化工进展 ›› 2021, Vol. 40 ›› Issue (10): 5708-5719.DOI: 10.16085/j.issn.1000-6613.2020-2055
环境中1,4-二 烷污染处理技术研究进展
烷污染处理技术研究进展
田坤1,2,3( ), 姚丹丹2,3, 赵元添2,3, 郭丽莉1, 董元华2,3, 刘云1,2,3(
), 姚丹丹2,3, 赵元添2,3, 郭丽莉1, 董元华2,3, 刘云1,2,3( )
)
- 1.污染场地安全修复技术国家工程实验室,北京 100015
2.中国科学院土壤环境与污染修复重点实验室(南京 土壤研究所),江苏 南京 210008
3.中国科学院大学,北京 100049
-
收稿日期:2020-10-13修回日期:2020-12-01出版日期:2021-10-10发布日期:2021-10-25 -
通讯作者:刘云 -
作者简介:田坤(1996—),男,博士研究生,研究方向为环境微生物学。E-mail:tiankun@issas.ac.cn 。 -
基金资助:国家自然科学基金(41877504);污染场地安全修复技术国家工程实验室开放基金(NEL-SRT201902)
Advances in 1,4-dioxane remediation methods: a review
TIAN Kun1,2,3( ), YAO Dandan2,3, ZHAO Yuantian2,3, GUO Lili1, DONG Yuanhua2,3, LIU Yun1,2,3(
), YAO Dandan2,3, ZHAO Yuantian2,3, GUO Lili1, DONG Yuanhua2,3, LIU Yun1,2,3( )
)
- 1.National Engineering Laboratory for Site Remediation Technologies, Beijing 100015, China
2.CAS Key Laboratory of Soil Environment and Pollution Remediation, Institute of Soil Science, Chinese Academy of Sciences, Nanjing 210008, Jiangsu, China
3.University of Chinese Academy of Sciences, Beijing 100049, China
-
Received:2020-10-13Revised:2020-12-01Online:2021-10-10Published:2021-10-25 -
Contact:LIU Yun
摘要:
新型污染物1,4-二 烷广泛分布于地表水、地下水和饮用水环境中。常规水处理手段对1,4-二
烷广泛分布于地表水、地下水和饮用水环境中。常规水处理手段对1,4-二 烷难以奏效,因而其污染处理技术成为了关注的热点。本文主要针对1,4-二
烷难以奏效,因而其污染处理技术成为了关注的热点。本文主要针对1,4-二 烷污染的处理方法进行了综述。首先对1,4-二
烷污染的处理方法进行了综述。首先对1,4-二 烷的性质及分布特征进行了总结,比较了国内外已有的污染调查状况和控制标准,然后重点从物理、化学和生物法三个方面介绍了近年来1,4-二
烷的性质及分布特征进行了总结,比较了国内外已有的污染调查状况和控制标准,然后重点从物理、化学和生物法三个方面介绍了近年来1,4-二 烷污染处理技术的最新研究进展,且分析了不同技术的作用机制、优缺点及可行性,并对不同技术的处理效率进行了归纳总结。文中特别指出了生物修复的难题,即氯代烃、重金属对微生物降解1,4-二
烷污染处理技术的最新研究进展,且分析了不同技术的作用机制、优缺点及可行性,并对不同技术的处理效率进行了归纳总结。文中特别指出了生物修复的难题,即氯代烃、重金属对微生物降解1,4-二 烷的抑制作用和机理及改进修复技术,最后对今后的研究方向进行了展望。
烷的抑制作用和机理及改进修复技术,最后对今后的研究方向进行了展望。
中图分类号:
引用本文
田坤, 姚丹丹, 赵元添, 郭丽莉, 董元华, 刘云. 环境中1,4-二 烷污染处理技术研究进展[J]. 化工进展, 2021, 40(10): 5708-5719.
烷污染处理技术研究进展[J]. 化工进展, 2021, 40(10): 5708-5719.
TIAN Kun, YAO Dandan, ZHAO Yuantian, GUO Lili, DONG Yuanhua, LIU Yun. Advances in 1,4-dioxane remediation methods: a review[J]. Chemical Industry and Engineering Progress, 2021, 40(10): 5708-5719.
| 属性 | 数值 | 结构 |
|---|---|---|
| 分子量/g·mol-1 | 88.11 |  |
| 密度/g·cm-3 | 1.033 | |
| 沸点(标准大气压下)/℃ | 101.1 | |
| 水溶性(25℃)/mg·L-1 | 4.31×105 | |
| 蒸气压(25℃)/mmHg | 38.1 | |
| 亨利定律常数(25℃)/atm·m3·mol-1 | 4.88×10-6 | |
| 正辛醇-水分配系数(lg Kow) | -0.27 | |
| 有机碳分配系数(lg Koc) | 1.23 | |
| 生物富集系数 | 0.2~0.7 |
表1 1,4-二烷的理化性质及结构
| 属性 | 数值 | 结构 |
|---|---|---|
| 分子量/g·mol-1 | 88.11 |  |
| 密度/g·cm-3 | 1.033 | |
| 沸点(标准大气压下)/℃ | 101.1 | |
| 水溶性(25℃)/mg·L-1 | 4.31×105 | |
| 蒸气压(25℃)/mmHg | 38.1 | |
| 亨利定律常数(25℃)/atm·m3·mol-1 | 4.88×10-6 | |
| 正辛醇-水分配系数(lg Kow) | -0.27 | |
| 有机碳分配系数(lg Koc) | 1.23 | |
| 生物富集系数 | 0.2~0.7 |
| 时间 | 吸附剂 | 浓度范围 | 吸附容量 | 参考文献 |
|---|---|---|---|---|
| 2007年 | 多孔TiO2颗粒 | 20.62~1.03×105mg·L-1 | 80.27mg·g-1(正己烷溶剂中) | [ |
| 2013年 | 碳质吸附剂 | 0.001~100mg·L-1 | 吸附等温线拟合最大值约50mg·g-1 | [ |
| 2014年 | AMBERSORB? 560 | 0.002~2000mg·L-1 | 吸附等温线拟合最大值约63mg·g-1 | [ |
| 2018年 | 活性炭Norit 1240 | 12.5~800mg·L-1 | 37.57mg·g-1 | [ |
| 2019年 | 钛硅沸石TS-1 | 25~500mg·L-1 | 85.17mg·g-1 | [ |
| 2019年 | 沸石分子筛ZSM-5 | 1~400mg·L-1 | 分子筛Si/Al=300时,Langmuir模型拟合最大值87.32mg·g-1 | [ |
表2 不同1,4-二烷吸附剂及吸附容量
| 时间 | 吸附剂 | 浓度范围 | 吸附容量 | 参考文献 |
|---|---|---|---|---|
| 2007年 | 多孔TiO2颗粒 | 20.62~1.03×105mg·L-1 | 80.27mg·g-1(正己烷溶剂中) | [ |
| 2013年 | 碳质吸附剂 | 0.001~100mg·L-1 | 吸附等温线拟合最大值约50mg·g-1 | [ |
| 2014年 | AMBERSORB? 560 | 0.002~2000mg·L-1 | 吸附等温线拟合最大值约63mg·g-1 | [ |
| 2018年 | 活性炭Norit 1240 | 12.5~800mg·L-1 | 37.57mg·g-1 | [ |
| 2019年 | 钛硅沸石TS-1 | 25~500mg·L-1 | 85.17mg·g-1 | [ |
| 2019年 | 沸石分子筛ZSM-5 | 1~400mg·L-1 | 分子筛Si/Al=300时,Langmuir模型拟合最大值87.32mg·g-1 | [ |
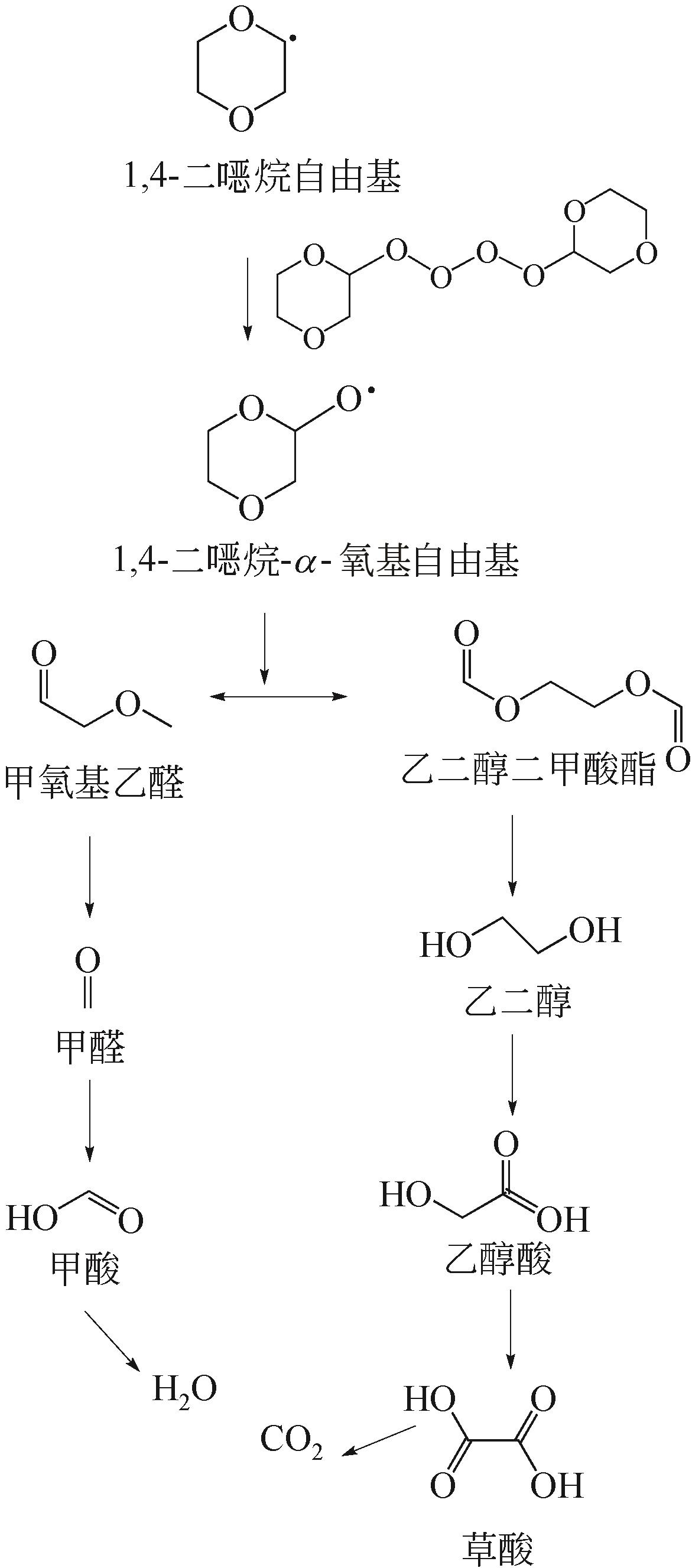
| 时间 | 高级氧化体系 | 活性物质 | 1,4-二 烷初始浓度 烷初始浓度 | 平均降解速率① | 参考文献 |
|---|---|---|---|---|---|
| 2006年 | 20kHz超声 | ·OH | 100mg·L-1 | 0.395mg·L-1·min-1 | [ |
| 2007年 | H2O2/UV体系 | ·OH | 0.36mg·L-1 | 0.5μgC·min-1 | [ |
| 2008年 | O3/电解工艺 | ·OH | 1.04mg·L-1 | 1.04mg·L-1·min-1 | [ |
| 2009年 | Fe0和紫外光类芬顿体系 | ·OH | 1~10mg·L-1 | 3.16×10-2mg·L-1·min-1 | [ |
| 2010年 | 硼掺杂金刚石电极阳极氧化 | ·OH | COD0为129molO2·m-3 | 约4.06molO2·m-3·h-1 | [ |
| 2010年 | 基于紫外线的光催化(TiO2) | ·OH | 36mg·L-1 | 12mg·L-1·min-1 | [ |
| 2013年 | O3/ H2O2体系 | ·OH | 150mg·L-1 | 约2.5mg·L-1·min-1 | [ |
