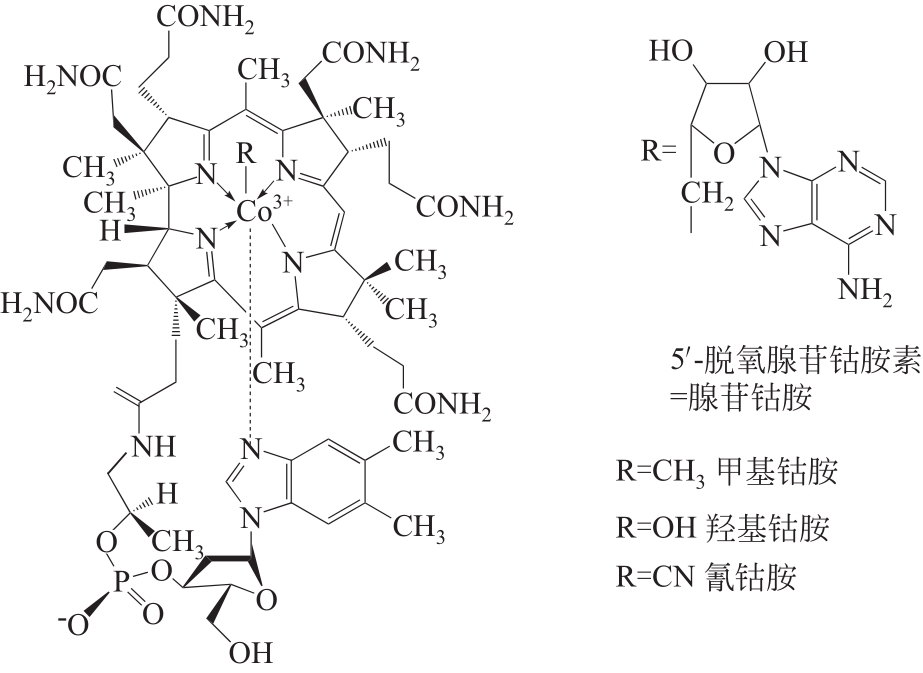
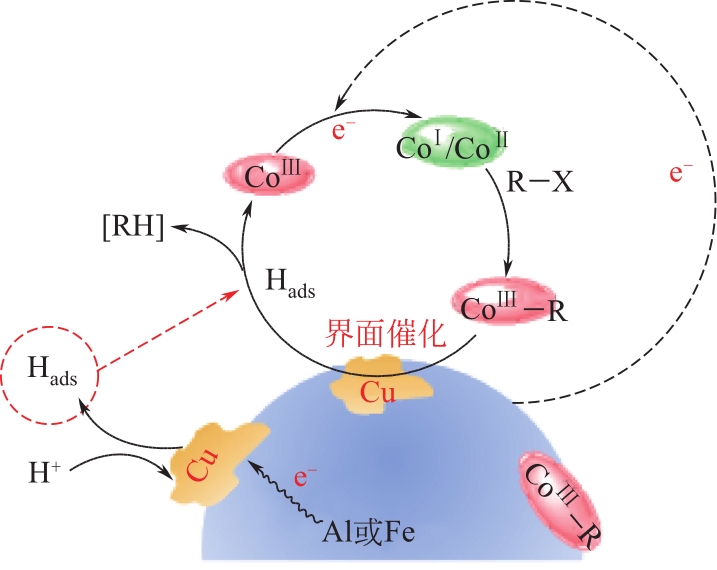
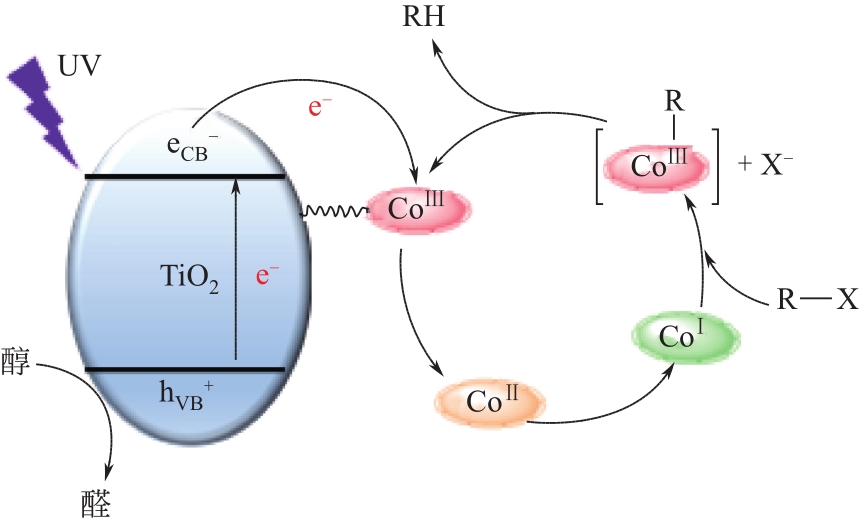
化工进展 ›› 2022, Vol. 41 ›› Issue (2): 708-720.DOI: 10.16085/j.issn.1000-6613.2021-0481
金属材料协同VB12催化卤代有机物降解研究进展
- 东华大学环境科学与工程学院,上海 201620
-
收稿日期:2021-03-10修回日期:2021-04-15出版日期:2022-02-05发布日期:2022-02-23 -
通讯作者:孙铸宇 -
作者简介:巫秀玲(1995—),女,硕士研究生,研究方向为水污染控制理论与技术。E-mail:wwxiuling@163.com 。 -
基金资助:国家自然科学基金青年基金(21906016);中央高校基本科研业务费专项资金项目(2232020D-25)
Catalytic degradation of halogenated organic compounds by synergistic system of metal materials and VB12: a review
WU Xiuling( ), ZHAO Xiaoxiang, SUN Zhuyu(
), ZHAO Xiaoxiang, SUN Zhuyu( )
)
- College of Environmental Science and Engineering, Donghua University, Shanghai 201620, China
-
Received:2021-03-10Revised:2021-04-15Online:2022-02-05Published:2022-02-23 -
Contact:SUN Zhuyu
摘要:
卤代有机物(HOCs)是环境领域的主要污染物类型之一,因其高持久性、毒性和生物累积性而受到广泛关注。还原脱卤技术是一种高效、低耗、易行的HOCs处理方式,而生物辅酶因子维生素B12(VB12)对HOCs的还原脱卤有很高的催化活性。本文总结了单金属、双金属、金属矿物及半导体等金属材料协同VB12催化降解HOCs的研究进展,讨论了每种金属材料与VB12的协同催化机制、降解HOCs机理特性、实际应用情况及局限性等。结果表明,金属材料协同VB12对HOCs还原脱卤具有加速电子转移、金属表面介导催化、活化碳-卤键等独特的优势和广阔的应用前景。最后,结合最新研究,指明目前该体系在机理研究、材料设计、实际应用、技术等方面所面临的挑战及未来的研究方向。
中图分类号:
引用本文
巫秀玲, 赵晓祥, 孙铸宇. 金属材料协同VB12催化卤代有机物降解研究进展[J]. 化工进展, 2022, 41(2): 708-720.
WU Xiuling, ZHAO Xiaoxiang, SUN Zhuyu. Catalytic degradation of halogenated organic compounds by synergistic system of metal materials and VB12: a review[J]. Chemical Industry and Engineering Progress, 2022, 41(2): 708-720.
