化工进展 ›› 2021, Vol. 40 ›› Issue (5): 2603-2612.DOI: 10.16085/j.issn.1000-6613.2020-0573
ZrO2-Al2O3复合氧化物催化反应精馏合成碳酸二甲酯
- 河北工业大学化工学院,天津 300130
-
收稿日期:2020-04-13出版日期:2021-05-06发布日期:2021-05-24 -
通讯作者:刘继东 -
作者简介:陶宁(1994—),女,硕士研究生,研究方向为绿色催化过程与工艺。E-mail:tn15222273160@126.com 。
ZrO2-Al2O3 composite oxide for synthesis of dimethyl carbonate in catalytic reactive distillation
TAO Ning( ), XU Yajin, FENG Yuchen, LYU Jianhua, LIU Jidong(
), XU Yajin, FENG Yuchen, LYU Jianhua, LIU Jidong( )
)
- College of Chemical Engineering, Hebei University of Technology, Tianjin 300130, China
-
Received:2020-04-13Online:2021-05-06Published:2021-05-24 -
Contact:LIU Jidong
摘要:
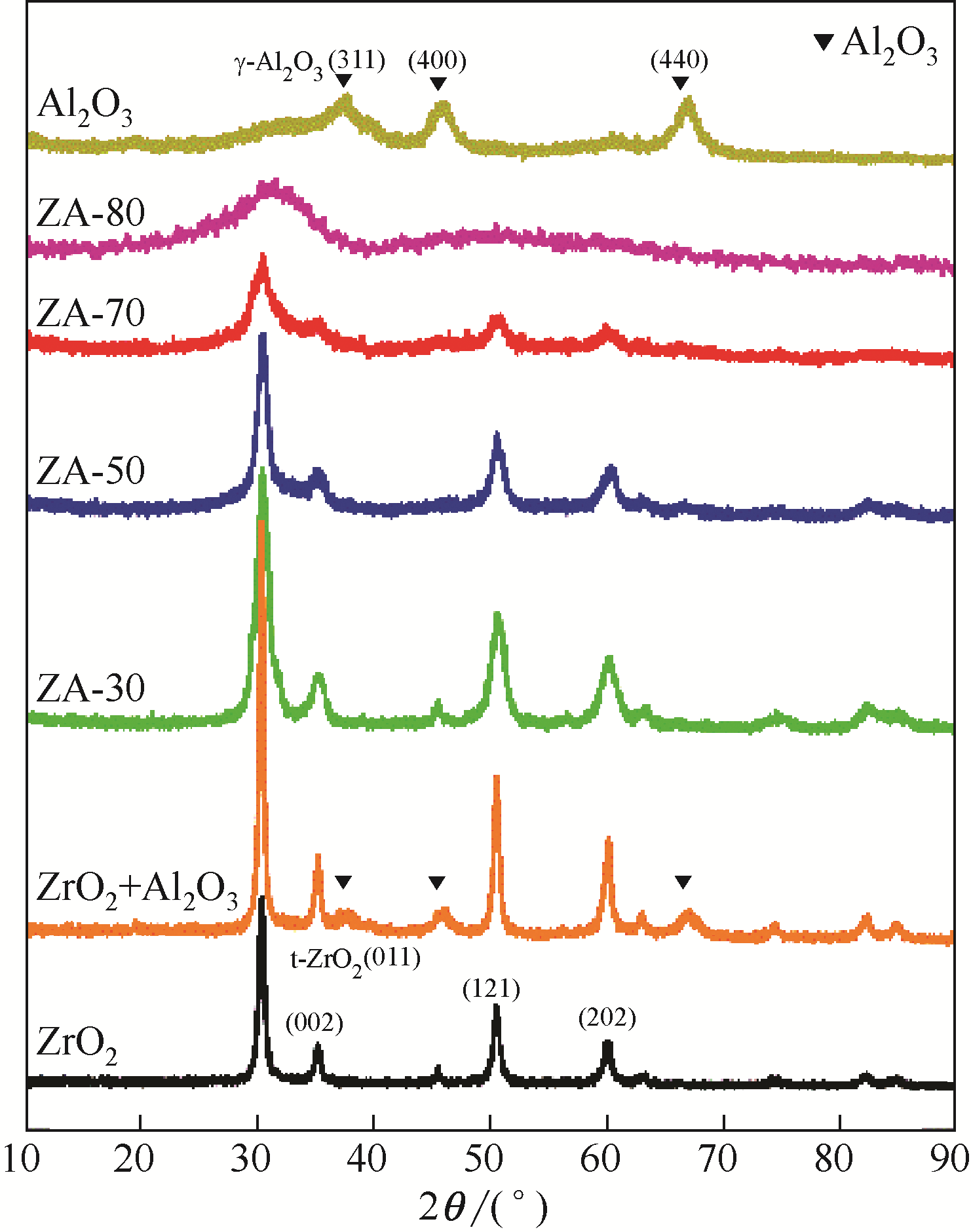
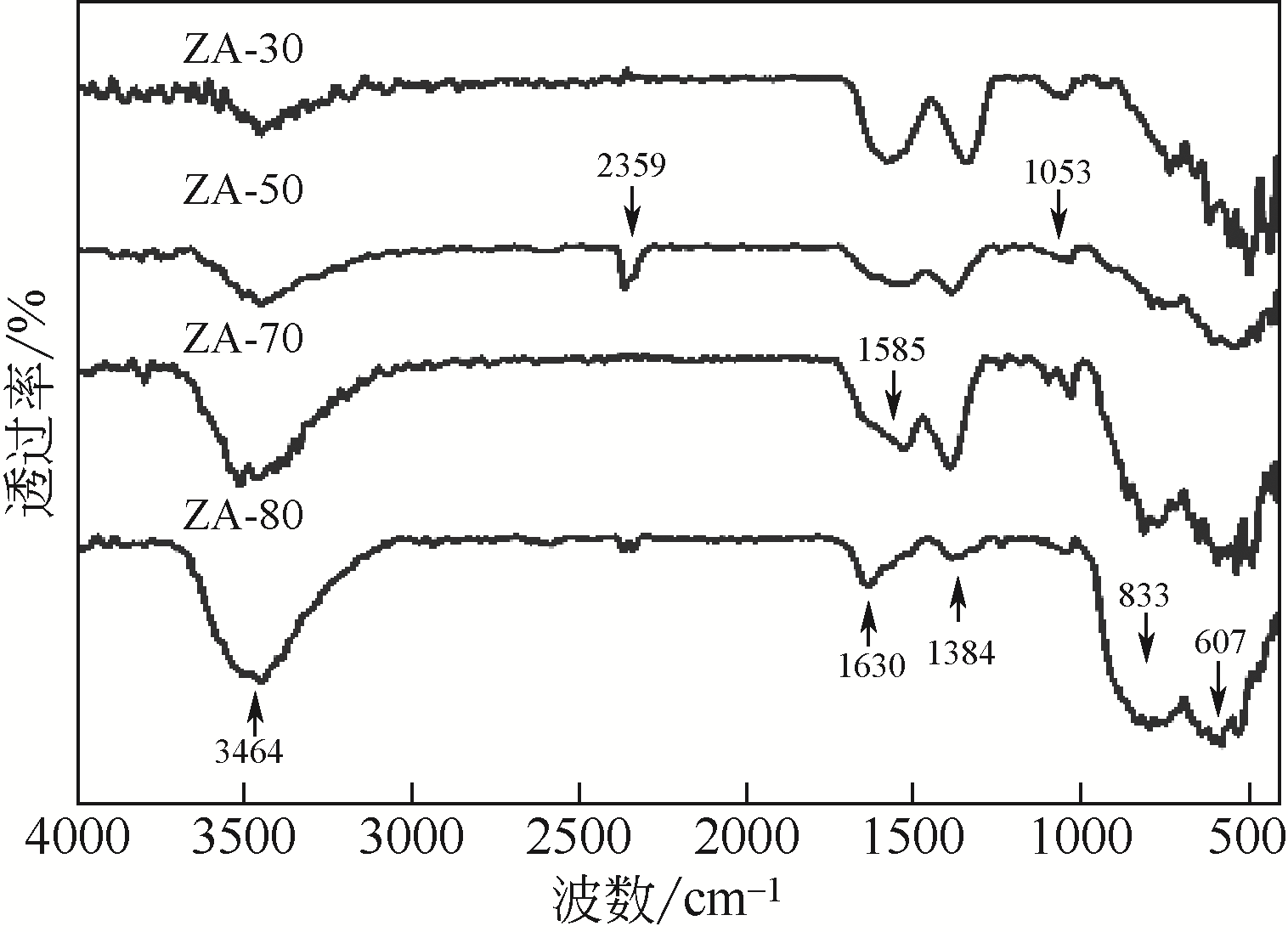
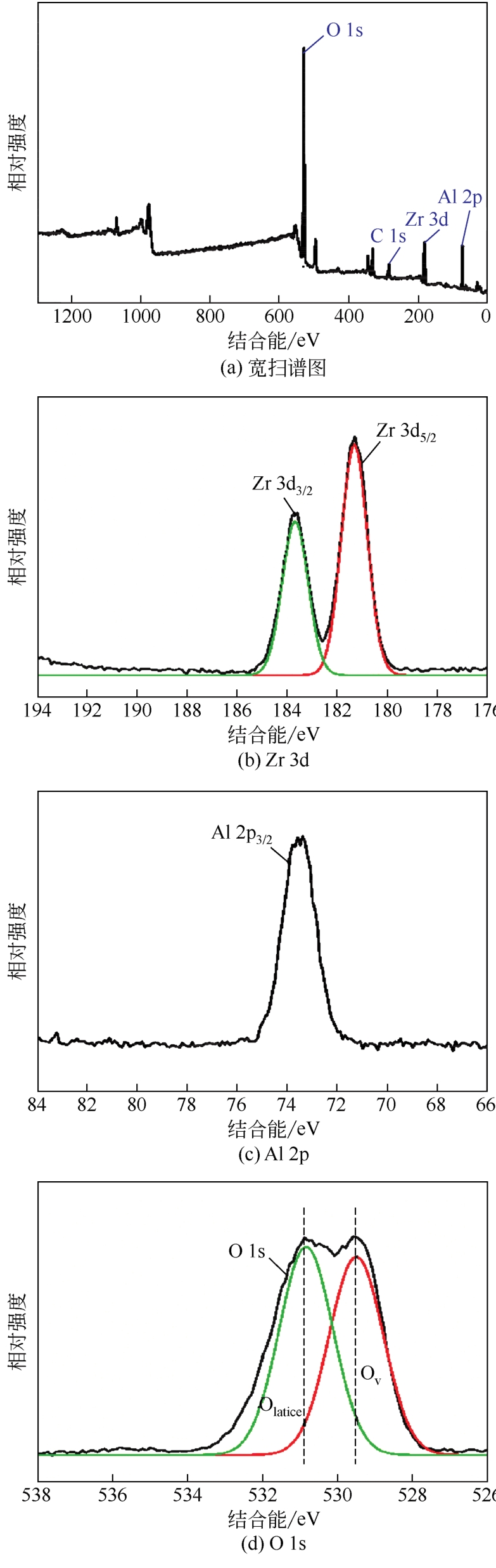
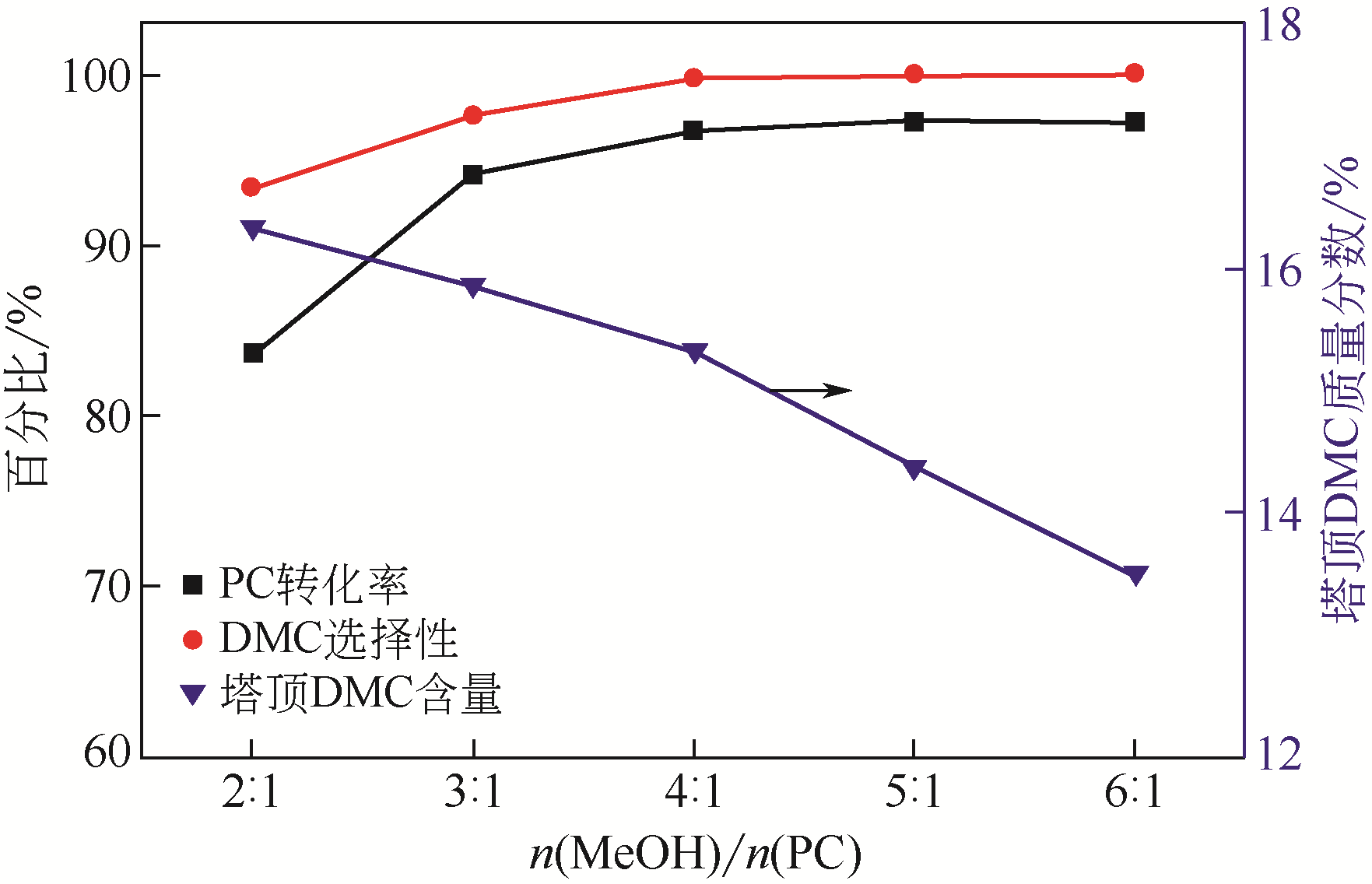
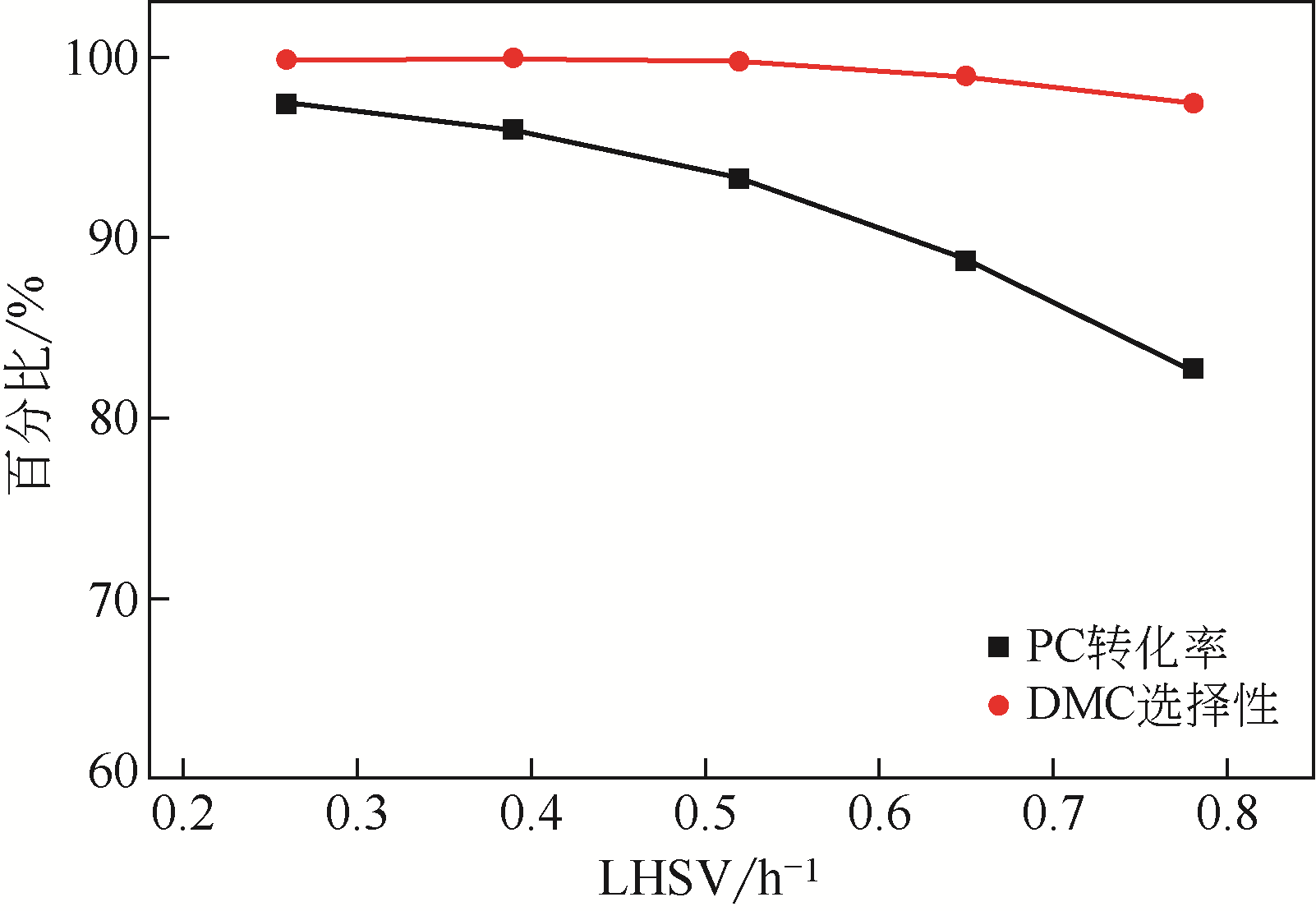
采用共沉淀法制备了一系列不同Al2O3含量的ZrO2-Al2O3复合氧化物,并在催化精馏实验装置中考察了该催化剂在碳酸丙烯酯(PC)与甲醇酯交换制备碳酸二甲酯(DMC)过程中的催化性能。通过X射线衍射(XRD)、红外光谱(FTIR)、X射线光电子能谱(XPS)、CO2程序升温脱附(CO2-TPD)和NH3程序升温脱附(NH3-TPD)等手段对所制备的催化剂进行了表征。结果表明,催化剂表面存在的酸碱性位点是制约PC与甲醇酯交换性能的重要因素。复合氧化物中Al2O3含量可以有效调控催化剂的结构特征和表面的酸碱性质,不同于ZrO2或Al2O3单金属催化剂,复合氧化物ZrO2-Al2O3在合成过程中形成了稳定的固溶体结构,导致催化剂表面弱酸量增加,并产生了强碱位点。数据分析表明,催化剂表面的强碱和弱酸含量高时,其催化活性高,说明该反应具有酸碱协同催化作用。当Zr/Al比为1时,弱酸和强碱量均达到最大值,PC的转化率和DMC选择性可达到98.14%和99.96%。催化剂在经过12次循环使用后依旧保持较高的活性,具有良好的结构稳定性。
中图分类号:
引用本文
陶宁, 徐亚津, 冯宇辰, 吕建华, 刘继东. ZrO2-Al2O3复合氧化物催化反应精馏合成碳酸二甲酯[J]. 化工进展, 2021, 40(5): 2603-2612.
TAO Ning, XU Yajin, FENG Yuchen, LYU Jianhua, LIU Jidong. ZrO2-Al2O3 composite oxide for synthesis of dimethyl carbonate in catalytic reactive distillation[J]. Chemical Industry and Engineering Progress, 2021, 40(5): 2603-2612.
| 样品 | 结合能/eV | 表面原子比① Al/(Zr+Al) | 体相中原子比② Al/(Zr+Al) | ||
|---|---|---|---|---|---|
| Zr 3d5/2 | Al 2p3/2 | O 1s | |||
| ZrO2 | 182.8 | — | 530.3 | — | — |
| ZA-30 | 182.5 | 73.8 | 530.5 | 0.27 | 0.26 |
| ZA-50 | 182.6 | 73.7 | 530.7 | 0.49 | 0.51 |
| ZA-70 | 182.4 | 73.7 | 531.3 | 0.68 | 0.69 |
