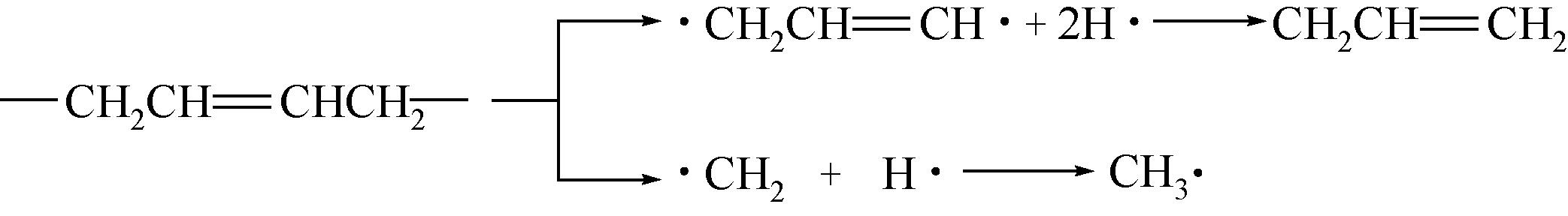化工进展 ›› 2021, Vol. 40 ›› Issue (6): 3119-3131.DOI: 10.16085/j.issn.1000-6613.2020-1421
基于分子动力学模拟的轮胎橡胶气相热解产物反应机理
于双鹏1( ), 杨启容1(
), 杨启容1( ), 陶礼1, 刘亭1, 杜威2, 姚尔人3
), 陶礼1, 刘亭1, 杜威2, 姚尔人3
- 1.青岛大学机电工程学院,山东 青岛 266071
2.海信(山东)空调有限公司,山东 青岛 266100
3.西安交通 大学能源动力与工程学院,陕西 西安 710049
-
收稿日期:2020-07-22修回日期:2020-10-19出版日期:2021-06-06发布日期:2021-06-22 -
通讯作者:杨启容 -