| 2013年 | O3/ UV体系 | ·OH | 150mg·L-1 | 约1.25mg·L-1·min-1 | [ |
| 2015年 | 铁屑原位活化过硫酸盐 | ·OH、·SO | 44mg·L-1 | 0.293mg·L-1·h-1 | [ |
| 2015年 | 电-过氧化物工艺 | ·OH | 200mg·L-1 | 6.67mg·L-1·min-1 | [ |
| 2016年 | 太阳光催化(NF-TiO2及TiO2) | ·OH | 135~140mg·L-1 | 2.8mgC·cm-2 ·h-1 | [ |
| 2016年 | 过氧化物活化过硫酸盐 | ·OH、·SO | 3.6~3.8mg·L-1 | 0.005mg·L-1·min-1 | [ |
| 2016年 | Fe0非均相光芬顿 | ·OH | 248mg·L-1 | 11.025mgO2· L-1·min-1 | [ |
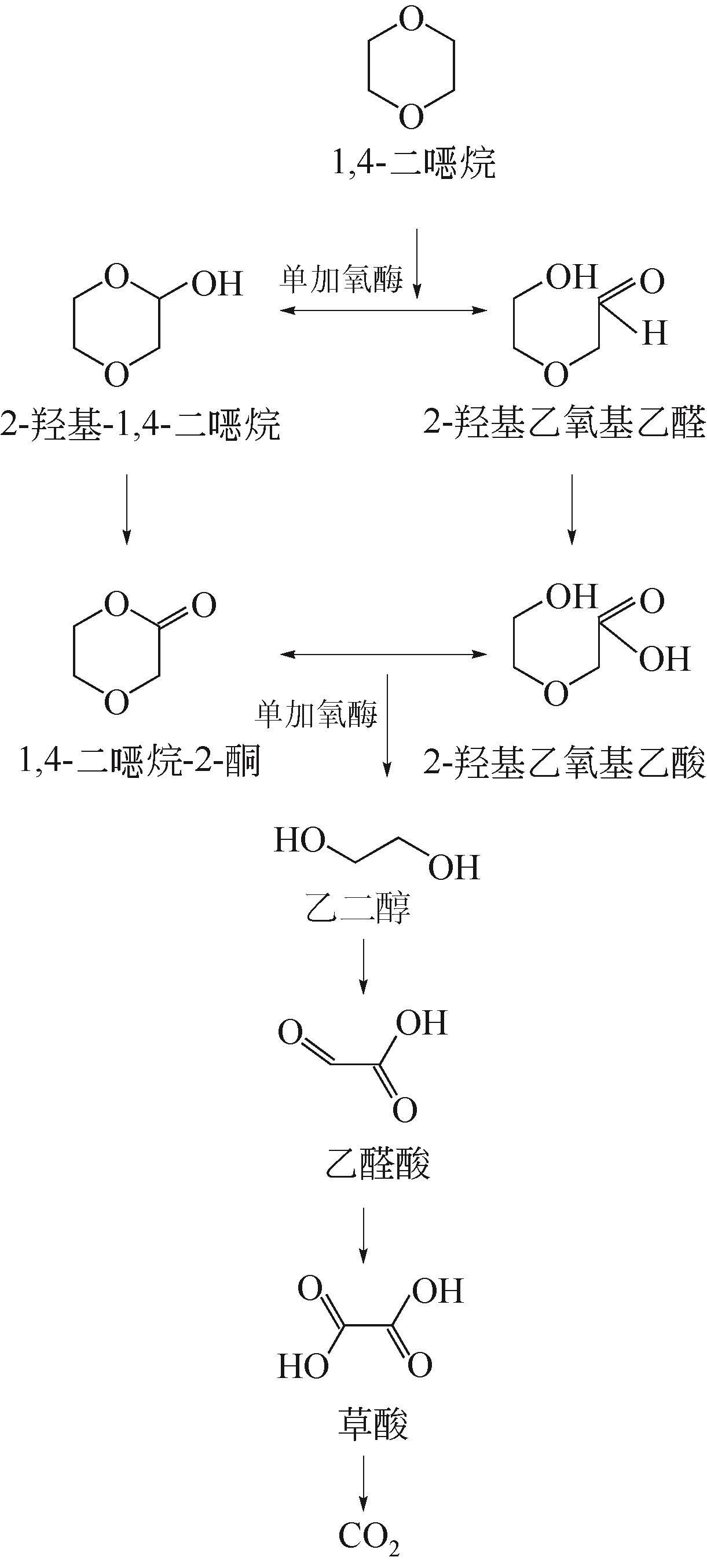
| 2016年 | 电芬顿法(阴极为活性炭) | ·OH、·SO | 40~100mg·L-1 | 0.28mg·L-1·min-1 | [ |
| 2016年 | 声活化过硫酸盐法 | ·OH、·SO | 1mg·L-1 | 0.003mg·L-1·min-1 | [ |
| 2017年 | 生物炭负载纳米Fe3O4活化过硫酸盐 | ·OH、·SO | 1.76mg·L-1 | 0.014mg·L-1·min-1 | [ |
| 2017年 | Fe0活化过硫酸盐 | ·OH、·SO | 500mg·L-1 | 4.17mg·L-1·min-1 | [ |
| 2017年 | Pd/Al2O3活化过硫酸盐 | ·OH、·SO | 48mg·L-1 | 0.81mg·L-1·min-1 | [ |
| 2018年 | 声活化过硫酸盐 | ·OH、·SO | 13mg·L-1 | 0.023mg·L-1·min-1 | [ |
| 2018年 | 纳米零价铁与常用氧化剂耦合 | ·OH、·SO | 44mg·L-1 | 0.0099g·mg-1·min-1 | [ |
| 2018年 | 紫外/电氯化 | ·OH、·Cl、·Cl | 88.11mg·L-1 | 约1.47mg·L-1·min-1 | [ |
| 2019年 | 生物炭活化过氧硫酸盐 | ·OH、·SO | 1.76mg·L-1 | 0.006mg·L-1·min-1 | [ |
| 2020年 | 液相等离子体增强光催化 | ·OH | 10~20mg·L-1 | 0.27mg·L-1·min-1 | [ |
表3 高级氧化法在处理1,4-二烷污染中的应用
| 时间 | 高级氧化体系 | 活性物质 | 1,4-二 烷初始浓度 烷初始浓度 | 平均降解速率① | 参考文献 |
|---|---|---|---|---|---|
| 2006年 | 20kHz超声 | ·OH | 100mg·L-1 | 0.395mg·L-1·min-1 | [ |
| 2007年 | H2O2/UV体系 | ·OH | 0.36mg·L-1 | 0.5μgC·min-1 | [ |
| 2008年 | O3/电解工艺 | ·OH | 1.04mg·L-1 | 1.04mg·L-1·min-1 | [ |
| 2009年 | Fe0和紫外光类芬顿体系 | ·OH | 1~10mg·L-1 | 3.16×10-2mg·L-1·min-1 | [ |
| 2010年 | 硼掺杂金刚石电极阳极氧化 | ·OH | COD0为129molO2·m-3 | 约4.06molO2·m-3·h-1 | [ |
| 2010年 | 基于紫外线的光催化(TiO2) | ·OH | 36mg·L-1 | 12mg·L-1·min-1 | [ |
| 2013年 | O3/ H2O2体系 | ·OH | 150mg·L-1 | 约2.5mg·L-1·min-1 | [ |
| 2013年 | O3/ UV体系 | ·OH | 150mg·L-1 | 约1.25mg·L-1·min-1 | [ |
| 2015年 | 铁屑原位活化过硫酸盐 | ·OH、·SO | 44mg·L-1 | 0.293mg·L-1·h-1 | [ |
| 2015年 | 电-过氧化物工艺 | ·OH | 200mg·L-1 | 6.67mg·L-1·min-1 | [ |
| 2016年 | 太阳光催化(NF-TiO2及TiO2) | ·OH | 135~140mg·L-1 | 2.8mgC·cm-2 ·h-1 | [ |
| 2016年 | 过氧化物活化过硫酸盐 | ·OH、·SO | 3.6~3.8mg·L-1 | 0.005mg·L-1·min-1 | [ |
| 2016年 | Fe0非均相光芬顿 | ·OH | 248mg·L-1 | 11.025mgO2· L-1·min-1 | [ |
| 2016年 | 电芬顿法(阴极为活性炭) | ·OH、·SO | 40~100mg·L-1 | 0.28mg·L-1·min-1 | [ |
| 2016年 | 声活化过硫酸盐法 | ·OH、·SO | 1mg·L-1 | 0.003mg·L-1·min-1 | [ |
| 2017年 | 生物炭负载纳米Fe3O4活化过硫酸盐 | ·OH、·SO | 1.76mg·L-1 | 0.014mg·L-1·min-1 | [ |
| 2017年 | Fe0活化过硫酸盐 | ·OH、·SO | 500mg·L-1 | 4.17mg·L-1·min-1 | [ |
| 2017年 | Pd/Al2O3活化过硫酸盐 | ·OH、·SO | 48mg·L-1 | 0.81mg·L-1·min-1 | [ |
| 2018年 | 声活化过硫酸盐 | ·OH、·SO | 13mg·L-1 | 0.023mg·L-1·min-1 | [ |
| 2018年 | 纳米零价铁与常用氧化剂耦合 | ·OH、·SO | 44mg·L-1 | 0.0099g·mg-1·min-1 | [ |
| 2018年 | 紫外/电氯化 | ·OH、·Cl、·Cl | 88.11mg·L-1 | 约1.47mg·L-1·min-1 | [ |
| 2019年 | 生物炭活化过氧硫酸盐 | ·OH、·SO | 1.76mg·L-1 | 0.006mg·L-1·min-1 | [ |
| 2020年 | 液相等离子体增强光催化 | ·OH | 10~20mg·L-1 | 0.27mg·L-1·min-1 | [ |
| 时间 | 植物种类 | 1,4-二 烷初始浓度 烷初始浓度 | 去除率 | 参考文献 |
|---|---|---|---|---|
| 2000年 | 杂交杨树 | 23mg·L-1 | 9天,去除54%±19% | [ |
| 2001年 | 杨树(降解菌CB1190强化) | 100mg·L-1 | 45天,完全去除 | [ |
| 2013年 | 针叶树 | 2.5mg·L-1 | 30天,去除约80% | [ |
| 2020年 | 杨树(降解菌PH-06强化) | 10mg·L-1 | 13天,完全去除 | [ |
| 2020年 | 浮萍植物L. gibba | 16mg·L-1·d-1 | 6天,去除54%±2.5% | [ |
表4 植物修复在处理1,4-二烷污染中的应用
| 时间 | 植物种类 | 1,4-二 烷初始浓度 烷初始浓度 | 去除率 | 参考文献 |
|---|---|---|---|---|
| 2000年 | 杂交杨树 | 23mg·L-1 | 9天,去除54%±19% | [ |
| 2001年 | 杨树(降解菌CB1190强化) | 100mg·L-1 | 45天,完全去除 | [ |
| 2013年 | 针叶树 | 2.5mg·L-1 | 30天,去除约80% | [ |
| 2020年 | 杨树(降解菌PH-06强化) | 10mg·L-1 | 13天,完全去除 | [ |
| 2020年 | 浮萍植物L. gibba | 16mg·L-1·d-1 | 6天,去除54%±2.5% | [ |
| 时间 | 菌种 | 生物降解速率 | 代谢途径 | 参考文献 |
|---|---|---|---|---|
| 1994年 | Actinomycete CB1190 | 0.33mg·min-1·mg-protein-1 | 代谢 | [ |
| 2001年 | Amycolata sp. CB1190 | 10mg·kg-soil-1 | 代谢 | [ |
| 2005年 | Cordyceps sinensis | 0.011mol·d-1 | 代谢 | [ |
| 2006年 | Pseudonocardia dioxanivorans CB1190 | (0.19±0.007)mg·h-1·mg-protein-1 | 代谢 | [ |
| 2006年 | Pseudonocardia benzenivorans B5 | (0.01±0.003)mg·h-1·mg-protein-1 | 代谢 | [ |
| 2006年 | Pseudonocardia K1 | (0.26±0.013)mg·h-1·mg-protein-1 | 共代谢 | [ |
| 2006年 | Burkholderia cepacia G4 | (0.1±0.006)mg·h-1·mg-protein-1 | 共代谢 | [ |
| 2006年 | Pseudomonas mendocina KR1 | (0.37±0.04)mg·h-1·mg-protein-1 | 共代谢 | [ |
| 2006年 | Mycobacterium austroafricanum JOB5 | (0.40±0.06)mg·h-1·mg-protein-1 | 共代谢 | [ |
