化工进展 ›› 2019, Vol. 38 ›› Issue (01): 576-585.DOI: 10.16085/j.issn.1000-6613.2018-1115
适应高滴度抗体制备的抗体捕集纯化技术研究进展
- 1. 天津大学化工学院,天津 300350
2. 系统生物工程教育部重点实验室,天津 300350
-
收稿日期:2018-05-30修回日期:2018-08-24出版日期:2019-01-05发布日期:2019-01-05 -
通讯作者:孙彦 -
作者简介:史清洪(1968—),男,工学博士,教授,博士生导师,研究方向为生物分离工程。E-mail:<email>qhshi@tju.edu.cn</email>。|孙彦,教授,博士生导师,研究方向为生物分子工程。E-mail:<email>ysun@tju.edu.cn</email>。 -
基金资助:国家自然科学基金面上项目(21476166, 21878221);国家自然科学基金创新群体项目(21621004);国家自然科学基金重点项目(21236005);国家自然科学基金面上项目(21476166, 21878221);国家自然科学基金创新群体项目(21621004);国家自然科学基金重点项目(21236005)。
Antibody capture technologies for high-titer antibody production
Qinghong SHI1,2( ),Yan SUN1,2(
),Yan SUN1,2( )
)
- 1. School of Chemical Engineering and Technology, Tianjin University, Tianjin 300350,China
2. Key Laboratory of Systems Bioengineering Ministry of Education, Tianjin 300350, China
-
Received:2018-05-30Revised:2018-08-24Online:2019-01-05Published:2019-01-05 -
Contact:Yan SUN
摘要:
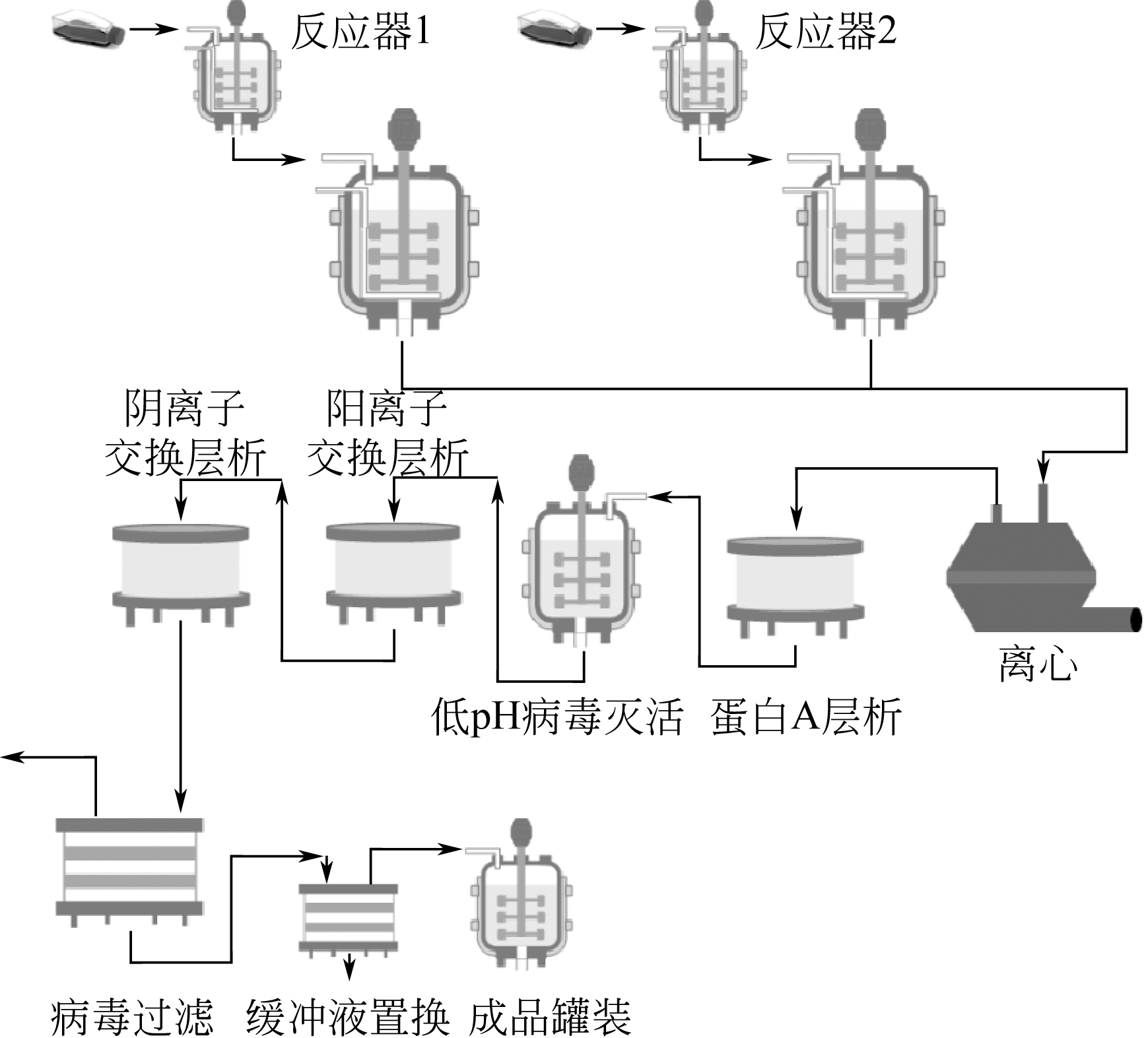
抗体捕集纯化是单克隆抗体和Fc融合蛋白药物制备过程的关键步骤。但相较于大规模动物细胞培养技术的迅猛发展,抗体的捕集纯化已经成为了制约单抗药物生产的主要“瓶颈”。本文回顾了蛋白A亲和层析和阳离子交换层析等当前抗体药物工业生产过程主要捕集纯化技术的发展现状和应用情况,介绍了近年来亲和肽层析和混合模式吸附层析等新型层析分离技术的发展以及膨胀床吸附和多柱串联吸附等过程集成方法在提高抗体捕集效率方面所展现的良好前景。在此基础上,指出了影响抗体捕集纯化的主要因素以及各技术发展中存在的问题,展望抗体捕集纯化技术的发展方向。
中图分类号:
引用本文
史清洪, 孙彦. 适应高滴度抗体制备的抗体捕集纯化技术研究进展[J]. 化工进展, 2019, 38(01): 576-585.
Qinghong SHI, Yan SUN. Antibody capture technologies for high-titer antibody production[J]. Chemical Industry and Engineering Progress, 2019, 38(01): 576-585.
| 介质名称 | 基质材料 | 配基种类 | 配基密度 /mg·mL-1 | 介质性能 | 参考 文献 | ||||
|---|---|---|---|---|---|---|---|---|---|
| 饱和吸附容量/mg·mL-1 | 解离常数 /mg·mL-1 | 动态吸附容量 /mg·mL-1 | |||||||
| GE Healthcare | |||||||||
| rProtein A Sepharose Fast Flow | 交联琼脂糖 | 4~6 | 55.1±1.5 | 0.037±0.007 | [18] | ||||
| Protein A Sepharose 4 Fast Flow | 交联琼脂糖 | 4~6 | 61.6±1.7 | 0.11±0.09 | [18] | ||||
| MabSelect | 高度交联琼脂糖 | 重组蛋白A | n.a. | 60.6±1.3 | 0.120±0.010 | 约30 | [31] | ||
| MabSelect SuRe | 高度交联琼脂糖 | 碱耐受性突变蛋白A | n.a. | 61.2±2.1 | 0.115±0.017 | 约30 | [31] | ||
| MabSelect Xtra | 高度交联琼脂糖 | 重组蛋白A | n.a. | 68.3±2.0 | 0.088±0.011 | 约40 | [31] | ||
| MabSelect PrismA | 高度交联琼脂糖 | 碱耐受性突变蛋白A | n.a. | 99.9 | — | >80 | |||
| Millipore | |||||||||
| ProSep-vA Ultra | 可控多孔玻璃材料 | 天然蛋白A | n.a. | 62.8±1.8 | 0.116±0.014 | 35 | [31] | ||
| ProSep-vA High capacity | 可控多孔玻璃材料 | n.a. | >40 | — | >20 | ||||
| ProSep-vA Ultra Plus | 可控多孔玻璃材料 | 重组蛋白A | n.a. | >67 | — | >50 | |||
| Eshmuno A resin | 亲水聚乙烯醚 | 重组C结构域 | n.a. | — | — | 40~55 | |||
| PerSecptive Biosystem | |||||||||
| POROS 50 A | 交联聚(苯乙烯-二乙烯基苯) | 重组蛋白A | n.a. | 25 | — | 17.5 | [ | [32] | |
| POROS MabCapture A | 交联聚(苯乙烯-二乙烯基苯) | 重组蛋白A | n.a. | — | — | >45 | |||
表1 常见蛋白A层析介质及其特性
| 介质名称 | 基质材料 | 配基种类 | 配基密度 /mg·mL-1 | 介质性能 | 参考 文献 | ||||
|---|---|---|---|---|---|---|---|---|---|
| 饱和吸附容量/mg·mL-1 | 解离常数 /mg·mL-1 | 动态吸附容量 /mg·mL-1 | |||||||