作者简介:于双鹏(1996—),男,硕士研究生,研究方向为能源开发与利用中的传热传质。E-mail:442134761@qq.com 。 -
基金资助:国家自然科学基金青年科学基金(51806136)
Gas phase pyrolysis products of tire rubber based on molecular dynamics simulation
YU Shuangpeng1( ), YANG Qirong1(
), YANG Qirong1( ), TAO Li1, LIU Ting1, DU Wei2, YAO Erren3
), TAO Li1, LIU Ting1, DU Wei2, YAO Erren3
- 1.College of Mechancial and Electrical Engineering, Qingdao University, Qingdao 266071, Shandong, China
2.Hisense (Shandong) Air-Conditioning Company Limited, Qingdao 266100, Shandong, China
3.School of Energy and Power Engineering, Xi’an Jiaotong University, Xi’an 710049, Shaanxi, China
-
Received:2020-07-22Revised:2020-10-19Online:2021-06-06Published:2021-06-22 -
Contact:YANG Qirong
摘要:
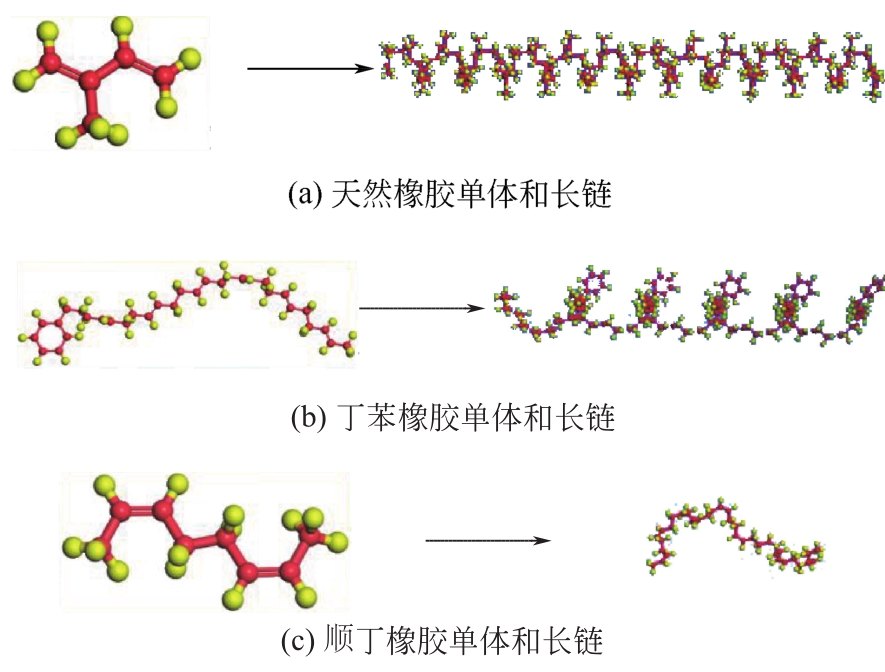
