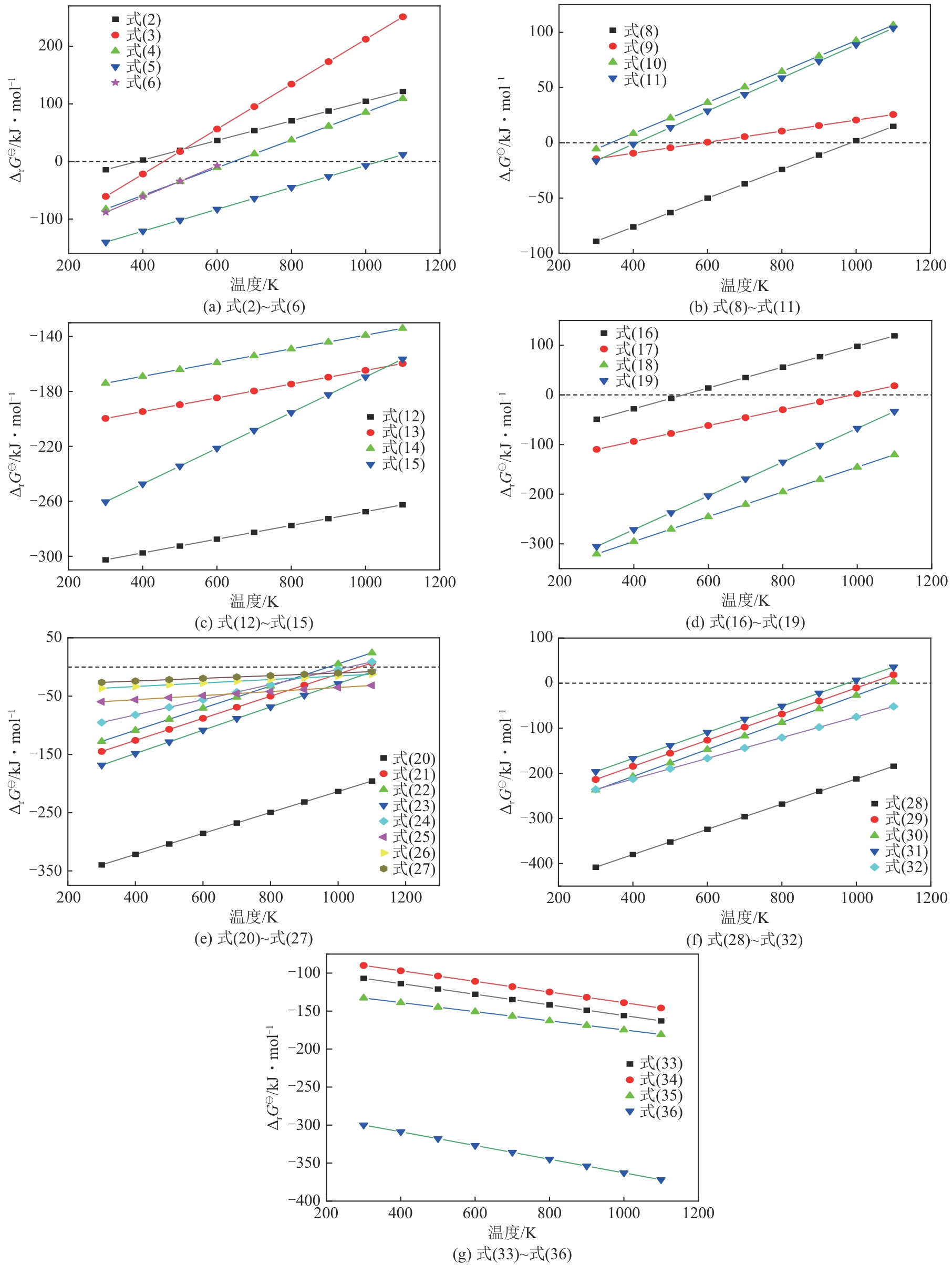
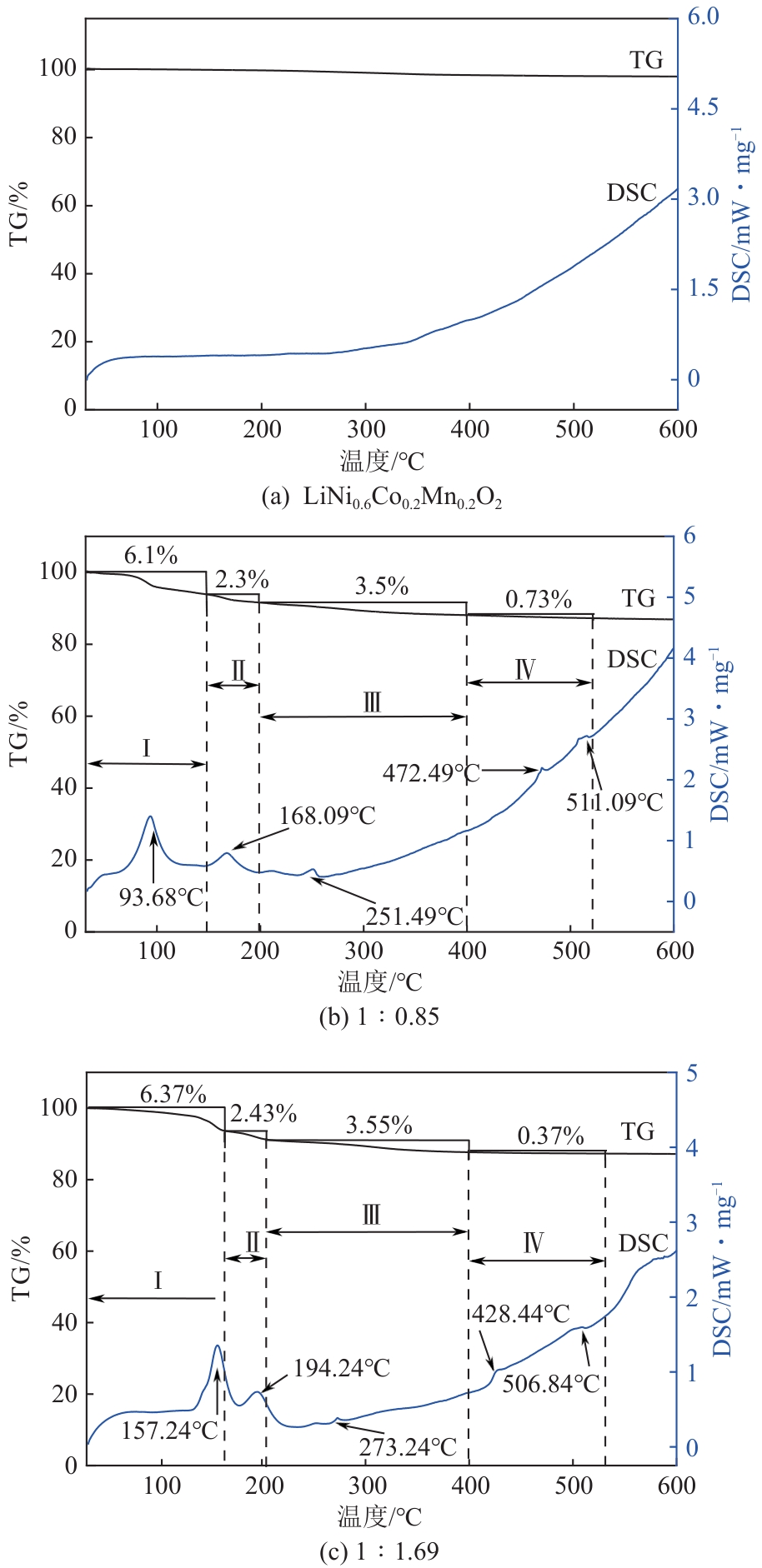
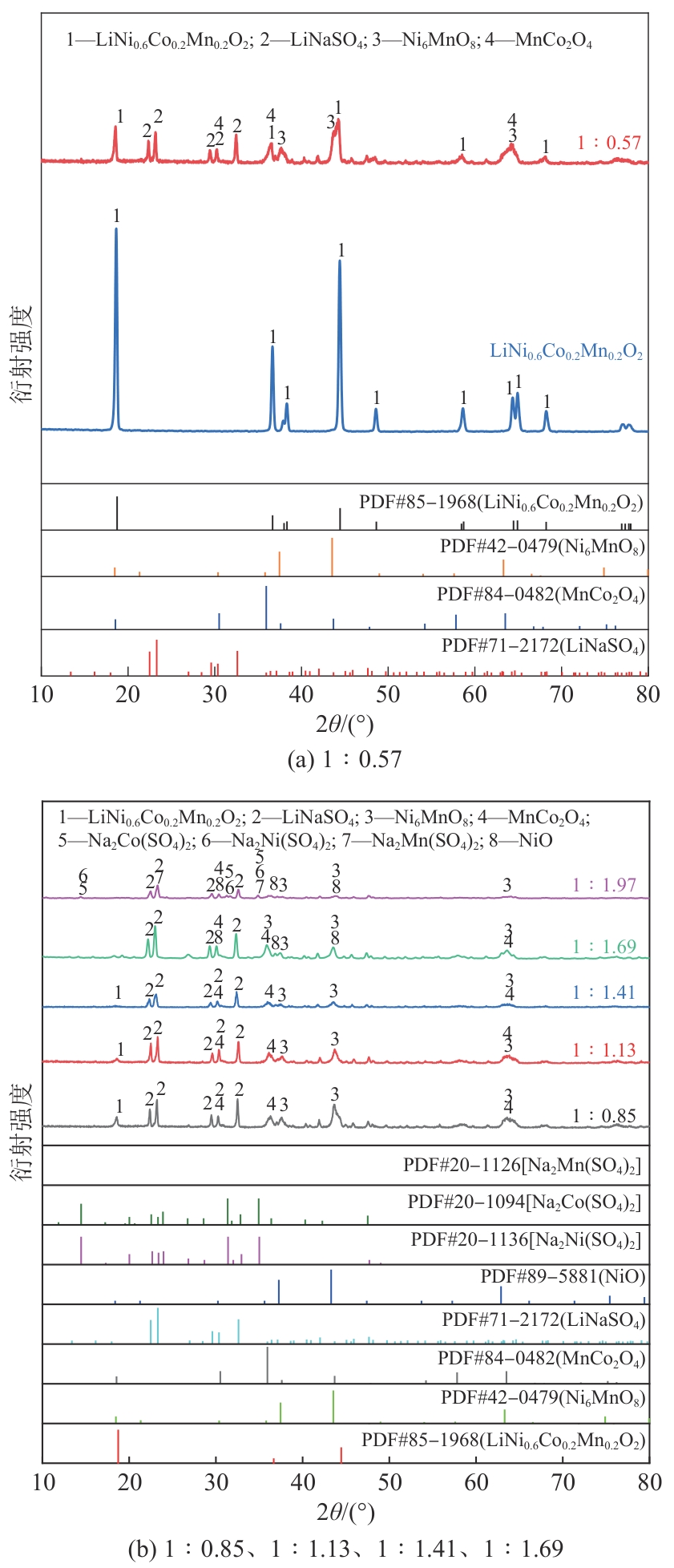
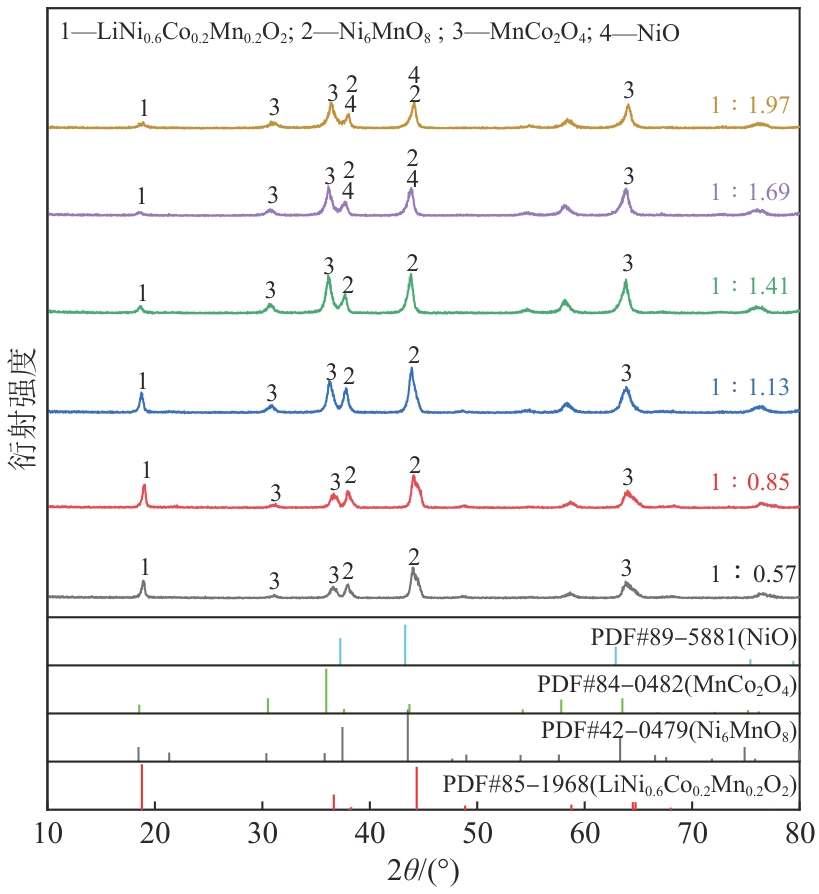
| [1] |
VIROLAINEN Sami, FALLAH FINI Mojtaba, LAITINEN Antero, et al. Solvent extraction fractionation of Li-ion battery leachate containing Li, Ni, and Co[J]. Separation and Purification Technology, 2017, 179: 274-282.
|
| [2] |
FAN Ersha, LI Li, WANG Zhenpo, et al. Sustainable recycling technology for Li-ion batteries and beyond: Challenges and future prospects[J]. Chemical Reviews, 2020, 120(14): 7020-7063.