| GE Healthcare | |||||||||
| rProtein A Sepharose Fast Flow | 交联琼脂糖 | 4~6 | 55.1±1.5 | 0.037±0.007 | [18] | ||||
| Protein A Sepharose 4 Fast Flow | 交联琼脂糖 | 4~6 | 61.6±1.7 | 0.11±0.09 | [18] | ||||
| MabSelect | 高度交联琼脂糖 | 重组蛋白A | n.a. | 60.6±1.3 | 0.120±0.010 | 约30 | [31] | ||
| MabSelect SuRe | 高度交联琼脂糖 | 碱耐受性突变蛋白A | n.a. | 61.2±2.1 | 0.115±0.017 | 约30 | [31] | ||
| MabSelect Xtra | 高度交联琼脂糖 | 重组蛋白A | n.a. | 68.3±2.0 | 0.088±0.011 | 约40 | [31] | ||
| MabSelect PrismA | 高度交联琼脂糖 | 碱耐受性突变蛋白A | n.a. | 99.9 | — | >80 | |||
| Millipore | |||||||||
| ProSep-vA Ultra | 可控多孔玻璃材料 | 天然蛋白A | n.a. | 62.8±1.8 | 0.116±0.014 | 35 | [31] | ||
| ProSep-vA High capacity | 可控多孔玻璃材料 | n.a. | >40 | — | >20 | ||||
| ProSep-vA Ultra Plus | 可控多孔玻璃材料 | 重组蛋白A | n.a. | >67 | — | >50 | |||
| Eshmuno A resin | 亲水聚乙烯醚 | 重组C结构域 | n.a. | — | — | 40~55 | |||
| PerSecptive Biosystem | |||||||||
| POROS 50 A | 交联聚(苯乙烯-二乙烯基苯) | 重组蛋白A | n.a. | 25 | — | 17.5 | [ | [32] | |
| POROS MabCapture A | 交联聚(苯乙烯-二乙烯基苯) | 重组蛋白A | n.a. | — | — | >45 | |||
| 1 | MORRISON C , LAHTEENMAKI R . Public biotech in 2016: the numbers[J]. Nature Biotechnology, 2017, 35(7): 623-629. |
| 2 | WANG W , SINGH S , ZENG D L , et al . Antibody structure, instability, and formulation[J]. Journal of Pharmaceutical Sciences, 2007, 96(1): 1-26. |
| 3 | SINGH S , KUMAR N , DWIWEDI P , et al . Monoclonal antibodies: a review[J]. Current Clinical Pharmacology, 2018, 13(2): 85-99. |
| 4 | GRONEMEYER P , DITZ R , STRUBE J . Trends in upstream and downstream process development for antibody manufacturing[J]. Bioengineering (Basel, Switzerland), 2014, 1(4): 188-212. |
| 5 | KUNERT R , REINHART D . Advances in recombinant antibody manufacturing[J]. Applied Microbiology and Biotechnology, 2016, 100(8): 3451-3461. |
| 6 | BOLTON G R , MEHTA K K . The role of more than 40 years of improvement in protein A chromatography in the growth of the therapeutic antibody industry[J]. Biotechnology Progress, 2016, 32(5): 1193-1202. |
| 7 | LOW D , O'LEARY R , PUJAR N S . Future of antibody purification[J]. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, 2007, 848(1): 48-63. |
| 8 | MIESEGAES G R , LUTE S , STRAUSS D M , et al . Monoclonal antibody capture and viral clearance by cation exchange chromatography[J]. Biotechnology and Bioengineering, 2012, 109(8): 2048-2058. |
| 9 | GRAILLE M , STURA E A , CORPER A L , et al . Crystal structure of a Staphylococcus aureus protein A domain complexed with the Fab fragment of a human IgM antibody: structural basis for recognition of B-cell receptors and superantigen activity[J]. Proceedings of the National Academy of Sciences of the United States of America, 2000, 97(10): 5399-5404. |
| 10 | JENSEN K . A normally occurring Staphylococcus antibody in human serum[J]. Acta Pathology et Microbiology Scandinavica, 1958, 44(5): 421-428. |