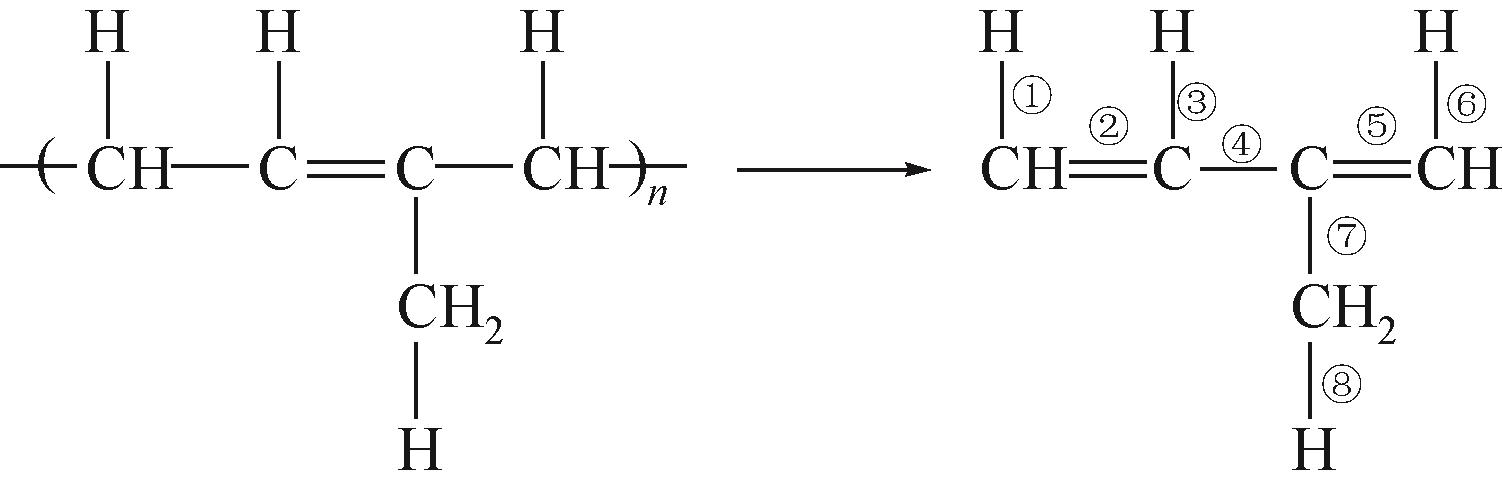
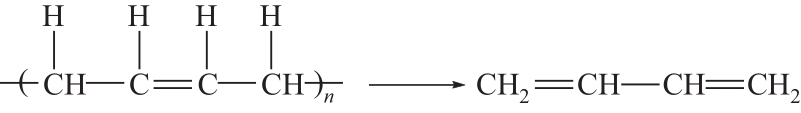
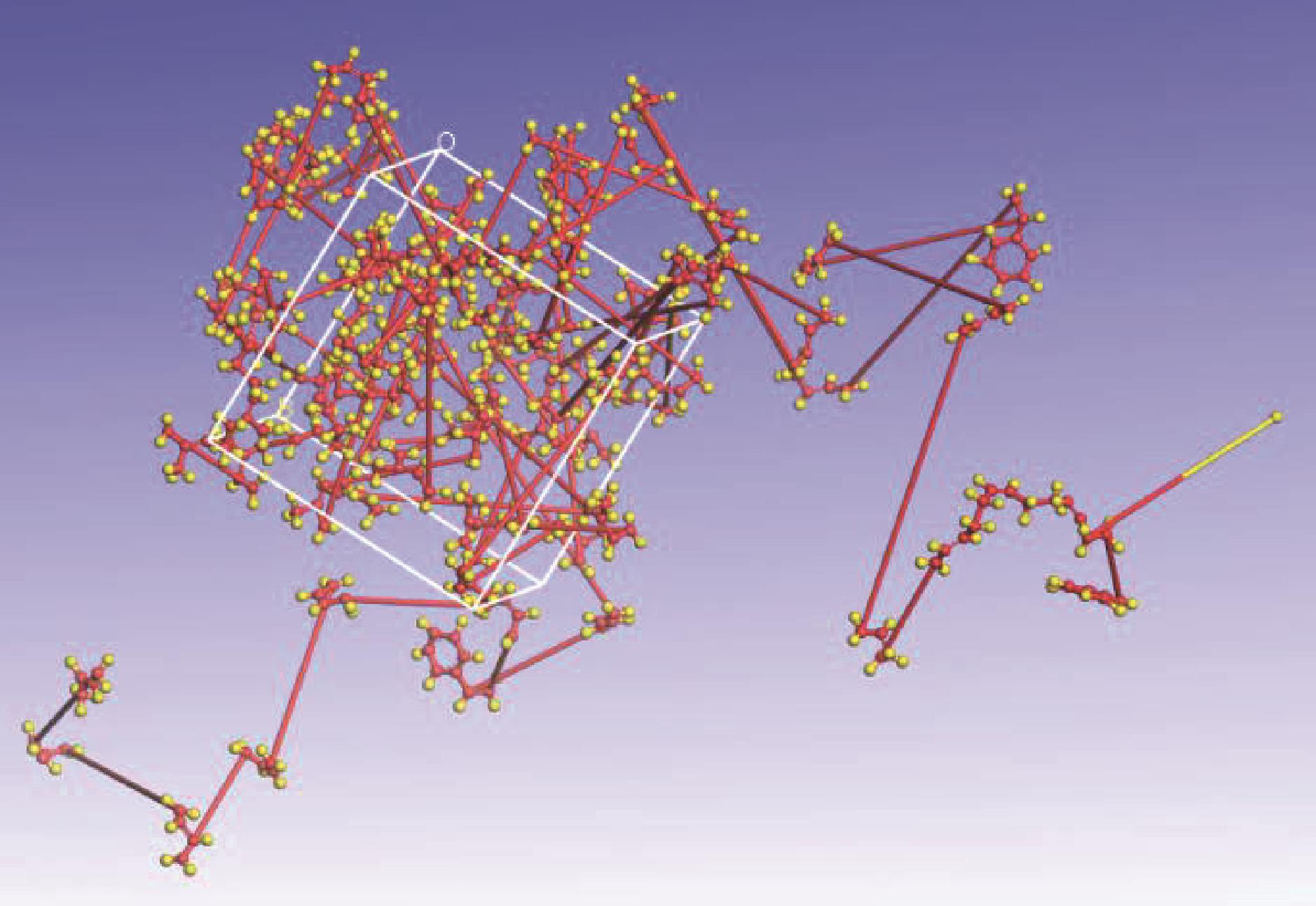
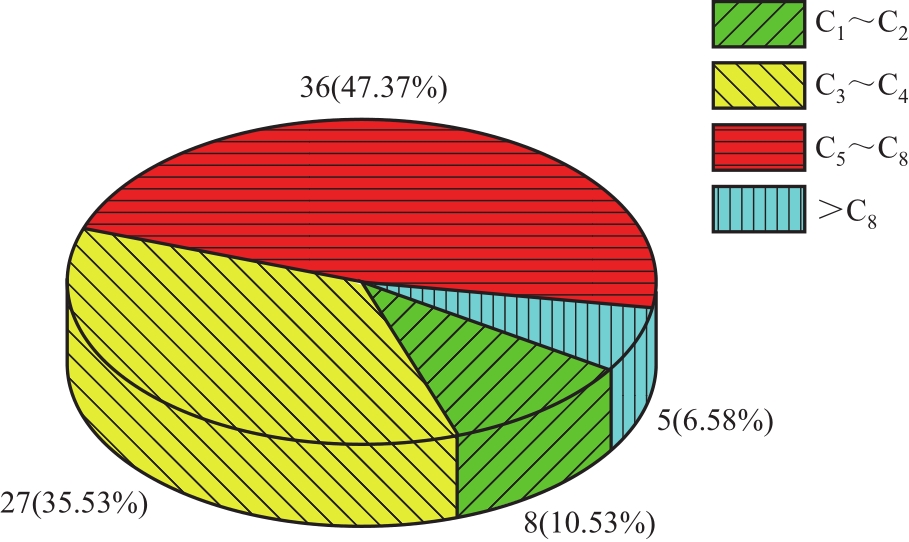
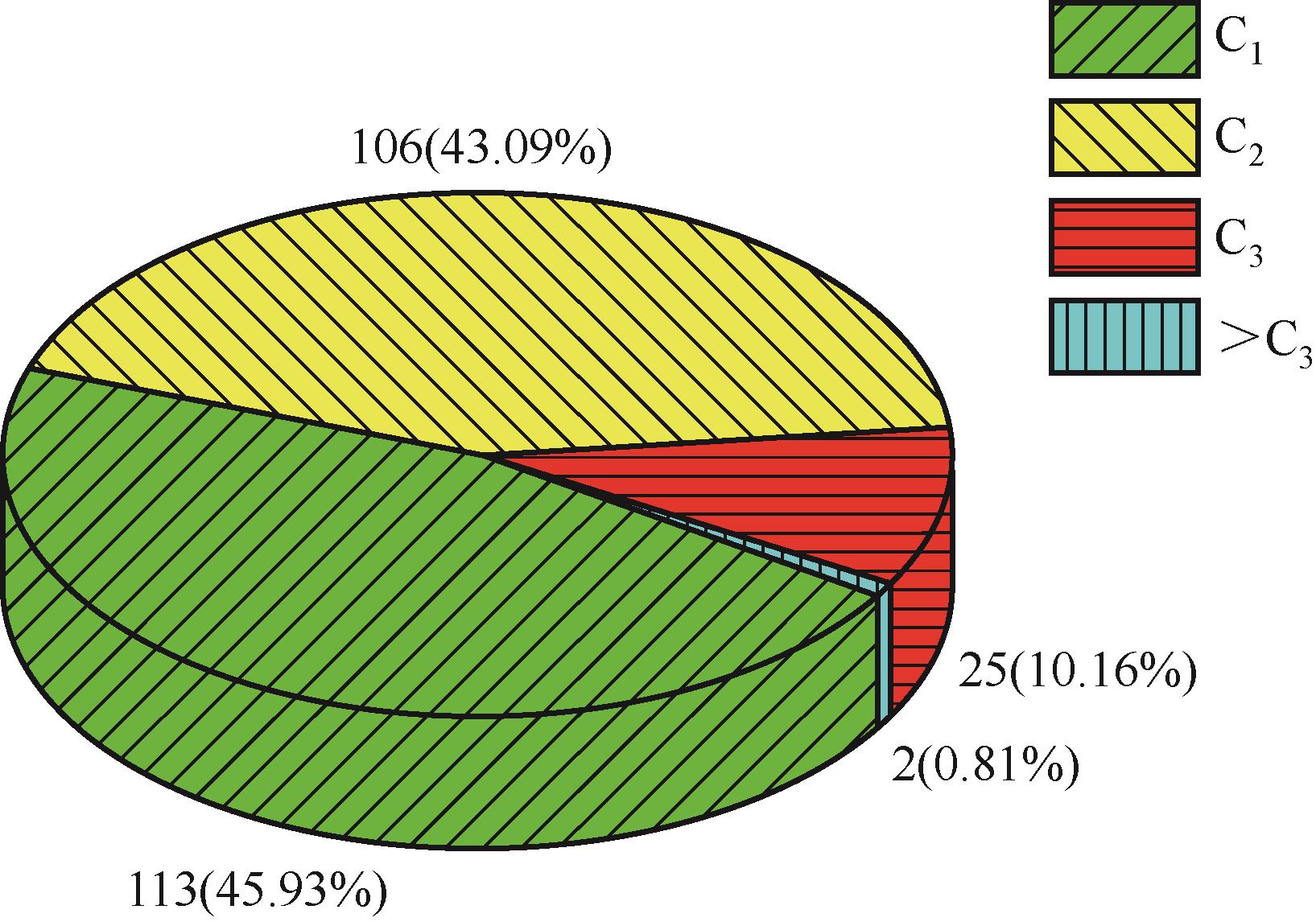
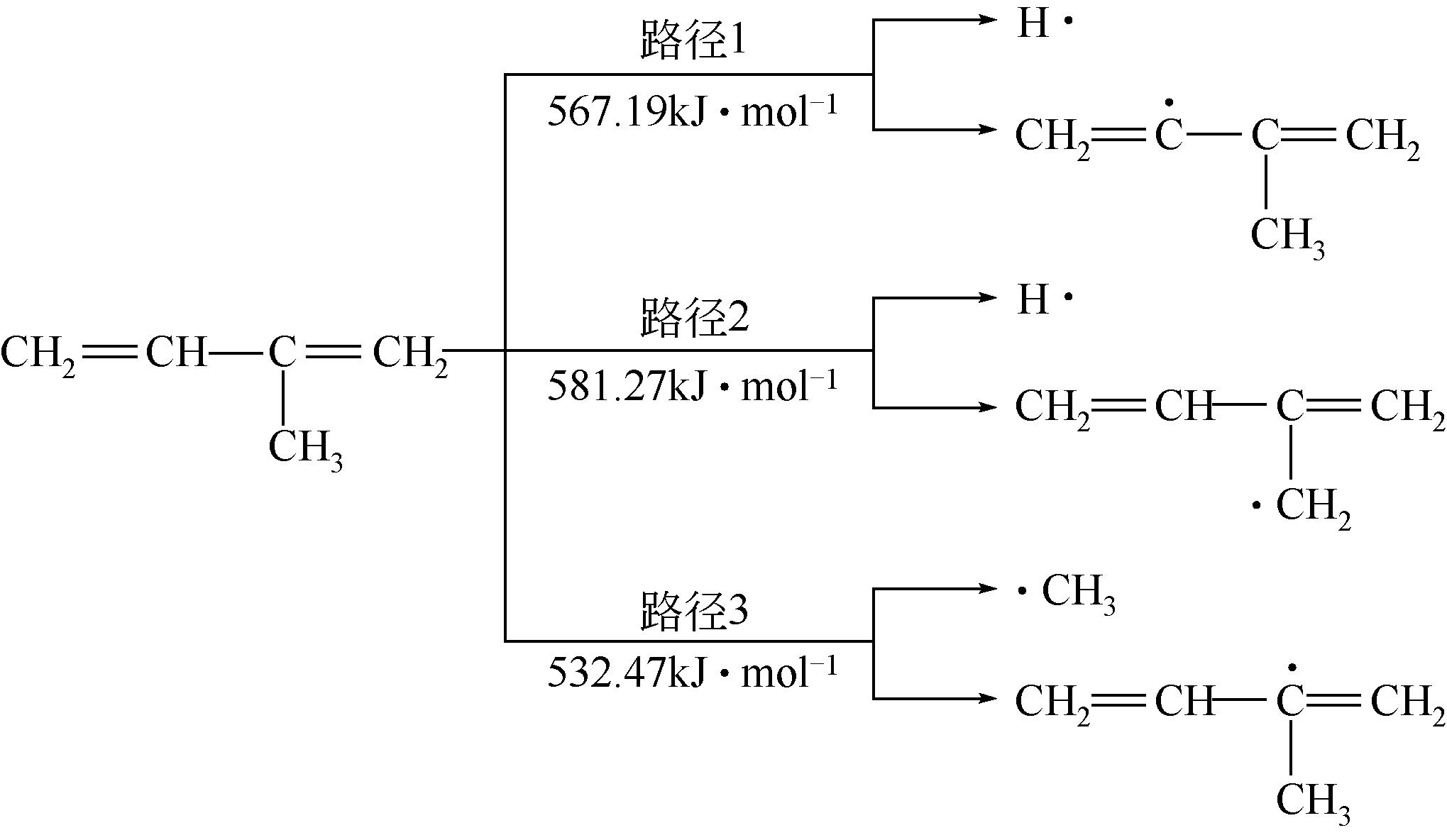
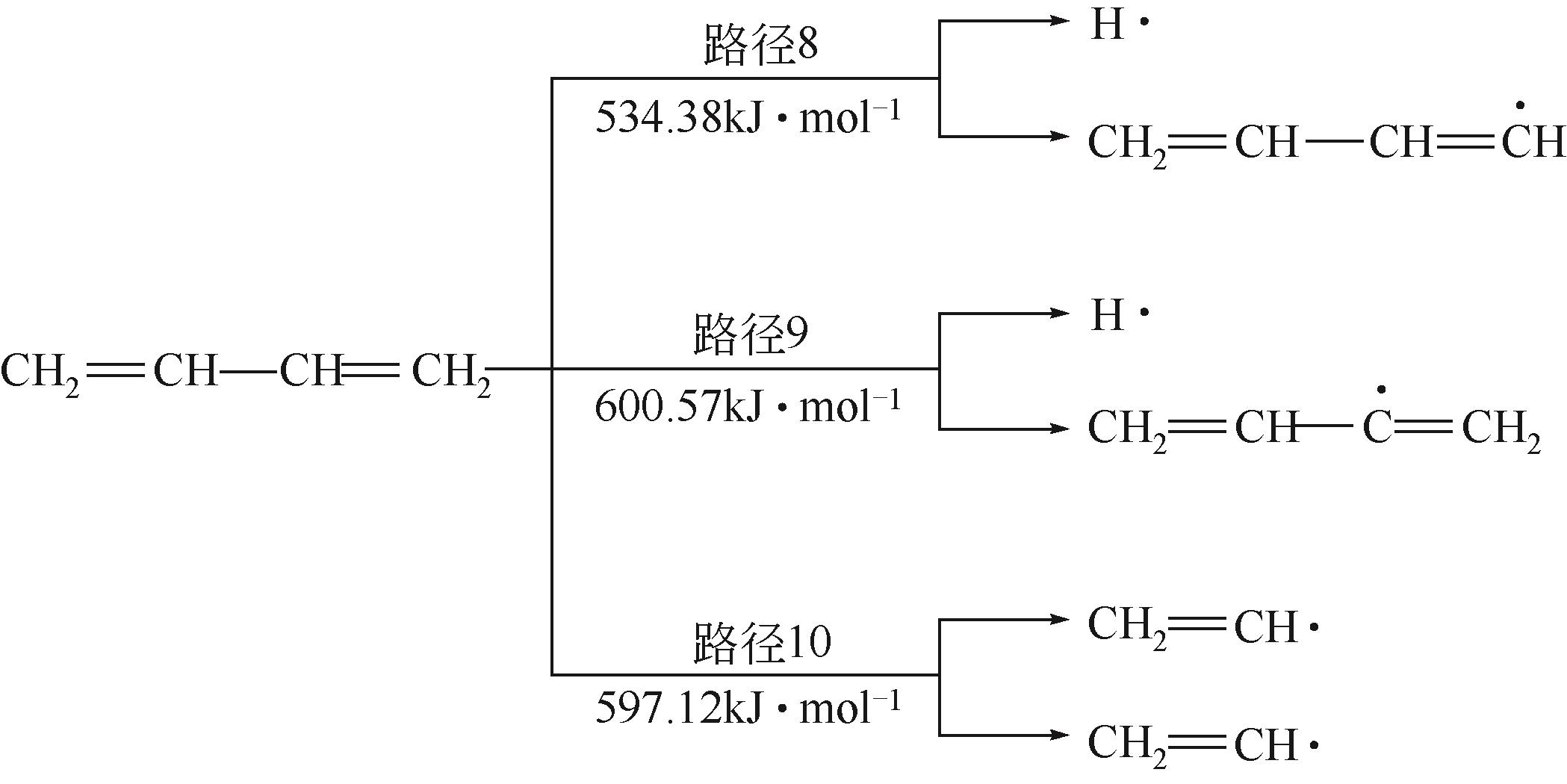
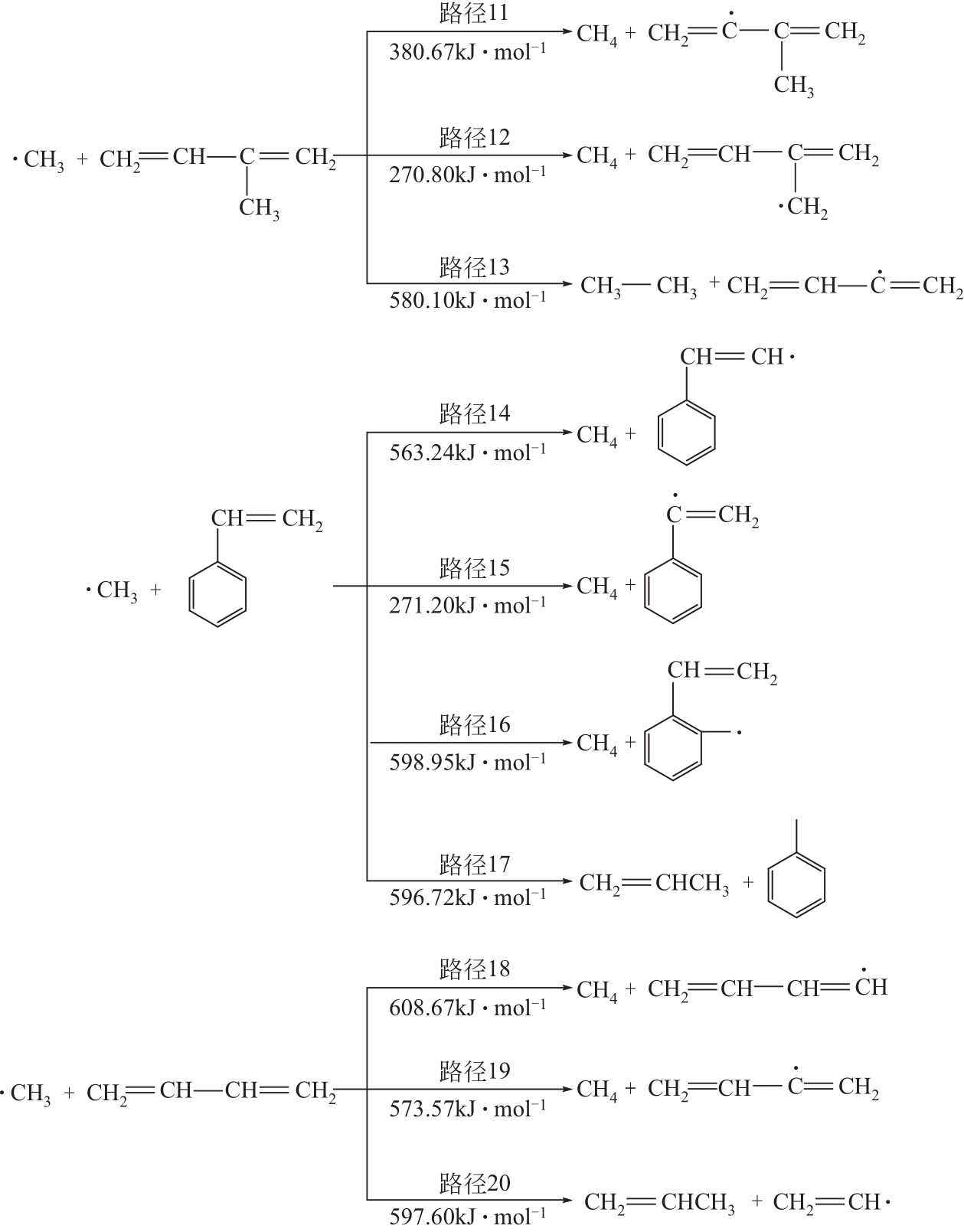
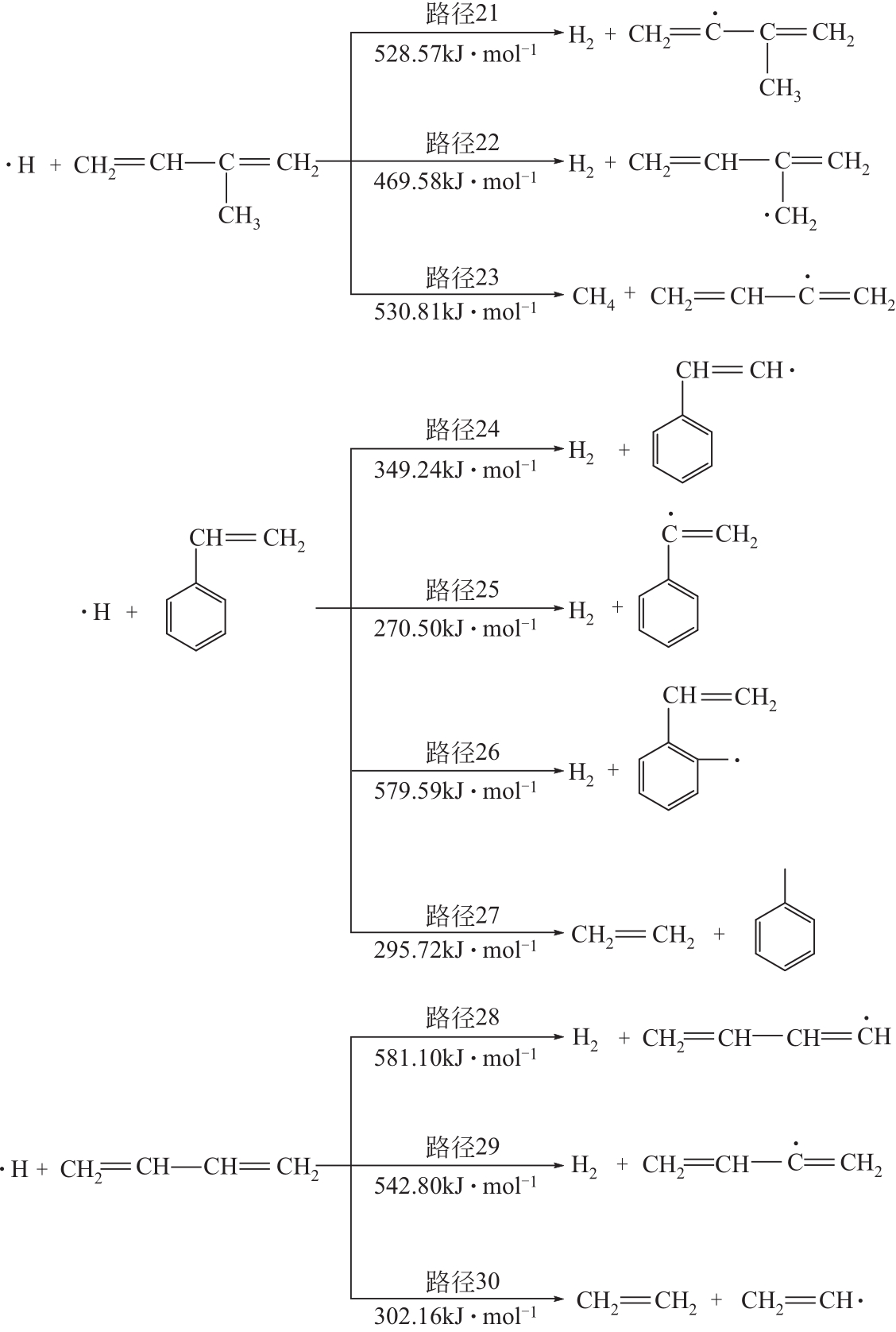
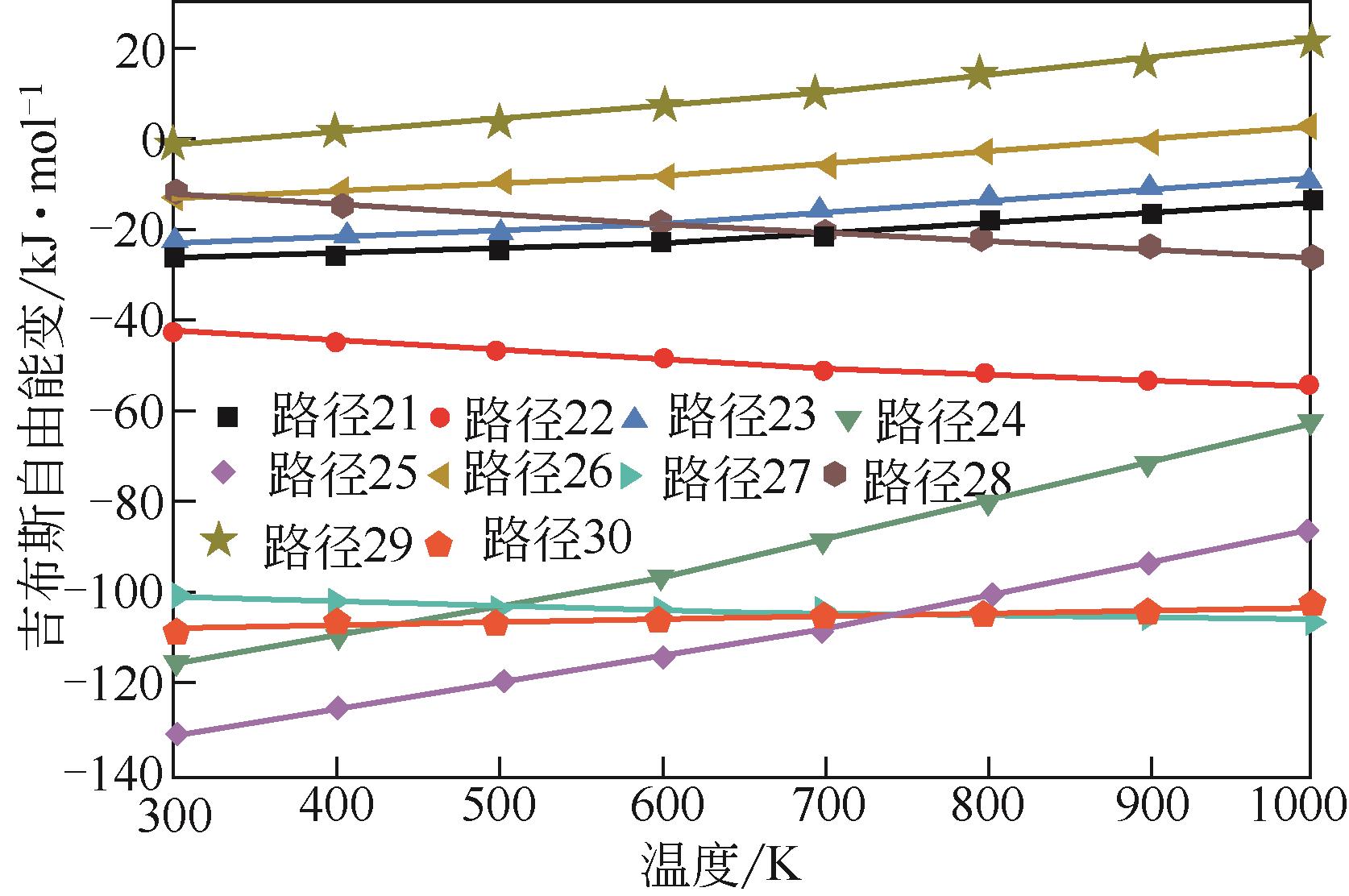