|
| [3] |
HUANG Bin, PAN Zhefei, SU Xiangyu, et al. Recycling of lithium-ion batteries: Recent advances and perspectives[J]. Journal of Power Sources, 2018, 399: 274-286.
|
| [4] |
HUANG Tao, LIU Longfei, ZHANG Shuwen. Recovery of cobalt, lithium, and manganese from the cathode active materials of spent lithium-ion batteries in a bio-electro-hydrometallurgical process[J]. Hydrometallurgy, 2019, 188: 101-111.
|
| [5] |
NIE Xuejiao, XI Xiaotong, YANG Yang, et al. Recycled LiMn2O4 from the spent lithium ion batteries as cathode material for sodium ion batteries: Electrochemical properties, structural evolution and electrode kinetics[J]. Electrochimica Acta, 2019, 320: 134626.
|
| [6] |
WU Guozhen, CHEN Huaijing, WANG Dahui, et al. Chemistry evolution of LiFePO4-NaHSO4·H2O system during roasting and recovery of Li and Fe[J]. Journal of Alloys and Compounds, 2024, 1007: 176376.
|
| [7] |
LI Li, FAN Ersha, GUAN Yibiao, et al. Sustainable recovery of cathode materials from spent lithium-ion batteries using lactic acid leaching system[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(6): 5224-5233.