| 11 | VERWEY W F . A type-specific antigenic protein derived from the Staphylococcus [J]. The Journal of Experimental Medicine, 1940, 71(5): 635-644. |
| 12 | ARNE G , BERIT M , PER O . Immunochemical studies on antigen preparations from Staphylococcus aureus [J]. Acta Pathologica Microbiologica Scandinavica, 1964, 61(4): 588-596. |
| 13 | EY P L , PROWSE S J , JENKIN C R . Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose[J]. Immunochemistry, 1978, 15(7): 429-436. |
| 14 | HUBER R , DEISENHOFER J , COLMAN P M , et al . Crystallographic structure studies of an IgG molecule and an Fc fragment[J]. Nature, 1976, 264(5585): 415-420. |
| 15 | DEISENHOFER J . Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9- and 2.8-A resolution[J]. Biochemistry, 1981, 20(9): 2361-2370. |
| 16 | NILSSON B , MOKS T , JANSSON B , et al . A synthetic IgG-binding domain based on staphylococcal protein A[J]. Protein Engineering, 1987, 1(2): 107-113. |
| 17 | FUGLISTALLER P . Comparison of immunoglobulin binding capacities and ligand leakage using eight different protein A affinity chromatography matrices[J]. Journal of Immunological Methods, 1989, 124(2): 171-177. |
| 18 | HAHN R , SCHLEGEL R , JUNGBAUER A . Comparison of protein A affinity sorbents[J]. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, 2003, 790(1/2): 35-51. |
| 19 | ULTSCH M , BRAISTED A , MAUN H R , et al . 3-2-1: Structural insights from stepwise shrinkage of a three-helix Fc-binding domain to a single helix[J]. Protein Engineering Design & Selection, 2017, 30(9): 619-625. |
| 20 | LYONS B A , TASHIRO M , CEDERGREN L , et al . An improved strategy for determining resonance assignments for isotopically enriched proteins and its application to an engineered domain of staphylococcal protein A[J]. Biochemistry, 1993, 32(31): 7839-7845. |
| 21 | JENDEBERG L , TASHIRO M , TEJERO R , et al . The mechanism of binding staphylococcal protein A to immunoglobin G does not involve helix unwinding[J]. Biochemistry, 1996, 35(1): 22-31. |
| 22 | LI R X , DOWD V , STEWART D J , et al . Design, synthesis, and application of a protein A mimetic[J]. Nature Biotechnology, 1998, 16(2): 190-195. |
| 23 | HUANG B , LIU F F , DONG X Y , et al . Molecular mechanism of the affinity interactions between protein A and human innmunoglobulin G1 revealed by molecular simulations[J]. Journal of Physical Chemistry B, 2011, 115(14): 4168-4176. |
| 24 | GOUDA H , TORIGOE H , SAITO A , et al . Three-dimensional solution structure of the B domain of staphylococcal protein A: comparisons of the solution and crystal structures[J]. Biochemistry, 1992, 31(40): 9665-9672. |
| 25 | TASHIRO M , TEJERO R , ZIMMERMAN D E , et al . High-resolution solution NMR structure of the Z domain of staphylococcal protein A[J]. Journal of molecular biology, 1997, 272(4): 573-590. |