| ZA-80 | 182.2 | 73.5 | 531.5 | 0.87 | 0.82 |
| Al2O3 | — | 73.3 | 531.7 | — | — |
表1 ZrO2-Al2O3系列催化剂的合成参数
| 样品 | 结合能/eV | 表面原子比① Al/(Zr+Al) | 体相中原子比② Al/(Zr+Al) | ||
|---|---|---|---|---|---|
| Zr 3d5/2 | Al 2p3/2 | O 1s | |||
| ZrO2 | 182.8 | — | 530.3 | — | — |
| ZA-30 | 182.5 | 73.8 | 530.5 | 0.27 | 0.26 |
| ZA-50 | 182.6 | 73.7 | 530.7 | 0.49 | 0.51 |
| ZA-70 | 182.4 | 73.7 | 531.3 | 0.68 | 0.69 |
| ZA-80 | 182.2 | 73.5 | 531.5 | 0.87 | 0.82 |
| Al2O3 | — | 73.3 | 531.7 | — | — |
| 样品 | 比表面积/m2·g-1 | 孔容/cm3·g-1 | 平均孔径/nm |
|---|---|---|---|
| ZrO2 | 8.4 | 0.02 | 1.19 |
| ZA-30 | 18.5 | 0.07 | 1.19 |
| ZA-50 | 80.7 | 0.18 | 1.15 |
| ZA-70 | 43.9 | 0.16 | 1.18 |
| ZA-80 | 20.6 | 0.09 | 1.19 |
| Al2O3 | 279.8 | 0.45 | 1.16 |
表2 ZA-x、ZrO2和Al2O3的结构参数
| 样品 | 比表面积/m2·g-1 | 孔容/cm3·g-1 | 平均孔径/nm |
|---|---|---|---|
| ZrO2 | 8.4 | 0.02 | 1.19 |
| ZA-30 | 18.5 | 0.07 | 1.19 |
| ZA-50 | 80.7 | 0.18 | 1.15 |
| ZA-70 | 43.9 | 0.16 | 1.18 |
| ZA-80 | 20.6 | 0.09 | 1.19 |
| Al2O3 | 279.8 | 0.45 | 1.16 |
| 样品 | ATotal/mmol·g-1 | AW/mmol·g-1 | AM/mmol·g-1 | AS/mmol·g-1 | BTotal/mmol·g-1 | BW/mmol·g-1 | BM/mmol·g-1 | BS/mmol·g-1 |
|---|---|---|---|---|---|---|---|---|
| ZrO2 | 2.92 | 1.98 | 0.64 | 0.30 | 1.34 | 0.88 | 0.35 | 0.11 |
| ZA-30 | 3.85 | 2.22 | 1.14 | 0.49 | 3.78 | 1.05 | 1.26 | 1.47 |
| ZA-50 | 3.95 | 2.24 | 1.17 | 0.54 | 4.53 | 1.12 | 1.54 | 1.87 |
| ZA-70 | 3.97 | 2.20 | 1.14 | 0.63 | 3.96 | 1.26 | 1.42 | 1.28 |
| ZA-80 | 4.03 | 2.16 | 1.15 | 0.72 | 3.36 | 1.55 | 0.77 | 1.04 |
| Al2O3 | 4.11 | 2.08 | 1.19 | 0.84 | 2.13 | 0.96 | 0.69 | 0.48 |
表3 ZrO2-Al2O3系列催化剂的酸-碱性质
| 样品 | ATotal/mmol·g-1 | AW/mmol·g-1 | AM/mmol·g-1 | AS/mmol·g-1 | BTotal/mmol·g-1 | BW/mmol·g-1 | BM/mmol·g-1 | BS/mmol·g-1 |
|---|---|---|---|---|---|---|---|---|
| ZrO2 | 2.92 | 1.98 | 0.64 | 0.30 | 1.34 | 0.88 | 0.35 | 0.11 |
| ZA-30 | 3.85 | 2.22 | 1.14 | 0.49 | 3.78 | 1.05 | 1.26 | 1.47 |
| ZA-50 | 3.95 | 2.24 | 1.17 | 0.54 | 4.53 | 1.12 | 1.54 | 1.87 |