|
| [8] |
ZHANG Xihua, CAO Hongbin, XIE Yongbing, et al. A closed-loop process for recycling LiNi1/3Co1/3Mn1/3O2 from the cathode scraps of lithium-ion batteries: Process optimization and kinetics analysis[J]. Separation and Purification Technology, 2015, 150: 186-195.
|
| [9] |
HE Tao, DAI Junjie, DONG Yangtao, et al. Green closed-loop regeneration of ternary cathode materials from spent lithium-ion batteries through deep eutectic solvent[J]. Ionics, 2023, 29(5): 1721-1729.
|
| [10] |
CHI Zhexi, LI Jian, WANG Lihua, et al. Direct regeneration method of spent LiNi1/3Co1/3Mn1/3O2 cathode materials via surface lithium residues[J]. Green Chemistry, 2021, 23(22): 9099-9108.
|
| [11] |
ZHANG Xiaodong, WANG Dahui, CHEN Huaijing, et al. Chemistry evolution of LiNi1/3Co1/3Mn1/3O2-NaHSO4·H2O system during roasting[J]. Solid State Ionics, 2019, 339: 114983.
|
| [12] |
ZENG Xianlai, LI Jinhui, LIU Lili. Solving spent lithium-ion battery problems in China: Opportunities and challenges[J]. Renewable and Sustainable Energy Reviews, 2015, 52: 1759-1767.
|
| [13] |
KU Heesuk, JUNG Yeojin, Minsang JO, et al. Recycling of spent lithium-ion battery cathode materials by ammoniacal leaching[J]. Journal of Hazardous Materials, 2016, 313: 138-146.
|
| [14] |
王昊, 霍进达, 曲国瑞, 等. 退役锂电池正极材料资源化回收技术研究进展[J]. 化工进展, 2023, 42(5): 2702-2716.
|
|
WANG Hao, HUO Jinda, QU Guorui, et al. Research progress of positive electrode material recycling technology for retired lithium batteries[J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2702-2716.
|
| [15] |
HE Lipo, SUN Shuying, SONG Xingfu, et al. Leaching process for recovering valuable metals from the LiNi1/3Co1/3Mn1/3O2 cathode of lithium-ion batteries[J]. Waste Management, 2017, 64: 171-181.
|
| [16] |
MA Liwen, NIE Zuoren, XI Xiaoli, et al. Cobalt recovery from cobalt-bearing waste in sulphuric and citric acid systems[J]. Hydrometallurgy, 2013, 136: 1-7.
|
| [17] |
MESHRAM Pratima, PANDEY B D, MANKHAND T R. Hydrometallurgical processing of spent lithium ion batteries (LIBs) in the presence of a reducing agent with emphasis on kinetics of leaching[J]. Chemical Engineering Journal, 2015, 281: 418-427.
|
| [18] |
CHEN Xiangping, CHEN Yongbin, ZHOU Tao, et al. Hydrometallurgical recovery of metal values from sulfuric acid leaching liquor of spent lithium-ion batteries[J]. Waste Management, 2015, 38: 349-356.
|
| [19] |
ZHUANG Luqi, SUN Conghao, ZHOU Tao, et al. Recovery of valuable metals from LiNi0.5Co0.2Mn0.3O2 cathode materials of spent Li-ion batteries using mild mixed acid as leachant[J]. Waste Management, 2019, 85: 175-185.
|
| [20] |
LIU Yenchun, LIU Minchi. Reproduction of Li battery LiNi x Mn y Co1- x- y O2 positive electrode material from the recycling of waste battery[J]. International Journal of Hydrogen Energy, 2017, 42(29): 18189-18195.
|
| [21] |
ZHENG Rujuan, WANG Wenhui, DAI Yunkun, et al. A closed-loop process for recycling LiNi x Co y Mn(1- x-y)O2 from mixed cathode materials of lithium-ion batteries[J]. Green Energy & Environment, 2017, 2(1): 42-50.
|
| [22] |
JOULIÉ M, LAUCOURNET R, BILLY E. Hydrometallurgical process for the recovery of high value metals from spent lithium nickel cobalt aluminum oxide based lithium-ion batteries[J]. Journal of Power Sources, 2014, 247: 551-555.
|
| [23] |
JI Guanjun, Xing OU, ZHAO Ruirui, et al. Efficient utilization of scrapped LiFePO4 battery for novel synthesis of Fe2P2O7/C as candidate anode materials[J]. Resources, Conservation and Recycling, 2021, 174: 105802.
|
| [24] |
LI Li, LU Jun, ZHAI Longyu, et al. A facile recovery process for cathodes from spent lithium iron phosphate batteries by using oxalic acid[J]. CSEE Journal of Power and Energy Systems, 2018, 4(2): 219-225.
|
| [25] |
刘子潇, 张家靓, 杨成, 等. 热力学研究在锂离子电池回收中的应用[J]. 化工进展, 2021, 40(10): 5325-5336.
|
|
LIU Zixiao, ZHANG Jialiang, YANG Cheng, et al. Applications of thermodynamic research in recycling of lithium ion battery[J]. Chemical Industry and Engineering Progress, 2021, 40(10): 5325-5336.