| 26 | YU F , JARVER P , NYGREN P A . Tailor-making a protein A-derived domain for efficient site-specific photocoupling to Fc of mouse IgG(1)[J]. PLoS One, 2013, 8(2): e56597. |
| 27 | TAJIMA N , TAKAI M , ISHIHARA K . Significance of antibody orientation unraveled: well-oriented antibodies recorded high binding affinity[J]. Analytical Chemistry, 2011, 83(6): 1969-1976. |
| 28 | VON ROMAN M F , BERENSMEIER S . Improving the binding capacities of protein A chromatographic materials by means of ligand polymerization[J]. Journal of Chromatography A, 2014, 1347: 80-86. |
| 29 | GHOSE S , HUBBARD B , CRAMER S M . Binding capacity differences for antibodies and Fc-fusion proteins on protein A chromatographic materials[J]. Biotechnology and Bioengineering, 2007, 96(4): 768-779. |
| 30 | KELLEY B . Very large scale monoclonal antibody purification: The case for conventional unit operations[J]. Biotechnology Progress, 2007, 23(5): 995-1008. |
| 31 | HAHN R , BAUERHANSL P , SHIMAHARA K , et al . Comparison of protein A affinity sorbents Ⅱ. Mass transfer properties[J]. Journal of Chromatography A, 2005, 1093(1/2): 98-110. |
| 32 | FAHRNER R L , WHITNEY D H , VANDERLAAN M , et al . Performance comparison of protein A affinity-chromatography sorbents for purifying recombinant monoclonal antibodies[J]. Biotechnology and Applied Biochemistry, 1999, 30: 121-128. |
| 33 | HAHN R , SHIMAHARA K , STEINDL F , et al . Comparison of protein A affinity sorbents Ⅲ. Life time study[J]. Journal of Chromatography A, 2006, 1102(1/2): 224-231. |
| 34 | MCCUE J T , KEMP G , LOW D , et al . Evaluation of protein-A chromatography media[J]. Journal of Chromatography A, 2003, 989(1): 139-153. |
| 35 | ZHAO L , ZHU K , HUANG Y , et al . Enhanced binding by dextran-grafting to protein A affinity chromatographic media[J]. Journal of Separation Science, 2017, 40(7): 1493-1499. |
| 36 | HORSTMANN B J , CHASE H A . Modeling the affinity adsorption of immoglobulin G to protein-A immobilized to agarose matrices[J]. Chemical Engineering Research and Design, 1989, 67(3): 243-254. |
| 37 | DENIZLI A , RAD A Y , PISKIN E . Protein A immobilized polyhydroxyethylmethacrylate beads for affinity sorption of human immunoglobulin G[J]. Journal of Chromatography. B, Biomedical Applications, 1995, 668(1): 13-19. |
| 38 | ZHANG X , DUAN Y , ZENG X . Improved performance of recombinant protein A immobilized on agarose beads by site-specific conjugation[J]. ACS Omega, 2017, 2(4): 1731-1737. |
| 39 | FAHRNER R L , IYER H V , BLANK G S . The optimal flow rate and column length for maximum production rate of protein A affinity chromatography[J]. Bioprocess Engineering, 1999, 21(4): 287-292. |
| 40 | MIESEGAES G , LUTE S , BRORSON K . Analysis of viral clearance unit operations for monoclonal antibodies[J]. Biotechnology and Bioengineering, 2010, 106(2): 238-246. |