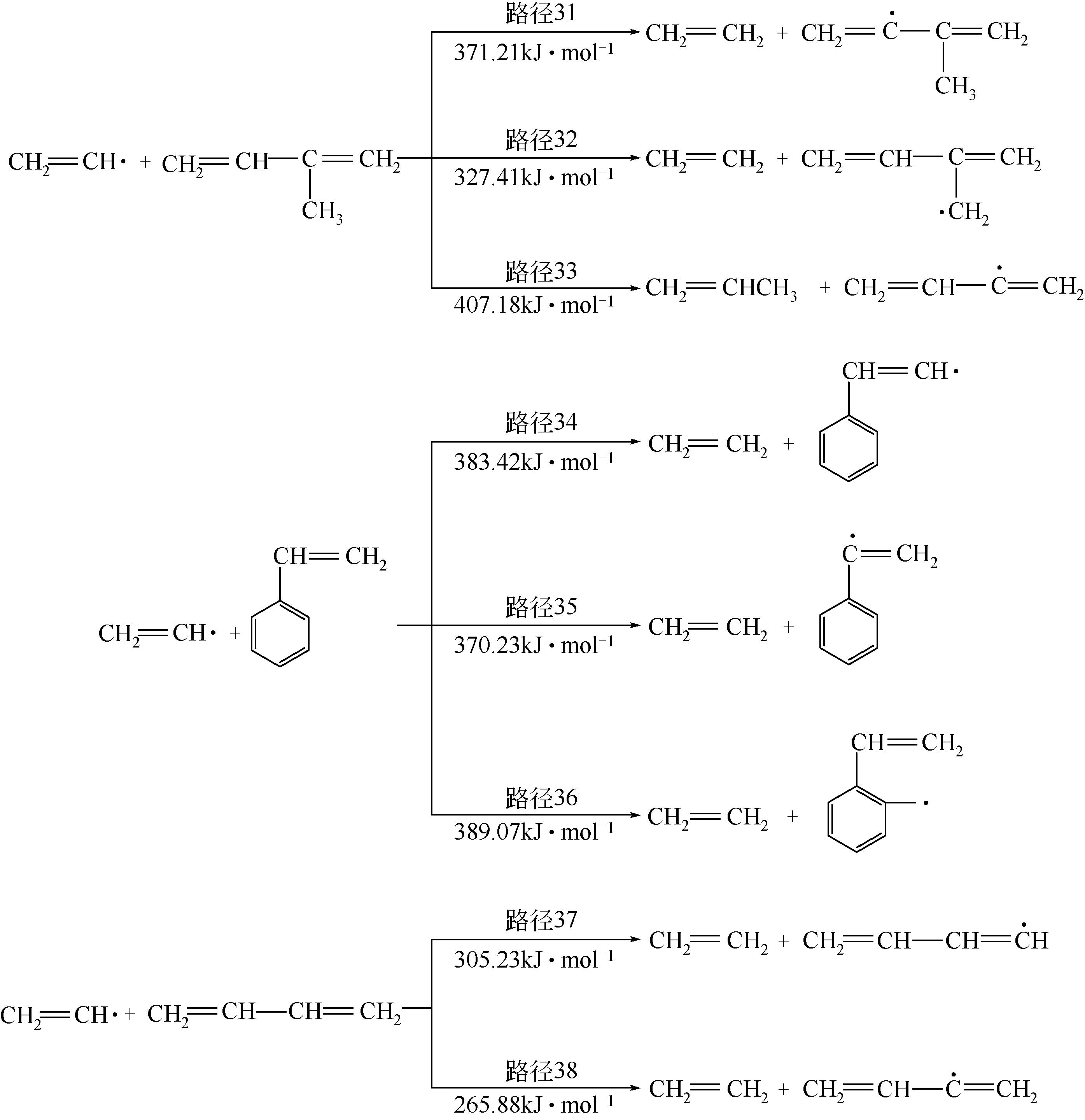
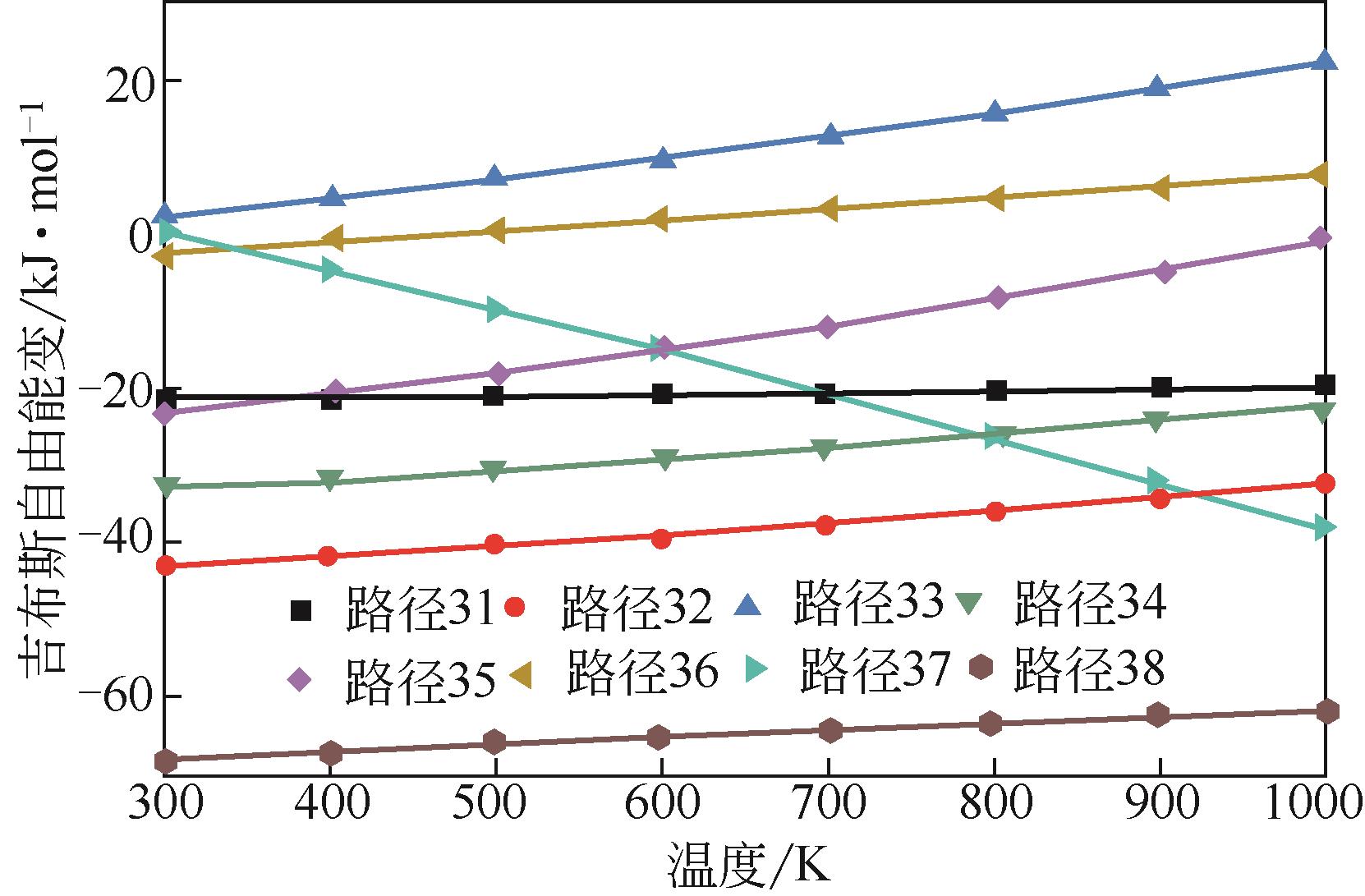
运用分子动力学的方法,对轮胎橡胶的热解过程进行了模拟,并结合模拟结果和密度泛函数对其气相产物的反应路径进行推测计算。模拟结果表明,热解过程主要分为两个阶段,低温热解阶段发生的主要反应是橡胶长链断裂形成单体,主要产物为异戊二烯、苯乙烯和1,3-丁二烯;高温热解阶段发生的主要反应是单体进一步生成小分子气体,产物中CH4、H2、C2H4占大部分,还有少量C2H6、C3H6。其中CH3·攻击异戊二烯和苯乙烯单体夺取特定位置的H·是生成CH4的最优路径,H·攻击苯乙烯单体夺取特定位置的H·是生成H2的最优路径,CH2CH·攻击1,3-丁二烯单体夺取特定位置的H·是生成CH2CH2的最优路径。本文还将热解产物分别跟天然橡胶单独热解和天然橡胶与丁苯橡胶共热解的热解产物做对比,为废旧轮胎橡胶热解得到特定的气相产物和催化热解提供理论依据。
中图分类号:
引用本文
于双鹏, 杨启容, 陶礼, 刘亭, 杜威, 姚尔人. 基于分子动力学模拟的轮胎橡胶气相热解产物反应机理[J]. 化工进展, 2021, 40(6): 3119-3131.
YU Shuangpeng, YANG Qirong, TAO Li, LIU Ting, DU Wei, YAO Erren. Gas phase pyrolysis products of tire rubber based on molecular dynamics simulation[J]. Chemical Industry and Engineering Progress, 2021, 40(6): 3119-3131.