| HOCs | 反应体系 | 反应条件 | 反应时间及 降解率 | 一级反应动力学 常数kobs | 降解产物 | 参考文献 |
|---|---|---|---|---|---|---|
四氯乙烯(PCE) 900μmol/L | Zn0 | 1g/L Zn0 | — | (0.186±0.028)d-1?m-2 | — | [ |
| Zn0-VB12 | 1g/L Zn0,45μmol/L VB12 | — | (1.44±0.23)d-1?m-2 | 三氯乙烯(TCE) | ||
| Fe0 | 1g/L Fe0 | — | (2.47±0.49)×10-3d-1?m-2 | — | ||
| Fe0-VB12 | 1g/L Fe0,45μmol/L VB12 | — | (3.00±0.21)×10-2d-1?m-2 | 三氯乙烯(TCE) | ||
三氯乙烯(TCE) 900μmol/L | Zn0 | 0.75g/L Zn0 | — | (1.15±0.8)×10-3d-1?m-2 | — | [ |
| Zn0-VB12 | 0.75g/L Zn0,45μmol/L VB12 | — | (0.645±0.035)d-1?m-2 | 1,2-二氯乙烯 (1,2-DCE) | ||
十氯酮(CLD) 10mg/L | DTT-VB12 | 25mL乙醇,13.56mg VB12,231mg DTT | 40min,76.0% | — | — | [ |
| Zn0-VB12 | 25mL乙醇,13.56mg VB12,65.9mg Zn0 | 20min,90.0% | — | 五氯茚 | ||
2,2',4,4'-四溴二苯(BDE47) 25mg/L | BM Al | 2g/L BM Al ,甲醇/水=40/60(体积比),pH=4 | 2h,15.6% | — | — | [ |
| BM Al-VB12 | 2g/L BM Al ,0.3mmol/L VB12,甲醇/水=40/60(体积比),pH=4 | 2h,97.6% | 2.94h-1 | 二苯醚 | ||
1,2,3-三氯丙烷 (1,2,3-TCP)12.27mg/L | μZVI | 0.5g/L μZVI | 未降解 | — | — | [ |
| μZVI-VB12 | 0.5g/L μZVI,1mmol/L VB12 | 7d,90.0% | (0.287±0.012)d-1 | 丙烯 | ||
1,2-二氯丙烷 (1,2-DCP)9.48mg/L | μZVI | 0.5g/L μZVI | 未降解 | — | — | |
| μZVI-VB12 | 0.5g/L μZVI,1mmol/L VB12 | 21d,70.0% | (0.0594±0.0060)d-1 | 丙烯 | ||
| 1,3-二氯丙烷(1,3-DCP)24.79mg/L | μZVI | 0.5g/L μZVI | 未降解 | — | — | |
| μZVI-VB12 | 0.5g/L μZVI,1mmol/L VB12 | 28d,90.0% | (0.0503±0.0038)d-1 | 环丙烷 | ||
1-氯丙烷(1-CP) 20.89mg/L | μZVI | 0.5g/L μZVI | 未降解 | — | — | |
| μZVI-VB12 | 0.5g/L μZVI,1mmol/L VB12 | 28d,40.0% | (0.0182±0.0044)d-1 | 丙烷 | ||
1,2-二氯乙烷 (1,2-DCA)13.52mg/L | μZVI | 0.5g/L μZVI | 未降解 | — | — | |
| μZVI-VB12 | 0.5g/L μZVI,1mmol/L VB12 | 28d,90.0% | 0.0847d-1 | 乙烯(未测到,基于计算) | ||
四氯乙烯(PCE) 0.1mmol/L | nZVI | 2g/L nFe0,pH= 8.2 | 未降解 | — | — | [ |
| nZVI-VB12 | 2g/L nZVI,0.5mmol/LVB12,pH=8.2 | 6h,80.0% | (0.25±0.01)h-1 | TCE和顺-DCE | ||
二氯甲烷(DCM) 26mg/L | TC | 0.177mmol/L TC,pH=9.6 | 未降解 | — | — | [ |
| TC-VB12 | 0.177mmol/L TC,0.004mmol/L VB12,pH=9.6 | 8h,80.0% | 0.24h-1 | — | ||
| TC-VB12-nCu0 | 0.177mmol/L TC,0.004mmol/L VB12,0.5g/L nCu0 ,pH=9.6 | 2h,99.0% | 1.35h-1 | 甲烷 | ||
全氟辛酸(PFOA) 50mg/L | NaBH4 | 11.25mmol/L NaBH4,pH=4.0,T=70℃ | 18h,3.1% | — | — | [ |
| NaBH4-nCu0-VB12 | 11.25mmol/L NaBH4,0.2mmol/L VB12,2g/L nCu0,pH=4.0,T=70oC | 18h,15.5% | — | — | ||
| nZVI-nCu0-VB12 | 25g/L nZVI,2g/L nCu0,0.2mmol/L VB12 ,pH=4.0,T=70oC | 18h,29.9% | — | — | ||
| TC | 45mmol/L TC,pH=4.0,T=70oC | 18h,34.5% | — | — | ||
| TC-nCu0-VB12 | 45mmol/L TC,2g/L nCu0,0.2mmol/L VB12,pH=4.0,T=70oC | 18h,65.0% | — | — | ||
全氟辛磺酸(PFOS) 200μmol/L | nZVI | 2g/L nZVI,T=70oC | 未降解 | — | — | [ |
| nZVI-VB12 | 2g/L nZVI,200μmol/L VB12,T=70oC | 10d,53.8% | 6.4×10-2d-1 | PFHxS、PFHpS、PFHxA、PFOA、PFUdA |
表1 单金属材料协同VB12催化还原降解HOCs
| HOCs | 反应体系 | 反应条件 | 反应时间及 降解率 | 一级反应动力学 常数kobs | 降解产物 | 参考文献 |
|---|---|---|---|---|---|---|
四氯乙烯(PCE) 900μmol/L | Zn0 | 1g/L Zn0 | — | (0.186±0.028)d-1?m-2 | — | [ |
| Zn0-VB12 | 1g/L Zn0,45μmol/L VB12 | — | (1.44±0.23)d-1?m-2 | 三氯乙烯(TCE) | ||
| Fe0 | 1g/L Fe0 | — | (2.47±0.49)×10-3d-1?m-2 | — | ||
| Fe0-VB12 | 1g/L Fe0,45μmol/L VB12 | — | (3.00±0.21)×10-2d-1?m-2 | 三氯乙烯(TCE) | ||
三氯乙烯(TCE) 900μmol/L | Zn0 | 0.75g/L Zn0 | — | (1.15±0.8)×10-3d-1?m-2 | — | [ |
| Zn0-VB12 | 0.75g/L Zn0,45μmol/L VB12 | — | (0.645±0.035)d-1?m-2 | 1,2-二氯乙烯 (1,2-DCE) | ||
十氯酮(CLD) 10mg/L | DTT-VB12 | 25mL乙醇,13.56mg VB12,231mg DTT | 40min,76.0% | — | — | [ |
| Zn0-VB12 | 25mL乙醇,13.56mg VB12,65.9mg Zn0 | 20min,90.0% | — | 五氯茚 | ||
2,2',4,4'-四溴二苯(BDE47) 25mg/L | BM Al | 2g/L BM Al ,甲醇/水=40/60(体积比),pH=4 | 2h,15.6% | — | — | [ |
| BM Al-VB12 | 2g/L BM Al ,0.3mmol/L VB12,甲醇/水=40/60(体积比),pH=4 | 2h,97.6% | 2.94h-1 | 二苯醚 | ||
1,2,3-三氯丙烷 (1,2,3-TCP)12.27mg/L | μZVI | 0.5g/L μZVI | 未降解 | — | — | [ |
| μZVI-VB12 | 0.5g/L μZVI,1mmol/L VB12 | 7d,90.0% | (0.287±0.012)d-1 | 丙烯 | ||
1,2-二氯丙烷 (1,2-DCP)9.48mg/L | μZVI | 0.5g/L μZVI | 未降解 | — | — | |
| μZVI-VB12 | 0.5g/L μZVI,1mmol/L VB12 | 21d,70.0% | (0.0594±0.0060)d-1 | 丙烯 | ||
| 1,3-二氯丙烷(1,3-DCP)24.79mg/L | μZVI | 0.5g/L μZVI | 未降解 | — | — | |
| μZVI-VB12 | 0.5g/L μZVI,1mmol/L VB12 | 28d,90.0% | (0.0503±0.0038)d-1 | 环丙烷 | ||
1-氯丙烷(1-CP) 20.89mg/L | μZVI | 0.5g/L μZVI | 未降解 | — | — | |
| μZVI-VB12 | 0.5g/L μZVI,1mmol/L VB12 | 28d,40.0% | (0.0182±0.0044)d-1 | 丙烷 | ||
1,2-二氯乙烷 (1,2-DCA)13.52mg/L | μZVI | 0.5g/L μZVI | 未降解 | — | — | |
| μZVI-VB12 | 0.5g/L μZVI,1mmol/L VB12 | 28d,90.0% | 0.0847d-1 | 乙烯(未测到,基于计算) | ||
四氯乙烯(PCE) 0.1mmol/L | nZVI | 2g/L nFe0,pH= 8.2 | 未降解 | — | — | [ |
| nZVI-VB12 | 2g/L nZVI,0.5mmol/LVB12,pH=8.2 | 6h,80.0% | (0.25±0.01)h-1 | TCE和顺-DCE | ||
二氯甲烷(DCM) 26mg/L | TC | 0.177mmol/L TC,pH=9.6 | 未降解 | — | — | [ |
| TC-VB12 | 0.177mmol/L TC,0.004mmol/L VB12,pH=9.6 | 8h,80.0% | 0.24h-1 | — | ||
| TC-VB12-nCu0 | 0.177mmol/L TC,0.004mmol/L VB12,0.5g/L nCu0 ,pH=9.6 | 2h,99.0% | 1.35h-1 | 甲烷 | ||
全氟辛酸(PFOA) 50mg/L | NaBH4 | 11.25mmol/L NaBH4,pH=4.0,T=70℃ | 18h,3.1% | — | — | [ |
| NaBH4-nCu0-VB12 | 11.25mmol/L NaBH4,0.2mmol/L VB12,2g/L nCu0,pH=4.0,T=70oC | 18h,15.5% | — | — | ||
| nZVI-nCu0-VB12 | 25g/L nZVI,2g/L nCu0,0.2mmol/L VB12 ,pH=4.0,T=70oC | 18h,29.9% | — | — | ||
| TC | 45mmol/L TC,pH=4.0,T=70oC | 18h,34.5% | — | — | ||
| TC-nCu0-VB12 | 45mmol/L TC,2g/L nCu0,0.2mmol/L VB12,pH=4.0,T=70oC | 18h,65.0% | — | — | ||
全氟辛磺酸(PFOS) 200μmol/L | nZVI | 2g/L nZVI,T=70oC | 未降解 | — | — | [ |
| nZVI-VB12 | 2g/L nZVI,200μmol/L VB12,T=70oC | 10d,53.8% | 6.4×10-2d-1 | PFHxS、PFHpS、PFHxA、PFOA、PFUdA |
| HOCs | 反应体系 | 反应条件 | 反应时间及降解率 | 一级反应动力学常数kobs/h-1 | 降解产物 | 参考文献 |
|---|---|---|---|---|---|---|
二氯甲烷(DCM) 10mg/L | VB12 | 20mg/L VB12,pH=8.2 | — | 0.005 | — | [ |
| Fe0 | 20g/L Fe0,pH=8.2 | — | 0.006 | — | ||
| Fe/Cu | 20g/L Cu/Fe[5%(质量分数)Cu],pH=8.2 | — | 0.018 | — | ||
| Fe0-VB12 | 20g/L Fe0,20mg/L VB12,pH=8.2 | — | 0.030 | — | ||
| Fe/Cu-VB12 | 20g/L Cu/Fe[5%(质量分数)Cu],20mg/L VB12,pH=8.2 | 8h,100.0% | 0.444 | 一氯甲烷、甲烷 | ||
二氯甲烷(DCM) 26mg/L | Al0 | 50g/L Al0,pH=10 | 未降解 | — | — | [ |
| Cu/Al0 | 60g/L Cu/Al0 [20%(质量分数)Cu],pH=10 | 4h,42.0% | 0.11 | — | ||