| 41 | SHUKLA A A , HUBBARD B , TRESSEL T , et al . Downstream processing of monoclonal antibodies—Application of platform approaches[J]. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, 2007, 848(1): 28-39. |
| 42 | VAZQUEZ-REY M , LANG D A . Aggregates in monoclonal antibody manufacturing processes[J]. Biotechnology and Bioengineering, 2011, 108(7): 1494-1508. |
| 43 | CHOLLANGI S , PARKER R , SINGH N , et al . Development of robust antibody purification by optimizing protein-A chromatography in combination with precipitation methodologies[J]. Biotechnology and Bioengineering, 2015, 112(11): 2292-2304. |
| 44 | GÜLICH S , UHLEN M , HOBER S . Protein engineering of an IgG-binding domain allows milder elution conditions during affinity chromatography[J]. Journal of Biotechnology, 2000, 76(2/3): 233-244. |
| 45 | PABST T M , PALMGREN R , FORSS A , et al . Engineering of novel staphylococcal protein A ligands to enable milder elution pH and high dynamic binding capacity[J]. Journal of Chromatography A, 2014, 1362: 180-185. |
| 46 | 万一, 訾静, 张琨, 等 . 金黄色葡萄球菌蛋白A(SpA)Z结构域串联体的克隆、表达和筛选[J]. 生物工程学报, 2012, 28(12): 1500-1510. |
| WAN Y , ZI J , ZHANG K , et al . Cloning, expression and screening tandem repeats of the Z domain of Staphylococcus aureus protein A[J]. Chinese Journal of Biotechnology, 2012, 28(12): 1500-1510. | |
| 47 | YANG X H , HUAN L M , CHU X S , et al . A comparative investigation of random and oriented immobilization of protein A ligands on the binding of immunoglobulin G[J]. Biochemical Engineering Journal, 2018, 139: 15-24. |
| 48 | MINAKUCHI K , MURATA D , OKUBO Y , et al . Remarkable alkaline stability of an engineered protein A as immunoglobulin affinity ligand: C domain having only one amino acid substitution[J]. Protein Science, 2013, 22(9): 1230-1238. |
| 49 | 管志龙, 白姝, 孙彦, 等 . 耐碱性蛋白A色谱配基的构建及性能评价[J]. 化工学报, 2017, 68(9): 3459-3465. |
| GUAN Z L , BAI S , SUN Y , et al . Construction and characteristics of alkali-tolerance mutants of Z domain for protein A chromatography[J]. CIESC Journal, 2017, 68(9): 3459-3465 | |
| 50 | LINHULT M , GULICH S , GRASLUND T , et al . Improving the tolerance of a protein a analogue to repeated alkaline exposures using a bypass mutagenesis approach[J]. Proteins-Structure Function and Bioinformatics, 2004, 55(2): 407-416. |
| 51 | PALMER B , ANGUS K , TAYLOR L , et al . Design of stability at extreme alkaline pH in streptococcal protein G[J]. Journal of Biotechnology, 2008, 134(3/4): 222-230. |
| 52 | PABST T M , THAI J , HUNTER A K . Evaluation of recent protein A stationary phase innovations for capture of biotherapeutics[J]. Journal of Chromatography A, 2018, 1554: 45-60. |
| 53 | KARLSSON E , HIRSH I . Ion exchange chromatography[M]// Protein purification: principles, high resolution methods, and applications. JANSON J C ed. New Jersey: John Wiley & Sons, Inc., 2011: 93-133. |