|
| [26] |
ALMEIDA Jenifer Rigo, MOURA Mayra Nicoli, BARRADA Renan Vicente, et al. Composition analysis of the cathode active material of spent Li-ion batteries leached in citric acid solution: A study to monitor and assist recycling processes[J]. Science of the Total Environment, 2019, 685: 589-595.
|
| [27] |
LI Li, BIAN Yifan, ZHANG Xiaoxiao, et al. Process for recycling mixed-cathode materials from spent lithium-ion batteries and kinetics of leaching[J]. Waste Management, 2018, 71: 362-371.
|
| [28] |
ZHANG Jialiang, HU Juntao, ZHANG Wenjuan, et al. Efficient and economical recovery of lithium, cobalt, nickel, manganese from cathode scrap of spent lithium-ion batteries[J]. Journal of Cleaner Production, 2018, 204: 437-446.
|
| [29] |
LIU Fupeng, PENG Chao, MA Quanxin, et al. Selective lithium recovery and integrated preparation of high-purity lithium hydroxide products from spent lithium-ion batteries[J]. Separation and Purification Technology, 2021, 259: 118181.
|
| [30] |
HOSSAIN Rumana, KUMAR Uttam, SAHAJWALLA Veena. Selective thermal transformation of value added cobalt from spent lithium-ion batteries[J]. Journal of Cleaner Production, 2021, 293: 126140.
|
| [31] |
XIAO Jiefeng, LI Jia, XU Zhenming. Novel approach for in situ recovery of lithium carbonate from spent lithium ion batteries using vacuum metallurgy[J]. Environmental Science & Technology, 2017, 51(20): 11960-11966.
|
| [32] |
YANG Yue, HUANG Guoyong, XU Shengming, et al. Thermal treatment process for the recovery of valuable metals from spent lithium-ion batteries[J]. Hydrometallurgy, 2016, 165: 390-396.
|
| [33] |
LIU Pengcheng, XIAO Li, CHEN Yifeng, et al. Recovering valuable metals from LiNi x Mn y Co1- x- y O2 cathode materials of spent lithium ion batteries via a combination of reduction roasting and stepwise leaching[J]. Journal of Alloys and Compounds, 2019, 783: 743-752.
|
| [34] |
FEI Zitong, SU Yongyou, ZHA Yunchun, et al. Selective lithium extraction of cathode materials from spent lithium-ion batteries via low-valent salt assisted roasting[J]. Chemical Engineering Journal, 2023, 464: 142534.
|
| [35] |
TANG Yiqi, ZHANG Beilei, XIE Hongwei, et al. Recovery and regeneration of lithium cobalt oxide from spent lithium-ion batteries through a low-temperature ammonium sulfate roasting approach[J]. Journal of Power Sources, 2020, 474: 228596.
|
| [36] |
DING Hongbing, SU Yang, WANG Xinlu, et al. Enhancing the cycling stability of nickel-rich oxide cathode materials through a multifunctional CeO2 coating[J]. Journal of Colloid and Interface Science, 2025, 687: 118-130.
|
| [37] |
郭学益, 蔡海燕, 毛高强, 等. 富镍三元正极材料的研究进展[J]. 工程科学学报, 2025, 47(4): 697-716.
|
|
GUO Xueyi, CAI Haiyan, MAO Gaoqiang, et al. Research progress of Ni-rich ternary cathode materials[J]. Chinese Journal of Engineering, 2025, 47(4): 697-716.
|
| [38] |
LIU Lixia, LI Xiaoqing, ZHOU Kuan, et al. Highly improved cyclic stability of high voltage LiNi0.6Co0.2Mn0.2O2/graphite pouch cells via a silicon-based electrolyte additive[J]. ACS Applied Materials & Interfaces, 2025, 17(8): 12105-12116.
|
| [39] |
牛萍健, 谢天, 袁安, 等. 锂离子电池正极材料LiNi0.6Co0.2Mn0.2O2的研究进展[J]. 功能材料, 2018, 49(12): 12007-12016.
|
|
NIU Pingjian, XIE Tian, YUAN An, et al. Research progress of the LiNi0.6Co0.2Mn0.2O2 as a cathode material of lithium ion batteries[J]. Journal of Functional Materials, 2018, 49(12): 12007-12016.
|
| [40] |
姚凤仪, 郭德威, 桂明德.氧 硫 硒分族[M]. 北京: 科学出版社, 1990.
|
|
YAO Fengyi, GUO Dewei, GUI Deming. Oxygen, Sulfur, Selenium Subgroups[M]. Beijing: Science Press, 1990.
|
| [41] |
LIANG Longwei, DU Ke, LU Wei, et al. Synthesis and characterization of concentration-gradient LiNi0.6Co0.2Mn0.2O2 cathode material for lithium ion batteries[J]. Journal of Alloys and Compounds, 2014, 613: 296-305.
|
| [42] |
CHEN Bo, LIU Tianhui, JIAO Huan, et al. Phase transitions and energy storage properties of some compositions in the (1-x)Li2SO4– x Na2SO4 system[J]. Phase Transitions, 2014, 87(7): 629-640.