| ZA-70 | 3.97 | 2.20 | 1.14 | 0.63 | 3.96 | 1.26 | 1.42 | 1.28 |
| ZA-80 | 4.03 | 2.16 | 1.15 | 0.72 | 3.36 | 1.55 | 0.77 | 1.04 |
| Al2O3 | 4.11 | 2.08 | 1.19 | 0.84 | 2.13 | 0.96 | 0.69 | 0.48 |
| 样品 | 比表面积 /m2·g-1 | 孔容 /cm3·g-1 | 平均孔径 /nm | 催化剂组分①/% | |
|---|---|---|---|---|---|
| Zr | Al | ||||
| 新鲜的ZA-50 | 80.77 | 0.18 | 1.15 | 50.12 | 49.88 |
| 使用过的ZA-50 | 67.89 | 0.17 | 1.17 | 50.28 | 49.72 |
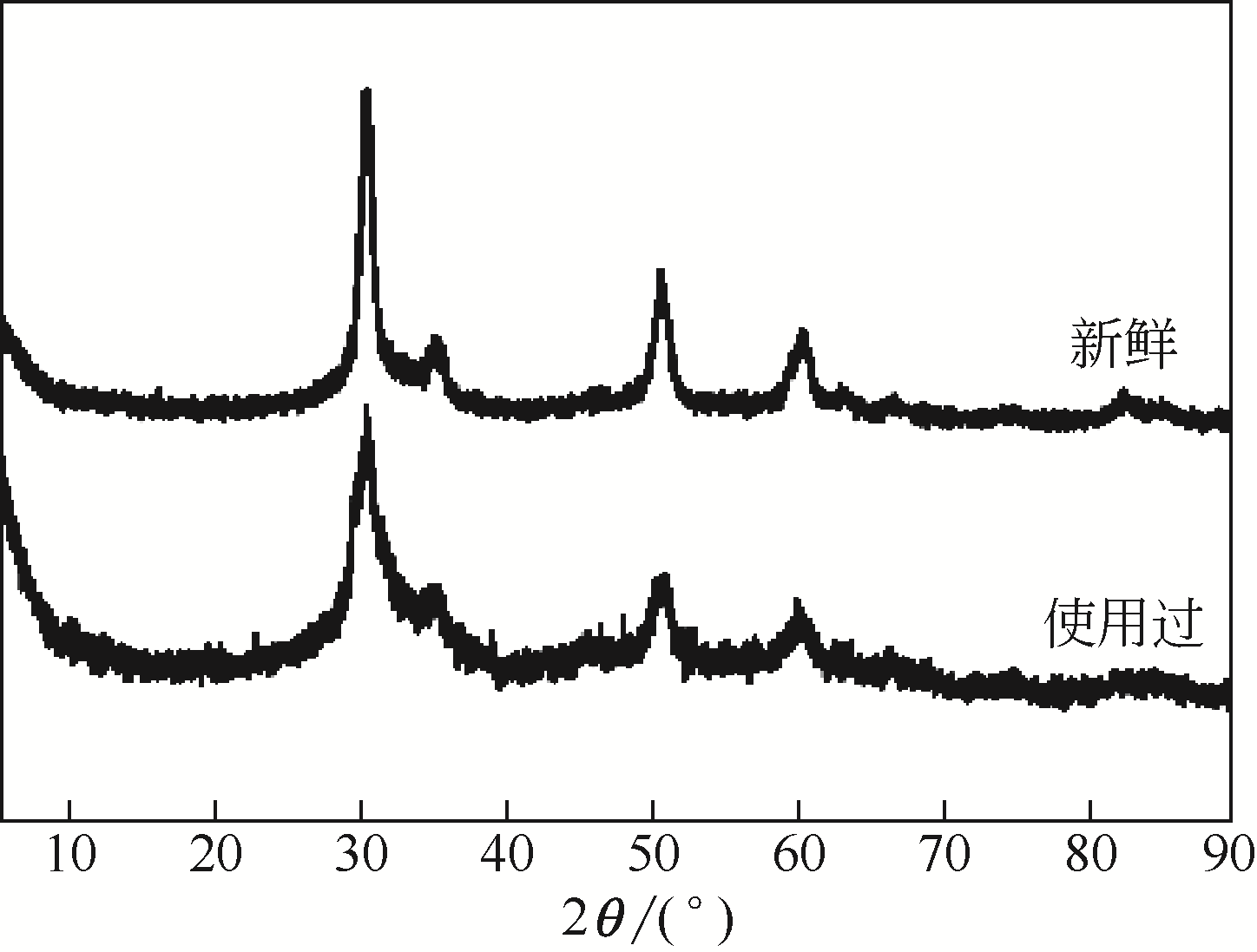
表4 新鲜的和使用过的ZA-50催化剂的主要结构参数和化学组成
| 样品 | 比表面积 /m2·g-1 | 孔容 /cm3·g-1 | 平均孔径 /nm | 催化剂组分①/% | |
|---|---|---|---|---|---|
| Zr | Al | ||||
| 新鲜的ZA-50 | 80.77 | 0.18 | 1.15 | 50.12 | 49.88 |
| 使用过的ZA-50 | 67.89 | 0.17 | 1.17 | 50.28 | 49.72 |
| 样品 | ATotal/mmol·g-1 | AW/mmol·g-1 | AM/mmol·g-1 | AS/mmol·g-1 | BTotal/mmol·g-1 | BW/mmol·g-1 | BM/mmol·g-1 | BS/mmol·g-1 |
|---|---|---|---|---|---|---|---|---|
| 新鲜的ZA-50 | 3.95 | 2.24 | 1.17 | 0.54 | 4.53 | 1.12 | 1.54 | 1.87 |
| 使用过的ZA-50 | 3.87 | 2.18 | 1.12 | 0.57 | 4.42 | 1.24 | 1.42 | 1.76 |
表5 新鲜的和使用过的ZA-50催化剂的酸-碱性质
| 样品 | ATotal/mmol·g-1 | AW/mmol·g-1 | AM/mmol·g-1 | AS/mmol·g-1 | BTotal/mmol·g-1 | BW/mmol·g-1 | BM/mmol·g-1 | BS/mmol·g-1 |
|---|---|---|---|---|---|---|---|---|
| 新鲜的ZA-50 | 3.95 | 2.24 | 1.17 | 0.54 | 4.53 | 1.12 | 1.54 | 1.87 |
| 使用过的ZA-50 | 3.87 | 2.18 | 1.12 | 0.57 | 4.42 | 1.24 | 1.42 | 1.76 |
| 1 | TAN H Z, WANG Z Q, XU Z N, et al. Review on the synthesis of dimethyl carbonate[J]. Catalysis Today, 2018, 316: 2-12. |
| 2 | 李波, 宋淑群, 汪志国. 碳酸二甲酯发展现状及前景[J]. 精细石油化工进展, 2011, 12(6): 38-41. |
| LI Bo, SONG Shuqun, WANG Zhiguo. Development and application prospect of dimethyl carbonate[J]. Advances in Fine Petrochemicals, 2011, 12(6): 38-41. | |
| 3 | PACHECO M A, MARSHALL C L. Review of dimethyl carbonate (DMC) manufacture and its characteristics as a fuel additive[J]. Energy & Fuels, 1997, 11(1): 2-29. |
| 4 | 杜治平, 黄丽明, 林志坤, 等. 甲醇氧化羰基化合成碳酸二甲酯反应机理研究进展[J]. 化工进展, 2012, 31(10): 2213-2220. |