| 54 | URMANN M , GRAALFS H , JOEHNCK M , et al . Cation-exchange chromatography of monoclonal antibodies characterization of a novel stationary phase designed for production-scale purification[J]. mAbs, 2010, 2(4): 395-404. |
| 55 | TAO Y Y , CARTA G . Rapid monoclonal antibody adsorption on dextran-grafted agarose media for ion-exchange chromatography[J]. Journal of Chromatography A, 2008, 1211(1/2): 70-79. |
| 56 | HARINARAYAN C , MUELLER J , LJUNGLOF A , et al . An exclusion mechanism in ion exchange chromatography[J]. Biotechnology and Bioengineering, 2006, 95(5): 775-787. |
| 57 | ZHANG S L , ZHAO M , YANG W , et al . A novel polymer-grafted cation exchanger for high-capacity protein chromatography: the role of polymer architecture[J]. Biochemical Engineering Journal, 2017, 128(Supplement C): 218-227. |
| 58 | STRAUSS D M , LUTE S , TEBAYKINA Z , et al . Understanding the mechanism of virus removal by q sepharose fast flow chromatography during the purification of CHO-cell derived biotherapeutics[J]. Biotechnology and Bioengineering, 2009, 104(2): 371-380. |
| 59 | FASSINA G , VERDOLIVA A , ODIERNA M R , et al . Protein A mimetic peptide ligand for affinity purification of antibodies[J]. Journal of Molecular Recognition, 1996, 9(5/6): 564-569. |
| 60 | FASSINA G , VERDOLIVA A , PALOMBO G , et al . Immunoglobulin specificity of TG19318: a novel synthetic ligand for antibody affinity purification[J]. Journal of Molecular Recognition, 1998, 11(1-6): 128-133. |
| 61 | FASSINA G , RUVO M , PALOMBO G , et al . Novel ligands for the affinity-chromatographic purification of antibodies[J]. Journal of Biochemical and Biophysical Methods, 2001, 49(1/2/3): 481-490. |
| 62 | YANG H , GURGEL P V , CARBONELL R G . Hexamer peptide affinity resins that bind the Fc region of human immunoglobulin G[J]. Journal of Peptide Research, 2005, 66: 120-137. |
| 63 | YANG H , GURGEL P V , CARBONELL R G . Purification of human immunoglobulin G via Fc-specific small peptide ligand affinity chromatography[J]. Journal of Chromatography A, 2009, 1216(6): 910-918. |
| 64 | EHRLICH G K , BAILON P . Identification of peptides that bind to the constant region of a humanized IgG(1) monoclonal antibody using phage display[J]. Journal of Molecular Recognition, 1998, 11(1/2/3/4/5/6): 121-125. |
| 65 | RAY P K , DATTA P K , MODAK D P , et al . Designing of peptides with immuno-modulatory properties using protein A as a probe[J]. Indian Journal of Biochemistry & Biophysics, 1995, 32(6): 372-377. |
| 66 | SENGUPTA J , SINHA P , MUKHOPADHYAY C , et al . Molecular modeling and experimental approaches toward designing a minimalist protein having Fc-binding activity of staphylococcal protein A[J]. Biochemical and Biophysical Research Communications, 1999, 256(1): 6-12. |