| 2008年 | Mycobacterium sp. PH-06 | 60mg·L-1·d-1 | 代谢 | [ |
| 2009年 | Graphium sp. (ATCC 58400) | (9±5)nmol·min-1·mg-1 dry weight | 共代谢 | [ |
| 2012年 | Pseudonocardia sp. strain ENV478 | 21mg·h-1·g-TSS-1 | 共代谢 | [ |
| 2013年 | Gram-negative Afipia sp.D1 | 0.052~0.263mg·mg-protein-1·h-1 | 代谢 | [ |
| 2014年 | Acinetobacter baumannii.DD1 | 2.38mg·h-1·L-1 | 代谢 | [ |
| 2015年 | Rhodococcus ruber ENV425 | 10mg·h-1·g-TSS-1 | 共代谢 | [ |
| 2015年 | Rhodanobacter AYS5 | 3.96mg·h-1·L-1 | 代谢/共代谢 | [ |
| 2016年 | Xanthobacter flavus DT8 | 与CB1190相近 | 代谢 | [ |
| 2016年 | Pseudonocardia carboxydivorans. RM-31 | 31.6mg·L-1·h-1 | 代谢 | [ |
| 2018年 | Rhodococcus aetherivorans JCM 14343 | 0.0073mg-dioxane·mg-protein-1·h-1 | 代谢/共代谢 | [ |
| 2020年 | Consortium N112 | 1.67mg·h-1·L-1 | 代谢 | [ |
表5 降解1,4-二烷的菌种及降解速率
| 时间 | 菌种 | 生物降解速率 | 代谢途径 | 参考文献 |
|---|---|---|---|---|
| 1994年 | Actinomycete CB1190 | 0.33mg·min-1·mg-protein-1 | 代谢 | [ |
| 2001年 | Amycolata sp. CB1190 | 10mg·kg-soil-1 | 代谢 | [ |
| 2005年 | Cordyceps sinensis | 0.011mol·d-1 | 代谢 | [ |
| 2006年 | Pseudonocardia dioxanivorans CB1190 | (0.19±0.007)mg·h-1·mg-protein-1 | 代谢 | [ |
| 2006年 | Pseudonocardia benzenivorans B5 | (0.01±0.003)mg·h-1·mg-protein-1 | 代谢 | [ |
| 2006年 | Pseudonocardia K1 | (0.26±0.013)mg·h-1·mg-protein-1 | 共代谢 | [ |
| 2006年 | Burkholderia cepacia G4 | (0.1±0.006)mg·h-1·mg-protein-1 | 共代谢 | [ |
| 2006年 | Pseudomonas mendocina KR1 | (0.37±0.04)mg·h-1·mg-protein-1 | 共代谢 | [ |
| 2006年 | Mycobacterium austroafricanum JOB5 | (0.40±0.06)mg·h-1·mg-protein-1 | 共代谢 | [ |
| 2008年 | Mycobacterium sp. PH-06 | 60mg·L-1·d-1 | 代谢 | [ |
| 2009年 | Graphium sp. (ATCC 58400) | (9±5)nmol·min-1·mg-1 dry weight | 共代谢 | [ |
| 2012年 | Pseudonocardia sp. strain ENV478 | 21mg·h-1·g-TSS-1 | 共代谢 | [ |
| 2013年 | Gram-negative Afipia sp.D1 | 0.052~0.263mg·mg-protein-1·h-1 | 代谢 | [ |
| 2014年 | Acinetobacter baumannii.DD1 | 2.38mg·h-1·L-1 | 代谢 | [ |
| 2015年 | Rhodococcus ruber ENV425 | 10mg·h-1·g-TSS-1 | 共代谢 | [ |
| 2015年 | Rhodanobacter AYS5 | 3.96mg·h-1·L-1 | 代谢/共代谢 | [ |
| 2016年 | Xanthobacter flavus DT8 | 与CB1190相近 | 代谢 | [ |
| 2016年 | Pseudonocardia carboxydivorans. RM-31 | 31.6mg·L-1·h-1 | 代谢 | [ |
| 2018年 | Rhodococcus aetherivorans JCM 14343 | 0.0073mg-dioxane·mg-protein-1·h-1 | 代谢/共代谢 | [ |
| 2020年 | Consortium N112 | 1.67mg·h-1·L-1 | 代谢 | [ |
| 1 | ANDERSON R H, ANDERSON J K, BOWER P A. Co-occurrence of 1,4-dioxane with trichloroethylene in chlorinated solvent groundwater plumes at US air force installations: fact or fiction[J]. Integr. Environ. Assess. Manag., 2012, 8(4): 731-737. |
| 2 | MOHR T K, STICKNEY J A, DIGUISEPPI W H. Environmental investigation and remediation: 1,4-dioxane and other solvent stabilizers[M]. Los Angeles: CRC Press, 2010. |
| 3 | USEPA. Integrated risk information system (IRIS) on 1,4-dioxane[R]. National Center for Environmental Assessment, Office of Research and Development, Washington D C, 2013. |
| 4 | ZENKER M J, BORDEN R C, BARLAZ M A. Occurrence and treatment of 1,4-dioxane in aqueous environments[J]. Environmental Engineering Science, 2003, 20(5): 423-432. |
| 5 | International Agency for Research on Cancer. Re-evaluation of some organic chemicals, hydrazine and hydrogen peroxide[C]//IARC. Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans. Lyon, 1999: 247. |
| 6 | STEPIEN D K, DIEHL P, HELM J, et al. Fate of 1,4-dioxane in the aquatic environment: from sewage to drinking water[J]. Water Research, 2014, 48: 406-419. |
| 7 | HOWARD P. Handbook of environmental fate and exposure data: for organic chemicals, volume Ⅲ pesticides[M]. Florida: Routledge & CRC Press, 1991: 216-221. |
| 8 | ADAMSON D T, PIÑA E A, CARTWRIGHT A E, et al. 1,4-Dioxane drinking water occurrence data from the third unregulated contaminant monitoring rule[J]. Science of the Total Environment, 2017, 596/597: 236-245. |
| 9 | ADAMSON D T, MAHENDRA S, WALKER K L, et al. A multisite survey to identify the scale of the 1,4-dioxane problem at contaminated groundwater sites[J]. Environmental Science & Technology Letters, 2014, 1(5): 254-258. |
| 10 | KARGES U, BECKER J, PÜTTMANN W. 1,4-Dioxane pollution at contaminated groundwater sites in western Germany and its distribution within a TCE plume[J]. Science of the Total Environment, 2018, 619/620: 712-720. |