| DU Z P, HUANG L M, LIN Z K, et.al. Mechanism for the synthesis of dimethyl carbonate from oxidative carbonylation of methanol[J]. Chemical Industry and Engineering Progress, 2012, 31(10): 2213-2220. | |
| 5 | 宋一兵, 罗爱国, 杜玉海, 等. 甲醇直接气相氧化羰基化合成碳酸二甲酯[J]. 化学进展, 2008, 20(S1): 221-226. |
| SONG Y B, LUO A G, DU Y H, et al. Synthesis of dimethyl carbonate by direct vapor-phase oxycarbonylation of methanol[J]. Progress in Chemistry, 2008, 20(S1): 221-226. | |
| 6 | 杨彩虹, 李文彬, 柳玉琴, 等. 甲醇和碳酸丙烯酯合成碳酸二甲酯的研究[J]. 燃料化学学报, 2002, 30(6): 551-554. |
| YANG C H, LI W B, LIU Y Q, et.al. Synthesis of dimethyl carbonate from methanol and propylene carbonate[J]. Journal of Fuel Chemistry and Technology, 2002, 30(6): 551-554. | |
| 7 | JEONG E S, KIM K H, PARK D W, et al. Synthesis of dimethyl carbonate and propylene glycol from transesterification of propylene carbonate and methanol using quaternary ammonium salt catalysts[J]. Reaction Kinetics and Catalysis Letters, 2005, 86(2): 241-248. |
| 8 | SEGOVIA-HERNÁNDEZ J G, HERNÁNDEZ S, BONILLA PETRICIOLET A. Reactive distillation: a review of optimal design using deterministic and stochastic techniques[J]. Chemical Engineering and Processing: Process Intensification, 2015, 97: 134-143. |
| 9 | 姜忠义, 王泳. 酯交换法合成碳酸二甲酯的催化精馏过程研究[J] .化学工程, 2001, 29(3): 29-32. |
| JIANG Z Y, WANG Y. Preparation of dimethyl carbonate through transesterification in a catalytic distillation column[J]. Chemical Engineering, 2001, 29(3): 29-32. | |
| 10 | HOLTBRUEGGE J, WIERSCJEM M, LUTZE P. Synthesis of dimethyl carbonate and propylene glycol in a membrane-assisted reactive distillation process pilot-scale experiments, modeling and process analysis[J]. Chemical Engineering and Processing: Process Intersification, 2014, 84(4): 54-70. |
| 11 | HOLTBRUEGGE J, LEIMBRINK M, LUTZE P, et al. Synthesis of dimethyl carbonate and propylene glycol by transesterification of propylene carbonate with methanol: catalyst screening, chemical equilibrium and reaction kinetics[J]. Chemical Engineering Science, 2013, 104(50): 347-360. |