| 成分 | 客车轮胎 | 卡车轮胎 | 说明 |
|---|---|---|---|
| 橡胶 | 47 | 45 | 使用了许多不同的天然橡胶和合成橡胶,例如,天然橡胶(聚异戊二烯)、丁苯橡胶、丁腈橡胶、氯丁橡胶、聚丁二烯橡胶 |
| 炭黑 | 21.5 | 22 | 用于增强橡胶的耐磨性 |
| 金属 | 16.5 | 21.5 | 用于增加轮胎强度 |
| 纺织材料 | 5.5 | — | 用于加固 |
| 氧化锌 | 1 | 2 | 与硬脂酸一起用于控制硫化过程并增强橡胶的物理性能 |
| 硫黄 | 1 | 1 | 用于交联橡胶内的聚合物链,增加硬度,防止在高温下过度变形 |
| 添加剂 | 7.5 | 5 | 用于部分替代炭黑的黏土或二氧化硅 |
表1 客车和卡车轮胎的典型组成及其质量分数 (%)
| 成分 | 客车轮胎 | 卡车轮胎 | 说明 |
|---|---|---|---|
| 橡胶 | 47 | 45 | 使用了许多不同的天然橡胶和合成橡胶,例如,天然橡胶(聚异戊二烯)、丁苯橡胶、丁腈橡胶、氯丁橡胶、聚丁二烯橡胶 |
| 炭黑 | 21.5 | 22 | 用于增强橡胶的耐磨性 |
| 金属 | 16.5 | 21.5 | 用于增加轮胎强度 |
| 纺织材料 | 5.5 | — | 用于加固 |
| 氧化锌 | 1 | 2 | 与硬脂酸一起用于控制硫化过程并增强橡胶的物理性能 |
| 硫黄 | 1 | 1 | 用于交联橡胶内的聚合物链,增加硬度,防止在高温下过度变形 |
| 添加剂 | 7.5 | 5 | 用于部分替代炭黑的黏土或二氧化硅 |
| 橡胶类型 | 主要成分 |
|---|---|
| 天然橡胶 | 顺-1,4-聚异戊二烯[(C5H8)n] |
| 丁苯橡胶 | 1,3-丁二烯[(CH2 苯乙烯[(C6H5—CH |
| 顺丁橡胶 | 顺-1,4-聚丁二烯[(C4H6)n] |
表2 各橡胶的主要成分
| 橡胶类型 | 主要成分 |
|---|---|
| 天然橡胶 | 顺-1,4-聚异戊二烯[(C5H8)n] |
| 丁苯橡胶 | 1,3-丁二烯[(CH2 苯乙烯[(C6H5—CH |
| 顺丁橡胶 | 顺-1,4-聚丁二烯[(C4H6)n] |
| 键序号 | 键长/nm | 键序号 | 键长/nm |
|---|---|---|---|
| ① | 1.140 | ⑤ | 1.439 |
| ② | 1.439 | ⑥ | 1.139 |
| ③ | 1.141 | ⑦ | 1.521 |
| ④ | 1.539 | ⑧ | 1.141 |
表3 天然橡胶单体的键长
| 键序号 | 键长/nm | 键序号 | 键长/nm |
|---|---|---|---|
| ① | 1.140 | ⑤ | 1.439 |
| ② | 1.439 | ⑥ | 1.139 |
| ③ | 1.141 | ⑦ | 1.521 |
| ④ | 1.539 | ⑧ | 1.141 |
| 键序号 | 键长/nm | 键序号 | 键长/nm |
|---|---|---|---|
| ⑨ | 1.072 | ? | 1.029 |
| ⑩ | 1.547 | ? | 1.540 |
| ? | 1.047 |  | 1.14 |
| ? | 1.621 |  | 1.539 |
| ? | 1.078 |  | 1.141 |
| ? | 1.517 |  | 1.14 |
| ? | 1.106 |  | 1.14 |
| ? | 1.371 |  | 1.54 |
| ? | 1.523 |  | 1.14 |
| ? | 1.424 |  | 1.54 |
表4 丁苯橡胶单体的键长
| 键序号 | 键长/nm | 键序号 | 键长/nm |
|---|---|---|---|
| ⑨ | 1.072 | ? | 1.029 |
| ⑩ | 1.547 | ? | 1.540 |
| ? | 1.047 |  | 1.14 |
| ? | 1.621 |  | 1.539 |
| ? | 1.078 |  | 1.141 |
| ? | 1.517 |  | 1.14 |
| ? | 1.106 |  | 1.14 |
| ? | 1.371 |  | 1.54 |
| ? | 1.523 |  | 1.14 |
| ? | 1.424 |  | 1.54 |
| 键序号 | 键长/nm | 键序号 | 键长/nm |
|---|---|---|---|
| ① | 1.094 | ? | 1.047 |
| ② | 1.347 | ? | 1.620 |
| ③ | 1.100 | ? | 1.078 |
| ④ | 1.473 | ? | 1.516 |
| ⑤ | 1.347 | ? | 1.106 |
| ⑥ | 1.094 | ? | 1.371 |
| ⑦ | 1.507 | ? | 1.521 |
| ⑧ | 1.104 | ? | 1.424 |
| ⑨ | 1.074 | ? | 1.029 |
| ⑩ | 1.547 |
表5 文献中丁苯橡胶和天然橡胶单体的键长[20-21]
| 键序号 | 键长/nm | 键序号 | 键长/nm |
|---|---|---|---|
| ① | 1.094 | ? | 1.047 |
| ② | 1.347 | ? | 1.620 |
| ③ | 1.100 | ? | 1.078 |
| ④ | 1.473 | ? | 1.516 |
| ⑤ | 1.347 | ? | 1.106 |
| ⑥ | 1.094 | ? | 1.371 |
| ⑦ | 1.507 | ? | 1.521 |
| ⑧ | 1.104 | ? | 1.424 |
| ⑨ | 1.074 | ? | 1.029 |
| ⑩ | 1.547 |
| 1 | THOMAS B S, GUPTA R C. A comprehensive review on the applications of waste tire rubber in cement concrete[J]. Renewable & Sustainable Energy Reviews, 2016, 54: 1323-1333. |
| 2 | SIENKIEWICZ M, KUCINSKA-LIPKA J, JANIK H, et al. Progress in used tyres management in the European Union: a review[J]. Waste Management, 2012, 32(10): 1742-1751. |
| 3 | MARTÍNEZ J D, PUY N, MURILLO R, et al. Waste tyre pyrolysis—A review[J]. Renewable and Sustainable Energy Reviews, 2013, 23: 179-213 |
| 4 | KANDASAMY J, GÖKALP I. Pyrolysis, combustion, and steam gasification of various types of scrap tires for energy recovery[J]. Energy & Fuels, 2015, 29(1): 346-354. |
| 5 | WILLIAMS P T W. Pyrolysis of waste tyres: a review[J]. Waste Management, 2013, 33(8): 1714-1728. |
| 6 | PRATHIBA R, SHRUTHI M, MIRANDA L R. Pyrolysis of polystyrene waste in the presence of activated carbon in conventional and microwave heating using modified thermocouple[J]. Waste Management, 2018, 76(6): 528-536. |
| 7 | KARATAS H, OLGUN H,AKGUN F,et al. Experimental results of gasification of waste tire with air in a bubbling fluidized bed gasifier[J]. Fuel Guildford, 2013, 105(3): 566-571. |
| 8 | VINCENT T, ANTHONY D G,TAHEREH H. Scrap tyre pyrolysis: modified chemical percolation devolatilization (M-CPD) to describe the influence of pyrolysis conditions on product yields[J]. Waste management, 2018: 76, 516-527. |
| 9 | MASTRAL A M, CALLÉN M S, GARCÍA T. Polycyclic aromatic hydrocarbons and organic matter associated to particulate matter emitted from atmospheric fluidized bed coal combustion[J]. Environmental Science & Technology, 1999, 33(18): 3177-3184. |
| 10 | AYLÓN E, FERNÁNDEZ-COLINO A, MURILLO R, et al. Valorisation of waste tyre by pyrolysis in a moving bed reactor[J]. Waste Management, 2010, 30(7): 1220-1224. |
| 11 | MALKOW T. Novel and innovative pyrolysis and gasification technologies for energy efficient and environmentally sound MSW disposal[J]. Waste Management, 2004, 24(1): 53-79. |
| 12 | MUI E L K, CHEUNG W H, MCKAY G. Tyre char preparation from waste tyre rubber for dye removal from effluents[J]. Journal of Hazardous Materials, 2010, 175(1/2/3): 151-158. |
| 13 | HIJAZI A, AL-MUHTASEB A H, AOUAD S, al et, Pyrolysis of waste rubber tires with palladium doped zeolite[J]. Journal of Environmental Chemical Engineering, 2019, 7(6): 103451. |
| 14 | GAUTHIER-MARADEI P, TAVERA RUIZ C P, CAPRON M. Oil and aromatic yield maximization during pyrolysis of scrap tire rubber[J]. Waste & Biomass Valorization, 2019, 10(12): 3723-3733. |