| Al0-VB12 | 50g/L Al0,1mmol/L VB12,pH=10 | — | 0.07 | — | ||
| Cu/Al0-VB12 | 60g/L Cu/Al0 [20%(质量分数)Cu],1mmol/L VB12,pH=10 | 2h,98.0% | 1.46 | 一氯甲烷、甲烷 | ||
五氯酚(PCP) 20mg/L | nFe0 | 5g/L nFe0,pH=8.5 | 8h,75.0% | 0.564 | 四氯酚 | [ |
| nFe/Cu | 5g/L nFe/Cu[10%(质量分数)Cu],pH=8.5 | 4h,100.0% | 2.375 | 四氯酚和三氯酚 | ||
| nFe/Cu-VB12 | 5g/L nFe/Cu[10%(质量分数)Cu],20mg/LVB12,pH=8.5 | 3h,100.0% | 3.272 | 二氯酚、氯酚和苯酚 |
表2 双金属材料协同VB12催化还原降解HOCs
| HOCs | 反应体系 | 反应条件 | 反应时间及降解率 | 一级反应动力学常数kobs/h-1 | 降解产物 | 参考文献 |
|---|---|---|---|---|---|---|
二氯甲烷(DCM) 10mg/L | VB12 | 20mg/L VB12,pH=8.2 | — | 0.005 | — | [ |
| Fe0 | 20g/L Fe0,pH=8.2 | — | 0.006 | — | ||
| Fe/Cu | 20g/L Cu/Fe[5%(质量分数)Cu],pH=8.2 | — | 0.018 | — | ||
| Fe0-VB12 | 20g/L Fe0,20mg/L VB12,pH=8.2 | — | 0.030 | — | ||
| Fe/Cu-VB12 | 20g/L Cu/Fe[5%(质量分数)Cu],20mg/L VB12,pH=8.2 | 8h,100.0% | 0.444 | 一氯甲烷、甲烷 | ||
二氯甲烷(DCM) 26mg/L | Al0 | 50g/L Al0,pH=10 | 未降解 | — | — | [ |
| Cu/Al0 | 60g/L Cu/Al0 [20%(质量分数)Cu],pH=10 | 4h,42.0% | 0.11 | — | ||
| Al0-VB12 | 50g/L Al0,1mmol/L VB12,pH=10 | — | 0.07 | — | ||
| Cu/Al0-VB12 | 60g/L Cu/Al0 [20%(质量分数)Cu],1mmol/L VB12,pH=10 | 2h,98.0% | 1.46 | 一氯甲烷、甲烷 | ||
五氯酚(PCP) 20mg/L | nFe0 | 5g/L nFe0,pH=8.5 | 8h,75.0% | 0.564 | 四氯酚 | [ |
| nFe/Cu | 5g/L nFe/Cu[10%(质量分数)Cu],pH=8.5 | 4h,100.0% | 2.375 | 四氯酚和三氯酚 | ||
| nFe/Cu-VB12 | 5g/L nFe/Cu[10%(质量分数)Cu],20mg/LVB12,pH=8.5 | 3h,100.0% | 3.272 | 二氯酚、氯酚和苯酚 |
| HOCs | 反应体系 | 反应条件 | 反应时间及 降解率 | 一级反应动力学 常数kobs/h-1 | 降解产物 | 参考 文献 |
|---|---|---|---|---|---|---|
四氯化碳(CT) 5mmol/L | FeS | 200mmol/L FeS,pH=8 | 30min,70.0% | — | — | [ |
| FeS-VB12 | 200mmol/L FeS,4mmol/L VB12,pH=8 | 30min,95.0% | 1.91 | 氯仿,二氯甲烷 | ||
四氯乙烯(PCE) 0.1mmol/L | mFeS | 4.17g/L mFeS,pH=8.3 | 120h,10.0% | — | — | [ |
| nFeS | 4.17g/L nFeS,pH=8.3 | 120h,20.0% | 0.0013±0.001 | — | ||
| mFeS-VB12 | 4.17g/L mFeS,0.1mmol/L VB12,pH=8.3 | 120h,60.0% | — | — | ||
| nFeS-VB12 | 4.17g/L nFeS,0.1mmol/L VB12,pH=8.3 | 120h,100.0% | 0.188±0.003 | 乙炔,1,3-丁二烯 | ||
| PCE 0.035mmol/L | nFeS | 4.17g/L nFeS,pH=12 | 120h,25.0% | — | — | [ |
| nFeS-VB12 | 4.17g/L nFeS,0.1mmol/L VB12 ,pH=12 | 15h,95.0% | 0.2036 | 乙炔,三氯乙烯,乙烯 | ||
| PCE 0.1mmol/L | S2- | 0.1mmol/LS2-,pH=8.3 | 未降解 | — | — | [ |
| nFeS | 4.17g/L nFeS,pH=8.3 | 220h,10.0% | — | — | ||
| nFeS-S2- | 0.1mmol/L S2-,4.17g/L nFeS,pH=8.3 | 220h,12.0% | — | — | ||
| VB12-S2- | 1mmol/L VB12,0.1mmol/L S2-,pH=8.3 | 220h,18.0% | — | — | ||
| nFeS-VB12 | 4.17g/L nFeS,1mmol/L VB12,pH=8.3 | 220h,32.0% | — | — | ||
| nFeS-VB12-S2- | 0.1mmol/L S2-,4.17g/L nFeS,1.0mmol/L VB12,pH=8.3 | 220h,82.0% | 0.008±0.001 | 乙炔,乙烯,1,3-丁二烯,三氯乙烯 | ||
| PCE 0.25mmol/L | nFeS | 4.17g/L nFeS,pH=12 | 5h,15.0% | — | — | [ |
| nFeS-水泥浆 | 4.17g/L nFeS,水泥与溶液的质量比为0.1,pH=12 | 5h,40.0% | — | — | ||
| nFeS-VB12 | 4.17g/L nFeS,0.1mmol/L VB12,pH=12 | 5h,40.0% | 0.10 | — | ||
| nFeS-VB12-水泥浆 | 4.17g/L nFeS,0.1mmol/LVB12,水泥与溶液的质量比为0.1,pH=12 | 5h,99.0% | 0.57 | 乙烯,乙炔和C3~C4烃 |
表3 金属矿物协同VB12催化还原降解HOCs
| HOCs | 反应体系 | 反应条件 | 反应时间及 降解率 | 一级反应动力学 常数kobs/h-1 | 降解产物 | 参考 文献 |
|---|---|---|---|---|---|---|
四氯化碳(CT) 5mmol/L | FeS | 200mmol/L FeS,pH=8 | 30min,70.0% | — | — | [ |
| FeS-VB12 | 200mmol/L FeS,4mmol/L VB12,pH=8 | 30min,95.0% | 1.91 | 氯仿,二氯甲烷 | ||
四氯乙烯(PCE) 0.1mmol/L | mFeS | 4.17g/L mFeS,pH=8.3 | 120h,10.0% | — | — | [ |
| nFeS | 4.17g/L nFeS,pH=8.3 | 120h,20.0% | 0.0013±0.001 | — | ||
| mFeS-VB12 | 4.17g/L mFeS,0.1mmol/L VB12,pH=8.3 | 120h,60.0% | — | — | ||
| nFeS-VB12 | 4.17g/L nFeS,0.1mmol/L VB12,pH=8.3 | 120h,100.0% | 0.188±0.003 | 乙炔,1,3-丁二烯 | ||
| PCE 0.035mmol/L | nFeS | 4.17g/L nFeS,pH=12 | 120h,25.0% | — | — | [ |
| nFeS-VB12 | 4.17g/L nFeS,0.1mmol/L VB12 ,pH=12 | 15h,95.0% | 0.2036 | 乙炔,三氯乙烯,乙烯 | ||
| PCE 0.1mmol/L | S2- | 0.1mmol/LS2-,pH=8.3 | 未降解 | — | — | [ |
| nFeS | 4.17g/L nFeS,pH=8.3 | 220h,10.0% | — | — | ||
| nFeS-S2- | 0.1mmol/L S2-,4.17g/L nFeS,pH=8.3 | 220h,12.0% | — | — | ||
| VB12-S2- | 1mmol/L VB12,0.1mmol/L S2-,pH=8.3 | 220h,18.0% | — | — | ||
| nFeS-VB12 | 4.17g/L nFeS,1mmol/L VB12,pH=8.3 | 220h,32.0% | — | — | ||
| nFeS-VB12-S2- | 0.1mmol/L S2-,4.17g/L nFeS,1.0mmol/L VB12,pH=8.3 | 220h,82.0% | 0.008±0.001 | 乙炔,乙烯,1,3-丁二烯,三氯乙烯 | ||
| PCE 0.25mmol/L | nFeS | 4.17g/L nFeS,pH=12 | 5h,15.0% | — | — | [ |
| nFeS-水泥浆 | 4.17g/L nFeS,水泥与溶液的质量比为0.1,pH=12 | 5h,40.0% | — | — | ||
| nFeS-VB12 | 4.17g/L nFeS,0.1mmol/L VB12,pH=12 | 5h,40.0% | 0.10 | — | ||
| nFeS-VB12-水泥浆 | 4.17g/L nFeS,0.1mmol/LVB12,水泥与溶液的质量比为0.1,pH=12 | 5h,99.0% | 0.57 | 乙烯,乙炔和C3~C4烃 |
| HOCs | 反应体系 | 反应条件 | 反应时间及 降解率 | 降解产物 | 参考文献 |
|---|---|---|---|---|---|
苯乙基溴(PhCH2CH2Br) 3mmol/L | TiO2 | 0.67g/L TiO2,λ=365nm,30mL EtOH ,N2氛围 | 微量 | 微量 | [ |
| TiO2-VB12 | 0.67g/L TiO2-VB12,λ=365nm,30mL EtOH ,N2氛围 | 24h,95.0% | 乙苯,2,3-二苯基丁烷 | ||
苄基溴(PhCH2Br) 3mmol/L | TiO2-VB12 | 0.67g/L TiO2-VB12,λ=365nm,30mL EtOH ,N2氛围 | 24h,99.0% | 甲苯,二芳基乙烷 | |
1,1-双(4-氯苯基)-2,2,2- 三氯乙烷(DDT)3mmol/L | TiO2-VB12 | 0.67g/L TiO2-VB12,λ=365nm,30mL EtOH ,N2氛围 | 24h,99.0% | DDD、TTDB、DDA乙酯 | |
1,1-双(4-氯苯基)-2,2- 二氯乙烷(DDD)3mmol/L | TiO2-VB12 | 0.67g/L TiO2-VB12,λ=365nm,30mL EtOH ,N2氛围 | 24h,82.0% | DDMU、DDMS、DCS | |
三氯甲苯(PhCCl3) 3mmol/L | TiO2 | 0.167g/L TiO2,λ=365nm,6mL MeOH,UV,空气 | 3h,3.0% | 苯甲酸甲酯 | [ |
| Pt-TiO2 | 0.167g/L Pt-TiO2[0.15%(质量分数)Pt],λ=365nm,6mL MeOH ,UV,空气 | 3h,10.0% | 苯甲酸甲酯 | ||
| TiO2-VB12 | 0.167g/L TiO2,0.0228mmol/L VB12,λ=365nm,6mL MeOH,UV,空气 | 3h,99.0% | 苯甲酸甲酯 | ||
| TiO2-VB12 | 0.167g/L TiO2,0.0228mmol/L VB12,λ=365nm,6mL MeOH,UV,O2 | 3h,91.0% | 苯甲酸甲酯 | ||
| DDT 2.4mmol/L | TiO2 | 0.6g/L TiO2,λ=420nm,0.2mmol/L TEOA,5mL MeOH | 未降解 | — | [ |
| TiO2-VB12 | 0.6g/L TiO2-VB12,λ=420nm,0.2mmol/L TEOA,5mL MeOH | 30min,24.0% | DDD | ||
| RuⅡ-TiO2 | 0.6g/L RuⅡ-TiO2,λ=420nm,0.2mmol/L TEOA,5mL MeOH | 30min,13.0% | DDD | ||
| VB12-RuⅡ-TiO2 | 0.6g/L VB12-RuⅡ-TiO2,λ=420nm,0.2mmol/L TEOA,5mL MeOH | 30min,100% | DDMU、DDMS、TTDB、DDA甲酯 |
表4 半导体材料协同VB12光催化还原降解HOCs
| HOCs | 反应体系 | 反应条件 | 反应时间及 降解率 | 降解产物 | 参考文献 |
|---|---|---|---|---|---|
苯乙基溴(PhCH2CH2Br) 3mmol/L | TiO2 | 0.67g/L TiO2,λ=365nm,30mL EtOH ,N2氛围 | 微量 | 微量 | [ |