| 11 | KARGES U, OTT D, DE BOER S, et al. 1,4-Dioxane contamination of German drinking water obtained by managed aquifer recharge systems: distribution and main influencing factors[J]. The Science of the Total Environment, 2020, 711: 134783. |
| 12 | KWAK Jin Il, Sun-Hwa NAM, AN Youn-Joo. Water quality standards for the protection of human health and aquatic ecosystems in Korea: current state and future perspective[J]. Environmental Science and Pollution Research, 2018, 25(4): 3108-3119. |
| 13 | AN Y-J, KWAK J, S-H NAM, et al. Development and implementation of surface water quality standards for protection of human health in Korea[J]. Environmental Science and Pollution Research, 2014, 21(1): 77-85. |
| 14 | TANABE A, TSUCHIDA Y, IBARAKI T, et al. Impact of 1,4-dioxane from domestic effluent on the Agano and Shinano Rivers, Japan[J]. Bulletin of Environmental Contamination and Toxicology, 2006, 76(1): 44-51. |
| 15 | POLLITT K J G, KIM Jae-Hong, PECCIA J, et al. 1,4-Dioxane as an emerging water contaminant: state of the science and evaluation of research needs[J]. Science of the Total Environment, 2019, 690: 853-866. |
| 16 | LIN Wan-Ting, CHEN Wun-Ling, CHENG Wei-Chih, et al. Determining the residual characteristics of alkylphenols, arsenic, and lead as well as assessing the exposures of 1,4-dioxane from household food detergents[J]. Journal of AOAC International, 2017, 100(4): 1086-1093. |
| 17 | KINGSTON J L T, DAHLEN P R, JOHNSON P C. State-of-the-practice review of in situ thermal technologies[J]. Groundwater Monitoring & Remediation, 2010, 30(4): 64-72. |
| 18 | CHEN Ruihuan, LIU Cun, JOHNSON N W, et al. Removal of 1,4-dioxane by titanium silicalite-1: separation mechanisms and bioregeneration of sorption sites[J]. Chemical Engineering Journal, 2019, 371: 193-202. |
| 19 | KISHIMOTO N, NAKAGAWA T, ASANO M, et al. Ozonation combined with electrolysis of 1,4-dioxane using a two-compartment electrolytic flow cell with solid electrolyte[J]. Water Research, 2008, 42(1): 379-385. |
| 20 | YAMAZAKI S, YAMABE N, NAGANO S, et al. Adsorption and photocatalytic degradation of 1,4-dioxane on TiO2[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2007, 185(2/3): 150-155. |
| 21 | EBERLE D, BALL R, BOVING T B. Peroxone activated persulfate treatment of 1,4-dioxane in the presence of chlorinated solvent co-contaminants[J]. Chemosphere, 2016, 144: 728-735. |
| 22 | ISACOFF E G, NICKELSEN M G. Removal of 1,4-dioxane from water using carbonaceous adsorbents: US20130220935A1[S]. 2013-08-29. |
| 23 | WOODARD S, MOHR T, NICKELSEN M G. Synthetic media: a promising new treatment technology for 1,4‐dioxane[J]. Remediation Journal, 2014, 24(4): 27-40. |
| 24 | MYERS M A, JOHNSON N W, MARIN E Z, et al. Abiotic and bioaugmented granular activated carbon for the treatment of 1,4-dioxane-contaminated water[J]. Environmental Pollution, 2018, 240: 916-924. |
| 25 | LIU Yun, JOHNSON N W, LIU Cun, et al. Mechanisms of 1,4-dioxane biodegradation and adsorption by bio-zeolite in the presence of chlorinated solvents: experimental and molecular dynamics simulation studies[J]. Environmental Science & Technology, 2019, 53(24): 14538-14547. |
| 26 | CORTÉS-ARRIAGADA D. Expanding the environmental applications of metal (Al, Ti, Mn, Fe) doped graphene: adsorption and removal of 1,4-dioxane[J]. Physical Chemistry Chemical Physics, 2016, 18(47): 32281-32292. |
| 27 | CORTÉS-ARRIAGADA D, MIRANDA-ROJAS S, ORTEGA D E, et al. Oxidized and Si-doped graphene: emerging adsorbents for removal of dioxane[J]. Physical Chemistry Chemical Physics, 2017, 19(27): 17587-17597. |
| 28 | IZAKMEHRI Z, ARDJMAND M, GANJI M D, et al. Removal of dioxane pollutants from water by using Al-doped single walled carbon nanotubes[J]. RSC Advances, 2015, 5(59): 48124-48132. |
| 29 | RÖDER A, SKOV A, BOGUSLAVSKIY A E, et al. VUV Excited-state dynamics of cyclic ethers as a function of ring size[J]. Physical Chemistry Chemical Physics, 2020, 22(45): 26241-26254. |
| 30 | COLEMAN H M, VIMONSES V, LESLIE G, et al. Degradation of 1,4-dioxane in water using TiO2 based photocatalytic and H2O2/UV processes[J]. Journal of Hazardous Materials, 2007, 146(3): 496-501. |