| 12 | WEI T, WANG M, WEI W. Synthesis of dimethyl carbonate by transesterification over CaO/carbon solid base catalysts[J]. Chinese Academic of Sciences, 2004, 153: 41-46. |
| 13 | SONG Z, JIN X, HU Y, et al. Intriguing catalyst (CaO) pretreatment effects and mechanistic insights during propylene carbonate transesterification with methanol[J]. ACS Sustainable Chem. Eng., 2017, 5: 4718-4729. |
| 14 | KUMAR P, SRIVASTAVA V C, MISHRA I M. Synthesis and characterization of Ce-La oxides for the formation of dimethyl carbonate by transesterification of propylene carbonate[J]. Catalysis Communications, 2015, 60: 27-31. |
| 15 | KUMAR, P, SRIVASTAVA V C, MISHRA I M. Dimethyl carbonate synthesis by transesterification of propylene carbonate with methanol: comparative assessment of Ce-M (M=Co, Fe, Cu and Zn) catalysts[J]. Renewable Energy, 2016, 88: 457-464. |
| 16 | KUMAR P, SRIVASTAVA V C, MISHRA I M. Dimethyl carbonate synthesis via transesterification of propylene carbonate with methanol by ceria-zinc catalysts: role of catalyst support and reaction parameters[J]. Korean Journal of Chemical Engineering, 2015, 32(9): 1774-1783. |
| 17 | SONG J H, JUN J O, KANG K H, et.al. Synthesis of dimethyl carbonate from propylene carbonate and methanol over Y2O3/CeO2-La2O3 catalysts[J]. Journal of Nanoscience and Nanotechnology, 2016, 16(10): 10810-10815. |
| 18 | WANG H, WANG M H, LIU S G, et al. Influence of preparation methods on the structure and performance of CaO-ZrO2 catalyst for the synthesis of dimethyl carbonate via transesterification[J]. Journal of Molecular Catalysis A: Chemical, 2006, 258: 308-312. |
| 19 | WANG H, SHI S, WANG Y, et al. CaF2-doped ZrO2: a base catalyst for synthesis of dimethyl carbonate[J]. Journal of Shanghai University, 2010, 14(4): 281-285. |
| 20 | MERCERA P D L, OMMEN J G VAN, DOESBURG E B M, et al. Zirconia as a support for catalysts Influence of additives on the thermal stability of the porous texture of monoclinic zirconia[J]. Applied Catalysis, 1991, 71(2): 363-391. |