| TiO2-VB12 | 0.67g/L TiO2-VB12,λ=365nm,30mL EtOH ,N2氛围 | 24h,95.0% | 乙苯,2,3-二苯基丁烷 | ||
苄基溴(PhCH2Br) 3mmol/L | TiO2-VB12 | 0.67g/L TiO2-VB12,λ=365nm,30mL EtOH ,N2氛围 | 24h,99.0% | 甲苯,二芳基乙烷 | |
1,1-双(4-氯苯基)-2,2,2- 三氯乙烷(DDT)3mmol/L | TiO2-VB12 | 0.67g/L TiO2-VB12,λ=365nm,30mL EtOH ,N2氛围 | 24h,99.0% | DDD、TTDB、DDA乙酯 | |
1,1-双(4-氯苯基)-2,2- 二氯乙烷(DDD)3mmol/L | TiO2-VB12 | 0.67g/L TiO2-VB12,λ=365nm,30mL EtOH ,N2氛围 | 24h,82.0% | DDMU、DDMS、DCS | |
三氯甲苯(PhCCl3) 3mmol/L | TiO2 | 0.167g/L TiO2,λ=365nm,6mL MeOH,UV,空气 | 3h,3.0% | 苯甲酸甲酯 | [ |
| Pt-TiO2 | 0.167g/L Pt-TiO2[0.15%(质量分数)Pt],λ=365nm,6mL MeOH ,UV,空气 | 3h,10.0% | 苯甲酸甲酯 | ||
| TiO2-VB12 | 0.167g/L TiO2,0.0228mmol/L VB12,λ=365nm,6mL MeOH,UV,空气 | 3h,99.0% | 苯甲酸甲酯 | ||
| TiO2-VB12 | 0.167g/L TiO2,0.0228mmol/L VB12,λ=365nm,6mL MeOH,UV,O2 | 3h,91.0% | 苯甲酸甲酯 | ||
| DDT 2.4mmol/L | TiO2 | 0.6g/L TiO2,λ=420nm,0.2mmol/L TEOA,5mL MeOH | 未降解 | — | [ |
| TiO2-VB12 | 0.6g/L TiO2-VB12,λ=420nm,0.2mmol/L TEOA,5mL MeOH | 30min,24.0% | DDD | ||
| RuⅡ-TiO2 | 0.6g/L RuⅡ-TiO2,λ=420nm,0.2mmol/L TEOA,5mL MeOH | 30min,13.0% | DDD | ||
| VB12-RuⅡ-TiO2 | 0.6g/L VB12-RuⅡ-TiO2,λ=420nm,0.2mmol/L TEOA,5mL MeOH | 30min,100% | DDMU、DDMS、TTDB、DDA甲酯 |
| 1 | WŁODARCZYK-MAKUŁA M, WIŚNIOWSKA E. Halogenated organic compounds in water and in wastewater[J]. Civil and Environmental Engineering Reports, 2019, 29(4): 236-247. |
| 2 | COVACI A, HARRAD S, ABDALLAH M A E, et al. Novel brominated flame retardants: a review of their analysis, environmental fate and behaviour[J]. Environment International, 2011, 37(2): 532-556. |
| 3 | STAPLETON H M, SHARMA S, GETZINGER G, et al. Novel and high volume use flame retardants in US couches reflective of the 2005 PentaBDE phase out[J]. Environmental Science & Technology, 2012, 46(24): 13432-13439. |
| 4 | TERÁN J E, ZAMBRANO C H, MORA J R, et al. Theoretical investigation of the mechanism for the reductive dehalogenation of methyl halides mediated by the CoI -based compounds cobalamin and cobaloxime[J]. Journal of Molecular Modeling, 2018, 24(11): 316. |
| 5 | ZHANG Meng, SHI Qin, SONG Xiaozhe, et al. Recent electrochemical methods in electrochemical degradation of halogenated organics: a review[J]. Environmental Science and Pollution Research International, 2019, 26(11): 10457-10486. |
| 6 | ZHANG Haifeng, YANG Min. Characterization of brominated disinfection byproducts formed during chloramination of fulvic acid in the presence of bromide[J]. Science of the Total Environment, 2018, 627: 118-124. |
| 7 | GE Linke, CHEN Jingwen, WEI Xiaoxuan, et al. Aquatic photochemistry of fluoroquinolone antibiotics: kinetics, pathways, and multivariate effects of main water constituents[J]. Environmental Science & Technology, 2010, 44(7): 2400-2405. |
| 8 | GUO Ning, WANG Yunkun, YAN Lei, et al. Effect of bio-electrochemical system on the fate and proliferation of chloramphenicol resistance genes during the treatment of chloramphenicol wastewater[J]. Water Research, 2017, 117: 95-101. |
| 9 | EL-ATHMAN F, JEKEL M, PUTSCHEW A. Reaction kinetics of corrinoid-mediated deiodination of iodinated X-ray contrast media and other iodinated organic compounds[J]. Chemosphere, 2019, 234: 971-977. |
| 10 | 李松旌, 樊向阳, 崔二苹, 等. PPCPs在土壤-作物系统行为特征及环境风险的研究进展[J]. 化工进展, 2021, 40(5): 2827-2838. |
| LI Songjing, FAN Xiangyang, CUI Erping, et al. Advances in behavioral characteristics and environmental risks of PPCPs in soil-crop systems[J]. Chemical Industry and Engineering Progress, 2021, 40(5): 2827-2838. | |
| 11 | HILDEBRANDT A, GUILLAMÓN M, LACORTE S, et al. Impact of pesticides used in agriculture and vineyards to surface and groundwater quality (North Spain)[J]. Water Research, 2008, 42(13): 3315-3326. |
| 12 | BOYD R A. Herbicides and herbicide degradates in shallow groundwater and the Cedar River near a municipal well field, Cedar Rapids, Iowa[J]. The Science of the Total Environment, 2000, 248(2/3): 241-253. |
| 13 | CEREJEIRA M J, VIANA P, BATISTA S, et al. Pesticides in Portuguese surface and ground waters[J]. Water Research, 2003, 37(5): 1055-1063. |
| 14 | XUE Nandong, XU Xiaobai, JIN Zuliang. Screening 31 endocrine-disrupting pesticides in water and surface sediment samples from Beijing Guanting reservoir[J]. Chemosphere, 2005, 61(11): 1594-1606. |
| 15 | 程荣, 王建龙, 张伟贤. 纳米金属铁降解有机卤化物的研究进展[J]. 化学进展, 2006, 18(1): 93-99. |
| CHENG Rong, WANG Jianlong, ZHANG Weixian. The research progress on degradation of halogenated organic compounds by nano iron[J]. Progress in Chemistry, 2006, 18(1): 93-99. | |
| 16 | BIGOT M, HAWKER D W, CROPP R, et al. Spring melt and the redistribution of organochlorine pesticides in the sea-ice environment: a comparative study between Arctic and Antarctic regions[J]. Environmental Science & Technology, 2017, 51(16): 8944-8952. |
| 17 | MARIN M, MIRANDA M A, MARIN M L. Correction: a comprehensive mechanistic study on the visible-light photocatalytic reductive dehalogenation of haloaromatics mediated by Ru(bpy)3Cl2[J]. Catalysis Science & Technology, 2019, 9(6): 1543. |
| 18 | MENG Pingping, DENG Shubo, LU Xinyu, et al. Role of air bubbles overlooked in the adsorption of perfluorooctanesulfonate on hydrophobic carbonaceous adsorbents[J]. Environmental Science & Technology, 2014, 48(23): 13785-13792. |
| 19 | KOLOMYTSEVA M, FERRARONI M, CHERNYKH A, et al. Structural basis for the substrate specificity and the absence of dehalogenation activity in 2-chloromuconate cycloisomerase from Rhodococcus opacus 1CP[J]. Biochimica et Biophysica Acta, 2014, 1844(9): 1541-1549. |
| 20 | YANG Bo, JIANG Chaojin, YU Gang, et al. Highly efficient electrochemical degradation of perfluorooctanoic acid (PFOA) by F-doped Ti/SnO2 electrode[J]. Journal of Hazardous Materials, 2015, 299: 417-424. |
| 21 | LUO Jin, HU Jiwei, WEI Xionghui, et al. Dehalogenation of persistent halogenated organic compounds: a review of computational studies and quantitative structure-property relationships[J]. Chemosphere, 2015, 131: 17-33. |