|
| [43] |
TALLMAN Killian R, WHEELER Garrett P, KERN Christopher J, et al. Nickel-rich nickel manganese cobalt (NMC622) cathode lithiation mechanism and extended cycling effects using operando X-ray absorption spectroscopy[J]. The Journal of Physical Chemistry C, 2021, 125(1): 58-73.
|
| [44] |
YUE Peng, WANG Zhixing, GUO Huajun, et al. A low temperature fluorine substitution on the electrochemical performance of layered LiNi0.8Co0.1Mn0.1O2- z F z cathode materials[J]. Electrochimica Acta, 2013, 92: 1-8.
|
| [45] |
SOHN Jong Rack. Correlation between acidic properties of nickel catalysts and catalytic activities for ethylene dimerization and butene isomerization[J]. Catalysis Surveys from Asia, 2004, 8(4): 249-263.
|
| [46] |
PARK Sanghyuk, KIM Duho, KU Heesuk, et al. The effect of Fe as an impurity element for sustainable resynthesis of Li [Ni1/3Co1/3Mn1/3]O2 cathode material from spent lithium-ion batteries[J]. Electrochimica Acta, 2019, 296: 814-822.
|
| [47] |
OKAZAKI Noriyasu, OSADA Seiji, TADA Akio. Deactivation by sulfur dioxide of alumina-based catalysts for selective catalytic reduction of nitrogen monoxide by ethene[J]. Applied Surface Science, 1997, 121/122: 396-399.
|
| [48] |
Katana NGALA J, CHERNOVA Natasha A, MA Miaomiao, et al. The synthesis, characterization and electrochemical behavior of the layered LiNi0.4Mn0.4Co0.2O2 compound[J]. Journal of Materials Chemistry, 2004, 14(2): 214-220.