| 31 | MIN Byoung Koun, Jung Eun HEO, YOUN Na Kyoung, et al. Tuning of the photocatalytic 1,4-dioxane degradation with surface plasmon resonance of gold nanoparticles on titania[J]. Catalysis Communications, 2009, 10(5): 712-715. |
| 32 | VESCOVI T, COLEMAN H M, AMAL R. The effect of pH on UV-based advanced oxidation technologies – 1,4-dioxane degradation[J]. Journal of Hazardous Materials, 2010, 182(1): 75-79. |
| 33 | KRUITHOF J C, KAMP P C, MARTIJN B J. UV/H2O2 Treatment: a practical solution for organic contaminant control and primary disinfection[J]. Ozone: Science & Engineering, 2007, 29(4): 273-280. |
| 34 | COOPER W J, CRAMER C J, MARTIN N H, et al. Free radical mechanisms for the treatment of methyl tert-butyl ether (MTBE) via advanced oxidation/reductive processes in aqueous solutions[J]. Chemical Reviews, 2009, 109(3): 1302-1345. |
| 35 | ZHANG Shu, GEDALANGA P B, MAHENDRA S. Advances in bioremediation of 1,4-dioxane-contaminated waters[J]. Journal of Environmental Management, 2017, 204: 765-774. |
| 36 | BARNDÕK H, HERMOSILLA D, HAN Changseok, et al. Degradation of 1,4-dioxane from industrial wastewater by solar photocatalysis using immobilized NF-TiO2 composite with monodisperse TiO2 nanoparticles[J]. Applied Catalysis B: Environmental, 2016, 180: 44-52. |
| 37 | BECKETT M A, HUA I. Elucidation of the 1,4-dioxane decomposition pathway at discrete ultrasonic frequencies[J]. Environmental Science & Technology, 2000, 34(18): 3944-3953. |
| 38 | BECKETT M A, HUA I. Enhanced sonochemical decomposition of 1,4-dioxane by ferrous iron[J]. Water Research, 2003, 37(10): 2372-2376. |
| 39 | Hyun-Seok SON, CHOI Seok-Bong, KHAN E, et al. Removal of 1,4-dioxane from water using sonication: effect of adding oxidants on the degradation kinetics[J]. Water Research, 2006, 40(4): 692-698. |
| 40 | CHOI Jong Young, LEE You-Jin, SHIN J, et al. Anodic oxidation of 1,4-dioxane on boron-doped diamond electrodes for wastewater treatment[J]. Journal of Hazardous Materials, 2010, 179(1): 762-768. |
| 41 | NAKAGAWA H, TAKAGI S, MAEKAWA J. Fered-Fenton process for the degradation of 1,4-dioxane with an activated carbon electrode: a kinetic model including active radicals[J]. Chemical Engineering Journal, 2016, 296: 398-405. |
| 42 | WANG Huijiao, BAKHEET B, YUAN Shi, et al. Kinetics and energy efficiency for the degradation of 1,4-dioxane by electro-peroxone process[J]. Journal of Hazardous Materials, 2015, 294: 90-98. |
| 43 | KISHIMOTO N, KATAYAMA Y, KATO M, et al. Technical feasibility of UV/electro-chlorine advanced oxidation process and pH response[J]. Chemical Engineering Journal, 2018, 334: 2363-2372. |
| 44 | ZHANG B T, ZHANG Y, TENG Y G, et al. Sulfate radical and its application in decontamination technologies[J]. Critical Reviews in Environmental Science and Technology, 2015, 45(16): 1756-1800. |
| 45 | 黄智辉, 纪志永, 陈希, 等. 过硫酸盐高级氧化降解水体中有机污染物研究进展[J]. 化工进展, 2019, 38(5): 2461-2470. |
| HUANG Zhihui, JI Zhiyong, CHEN Xi, et al. Degradation of organic pollutants in water by persulfate advanced oxidation[J]. Chemical Industry and Engineering Progress, 2019, 38(5): 2461-2470. | |
| 46 | ZHU Jiang, LI Bingzhi. Degradation kinetic and remediation effectiveness of 1,4-dioxane-contaminated groundwater by a sono-activated persulfate process[J]. Journal of Environmental Engineering, 2018, 144(10): 04018098. |
| 47 | Hyun-Seok SON, Jong-Kwon IM, Kyung-Duk ZOH. A Fenton-like degradation mechanism for 1,4-dioxane using zero-valent iron (Fe0) and UV light[J]. Water Research, 2009, 43(5): 1457-1463. |
| 48 | TAKAHASHI N, HIBINO T, TORII H, et al. Evaluation of O3/UV and O3/H2O2 as practical advanced oxidation processes for degradation of 1,4-dioxane[J]. Ozone: Science and Engineering, 2013, 35(5): 331-337. |
| 49 | ZHONG Hua, BRUSSEAU M L, WANG Yake, et al. In-situ activation of persulfate by iron filings and degradation of 1,4-dioxane[J]. Water Research, 2015, 83: 104-111. |
| 50 | BARNDÕK H, BLANCO L, HERMOSILLA D, et al. Heterogeneous photo-Fenton processes using zero valent iron microspheres for the treatment of wastewaters contaminated with 1,4-dioxane[J]. Chemical Engineering Journal, 2016, 284: 112-121. |
| 51 | LI Bingzhi, ZHU Jiang. Simultaneous degradation of 1,1,1-trichloroethane and solvent stabilizer 1,4-dioxane by a sono-activated persulfate process[J]. Chemical Engineering Journal, 2016, 284: 750-763. |