| 22 | LI Xuchun, MA Jun, LIU Guifang, et al. Efficient reductive dechlorination of monochloroacetic acid by sulfite/UV process[J]. Environmental Science & Technology, 2012, 46(13): 7342-7349. |
| 23 | 李长芳. 纳米Pd/Fe催化还原降解2,2',4,4'-四溴联苯醚的研究[D]. 广州: 华南理工大学, 2012. |
| LI Changfang. Study on catalytic debromination of2,2',4,4'-tetrabromodiphenyl ether by nanoscale Pd/Fe bimetallic particles[D]. Guangzhou: South China University of Technology, 2012. | |
| 24 | 张峰振, 吴超飞, 胡芸, 等. 卤代有机污染物的光化学降解[J]. 化学进展, 2014, 26(6): 1079-1098. |
| ZHANG Fengzhen, WU Chaofei, HU Yun, et al. Photochemical degradation of halogenated organic contaminants[J]. Progress in Chemistry, 2014, 26(6): 1079-1098. | |
| 25 | FANG Huan, KANG Jie, ZHANG Dawei. Microbial production of vitamin B12: a review and future perspectives[J]. Microbial Cell Factories, 2017, 16(1): 15. |
| 26 | GHARAGOZLOU M, NAGHIBI S. Sensitization of ZnO nanoparticle by vitamin B12: investigation of microstructure, FTIR and optical properties[J]. Materials Research Bulletin, 2016, 84: 71-78. |
| 27 | GANTZER C J, WACKETT L P. Reductive dechlorination catalyzed by bacterial transition-metal coenzymes[J]. Environmental Science & Technology, 1991, 25(4): 715-722. |
| 28 | ZOU Siwei, STENSEL H D, FERGUSON J F. Carbon tetrachloride degradation: effect of microbial growth substrate and vitamin B12 content[J]. Environmental Science & Technology, 2000, 34(9): 1751-1757. |
| 29 | KRONE U E, LAUFER K, THAUER R K, et al. Coenzyme F430 as a possible catalyst for the reductive dehalogenation of chlorinated C1 hydrocarbons in methanogenic bacteria[J]. Biochemistry, 1989, 28(26): 10061-10065. |
| 30 | ZHENG Kaiyuan, NGO P D, OWENS V L, et al. The biosynthetic pathway of coenzyme F430 in methanogenic and methanotrophic Archaea[J]. Science, 2016, 354(6310): 339-342. |
| 31 | CHIU P C, REINHARD M. Metallocoenzyme-mediated reductive transformation of carbon tetrachloride in titanium(Ⅲ) citrate aqueous solution[J]. Environmental Science & Technology, 1995, 29(3): 595-603. |
| 32 | 陈轶丹. 过渡金属辅酶仿生系统催化还原降解全氟辛磺酸[D]. 泉州: 华侨大学, 2017. |
| CHEN Yidan. Reductive degradation of perfluorooctane sulfonate catalyzed by transition metal coenzyme in a bionic system[D]. Quanzhou: Huaqiao University, 2017. | |
| 33 | 粟强发. 维生素B12催化还原降解全氟辛磺酸[D]. 泉州: 华侨大学, 2018. |
| SU Qiangfa. Reductive degradation of perfluorooctane sulfonate catalyzed by vitamin B12[D]. Quanzhou: Huaqiao University, 2018. | |
| 34 | 杨宁, 李飞, 杨志敏, 等. 维生素B12催化纳米零价铁仿生降解全氟辛磺酸[J]. 中国环境科学, 2020, 40(11): 4770-4778. |
| YANG Ning, LI Fei, YANG Zhimin, et al. Biomimetic degradation of PFOS catalyzed by vitamin B12 using nanoscale zero-valent iron as reductants[J]. China Environmental Science, 2020, 40(11): 4770-4778. | |
| 35 | SCHANKE C A, WACKETT L P. Environmental reductive elimination-reactions of polychlorinated ethanes mimicked by transition-metal coenzymes[J]. Environmental Science & Technology, 1992, 26(4): 830-833. |
| 36 | AMIR A, LEE W. Enhanced reductive dechlorination of tetrachloroethene during reduction of cobalamin(Ⅲ) by nano-mackinawite[J]. Journal of Hazardous Materials, 2012, 235/236: 359-366. |
| 37 | HOLLIGER C, SCHRAA G, STUPPERICH E, et al. Evidence for the involvement of corrinoids and factor F430 in the reductive dechlorination of 1,2-dichloroethane by Methanosarcina barkeri[J]. Journal of Bacteriology, 1992, 174(13): 4427-4434. |
| 38 | TRATNYEK P G, SCHERER M M, DENG Baolin, et al. Effects of natural organic matter, anthropogenic surfactants, and model quinones on the reduction of contaminants by zero-valent iron[J]. Water Research, 2001, 35(18): 4435-4443. |
| 39 | GIEDYK M, GOLISZEWSKA K, GRYKO D. Vitamin B12 catalysed reactions[J]. Chemical Society Reviews, 2015, 44(11): 3391-3404. |
| 40 | MARTENS J H, BARG H, WARREN M J, et al. Microbial production of vitamin B12[J]. Applied Microbiology and Biotechnology, 2002, 58(3): 275-285. |
| 41 | RAUX E, SCHUBERT H L, WARREN M J. Biosynthesis of cobalamin (vitamin B12): a bacterial conundrum[J]. Cellular and Molecular Life Sciences, 2000, 57(13/14): 1880-1893. |
| 42 | HAZRA A B, HAN A W, MEHTA A P, et al. Anaerobic biosynthesis of the lower ligand of vitamin B12[J]. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(34): 10792-10797. |
| 43 | KOENIG Joanna, LEE Matthew, MANEFIELD Mike. Aliphatic organochlorine degradation in subsurface environments[J]. Reviews in Environmental Science and Bio/Technology, 2015, 14(1): 49-71. |
| 44 | ÓPROINSIAS Keith O, GIEDYK Maciej, GRYKO Dorota. Vitamin B12: chemical modifications[J]. Chemical Society Reviews, 2013, 42(16): 6605-6619. |
| 45 | ASSAF-ANID N, NIES L, VOGEL T M. Reductive dechlorination of a polychlorinated biphenyl congener and hexachlorobenzene by vitamin B12[J]. Applied and Environmental Microbiology, 1992, 58(3): 1057-1060. |
| 46 | CHEN Chun, PUHAKKA J A, FERGUSON J F. Transformations of 1, 1,2,2-tetrachloroethane under methanogenic conditions[J]. Environmental Science & Technology, 1996, 30(2): 542-547. |
| 47 | 韩燕妮. 金属配位催化还原降解2,2',4,4'-四溴联苯醚(BDE-47)的研究[D]. 深圳: 深圳大学, 2017. |
| HAN Yanni. Study on the catalytic reduction of2,2',4,4'-tetrabromodiphenyl ether (BDE-47) by metal coordination[D]. Shenzhen: Shenzhen University, 2017. | |
| 48 | HOLLIGER C, SCHRAA G, STUPPERICH E, et al. Evidence for the involvement of corrinoids and factor F430 in the reductive dechlorination of 1, 2-dichloroethane by Methanosarcina barkeri[J]. Journal of Bacteriology, 1992, 174(13): 4427-4434. |
| 49 | CHIU P C, REINHARD M. Metallocoenzyme-mediated reductive transformation of carbon tetrachloride in titanium(Ⅲ) citrate aqueous solution[J]. Environmental Science & Technology, 1995, 29(3): 595-603. |
| 50 | BURRIS D R, DELCOMYN C A, SMITH M H, et al. Reductive dechlorination of tetrachloroethylene and trichloroethylene catalyzed by vitamin B12 in homogeneous and heterogeneous systems[J]. Environmental Science & Technology, 1996, 30(10): 3047-3052. |