| 21 | DOMINGUEZ J M, HERNANDEZ J L, SANDOVAL G. Surface and catalytic properties of Al2O3-ZrO2 solid solutions prepared by sol-gel methods[J]. Applied Catalysis A: General, 2000, 197: 119-130. |
| 22 | KUŚTROWSKI P, CHMIELARZ L, BOŹEK E, et al. Acidity and basicity of hydrotalcite derived mixed Mg-Al oxides studied by test reaction of MBOH conversion and temperature programmed desorption of NH3 and CO2[J]. Materials Research Bulletin, 2004, 39(2): 263-281. |
| 23 | VÁZQUEZ A, L􀆕PEZ T, GMEZ R,et al. X-ray diffraction, FTIR, and NMR characterization of sol-gel alumina doped with lanthanum and cerium[J]. Journal of Solid State Chemistry, 1997, 128(2): 161-168. |
| 24 | MORÁN-PINEDA M, CASTILLO S, L􀆕PEZ T,et al. Synthesis, characterization and catalytic activity in the reduction of NO by CO on alumina-zirconia sol-gel derived mixed oxides[J]. Applied Catalysis B: Environmental, 1999, 21(2): 79-88. |
| 25 | WANG J J, TANG C Z, LI G N, et al. High performance MaZrOx(Ma=Cd, Ga) solid solution catalysts for CO2 hydrogenation to methanol[J]. ACS Catalysis, 2019, 9: 10253-10259. |
| 26 | TAMBOLI A H, CHAUGULE A A, GOSAVI S W, et al. CexZr1-xO2 solid solutions for catalytic synthesis of dimethyl carbonate from CO2: reaction mechanism and the effect of catalyst morphology on catalytic activity[J]. Fuel, 2018, 216: 245-254. |
| 27 | LI G R, LI W, ZHANG M H, et al. Characterization and catalytic application of homogeneous nano-composite oxides ZrO2-Al2O3[J]. Catalysis Today, 2004, 93-95: 595-601. |
| 28 | PRESOTT H A, LI Z J, KEMNIZ E, et al. Application of calcined Mg-Al hydrotalcites for Michael additions: an investigation of catalytic activity and acid-base properties[J]. Journal of Catalysis, 2005, 234(1): 119-130. |
| 29 | MEHER L C, GOPINATH R, NAIK S N, et al. Catalytic hydrogenolysis of glycerol to propylene glycol over mixed oxides derived from a hydrotalcite-type precursor[J]. Industrial & Engineering Chemistry Research, 2009, 48(4): 1840-1846. |
