |
| 30 | ZENG Rongying, TANG Wenqing, DING Chunxia, et al. Preparation of anionic-cationic co-substituted hydroxyapatite for heavy metal removal: performance and mechanisms[J]. Journal of Solid State Chemistry, 2019, 280: 120960. |
| 31 | HARPER R A, SHELTON R M, JAMES J D, et al. Acid-induced demineralisation of human enamel as a function of time and pH observed using X-ray and polarised light imaging[J]. Acta Biomaterialia, 2021, 120: 240-248. |
| 32 | WANG Shengdan, KONG Lingjun, LONG Jianyou, et al. Adsorption of phosphorus by calcium-flour biochar: Isotherm, kinetic and transformation studies[J]. Chemosphere, 2018, 195: 666-672. |
| 33 | KIM J A, VIJAYARAGHAVAN K, REDDY D H K, et al. A phosphorus-enriched biochar fertilizer from bio-fermentation waste: a potential alternative source for phosphorus fertilizers[J]. Journal of Cleaner Production, 2018, 196: 163-171. |
| 34 | 刘凌青, 肖细元, 郭朝晖, 等. 锌冶炼地块剖面土壤对镉、铅的吸附特征及机制[J]. 环境科学, 2021, 42(8): 4015-4023. |
| LIU Lingqing, XIAO Xiyuan, GUO Zhaohui, et al. Adsorption characteristics and mechanism of Cd and Pb in tiered soil profiles from a zinc smelting site[J]. Environmental Science, 2021, 42(8): 4015-4023. | |
| 35 | SONG Zhuoyue, HU Youdong, QI Longkai, et al. An effective and recyclable deproteinization method for polysaccharide from oyster by magnetic chitosan microspheres[J]. Carbohydrate Polymers, 2018, 195: 558-565. |
| 36 | 杨放, 毛志强, 李阳, 等. 纳米羟基磷灰石修复硫铁矿区镉锌复合污染土壤的效果研究[J]. 长江流域资源与环境, 2020, 29(5): 1216-1223. |
| YANG Fang, MAO Zhiqiang, LI Yang, et al. Effect assessment of nano-hydroxyapatite for remediation of Cd and Zn contaminated soil in pyrite areas[J]. Resources and Environment in the Yangtze Basin, 2020, 29(5): 1216-1223. | |
| 37 | 孔祥武, 姜恒, 宫红. 氧化钇对磷酸根的吸附及机理研究[J]. 应用化工, 2014, 43(12): 2202-2205, 2209. |
| KONG Xiangwu, JIANG Heng, GONG Hong. Adsorption and mechanism of yttrium oxide for phosphate[J]. Applied Chemical Industry, 2014, 43(12): 2202-2205, 2209. |
| [1] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [2] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [3] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [4] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [5] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [6] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [7] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [8] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| [9] | 张振, 李丹, 陈辰, 吴菁岚, 应汉杰, 乔浩. 吸附树脂对唾液酸的分离纯化[J]. 化工进展, 2023, 42(8): 4153-4158. |
| [10] | 姜晶, 陈霄宇, 张瑞妍, 盛光遥. 载锰生物炭制备及其在环境修复中应用研究进展[J]. 化工进展, 2023, 42(8): 4385-4397. |
| [11] | 于静文, 宋璐娜, 刘砚超, 吕瑞东, 武蒙蒙, 冯宇, 李忠, 米杰. 一种吲哚基超交联聚合物In-HCP对水中碘的吸附作用[J]. 化工进展, 2023, 42(7): 3674-3683. |
| [12] | 李艳玲, 卓振, 池亮, 陈曦, 孙堂磊, 刘鹏, 雷廷宙. 氮掺杂生物炭的制备与应用研究进展[J]. 化工进展, 2023, 42(7): 3720-3735. |
| [13] | 白亚迪, 邓帅, 赵睿恺, 赵力, 杨英霞. 变温吸附碳捕集机组标准化测试方案探讨及性能实验[J]. 化工进展, 2023, 42(7): 3834-3846. |
| [14] | 张雪伟, 黄亚继, 许月阳, 程好强, 朱志成, 李金壘, 丁雪宇, 王圣, 张荣初. 碱性吸附剂对燃煤烟气中SO3的吸附特性[J]. 化工进展, 2023, 42(7): 3855-3864. |
| [15] | 陆洋, 周劲松, 周启昕, 王瑭, 刘壮, 李博昊, 周灵涛. CeO2/TiO2吸附剂煤气脱汞产物的浸出规律[J]. 化工进展, 2023, 42(7): 3875-3883. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||