| 51 | OCHOA-HERRERA V, SIERRA-ALVAREZ R, SOMOGYI A, et al. Reductive defluorination of perfluorooctane sulfonate[J]. Environmental Science & Technology, 2008, 42(9): 3260-3264. |
| 52 | ASSAF-ANID N, NIES L, VOGEL T M. Reductive dechlorination of a polychlorinated biphenyl congener and hexachlorobenzene by vitamin B12[J]. Applied and Environmental Microbiology, 1992, 58(3): 1057-1060. |
| 53 | CHIU P C, REINHARD M. Transformation of carbon tetrachloride by reduced vitamin B12 in aqueous cysteine solution[J]. Environmental Science & Technology, 1996, 30(6): 1882-1889. |
| 54 | PARK S, DE PERRE C, LEE L S. Alternate reductants with VB12 to transform C8 and C6 perfluoroalkyl sulfonates: limitations and insights into isomer-specific transformation rates, products and pathways[J]. Environmental Science & Technology, 2017, 51(23): 13869-13877. |
| 55 | HUANG C C, LO S L, LIEN H L. Synergistic effect of zero-valent copper nanoparticles on dichloromethane degradation by vitamin B12 under reducing condition[J]. Chemical Engineering Journal, 2013, 219: 311-318. |
| 56 | HUANG C C, LO S L, LIEN H L. Vitamin B12-mediated hydrodechlorination of dichloromethane by bimetallic Cu/Al particles[J]. Chemical Engineering Journal, 2015, 273: 413-420. |
| 57 | 邢悦, 孙力平, 张婷婷, 等. 维生素B12协同纳米Fe/Cu双金属对五氯酚的催化降解与机理[J]. 环境工程学报, 2017, 11(9): 4937-4943. |
| XING Yue, SUN Liping, ZHANG Tingting, et al. Catalytic degradation and mechanism of pentachlorophenol by nano Fe/Cu bimetal and vitamin B12[J]. Chinese Journal of Environmental Engineering, 2017, 11(9): 4937-4943. | |
| 58 | IM J, WALSHE-LANGFORD G E, MOON J W, et al. Environmental fate of the next generation refrigerant 2, 3, 3, 3-tetrafluoropropene (HFO-1234yf)[J]. Environmental Science & Technology, 2014, 48(22): 13181-13187. |
| 59 | KIM S, PARK T, LEE W. Enhanced reductive dechlorination of tetrachloroethene by nano-sized mackinawite with cyanocobalamin in a highly alkaline condition[J]. Journal of Environmental Management, 2015, 151: 378-385. |
| 60 | SHIMAKOSHI H, SAKUMORI E, KANEKO K, et al. B12-TiO2 Hybrid catalyst for dehalogenation of organic halides[J]. Chemistry Letters, 2009, 38(5): 468-469. |
| 61 | GHARAGOZLOU M, NAGHIBI S. Surface modification of TiO2 nanoparticles with vitamin B12: relationships between vitamin B12 content and its optical properties[J]. Journal of the Chinese Chemical Society, 2015, 62(12): 1128-1136. |
| 62 | GUO Min, CHEN Yinguang. Coenzyme cobalamin: biosynthesis, overproduction and its application in dehalogenation—A review[J]. Reviews in Environmental Science and Bio/Technology, 2018, 17(2): 259-284. |
| 63 | SOREL D, LESAGE S, BROWN S, et al. Vitamin B12 and reduced titanium for remediation of residual chlorinated solvents: field experiment[J]. Groundwater Monitoring & Remediation, 2001, 21(4): 140-148. |
| 64 | XU Yi, WANG Chao, HOU Jun, et al. Application of zero valent iron coupling with biological process for wastewater treatment: a review[J]. Reviews in Environmental Science and Bio/Technology, 2017, 16(4): 667-693. |
| 65 | GILLHAM R W, O’HANNESIN S F. Enhanced degradation of halogenated aliphatics by zero-valent iron[J]. Ground Water, 1994, 32(6): 958-967. |
| 66 | MATHESON L J, TRATNYEK P G. Reductive dehalogenation of chlorinated methanes by iron metal[J]. Environmental Science & Technology, 1994, 28(12): 2045-2053. |
| 67 | KIM Y H, CARRAWAY E R. Reductive dechlorination of PCE and TCE by vitamin B12 and ZVMs[J]. Environmental Technology, 2002, 23(10): 1135-1145. |
| 68 | RANGUIN R, DURIMEL A, KARIOUA R, et al. Study of chlordecone desorption from activated carbons and subsequent dechlorination by reduced cobalamin[J]. Environmental Science and Pollution Research, 2017, 24(33): 25488-25499. |
| 69 | YANG Bo, DENG Jianping, WEI Liyan, et al. Synergistic effect of ball-milled Al micro-scale particles with vitamin B12 on the degradation of 2,2',4,4'-tetrabromodiphenyl ether in liquid system[J]. Chemical Engineering Journal, 2018, 333: 613-620. |
| 70 | LAPEYROUSE Nicole, YESTREBSKY Cherie, BOOTH Greg. Remediation of chlorinated alkanes by zero-valent iron and vitamin B12[J]. Abstracts of Papers of the American Chemical Society, 2019, 2019: 8. |
| 71 | WANG Chuanbao, ZHANG Weixian. Synthesizing nanoscale iron particles for rapid and complete dechlorination of TCE and PCBs[J]. Environmental Science & Technology, 1997, 31(7): 2154-2156. |
| 72 | 芮延年, 刘文杰, 王明娣, 等. 高浓有机废水的纳米催化超声裂解处理[J]. 中国给水排水, 2003, 19(2): 64-66. |
| RUI Yannian, LIU Wenjie, WANG Mingdi, et al. High-strength organic wastewater treatment by using nanon catalytic ultrasonic cracking process[J]. China Water & Wastewater, 2003, 19(2): 64-66. | |
| 73 | 张健, 孟昭虹, 刘惠玲. 零价铁用于水中氯代有机物还原脱氯的现状与发展[J]. 环境保护科学, 2010, 36(1): 7-10. |
| ZHANG Jian, MENG Zhaohong, LIU Huiling. Status and development of zero-valent iron for reductive dechlorination of chlorinated organics[J]. Environmental Protection Science, 2010, 36(1): 7-10. | |
| 74 | AMIR A, LEE W. Enhanced reductive dechlorination of tetrachloroethene by nano-sized zero valent iron with vitamin B12[J]. Chemical Engineering Journal, 2011, 170(2/3): 492-497. |
| 75 | HUANG C C, LO S L, LIEN H L. Synergistic effect of zero-valent copper nanoparticles on dichloromethane degradation by vitamin B12 under reducing condition[J]. Chemical Engineering Journal, 2013, 219: 311-318. |
| 76 | LEE Yuchi, CHEN Yipei, CHEN Mengjia, et al. Reductive defluorination of perfluorooctanoic acid by titanium(Ⅲ) citrate with vitamin B12 and copper nanoparticles[J]. Journal of Hazardous Materials, 2017, 340: 336-343. |
| 77 | OCHOA-HERRERA V, SIERRA-ALVAREZ R, SOMOGYI A, et al. Reductive defluorination of perfluorooctane sulfonate[J]. Environmental Science & Technology, 2008, 42(9): 3260-3264. |