| 30 | CANTRELL D G, GILLIE L J, LEE A F, et al. Structure-reactivity correlations in MgAl hydrotalcite catalysts for biodiesel synthesis[J]. Applied Catalysis A: General, 2005, 287(2): 183-190. |
| 31 | 李东. 介孔Ni-Al复合氧化物的结构及其催化乙烷氧化脱氢反应方法的分析[D]. 沈阳: 沈阳师范大学, 2019. |
| LI D. Structure of mesoporous Ni-Al composite oxides and its catalytic dehydrogenation of ethane[D]. Shenyang: Shenyang Normal University, 2019. | |
| 32 | YAN T, BING W, XU M, et al. Acid-base sites synergistic catalysis over Mg-Zr-Al mixed metal oxide toward synthesis of diethyl carbonate[J]. RSC Advances, 2018, 8(9): 4695-4702. |
| 33 | LIAO Y H, LI F, DAI X, et al. Dimethyl carbonate synthesis over solid base catalysts derived from Ca-Al layered double hydroxides[J]. Chemical Papers, 2018, 72: 1963-1971. |
| 34 | WANG D F, ZHANG X L, CONG X S, et al. Influence of Zr on the performance of Mg-Al catalysts via hydrotalcite-like precursors for the synthesis of glycerol carbonate from urea and glycerol[J]. Applied Catalysis A: General, 2018, 555: 36-46. |
| 35 | ZHANG J, YAN T, YANG Y, et al. Zn-Zr-Al oxides derived from hydrotalcite precursors for ethanol conversion to diethyl carbonate[J]. Chinese Journal of Catalysis, 2019, 40(4): 515-522. |
| 36 | LI C, HE B, LING Y, et al. Glycerol hydrogenolysis to n-propanol over Zr-Al composite oxide-supported Pt catalysts[J]. Chinese Journal of Catalysis, 2018, 39(6): 1121-1128. |
| 37 | XU J, WU H T, MA C M, et al. Ionic liquid immobilized on mesocellular silica foam as an efficient heterogeneous catalyst for the synthesis of dimethyl carbonate via transesterification[J]. Applied Catalysis A: General, 2013, 464/465: 357-363. |
| 38 | WANG H, WANG M, ZHANG W, et al. Synthesis of dimethyl carbonate from propylene carbonate and methanol using CaO-ZrO2 solid solutions as highly stable catalysts[J]. Catalysis Today, 2006, 115(1-4): 107-110. |