| 67 | ZHAO W W , LIU F F , SHI Q H , et al . Biomimetic design of affinity peptide ligands for human IgG based on protein A-IgG complex[J]. Biochem. Eng. J., 2014, 88: 1-11. |
| 68 | ZHAO W W , LIU F F , SHI Q H , et al . Octapeptide-based affinity chromatography of human immunoglobulin G: comparisons of three different ligands[J]. Journal of Chromatography A, 2014, 1359: 100-111. |
| 69 | ZHAO W W , SHI Q H , SUN Y . FYWHCLDE-based affinity chromatography of IgG: effect of ligand density and purifications of human IgG and monoclonal antibody[J]. Journal of Chromatography A, 2014, 1355: 107-114. |
| 70 | ZHAO W W , SHI Q H , SUN Y . Dual-ligand affinity systems with octapeptide ligands for affinity chromatography of hIgG and monoclonal antibody[J]. Journal of Chromatography A, 2014, 1369: 64-72. |
| 71 | YON R J . Chromatography of lipophilic proteins on adsorbents containing mixed hydrophobic and ionic groups[J]. The Biochemical journal, 1972, 126(3): 765-767. |
| 72 | TENG S F , SPROULE K , HUSAIN A , et al . Affinity chromatography on immobilized "biomimetic" ligands synthesis, immobilization and chromatographic assessment of an immunoglobulin G-binding ligand[J]. Journal of Chromatography B, 2000, 740(1): 1-15. |
| 73 | CHEN J , TETRAULT J , LEY A . Comparison of standard and new generation hydrophobic interaction chromatography resins in the monoclonal antibody purification process[J]. Journal of Chromatography A, 2008, 1177(2): 272-281. |
| 74 | ARAKAWA T , KITA Y , SATO H , et al . MEP chromatography of antibody and Fc-fusion protein using aqueous arginine solution[J]. Protein Expression and Purification, 2009, 63(2): 158-163. |
| 75 | TONG H F , LIN D Q , YUAN X M , et al . Enhancing IgG purification from serum albumin containing feedstock with hydrophobic charge-induction chromatography[J]. Journal of Chromatography A, 2012, 1244: 116-122. |
| 76 | SCHWARTZ W , JUDD D , WYSOCKI M , et al . Comparison of hydrophobic charge induction chromatography with affinity chromatography on protein A for harvest and purification of antibodies[J]. Journal of Chromatography A, 2001, 908(1/2): 251-263. |
| 77 | 李菁, 林东强, 童红飞, 等 . 疏水性电荷诱导色谱分离抗HER2单克隆抗体[J]. 化工学报, 2014, 65(10): 3931-3937. |
| LI J , LIN D , TONG H , et al . Separation and purification of anti-HER2 monoclonal antibody with hydrophobic charge-induction chromatography[J]. CIESC Journal, 2014, 65(10): 3931-3937. | |
| 78 | XIA H F , LIN D Q , WANG L P , et al . Preparation and evaluation of cellulose adsorbents for hydrophobic charge induction chromatography[J]. Industrial & Engineering Chemistry Research, 2008, 47(23): 9566-9572. |
| 79 | SHI Q H , CHENG Z , SUN Y . 4-(1H-imidazol-1-yl) aniline: a new ligand of mixed-mode chromatography for antibody purification[J]. Journal of Chromatography A, 2009, 1216(33): 6081-6087. |