| 78 | HUANG Qiang, LIU Wen, PENG Ping’an, et al. Reductive dechlorination of tetrachlorobisphenol A by Pd/Fe bimetallic catalysts[J]. Journal of Hazardous Materials, 2013, 262: 634-641. |
| 79 | CHEN Li-Hua, HUANG Chang-Chieh, LIEN Hsing-Lung. Bimetallic iron-aluminum particles for dechlorination of carbon tetrachloride[J]. Chemosphere, 2008, 73(5): 692-697. |
| 80 | FU Fenglian, CHENG Zihang, LU Jianwei. Synthesis and use of bimetals and bimetal oxides in contaminants removal from water: a review[J]. RSC Advances, 2015, 5(104): 85395-85409. |
| 81 | LIN C J, LIOU Y H, LO S L. Supported Pd/Sn bimetallic nanoparticles for reductive dechlorination of aqueous trichloroethylene[J]. Chemosphere, 2009, 74(2): 314-319. |
| 82 | MA L M, DING Z G, GAO T Y, et al. Discoloration of methylene blue and wastewater from a plant by a Fe/Cu bimetallic system[J]. Chemosphere, 2004, 55(9): 1207-1212. |
| 83 | JIANG Chaojin, JIANG Xiaoqian, ZHANG Lixun, et al. Enhanced debromination of 2,2',4,4'-tetrabromodiphenyl ether (BDE-47) by zero-valent zinc with ascorbic acid[J]. Frontiers of Environmental Science & Engineering, 2020, 14(3): 1-13. |
| 84 | DROR I, JACOV O M, CORTIS A, et al. Catalytic transformation of persistent contaminants using a new composite material based on nanosized zero-valent iron[J]. ACS Applied Materials & Interfaces, 2012, 4(7): 3416-3423. |
| 85 | OTTO M, FLOYD M, BAJPAI S. Nanotechnology for site remediation[J]. Remediation Journal, 2008, 19(1): 99-108. |
| 86 | KIM J H, TRATNYEK P G, CHANG Y S. Rapid dechlorination of polychlorinated dibenzo-p-dioxins by bimetallic and nanosized zerovalent iron[J]. Environmental Science & Technology, 2008, 42(11): 4106-4112. |
| 87 | LIEN H L, ZHANG Weixian. Enhanced dehalogenation of halogenated methanes by bimetallic Cu/Al[J]. Chemosphere, 2002, 49(4): 371-378. |
| 88 | CHANG Chun, LIAN Fei, ZHU Lingyan. Simultaneous adsorption and degradation of γ-HCH by nZVI/Cu bimetallic nanoparticles with activated carbon support[J]. Environmental Pollution, 2011, 159(10): 2507-2514. |
| 89 | AGARWAL S, AL-ABED S R, DIONYSIOU D D. Enhanced corrosion-based Pd/Mg bimetallic systems for dechlorination of PCBs[J]. Environmental Science & Technology, 2007, 41(10): 3722-3727. |
| 90 | XU Yue, ZHANG Weixian. Subcolloidal Fe/Ag particles for reductive dehalogenation of chlorinated benzenes[J]. Industrial & Engineering Chemistry Research, 2000, 39(7): 2238-2244. |
| 91 | GHAUCH A, TUQAN A. Reductive destruction and decontamination of aqueous solutions of chlorinated antimicrobial agent using bimetallic systems[J]. Journal of Hazardous Materials, 2009, 164(2/3): 665-674. |
| 92 | TONG Shaoping, WEI Hong, MA Chun’an, et al. Rapid dechlorination of chlorinated organic compounds by nickel/iron bimetallic system in water[J]. Journal of Zhejiang University-Science A, 2005, 6(7): 627-631. |
| 93 | MUFTIKIAN R, FERNANDO Q, KORTE N. A method for the rapid dechlorination of low molecular weight chlorinated hydrocarbons in water[J]. Water Research, 1995, 29(10): 2434-2439. |
| 94 | ZHANG Weixian, WANG Chuanbao, LIEN Hsinglung. Treatment of chlorinated organic contaminants with nanoscale bimetallic particles[J]. Catalysis Today, 1998, 40(4): 387-395. |
| 95 | WANG Xiangyu, CHEN Chao, LIU Huiling, et al. Characterization and evaluation of catalytic dechlorination activity of Pd/Fe bimetallic nanoparticles[J]. Industrial & Engineering Chemistry Research, 2008, 47(22): 8645-8651. |
| 96 | 林英杰, 张硕, 孙力平, 等. VB12协同Fe/Cu双金属去除二氯甲烷过程调控与机理[J]. 中国环境科学, 2016, 36(9): 2650-2657. |
| LIN Yingjie, ZHANG Shuo, SUN Liping, et al. Synergistic effect of vitamin B12 and Fe/Cu bimetal on reduction of dichloromethane: mechanism study and process control[J]. China Environmental Science, 2016, 36(9): 2650-2657. | |
| 97 | 张婷婷, 孙力平, 林英杰, 等. 铜负载率对纳米Fe/Cu双金属+维生素B12体系中二氯甲烷还原速率的影响及机理[J]. 应用化工, 2018, 47(10): 2124-2128. |
| ZHANG Tingting, SUN Liping, LIN Yingjie, et al. Effects of copper loading on the reduction rate of dichloromethane by bimetallic Fe/Cu nanoparticles + vitamin B12 and mechanism[J]. Applied Chemical Industry, 2018, 47(10): 2124-2128. | |
| 98 | YANG M X, SARKAR S, BENT B E, et al. Degradation of multiply-chlorinated hydrocarbons on Cu(100)[J]. Langmuir, 1997, 13(2): 229-242. |
| 99 | O’CARROLL D, SLEEP B, KROL M, et al. Nanoscale zero valent iron and bimetallic particles for contaminated site remediation[J]. Advances in Water Resources, 2013, 51: 104-122. |
| 100 | XIE Jituo, LEI Chao, CHEN Wenqian, et al. Catalytic properties of transition metals modified nanoscale zero-valent iron for simultaneous removal of 4-chlorophenol and Cr(Ⅵ): efficacy, descriptor and reductive mechanisms[J]. Journal of Hazardous Materials, 2021, 403: 123827. |
| 101 | GUNAWARDANA B, SINGHAL N, SWEDLUND P. Degradation of chlorinated phenols by zero valent iron and bimetals of iron: a review[J]. Environmental Engineering Research, 2011, 16(4): 187-203. |
| 102 | GHAUCH A, ASSI H A, BAYDOUN H, et al. Fe0-based trimetallic systems for the removal of aqueous diclofenac: mechanism and kinetics[J]. Chemical Engineering Journal, 2011, 172(2/3): 1033-1044. |
| 103 | HUANG C C, LIEN H L. Trimetallic Pd/Fe/Al particles for catalytic dechlorination of chlorinated organic contaminants[J]. Water Science and Technology, 2010, 62(1): 202-208. |
| 104 | SUWANNARAT K, THONGTHAI K, ANANTA S, et al. Synthesis of hollow trimetallic Ag/Au/Pd nanoparticles for reduction of 4-nitrophenol[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2018, 540: 73-80. |
| 105 | KRIEGMAN-KING M R, REINHARD M. Transformation of carbon tetrachloride by pyrite in aqueous solution[J]. Environmental Science & Technology, 1994, 28(4): 692-700. |
| 106 | LEE W, BATCHELOR B. Abiotic reductive dechlorination of chlorinated ethylenes by iron-bearing soil minerals. 2. Green rust[J]. Environmental Science & Technology, 2002, 36(24): 5348-5354. |