| 52 | OUYANG Da, YAN Jingchun, QIAN Linbo, et al. Degradation of 1,4-dioxane by biochar supported nano magnetite particles activating persulfate[J]. Chemosphere, 2017, 184: 609-617. |
| 53 | KAMBHU A, GREN M, TANG Wei, et al. Remediating 1,4-dioxane-contaminated water with slow-release persulfate and zerovalent iron[J]. Chemosphere, 2017, 175: 170-177. |
| 54 | FENG Yong, LEE Po-Heng, WU Deli, et al. Surface-bound sulfate radical-dominated degradation of 1,4-dioxane by alumina-supported palladium (Pd/Al2O3) catalyzed peroxymonosulfate[J]. Water Research, 2017, 120: 12-21. |
| 55 | KANG Yu-Gyeong, YOON Hakwon, LEE Woojin, et al. Comparative study of peroxide oxidants activated by nZVI: removal of 1,4-dioxane and arsenic(Ⅲ) in contaminated waters[J]. Chemical Engineering Journal, 2018, 334: 2511-2519. |
| 56 | OUYANG Da, CHEN Yun, YAN Jingchun, et al. Activation mechanism of peroxymonosulfate by biochar for catalytic degradation of 1,4-dioxane: important role of biochar defect structures[J]. Chemical Engineering Journal, 2019, 370: 614-624. |
| 57 | PARK Young-kwon, CHUNG Kyong-Hwan, PARK In-Soo, et al. Photocatalytic degradation of 1,4-dioxane using liquid phase plasma on visible light photocatalysts[J]. Journal of Hazardous Materials, 2020, 399: 123087. |
| 58 | ZENKER M J, BORDEN R C, BARLAZ M A. Mineralization of 1,4-dioxane in the presence of a structural analog[J]. Biodegradation, 2000, 11(4): 239-246. |
| 59 | SEI K, KAKINOKI T, INOUE D, et al. Evaluation of the biodegradation potential of 1,4-dioxane in river, soil and activated sludge samples[J]. Biodegradation, 2010, 21(4): 585-591. |
| 60 | CHIANG S Y D, MORA R, DIGUISEPPI W H, et al. Characterizing the intrinsic bioremediation potential of 1,4-dioxane and trichloroethene using innovative environmental diagnostic tools[J]. Journal of Environmental Monitoring, 2012, 14(9): 2317-2326. |
| 61 | LI M Y, ORDEN E T VAN, DEVRIES D J, et al. Bench-scale biodegradation tests to assess natural attenuation potential of 1,4-dioxane at three sites in California[J]. Biodegradation, 2015, 26(1): 39-50. |
| 62 | FUTUGHE A E, PURCHASE D, JONES H. Phytoremediation: phytoremediation using native plants[M]. Switzerland: Springer, Cham, 2020: 285-327. |
| 63 | CRISTALDI A, CONTI G O, Eun Hea JHO, et al. Phytoremediation of contaminated soils by heavy metals and PAHs. A brief review[J]. Environmental Technology & Innovation, 2017, 8: 309-326. |
| 64 | AITCHISON E W, KELLEY S L, ALVAREZ P J J, et al. Phytoremediation of 1,4-dioxane by hybrid poplar trees[J]. Water Environment Research, 2000, 72(3): 313-321. |
| 65 | KELLEY S L, AITCHISON E W, DESHPANDE M, et al. Biodegradation of 1,4-dioxane in planted and unplanted soil: effect of bioaugmentation with Amycolata sp. CB1190[J]. Water Research, 2001, 35(16): 3791-3800. |
| 66 | FERRO A M, KENNEDY J, LARUE J C. Phytoremediation of 1,4-dioxane-containing recovered groundwater[J]. International Journal of Phytoremediation, 2013, 15(10): 911-923. |
| 67 | SIMMER R, MATHIEU J, SILVA M L B DA, et al. Bioaugmenting the poplar rhizosphere to enhance treatment of 1,4-dioxane[J]. Science of the Total Environment, 2020, 744: 140823. |
| 68 | OSAMA R, AWAD H M, ZHA Shanshan, et al. Greenhouse gases emissions from duckweed pond system treating polyester resin wastewater containing 1,4-dioxane and heavy metals[J]. Ecotoxicology and Environmental Safety, 2020, 207: 111253. |
| 69 | OUYANG Ying. Phytoremediation: modeling plant uptake and contaminant transport in the soil-plant-atmosphere continuum[J]. Journal of Hydrology, 2002, 266(1): 66-82. |
| 70 | SORENSEN H. 1,4-Dioxane and the application of phytoremediation at North Carolina hazardous waste groundwater contaminated sites[D]. North Carolina State University, 2013. |
| 71 | 沈源源, 滕应, 骆永明, 等. 几种豆科、禾本科植物对多环芳烃复合污染土壤的修复[J]. 土壤, 2011, 43(2): 253-257. |
| SHEN Yuanyuan, TENG Ying, LUO Yongming, et al. Remediation efficiency of several legumes and grasses in PAH-contaminated soils[J]. Soil, 2011, 43(2): 253-257. | |
| 72 | 陈静. 小麦草对芘-镍污染土壤的修复潜力及耐性机理研究[D]. 上海: 上海大学, 2019. |
| CHEN Jing. Study on the remediation potential and tolerance mechanism of wheatgrass to pyrene-Ni contaminated soil[D]. Shanghai: Shanghai University, 2019. | |