| 15 | SINGH R K, MONDAL S, RUJ B, et al. Interaction of three categories of tyre waste during co-pyrolysis: effect on product yield and quality[J]. Journal of Analytical and Applied Pyrolysis, 2019, 141: 104618 |
| 16 | PU Y, LIU C, LI Q B, et al. Pyrolysis mechanism of HFO-1234yf with R32 by ReaxFF MD and DFT method[J]. International Journal of Refrigeration, 2020, 109: 82-91. |
| 17 | ZARAS A M, DAGAUT P, SERINYEL Z. Computational kinetic study for the unimolecular decomposition path ways of cyclohexanone[J]. The Journal of Physical Chemistry A, 2015, 119(28): 7138-7144. |
| 18 | 霍二光, 刘朝, 李期斌, 等. 基于ReaxFF模拟的正戊烷热分解机理研究[J]. 工程热物理学报, 2020, 41(1): 61-67. |
| HUO Erguang, LIU Chao, LI Qibin, et al. Thermal decomposition mechanism of n-pentane by ReaxFF simulations[J]. Journal of Engineering Thermophysics, 2020, 41(1): 61-67 | |
| 19 | 于清溪. 轮胎工业用橡胶材料现状与发展(三)[J]. 橡胶科技市场, 2008, 6(11): 1-5. |
| YU Qingxi. Present situation and development of rubber materials for tire industry(3)[J]. Rubber Science and Technology Market, 2008, 6(11): 1-5. | |
| 20 | 杨启容, 邹瀚森, 魏鑫, 等. 天然橡胶热解产物反应机理研究[J]. 西安交通大学学报, 2019, 53(1): 114-121. |
| YANG Qirong, ZOU Hansen, WEI Xin, et al. Study on the reaction Mechanism of natural rubber primary pyrolysis products[J]. Journal of Xi’an Jiaotong University, 2019, 53(1): 114-121. | |
| 21 | 钟浩文, 宫薛菲, 杨启容, 等. 丁苯橡胶气相热解产物反应机理的动力学计算与模型[J]. 热科学与技术, 2020, 19(2): 159-169. |
| ZHONG Haowen, GONG Xuefei, YANG Qirong, et al. Kinetic calculation and model about reaction mechanism of styrene-butadiene rubber pyrolysis gaseous products[J]. Journal of Thermal Science and Technology, 2020, 19(2): 159-169. | |
| 22 | 江德正, 刘朝, 魏顺安, 等. 纤维素热解过程的分子动力学模拟[J]. 工程热物理学报, 2009, 30(12): 1986-1990. |
| JIANG Dezheng,LIU Chao,WEI Shun’an, et al. Simulation of molecular dynamics incellulose pyrolysis[J]. Journal of Engineering Thermophysics, 2009, 30(12): 1986-1990 | |
| 23 | VAN D A, DASGUPTA S, LORANT F, et al. ReaxFF: a reactive force field for hydrocarbons[J]. The Journal of Physical Chemistry A, 2001, 105(41): 9396-9409. |
| 24 | LIU Y L, DING J X, HAN K L. Molecular dynamics simulation of the high-temperature pyrolysis of methylcyclohexane[J]. Fuel, 2018, 217: 185-192. |
| 25 | HU S, SUN W, FU J, et al. Initiation mechanisms and kinetic analysis of the isothermal decomposition of poly(α-methylstyrene): a ReaxFF molecular dynamics study[J]. RSC Advances, 2018, 8(7): 3423-3432. |
| 26 | 李震宇, 贺伟, 杨金龙. 密度泛函理论及其数值方法新进展[J]. 化学进展, 2005, 17(2): 192-202. |
| LI Zhenyu, HE Wei, YANG Jinlong. Recent progress in density functional theory and its numerical methods[J]. Progress in Chemistry, 2005, 17(2): 192-202. | |
| 27 | 傅献彩, 沈文霞, 姚天扬. 物理化学[M]. 4版. 北京: 高等教育出版社, 1990: 798-812. |
| FU Xiancai, SHEN Wenxia, YAO Tianyang. Physical chemistry[M]. 4th ed. Beijing: Higher Education Press, 1990: 798-812. | |
| 28 | 郝玉兰, 张红梅, 李金莲, 等. 1-丁烯热裂解自由基反应模型的建立及验证[J]. 化工科技, 2018, 26(1): 36-40, 56. |
| HAO Yulan, ZHANG Hongmei, LI Jinlian, et al.Establishment and verification of steam cracking radical reaction model of 1-butene[J]. Science & Technology in Chemical Industry, 2018, 26(1): 36-40, 56. | |
| 29 | CZAJCZYŃSKA D, KRZYŻYŃSKA R, JOUHARA H, et al. Use of pyrolytic gas from waste tire as a fuel: a review[J]. Energy, 2017, 134: 1121-1131. |
| 30 | WEI X, ZHONG H W, YANG Q R, et al. Studying the mechanisms of natural rubber pyrolysis gas generation using RMD simulations and TG-FTIR experiments[J]. Energy Conversion & Management, 2019, 189: 43-152. |
| 31 | YANG Q R, YU S P, ZHONG H W, et al. Gas products generation mechanism during co-pyrolysis of styrene-butadiene rubber and natural rubber[J]. Journal of Hazardous Materials, 2020, 401: 123302. |
| [1] | 王家庆, 宋广伟, 李强, 郭帅成, DAI Qingli. 橡胶混凝土界面改性方法及性能提升路径[J]. 化工进展, 2023, 42(S1): 328-343. |
| [2] | 邵博识, 谭宏博. 锯齿波纹板对挥发性有机物低温脱除过程强化模拟分析[J]. 化工进展, 2023, 42(S1): 84-93. |
| [3] | 邵志国, 任雯, 许世佩, 聂凡, 许毓, 刘龙杰, 谢水祥, 李兴春, 王庆吉, 谢加才. 终温对油基钻屑热解产物分布和特性影响[J]. 化工进展, 2023, 42(9): 4929-4938. |
| [4] | 李志远, 黄亚继, 赵佳琪, 于梦竹, 朱志成, 程好强, 时浩, 王圣. 污泥与聚氯乙烯共热解重金属特性[J]. 化工进展, 2023, 42(9): 4947-4956. |
| [5] | 李海东, 杨远坤, 郭姝姝, 汪本金, 岳婷婷, 傅开彬, 王哲, 何守琴, 姚俊, 谌书. 炭化与焙烧温度对植物基铁碳微电解材料去除As(Ⅲ)性能的影响[J]. 化工进展, 2023, 42(7): 3652-3663. |
| [6] | 姚丽铭, 王亚琢, 范洪刚, 顾菁, 袁浩然, 陈勇. 餐厨垃圾处理现状及其热解技术研究进展[J]. 化工进展, 2023, 42(7): 3791-3801. |
| [7] | 张杉, 仲兆平, 杨宇轩, 杜浩然, 李骞. 磷酸盐改性高岭土对生活垃圾热解过程中重金属的富集[J]. 化工进展, 2023, 42(7): 3893-3903. |
| [8] | 李若琳, 何少林, 苑宏英, 刘伯约, 纪冬丽, 宋阳, 刘博, 余绩庆, 徐英俊. 原位热解对油页岩物性及地下水水质影响探索[J]. 化工进展, 2023, 42(6): 3309-3318. |
| [9] | 李栋先, 王佳, 蒋剑春. 皂脚热解-催化气态加氢制备生物燃料[J]. 化工进展, 2023, 42(6): 2874-2883. |
| [10] | 王志伟, 郭帅华, 吴梦鸽, 陈颜, 赵俊廷, 李辉, 雷廷宙. 生物质与塑料催化共热解技术研究进展[J]. 化工进展, 2023, 42(5): 2655-2665. |
| [11] | 梁贻景, 马岩, 卢展烽, 秦福生, 万骏杰, 王志远. La1-x Sr x MnO3钙钛矿涂层的抗结焦性能[J]. 化工进展, 2023, 42(4): 1769-1778. |
| [12] | 刘静, 林琳, 张健, 赵峰. 生物质基炭材料孔径调控及电化学性能研究进展[J]. 化工进展, 2023, 42(4): 1907-1916. |
| [13] | 杨自强, 李风海, 郭卫杰, 马名杰, 赵薇. 市政污泥热处理过程中磷迁移转化的研究进展[J]. 化工进展, 2023, 42(4): 2081-2090. |
| [14] | 赵佳琪, 黄亚继, 李志远, 丁雪宇, 祁帅杰, 张煜尧, 刘俊, 高嘉炜. 污泥和聚氯乙烯共热解三相产物特性[J]. 化工进展, 2023, 42(4): 2122-2129. |
| [15] | 潘宇涵, 徐俊, 赵光杰, 林诚乾, 金亮, 薛志亮, 周永刚, 黄群星. 废轮胎梯级热解中试装置开发与产物特性分析[J]. 化工进展, 2023, 42(3): 1240-1247. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||