| 80 | CHAI D S , SUN Y , WANG X N , et al . Improved purification of immunoglobulin G from plasma by mixed-mode chromatography[J]. Journal of Separation Science, 2014, 37(23): 3461-3472. |
| 81 | SPALDING B J . Downstream processing: key to slashing production costs 100 fold[J]. Bio/technology (Nature Publishing Company), 1991, 9(3): 229-235. |
| 82 | THOMMES J , HALFAR M , LENZ S , et al . Purification of monoclonal antibodies from whole hybridoma fermentation broth by fluidized bed adsorption[J]. Biotechnology and Bioengineering, 1995, 45(3): 205-211. |
| 83 | THOMMES J , BADER A , HALFAR M , et al . Isolation of monoclonal antibodies from cell containing hybridoma broth using a protein A coated adsorbent in expanded beds[J]. Journal of Chromatography A, 1996, 752(1/2): 111-122. |
| 84 | OHASHI R , OTERO J M , CHWISTEK A , et al . On-line purification of monoclonal antibodies using an integrated stirred-tank reactor/expanded-bed adsorption system[J]. Biotechnology Progress, 2002, 18(6): 1292-1300. |
| 85 | GONZÁLEZ Y , IBARRA N , GOMEZ H , et al . Expanded bed adsorption processing of mammalian cell culture fluid: comparison with packed bed affinity chromatography[J]. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, 2003, 784(1): 183-187. |
| 86 | MAHAJAN E , GEORGE A , WOLK B . Improving affinity chromatography resin efficiency using semi-continuous chromatography[J]. Journal of Chromatography A, 2012, 1227: 154-162. |
| 87 | HUANG S Y , LIN C K , CHANG W H , et al . Enzyme purification and concentration by simulated moving bed chromatography: an experimental study[J]. Chemical Engineering Communications, 1986, 45(1/2/3/4/5/6): 291-309. |
| 88 | GOTTSCHLICH N , KASCHE V . Purification of monoclonal antibodies by simulated moving-bed chromatography[J]. Journal of Chromatography A, 1997, 765(2): 201-206. |
| 89 | IMAMOGLU S . Simulated moving bed chromatography (SMB) for application in bioseparation[J]. Advances in Biochemical Engineering/Biotechnology, 2002, 76: 211-231. |
| 90 | KEβLER L C , GUEORGUIEVA L , RINAS U , et al . Step gradients in 3-zone simulated moving bed chromatography application to the purification of antibodies and bone morphogenetic protein-2[J]. Journal of Chromatography A, 2007, 1176(1/2): 69-78. |
| 91 | NG C K S , ROUSSET F , VALERY E , et al . Design of high productivity sequential multi-column chromatography for antibody capture[J]. Food and Bioproducts Processing, 2014, 92(C2): 233-241. |
| [1] | 董晓宇. 酿酒酵母钙通道膜蛋白单克隆抗体制备及鉴定[J]. 化工进展, 2021, 40(S1): 334-343. |
| [2] | 杜晓芳,李兆周,陈秀金,王耀,高红丽,李道敏,任国艳,吕璞. 喹诺酮类药物印迹仿生抗体的研制及应用进展[J]. 化工进展, 2020, 39(4): 1447-1457. |
| [3] | 郭飞鸽,张秋禹,张和鹏,张宝亮. Fe3O4/P(AA-MMA-GMA) 磁性复合微球的制备及其 [J]. 化工进展, 2010, 29(9): 1693-. |
| [4] | 朱友双,孙庆元,蒙 敏,朱 强,陈 曦. 免疫淀粉微球的制备及其结合特性 [J]. 化工进展, 2008, 27(7): 1052-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||