| 73 | 朱灿, 刘慧刚, 顾彦, 等. 外源硒对萘胁迫下车前草生长及土壤修复能力的影响[J]. 农业环境科学学报, 2019, 38(11): 2511-2519. |
| ZHU Can, LIU Huigang, GU Yan, et al. Effects of selenium on the growth and phytoremediation efficiency of Plantago asiatica L. in soil exposed to naphthalene[J]. Journal of Agro-Environment Science, 2019, 38(11): 2511-2519. | |
| 74 | GLICK B R. Using soil bacteria to facilitate phytoremediation[J]. Biotechnology Advances, 2010, 28(3): 367-374. |
| 75 | PARALES R E, ADAMUS J E, WHITE N, et al. Degradation of 1,4-dioxane by an actinomycete in pure culture[J]. Applied and Environmental Microbiology, 1994, 60(12): 4527. |
| 76 | MAHENDRA S, ALVAREZ-COHEN L. Pseudonocardia dioxanivorans sp. nov., a novel actinomycete that grows on 1,4-dioxane[J]. International Journal of Systematic and Evolutionary Microbiology, 2005, 55(2): 593-598. |
| 77 | NAKAMIYA K, HASHIMOTO S, ITO H, et al. Degradation of 1,4-dioxane and cyclic ethers by an isolated fungus[J]. Applied and Environmental Microbiology, 2005, 71(3): 1254. |
| 78 | MAHENDRA S, ALVAREZ-COHEN L. Kinetics of 1,4-dioxane biodegradation by monooxygenase-expressing bacteria[J]. Environmental Science & Technology, 2006, 40(17): 5435-5442. |
| 79 | KIM Young Mo, JEON Jong Rok, MURUGESAN K, et al. Biodegradation of 1,4-dioxane and transformation of related cyclic compounds by a newly isolated Mycobacterium sp. PH-06[J]. Biodegradation, 2008, 20(4): 511. |
| 80 | SKINNER K, CUIFFETTI L, HYMAN M. Metabolism and cometabolism of cyclic ethers by a filamentous fungus, a Graphium sp[J]. Applied and Environmental Microbiology, 2009, 75(17): 5514. |
| 81 | MASUDA H, MCCLAY K, STEFFAN R J, et al. Biodegradation of tetrahydrofuran and 1,4-dioxane by soluble diiron monooxygenase in Pseudonocardia sp. strain ENV478[J]. J. Mol. Microbiol. Biotechnol., 2012, 22(5): 312-316. |
| 82 | SEI K, MIYAGAKI K, KAKINOKI T, et al. Isolation and characterization of bacterial strains that have high ability to degrade 1,4-dioxane as a sole carbon and energy source[J]. Biodegradation, 2013, 24(5): 665-674. |
| 83 | HUANG Huanlin, SHEN Dongsheng, LI Na, et al. Biodegradation of 1,4-dioxane by a novel strain and its biodegradation pathway[J]. Water, Air, & Soil Pollution, 2014, 225(9): 2135. |
| 84 | LIPPINCOTT D, STREGER S H, SCHAEFER C E, et al. Bioaugmentation and propane biosparging for in situ biodegradation of 1,4-dioxane[J]. Groundwater Monitoring and Remediation, 2015, 35(2): 81-92. |
| 85 | PUGAZHENDI A, RAJESH BANU J, DHAVAMANI J, et al. Biodegradation of 1,4-dioxane by Rhodanobacter AYS5 and the role of additional substrates[J]. Annals of Microbiology, 2015, 65(4): 2201-2208. |
| 86 | CHEN Dongzhi, JIN Xiaojun, CHEN Jing, et al. Intermediates and substrate interaction of 1,4-dioxane degradation by the effective metabolizer Xanthobacter flavus DT8[J]. International Biodeterioration & Biodegradation, 2016, 106: 133-140. |
| 87 | MATSUI R, TAKAGI K, SAKAKIBARA F, et al. Identification and characterization of 1,4-dioxane-degrading microbe separated from surface seawater by the seawater-charcoal perfusion apparatus[J]. Biodegradation, 2016, 27(2): 155-163. |
| 88 | INOUE D, TSUNODA T, YAMAMOTO N, et al. 1,4-Dioxane degradation characteristics of Rhodococcus aetherivorans JCM 14343[J]. Biodegradation, 2018, 29(3): 301-310. |
| 89 | TUSHER T R, SHIMIZU T, INOUE C, et al. Enrichment and analysis of stable 1,4-dioxane-degrading microbial consortia consisting of novel dioxane-degraders[J]. Microorganisms, 2020, 8(1): 50. |
| 90 | SHEN Weirong, CHEN Hong, PAN Shanshan. Anaerobic biodegradation of 1,4-dioxane by sludge enriched with iron-reducing microorganisms[J]. Bioresource Technology, 2008, 99(7): 2483-2487. |
| 91 | RODRIGUEZ F J B. Evaluation of 1,4-dioxane biodegradation under aerobic and anaerobic conditions[D]. Clemson: Clemson University, 2016. |
| 92 | MAHENDRA S, PETZOLD C J, BAIDOO E E, et al. Identification of the intermediates of in vivo oxidation of 1,4-dioxane by monooxygenase-containing bacteria[J]. Environmental Science & Technology, 2007, 41(21): 7330-7336. |
| 93 |
金小君, 陈东之, 朱润晔, 等. Xanthobacter flavus DT8降解二 烷的特性研究[J]. 环境科学, 2012, 33(5): 1657-1662. 烷的特性研究[J]. 环境科学, 2012, 33(5): 1657-1662.