|
| [49] |
MAO Jiakai, LI Jia, XU Zhengming. Coupling reactions and collapsing model in the roasting process of recycling metals from LiCoO2 batteries[J]. Journal of Cleaner Production, 2018, 205: 923-929.
|
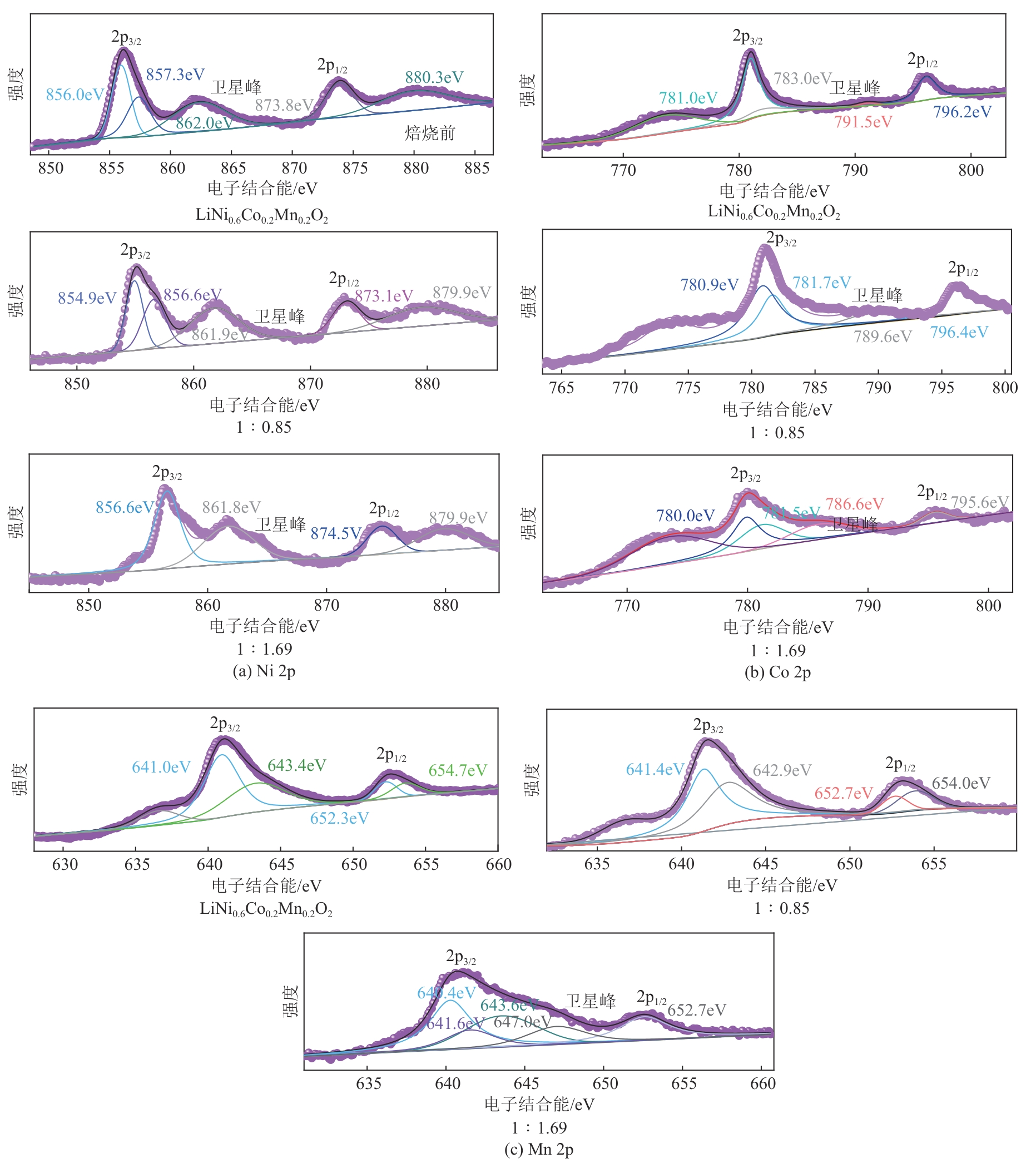
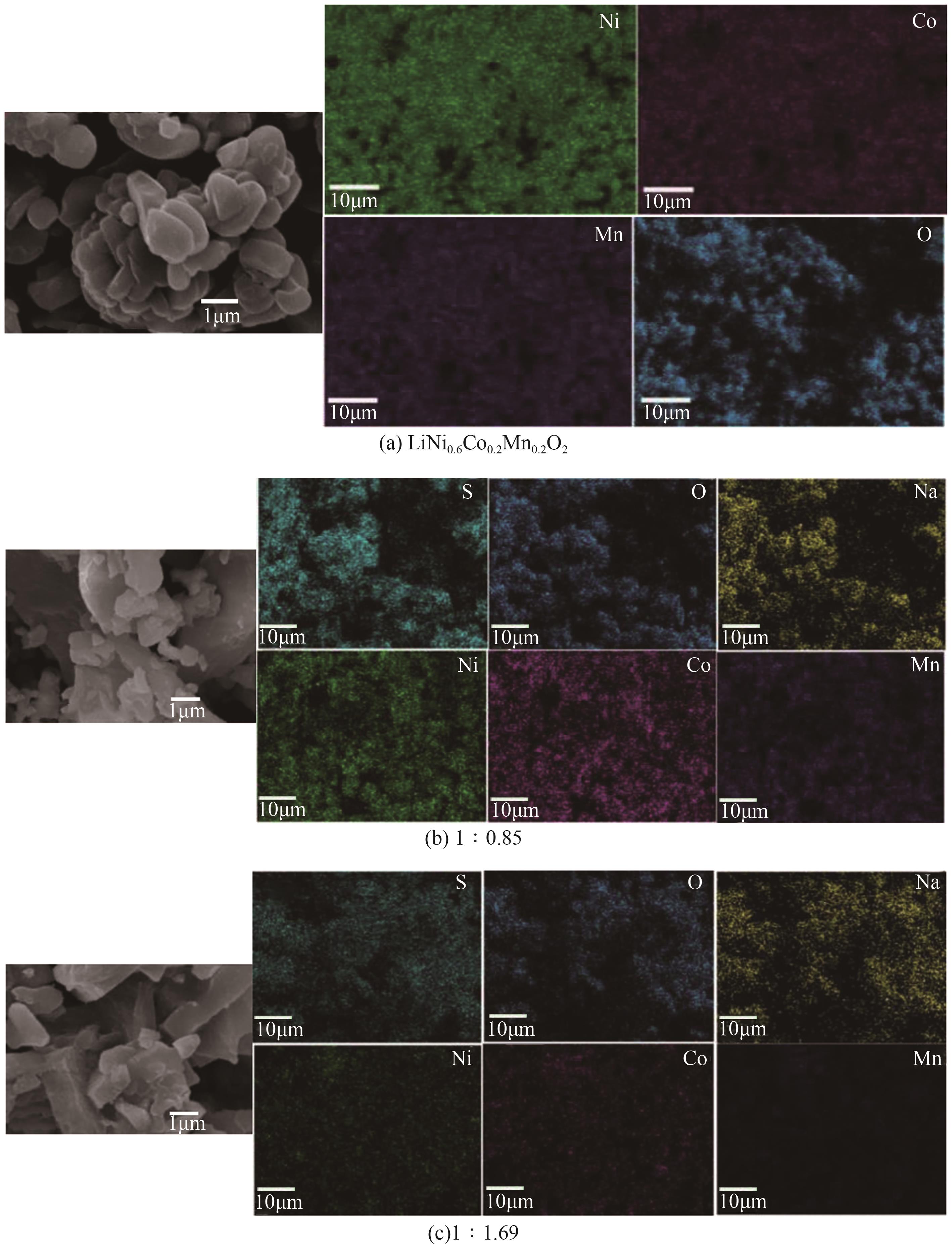
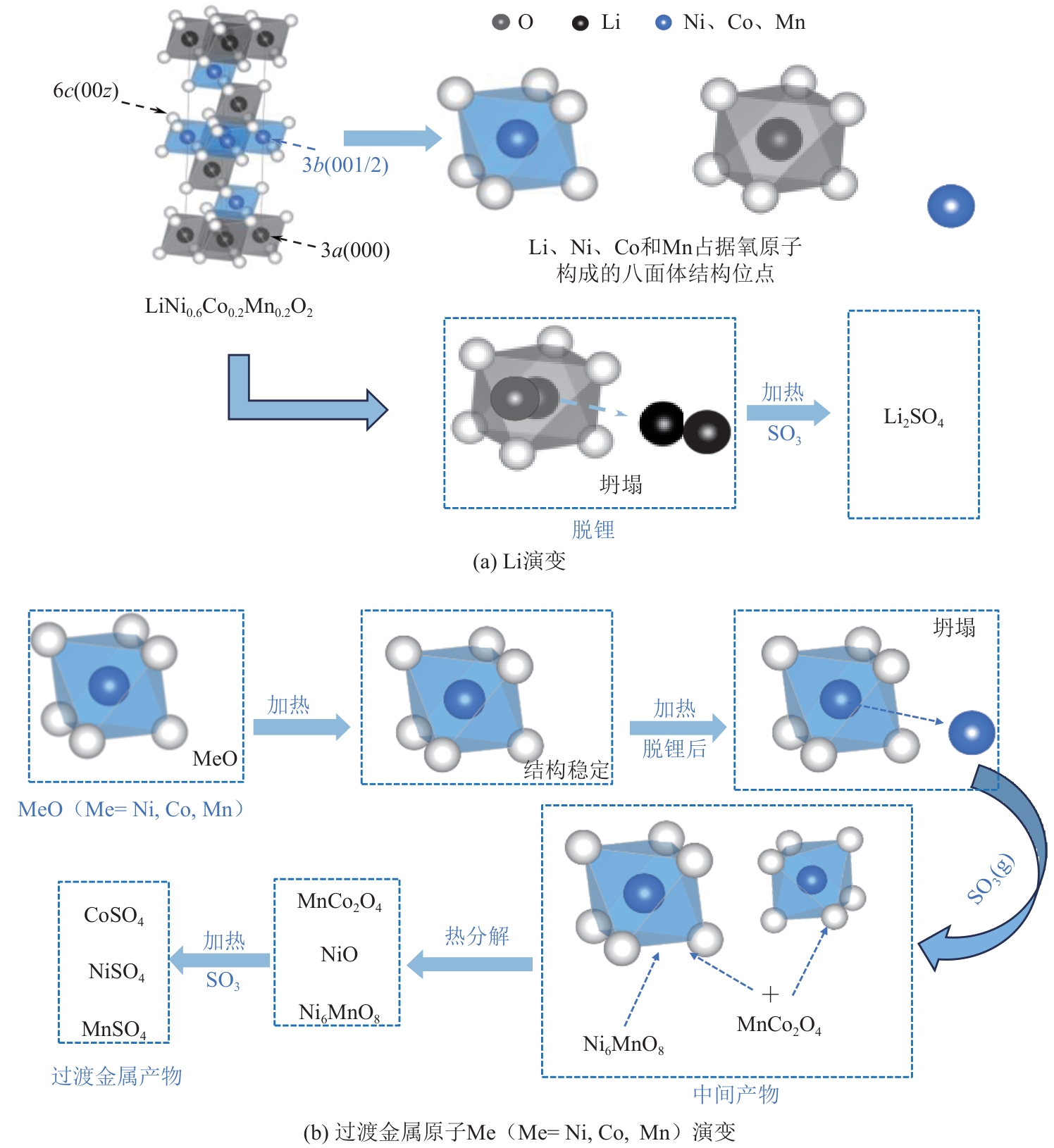
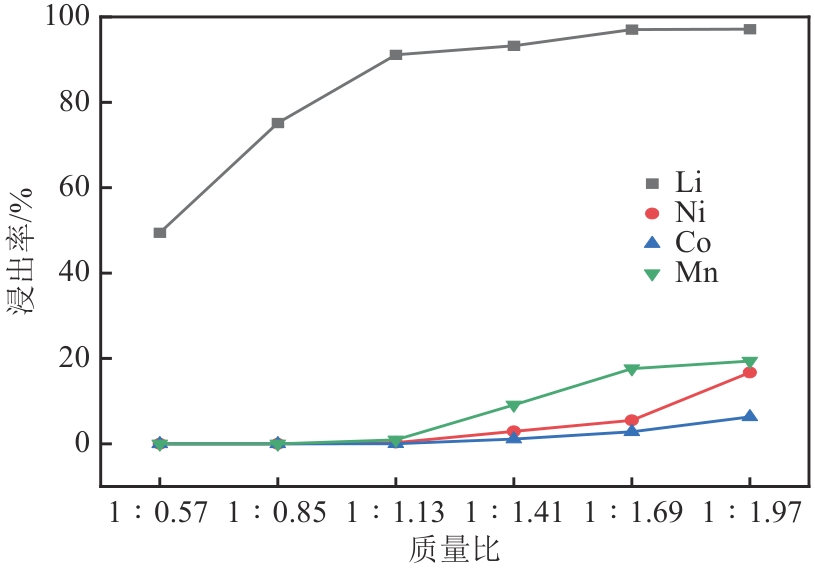
 ), 李彦强1(
), 李彦强1( ), 王大辉1(
), 王大辉1( ), 彭小平1, 宋晓龙1
), 彭小平1, 宋晓龙1
 ), LI Yanqiang1(
), LI Yanqiang1( ), WANG Dahui1(
), WANG Dahui1( ), PENG Xiaoping1, SONG Xiaolong1
), PENG Xiaoping1, SONG Xiaolong1