| 39 | CROCELLA V, TABANELLI T, VITILLO J G, et al. A multi-technique approach to disclose the reaction mechanism of dimethyl carbonate synthesis over amino-modified SBA-15 catalysts[J]. Applied Catalysis B: Environmental, 2017, 211: 323-336. |
| 40 | SONG Z, SUBRAMANIAM B, CHAUDHARI R V. Transesterification of propylene carbonate with methanol using Fe-Mn double metal cyanide catalyst[J]. ACS Sustain. Chem. Eng.,2019, 7: 5698-5710. |
| 41 | TAYLOR R, KRISHNA R. Modelling reactive distillation[J]. Chemical Engineering Science, 2000, 55(22): 5183-5229. |
| [1] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [2] | 陈丹阳, 朱建宇, 吴勤, 王自庆, 张金利. KF/MgO催化甘油和碳酸二甲酯酯交换合成甘油碳酸酯[J]. 化工进展, 2022, 41(4): 2082-2089. |
| [3] | 林子昕, 田伟, 安维中. 热泵辅助变压精馏分离碳酸二甲酯/甲醇工艺及系统模拟优化[J]. 化工进展, 2022, 41(11): 5722-5730. |
| [4] | 朱长辉, 朱文超, 罗嘉, 田保河, 孙佳琳, 邹志云. 微波强化酯交换反应制备生物柴油研究进展[J]. 化工进展, 2022, 41(10): 5145-5154. |
| [5] | 岳倩倩, 高李璟, 肖国民, 魏瑞平, 雷严. 生物柴油连续化生产设备及工艺进展[J]. 化工进展, 2021, 40(S2): 81-88. |
| [6] | 王玉春, 张志浩, 高源, 李忠, 郑华艳. DMC-甲醇-水三元混合物的萃取精馏分离工艺[J]. 化工进展, 2021, 40(8): 4196-4204. |
| [7] | 孔会娜, 郑卫, 曹海龙, 黄贵明. 煤制乙二醇装置联产碳酸二甲酯精馏工艺优化[J]. 化工进展, 2021, 40(7): 3626-3631. |
| [8] | 王玉春, 刘赵荣, 谭超, 孙鸿, 李忠. 蒸气浸渍法制备完全无氯CuY催化剂及其氧化羰基化性能[J]. 化工进展, 2021, 40(1): 242-246. |
| [9] | 王军, 赵妍, 邹欣, 柳娜, 许杰, 薛冰. KF改性MgAl水滑石催化酯交换合成碳酸乙烯酯[J]. 化工进展, 2020, 39(7): 2670-2676. |
| [10] | 张洁, 贾爱忠, 李芳, 赵新强, 王延吉. 酯交换法合成碳酸甲乙酯催化剂的研究进展[J]. 化工进展, 2020, 39(11): 4435-4445. |
| [11] | 孙宇辰,张国强,刘斌,李忠,上官炬,刘守军,史鹏政. CO2和甲醇直接合成碳酸二甲酯耦合脱水体系研究进展[J]. 化工进展, 2019, 38(11): 5127-5135. |
| [12] | 贾东森,张国强,尹娇,张亮亮,赵丹,郑华艳,李忠. 碳球表面缺陷密度对其负载铜催化剂甲醇氧化羰基化反应性能的影响[J]. 化工进展, 2019, 38(08): 3701-3710. |
| [13] | 邹建军, 鲁婷, 王家喜. 氨基甲酸酯基丙烯酸酯的合成及其光固化性能[J]. 化工进展, 2019, 38(04): 1947-1952. |
| [14] | 彭向聪,王志苗,李红芹,薛伟,李芳,王延吉. 溶剂热浸渍法制备高稳定性Zn(OAc)2/SiO2催化剂及其催化合成苯氨基甲酸甲酯[J]. 化工进展, 2019, 38(03): 1396-1402. |
| [15] | 袁川, 鲁厚芳, 刘长军, 蒋炜, 刘颖颖, 梁斌. 水和游离脂肪酸对DBU催化制备生物柴油的影响[J]. 化工进展, 2018, 37(09): 3386-3392. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||