| 107 | LEE W, BATCHELOR B. Abiotic reductive dechlorination of chlorinated ethylenes by iron-bearing soil minerals. 1. Pyrite and magnetite[J]. Environmental Science & Technology, 2002, 36(23): 5147-5154. |
| 108 | GROOS P G K VAN, HATZINGER P B, STREGER S H, et al. Carbon isotope fractionation of 1,2-dibromoethane by biological and abiotic processes[J]. Environmental Science & Technology, 2018, 52(6): 3440-3448. |
| 109 | BJERG P L, RUEGGE K, PEDERSEN J K, et al. Distribution of redox-sensitive groundwater quality parameters downgradient of a landfill (Grindsted, Denmark)[J]. Environmental Science & Technology, 1995, 29(5): 1387-1394. |
| 110 | ELSNER M, SCHWARZENBACH R P, HADERLEIN S B. Reactivity of Fe(Ⅱ)-bearing minerals toward reductive transformation of organic contaminants[J]. Environmental Science & Technology, 2004, 38(3): 799-807. |
| 111 | 廖高明, 马杰, 谷春云, 等. 污染场地卤代烃非生物自然衰减研究进展[J]. 环境科学研究, 2021, 34(3): 742-754. |
| LIAO Gaoming, MA Jie, GU Chunyun, et al. Research progress on abiotic natural attenuation of halogenated hydrocarbons at contaminated sites[J]. Research of Environmental Sciences, 2021, 34(3): 742-754. | |
| 112 | JEONG H Y, HAYES K F. Impact of transition metals on reductive dechlorination rate of hexachloroethane by mackinawite[J]. Environmental Science & Technology, 2003, 37(20): 4650-4655. |
| 113 | ASSAF-ANID N, LIN Kunyu. Carbon tetrachloride reduction by Fe2+, S2-, and FeS with vitamin B12 as organic amendment[J]. Journal of Environmental Engineering, 2002, 128(1): 94-99. |
| 114 | KYUNG D, AMIR A, CHOI K, et al. Reductive transformation of tetrachloroethene catalyzed by sulfide-cobalamin in nano-mackinawite suspension[J]. Industrial & Engineering Chemistry Research, 2015, 54(5): 1439-1446. |
| 115 | KYUNG D, SIHN Y, KIM S, et al. Synergistic effect of nano-sized mackinawite with cyano-cobalamin in cement slurries for reductive dechlorination of tetrachloroethylene[J]. Journal of Hazardous Materials, 2016, 311: 1-10. |
| 116 | BUTLER E C, HAYES K F. Factors influencing rates and products in the transformation of trichloroethylene by iron sulfide and iron metal[J]. Environmental Science & Technology, 2001, 35(19): 3884-3891. |
| 117 | HWANG I, BATCHELOR B. Reductive dechlorination of tetrachloroethylene in soils by Fe(Ⅱ)-based degradative solidification/stabilization[J]. Environmental Science & Technology, 2001, 35(18): 3792-3797. |
| 118 | BROWN K L. Chemistry and enzymology of vitamin B12[J]. Chemical Reviews, 2005, 105(6): 2075-2149. |
| 119 | ASAFTEI S, WALDER L. Modification of mesoporous TiO2 electrodes with cross-linkable B12 derivatives[J]. Langmuir, 2006, 22(13): 5544-5547. |
| 120 | NICOTRA G, RAMASSE Q M. Material science in semiconductor processing[J]. Materials Science in Semiconductor Processing, 2017, 65: 1. |
| 121 | GHARAGOZLOU M, NAGHIBI S. Sensitization of ZnO nanoparticle by vitamin B12: investigation of microstructure, FTIR and optical properties[J]. Materials Research Bulletin, 2016, 84: 71-78. |
| 122 | SHIMAKOSHI H, HISAEDA Y. Oxygen-controlled catalysis by vitamin B12-TiO2: formation of esters and amides from trichlorinated organic compounds by photoirradiation[J]. Angewandte Chemie International Edition, 2015, 54(51): 15439-15443. |
| 123 | SHICHIJO K, FUJITSUKA M, HISAEDA Y, et al. Visible light-driven photocatalytic duet reaction catalyzed by the B12-rhodium-titanium oxide hybrid catalyst[J]. Journal of Organometallic Chemistry, 2020, 907: 121058. |
| 124 | IZUMI S I, SHIMAKOSHI H, ABE M, et al. Photo-induced ring-expansion reactions mediated by B12-TiO2 hybrid catalyst[J]. Dalton Transactions, 2010, 39(13): 3302-3307. |
| 125 | TIAN H, SHIMAKOSHI H, IMAMURA K, et al. Photocatalytic alkene reduction by a B12-TiO2 hybrid catalyst coupled with C—F bond cleavage for gem-difluoroolefin synthesis[J]. Chemical Communications, 2017, 53(68): 9478-9481. |
| 126 | SHIMAKOSHI H, HISAEDA Y. B12-TiO2 Hybrid catalyst for light-driven hydrogen production and hydrogenation of C—C multiple bonds[J]. ChemPlusChem, 2014, 79(9): 1250-1253. |
| 127 | SHIMAKOSHI H, SAKUMORI E, KANEKO K, et al. B12-TiO2 Hybrid catalyst for dehalogenation of organic halides[J]. Chemistry Letters, 2009, 38(5): 468-469. |
| 128 | GHARAGOZLOU M, BAYATI R. Photocatalytic characteristics of single phase Fe-doped anatase TiO2 nanoparticles sensitized with vitamin B12[J]. Materials Research Bulletin, 2015, 61: 340-347. |
| 129 | SUN Ying, ZHANG Wei, MA Tianyi, et al. Enhanced photocatalytic activity of a B12-based catalyst co-photosensitized by TiO2 and Ru(Ⅱ) towards dechlorination[J]. RSC Advances, 2018, 8(2): 662-670. |
| 130 | GE Yanhui, LUO Hao, HUANG Juanru, et al. Visible-light-active TiO2 photocatalyst for efficient photodegradation of organic dyes[J]. Optical Materials, 2021, 115: 111058. |
| 131 | TIAN H, SHIMAKOSHI H, ONO T, et al. Visible-light-driven, one-pot amide synthesis catalyzed by the B12 model complex under aerobic conditions[J]. ChemPlusChem, 2019, 84(3): 236. |
| 132 | IZUMI S I, SHIMAKOSHI H, ABE M, et al. Photo-induced ring-expansion reactions mediated by B12-TiO2 hybrid catalyst[J]. Dalton Transactions, 2010, 39(13): 3302-3307. |
| 133 | SHIMAKOSHI H, HISAEDA Y. A hybrid catalyst for light-driven green molecular transformations[J]. ChemPlusChem, 2017, 82(1): 18-29. |
| 134 | 孙亚秋, 邓国志, 田欣, 等. TiO2纳米光催化材料的研究进展[J]. 天津师范大学学报(自然科学版), 2019, 39(5): 1-6. |
| SUN Yaqiu, DENG Guozhi, TIAN Xin, et al. Research progress of TiO2 nanophotocatalytic materials[J]. Journal of Tianjin Normal University (Natural Science Edition), 2019, 39(5): 1-6. |
| [1] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [2] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [3] | 徐伟, 李凯军, 宋林烨, 张兴惠, 姚舜华. 光催化及其协同电化学降解VOCs的研究进展[J]. 化工进展, 2023, 42(7): 3520-3531. |
| [4] | 杨福, 刘梦婷, 马淑兰, 魏祎暄, 欧锐, 王旭裕, 李露露, 张武翔, 潘建明. 挥发性有机化合物催化消除前沿技术及研究进展[J]. 化工进展, 2022, 41(9): 4801-4812. |
| [5] | 陈 瑛,宋存义,张建祺. 协同催化臭氧化工艺对水中微量有机污染物的降解 [J]. 化工进展, 2006, 25(9): 1069-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||