|
| JIN Xiaojun, CHEN Dongzhi, ZHU Runye, et al. Characteristics of 1,4-dioxane degradation by Xanthobacter flavus DT8.[J]. Environmental Science, 2012, 33(5): 1657-1662. | |
| 94 | ZENKER M J, BORDEN R C, BARLAZ M A. Biodegradation of 1,4-dioxane using trickling filter[J]. Journal of Environmental Engineering, 2004, 130(9): 926-931. |
| 95 | SAUL M T. Bioaugmentation to remediate dioxane in groundwater: US8241500B2[P]. 2012-08-14. |
| 96 | Ji-Hyun NAM, VENTURA J-R S, YEOM Ick Tae, et al. Structural and kinetic characteristics of 1,4-dioxane-degrading bacterial consortia containing the Phylum TM7[J]. Journal of Microbiology and Biotechnology, 2016, 26(11): 1951-1964. |
| 97 | SEKAR R, DICHRISTINA T J. Microbially driven Fenton reaction for degradation of the widespread environmental contaminant 1,4-dioxane[J]. Environmental Science & Technology, 2014, 48(21): 12858-12867. |
| 98 | MAHENDRA S, GROSTERN A, ALVAREZ-COHEN L. The impact of chlorinated solvent co-contaminants on the biodegradation kinetics of 1,4-dioxane[J]. Chemosphere, 2013, 91(1): 88-92. |
| 99 | ZHANG Shu, GEDALANGA P B, MAHENDRA S. Biodegradation kinetics of 1,4-dioxane in chlorinated solvent mixtures[J]. Environmental Science & Technology, 2016, 50(17): 9599-9607. |
| 100 | MATTES T E, ALEXANDER A K, COLEMAN N V. Aerobic biodegradation of the chloroethenes: pathways, enzymes, ecology, and evolution[J]. FEMS Microbiology Reviews, 2010, 34(4): 445-475. |
| 101 | PORNWONGTHONG P, MULCHANDANI A, GEDALANGA P B, et al. Transition metals and organic ligands influence biodegradation of 1,4-dioxane[J]. Applied Biochemistry and Biotechnology, 2014, 173(1): 291-306. |
| 102 | YAN Ni, LIU Fei, LIU Boyang, et al. Treatment of 1,4-dioxane and trichloroethene co-contamination by an activated binary persulfate-peroxide oxidation process[J]. Environmental Science and Pollution Research, 2018, 25(32): 32088-32095. |
| 103 | POLASKO A L, ZULLI A, GEDALANGA P B, et al. A mixed microbial community for the biodegradation of chlorinated ethenes and 1,4-dioxane[J]. Environmental Science & Technology Letters, 2019, 6(1): 49-54. |
| 104 | SEKAR R, TAILLEFERT M, DICHRISTINA T J. Simultaneous transformation of commingled trichloroethylene, tetrachloroethylene, and 1,4-dioxane by a microbially driven Fenton reaction in batch liquid cultures[J]. Applied and Environmental Microbiology, 2016, 82(21): 6335. |
| 105 | DENG Daiyong, PHAM Dung Ngoc, LI Fei, et al. Discovery of an inducible toluene monooxygenase that cooxidizes 1,4-dioxane and 1,1-dichloroethylene in propanotrophic Azoarcus sp. strain DD4[J]. Applied and Environmental Microbiology, 2020, 86(17). |
| 106 | JASMANN J R, GEDALANGA P B, BORCH T, et al. Synergistic treatment of mixed 1,4-dioxane and chlorinated solvent contaminations by coupling electrochemical oxidation with aerobic biodegradation[J]. Environmental Science & Technology, 2017, 51(21): 12619-12629. |
| 107 | MIAO Yu, JOHNSON N W, GEDALANGA P B, et al. Response and recovery of microbial communities subjected to oxidative and biological treatments of 1,4-dioxane and co-contaminants[J]. Water Research, 2019, 149: 74-85. |
| 108 | MIAO Yu, JOHNSON N W, PHAN Thien, et al. Monitoring, assessment, and prediction of microbial shifts in coupled catalysis and biodegradation of 1,4-dioxane and co-contaminants[J]. Water Research, 2020, 173: 115540. |
| [1] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [2] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [3] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [4] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [5] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [6] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [7] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [8] | 邵志国, 任雯, 许世佩, 聂凡, 许毓, 刘龙杰, 谢水祥, 李兴春, 王庆吉, 谢加才. 终温对油基钻屑热解产物分布和特性影响[J]. 化工进展, 2023, 42(9): 4929-4938. |
| [9] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| [10] | 高聪, 陈城虎, 陈修来, 刘立明. 代谢工程改造微生物合成生物基单体的进展与挑战[J]. 化工进展, 2023, 42(8): 4123-4135. |
| [11] | 杨静, 李博, 李文军, 刘晓娜, 汤刘元, 刘月, 钱天伟. 焦化污染场地中萘降解菌的分离及降解特性[J]. 化工进展, 2023, 42(8): 4351-4361. |
| [12] | 姜晶, 陈霄宇, 张瑞妍, 盛光遥. 载锰生物炭制备及其在环境修复中应用研究进展[J]. 化工进展, 2023, 42(8): 4385-4397. |
| [13] | 张振, 李丹, 陈辰, 吴菁岚, 应汉杰, 乔浩. 吸附树脂对唾液酸的分离纯化[J]. 化工进展, 2023, 42(8): 4153-4158. |
| [14] | 王知彩, 刘伟伟, 周璁, 潘春秀, 闫洪雷, 李占库, 颜井冲, 任世彪, 雷智平, 水恒福. 基于煤基腐殖酸的高效减水剂合成与性能表征[J]. 化工进展, 2023, 42(7): 3634-3642. |
| [15] | 于静文, 宋璐娜, 刘砚超, 吕瑞东, 武蒙蒙, 冯宇, 李忠, 米杰. 一种吲哚基超交联聚合物In-HCP对水中碘的吸附作用[J]. 化工进展, 2023, 42(7): 3674-3683. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||